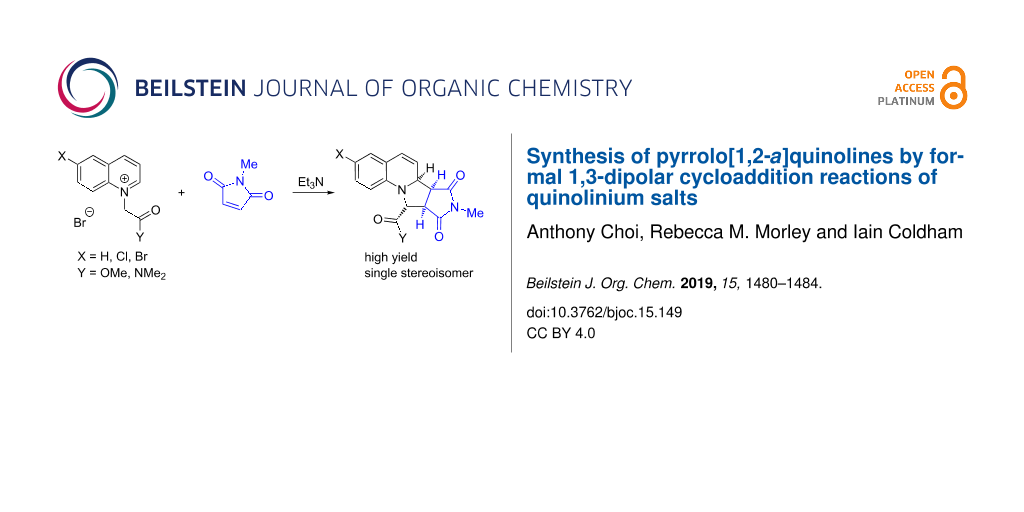
Abstract
Quinolinium salts, Q+-CH2-CO2Me Br− and Q+-CH2-CONMe2 Br− (where Q = quinoline), were prepared from quinolines. Deprotonation of these salts with triethylamine promoted the reaction of the resulting quinolinium ylides (formally azomethine ylides) with electron-poor alkenes by conjugate addition followed by cyclization or by [3 + 2] dipolar cycloaddition. The pyrroloquinoline products were formed as single regio- and stereoisomers. These could be converted to other derivatives by Suzuki–Miyaura coupling, reduction or oxidation reactions.
Graphical Abstract
Introduction
Cycloaddition reactions of azomethine ylides are an important class of pericyclic reactions that give rise to pyrrolidine rings, prevalent in a large number of natural products and bioactive compounds. Many methods have been used to prepare azomethine ylides that undergo cycloaddition with π-systems, especially electron-poor alkenes to give pyrrolidine products [1-4]. Azomethine ylides can be classed either as stabilised or non-stabilised, depending on the presence or absence of an electron-withdrawing substituent such as a carbonyl group. The most common method for their preparation is by condensation of a secondary amine with an aldehyde to give an iminium ion that loses a proton to give the ylide, or by condensation of a primary amine with an aldehyde to give an imine followed by prototropy or deprotonation to give N-metalated azomethine ylides (see, for example, [5-18]). An alternative method is to prepare a salt of a heterocycle, typically by N-alkylation of a pyridine [19-27], isoquinoline [26-32], or related structures [33-35], followed by deprotonation. Such ylides are formally azomethine structures assuming reactivity of the aromatic ring as an iminium ion, although the reaction with electron-poor alkenes occurs through a stepwise conjugate addition–cyclization process [23]. We were interested in the related quinolinium ylides that, on (formal) cycloaddition would provide pyrrolo[1,2-a]quinolines as products. These are tricyclic compounds consisting of a pyrrole ring fused with a quinoline. Pyrroloquinolines have been found to show antibacterial and antifungal activity, to be ligands for the NK1 receptor, and to be effective against the Hif hypoxia pathway in cancer cell lines [36-38]. Almost all of the examples of dipolar cycloaddition reactions involving quinolinium salts that have been reported in the literature involve ketones as electron-withdrawing groups to stabilise the intermediate ylide [39-49]; for example, the ketone 1 is known to undergo reaction with alkenes 2 (Z = electron-withdrawing group) to give the tricyclic products 3 (Scheme 1) [41,49]. Similar examples with phenanthridinium and related ylides make use of ketones to stabilise the ylide [50-54]. The only exception (as far as we are aware) to the use of quinolinium ylides with ketones as stabilising groups are isolated reports with a carboxylic acid derivative, particular an ethyl ester group [55-59]. Here we describe a wider scope that extends the examples to alternative carbonyl derivatives and alternative alkenes, hence providing novel pyrroloquinoline compounds.
Scheme 1: Reaction of ketone 1 with electron-deficient alkenes 2.
Scheme 1: Reaction of ketone 1 with electron-deficient alkenes 2.
Results and Discussion
To test the feasibility of the reaction of quinolinium salts bearing electron-withdrawing groups other than ketones, we prepared ester 4 [55] and amide 5 by alkylation of quinoline. Arylidenemalononitriles such as 6a are known to undergo related chemistry [41], so we heated this compound with the quinolinium salts in the presence of triethylamine and were pleased to obtain good yields of the adducts 7a–c and 8a,b (Scheme 2).
Scheme 2: Reactions of ester 4 and amide 5 with electron-deficient alkenes 6.
Scheme 2: Reactions of ester 4 and amide 5 with electron-deficient alkenes 6.
In each case, the products 7a–c and 8a,b were formed as a single regioisomer and stereoisomer. The selectivity in favour of the isomer drawn in Scheme 2 was verified by single crystal X-ray analysis of the adduct 7c (Figure 1). The other isomers had similar coupling constants between adjacent protons and were assumed to have the same relative configuration.
![[1860-5397-15-149-1]](/bjoc/content/figures/1860-5397-15-149-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Single crystal X-ray structure for 7c.
Figure 1: Single crystal X-ray structure for 7c.
A dipolarophile that has not been reported for reaction with quinolinium salts is N-methylmaleimide. We therefore tested the ability of this unsaturated compound to undergo reaction with the ylides derived from the salts 4 and 5. In both cases, very good yields of the tetracyclic adducts 9 and 10 were obtained after heating for only 1 h (Scheme 3). The relative stereochemistry of the adduct 9 was determined by single crystal X-ray analysis (Figure 2).
Scheme 3: Reactions of ester 4 and amide 5 with N-methylmaleimide.
Scheme 3: Reactions of ester 4 and amide 5 with N-methylmaleimide.
![[1860-5397-15-149-2]](/bjoc/content/figures/1860-5397-15-149-2.png?scale=1.7&max-width=1024&background=FFFFFF)
Figure 2: Single crystal X-ray structure for 9.
Figure 2: Single crystal X-ray structure for 9.
To explore the diversity of products that could be obtained from these adducts, we carried out a reduction of the alkene in compounds 9 and 10 by using hydrogen and palladium on charcoal (Scheme 4). This provides the tetrahydroquinoline adducts 11 and 12. Additionally, and in contrast, oxidation of the adduct 10 was performed using the oxidant 2,3-dichloro-5,6-dicyanoquinone (DDQ) to give the fully unsaturated product 13.
Scheme 4: Reduction and oxidation of adducts 9 and 10.
Scheme 4: Reduction and oxidation of adducts 9 and 10.
To expand the range of products and explore the scope of the reaction further, we prepared the salts 14a and 14b (from 6-chloroquinoline and 6-bromoquinoline) and these were heated with N-methylmaleimide in the presence of triethylamine in methanol to give the desired adducts 15a and 15b as single stereoisomers (Scheme 5). The stereochemistry of product 15a was confirmed by single crystal X-ray analysis (see Supporting Information File 1) and matches the relative configuration of the adducts 9 and 10. The bromide 15b was coupled with phenylboronic acid using palladium catalysis to give derivative 16 (the chloride 15a was inert under these conditions). The ability to prepare halogenated derivatives and to undergo palladium coupling demonstrates further versatility of these types of products.
Scheme 5: Formation of amides 15a and 15b and Suzuki–Miyaura coupling to yield 16.
Scheme 5: Formation of amides 15a and 15b and Suzuki–Miyaura coupling to yield 16.
Conclusion
In conclusion, we have found that carboxylic ester and amide-stabilised anions derived from quinolinium salts react with arylidenemalononitriles and N-methylmaleimide to give adducts in good yields and with very high stereoselectivity (single isomer products were isolated). The adducts could be reduced, oxidised, or could undergo Suzuki–Miyaura coupling to give different substituted dihydro- and tetrahydroquinoline derivatives.
Supporting Information
| Supporting Information File 1: Experimental procedures, spectroscopic and X-ray data (CCDC 1907018–1907020 for compounds 7c, 9 and 15a) and copies of NMR spectra. | ||
| Format: PDF | Size: 1.9 MB | Download |
References
-
Li, J.; Ye, Y.; Zhang, Y. Org. Chem. Front. 2018, 5, 864–892. doi:10.1039/c7qo01077j
Return to citation in text: [1] -
Meyer, A. G.; Ryan, J. H. Molecules 2016, 21, 935. doi:10.3390/molecules21080935
Return to citation in text: [1] -
Moyano, A.; Rios, R. Chem. Rev. 2011, 111, 4703–4832. doi:10.1021/cr100348t
Return to citation in text: [1] -
Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765–2810. doi:10.1021/cr040004c
Return to citation in text: [1] -
Xiong, Y.; Du, Z.; Chen, H.; Yang, Z.; Tan, Q.; Zhang, C.; Zhu, L.; Lan, Y.; Zhang, M. J. Am. Chem. Soc. 2019, 141, 961–971. doi:10.1021/jacs.8b10939
Return to citation in text: [1] -
Filatov, A. S.; Knyazev, N. A.; Shmakov, S. V.; Bogdanov, A. A.; Ryazantsev, M. N.; Shtyrov, A. A.; Starova, G. L.; Molchanov, A. P.; Larina, A. G.; Boitsov, V. M.; Stepakov, A. V. Synthesis 2019, 51, 713–729. doi:10.1055/s-0037-1611059
Return to citation in text: [1] -
Jia, Z.-J.; Shan, G.; Daniliuc, C. G.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2018, 57, 14493–14497. doi:10.1002/anie.201712882
Return to citation in text: [1] -
Feng, B.; Lu, L.-Q.; Chen, J.-R.; Feng, G.; He, B.-Q.; Lu, B.; Xiao, W.-J. Angew. Chem., Int. Ed. 2018, 57, 5888–5892. doi:10.1002/anie.201802492
Return to citation in text: [1] -
Xu, S.; Zhang, Z.-M.; Xu, B.; Liu, B.; Liu, Y.; Zhang, J. J. Am. Chem. Soc. 2018, 140, 2272–2283. doi:10.1021/jacs.7b12137
Return to citation in text: [1] -
Williams, B. M.; Trauner, D. J. Org. Chem. 2018, 83, 3061–3068. doi:10.1021/acs.joc.8b00192
Return to citation in text: [1] -
Zhang, Q.; Zhang, Z.; Huang, Z.; Zhang, C.; Xi, S.; Zhang, M. Angew. Chem., Int. Ed. 2018, 57, 937–941. doi:10.1002/anie.201711414
Return to citation in text: [1] -
Otero-Fraga, J.; Suárez-Pantiga, S.; Montesinos-Magraner, M.; Rhein, D.; Mendoza, A. Angew. Chem., Int. Ed. 2017, 56, 12962–12966. doi:10.1002/anie.201706682
Return to citation in text: [1] -
Erguven, H.; Leitch, D. C.; Keyzer, E. N.; Arndtsen, B. A. Angew. Chem., Int. Ed. 2017, 56, 6078–6082. doi:10.1002/anie.201609726
Return to citation in text: [1] -
Hauduc, C.; Bélanger, G. J. Org. Chem. 2017, 82, 4703–4712. doi:10.1021/acs.joc.7b00345
Return to citation in text: [1] -
Boissarie, P.; Bélanger, G. Org. Lett. 2017, 19, 3739–3742. doi:10.1021/acs.orglett.7b01566
Return to citation in text: [1] -
Liu, Y.; Hu, H.; Wang, X.; Zhi, S.; Kan, Y.; Wang, C. J. Org. Chem. 2017, 82, 4194–4202. doi:10.1021/acs.joc.7b00180
Return to citation in text: [1] -
Wang, H.; Regan, C. J.; Codelli, J. A.; Romanato, P.; Puchlopek-Dermenci, A. L. A.; Reisman, S. E. Org. Lett. 2017, 19, 1698–1701. doi:10.1021/acs.orglett.7b00418
Return to citation in text: [1] -
Dong, Z.; Zhu, Y.; Li, B.; Wang, C.; Yan, W.; Wang, K.; Wang, R. J. Org. Chem. 2017, 82, 3482–3490. doi:10.1021/acs.joc.6b02949
Return to citation in text: [1] -
Motornov, V. A.; Tabolin, A. A.; Nelyubina, Y. V.; Nenajdenko, V. G.; Ioffe, S. L. Org. Biomol. Chem. 2019, 17, 1442–1454. doi:10.1039/c8ob03126f
Return to citation in text: [1] -
Zhang, D.; Lin, L.; Yang, J.; Liu, X.; Feng, X. Angew. Chem., Int. Ed. 2018, 57, 12323–12327. doi:10.1002/anie.201806630
Return to citation in text: [1] -
Day, J.; McKeever-Abbas, B.; Dowden, J. Angew. Chem., Int. Ed. 2016, 55, 5809–5813. doi:10.1002/anie.201511047
Return to citation in text: [1] -
Brioche, J.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 2800–2803. doi:10.1021/acs.orglett.5b01205
Return to citation in text: [1] -
Allgäuer, D. S.; Mayr, H. Eur. J. Org. Chem. 2013, 6379–6388. doi:10.1002/ejoc.201300784
Return to citation in text: [1] [2] -
Kucukdisli, M.; Opatz, T. Eur. J. Org. Chem. 2012, 4555–4564. doi:10.1002/ejoc.201200424
Return to citation in text: [1] -
Jacobs, J.; Van Hende, E.; Claessens, S.; De Kimpe, N. Curr. Org. Chem. 2011, 15, 1340–1362. doi:10.2174/138527211795378209
Return to citation in text: [1] -
Tsuge, O.; Kanemasa, S.; Takenaka, S. Bull. Chem. Soc. Jpn. 1985, 58, 3137–3157. doi:10.1246/bcsj.58.3137
Return to citation in text: [1] [2] -
Tsuge, O.; Kanemasa, S.; Takenaka, S. Bull. Chem. Soc. Jpn. 1985, 58, 3320–3336. doi:10.1246/bcsj.58.3320
Return to citation in text: [1] [2] -
An, J.; Yang, Q.-Q.; Wang, Q.; Xiao, W.-J. Tetrahedron Lett. 2013, 54, 3834–3837. doi:10.1016/j.tetlet.2013.05.053
Return to citation in text: [1] -
Fernández, N.; Carrillo, L.; Vicario, J. L.; Badía, D.; Reyes, E. Chem. Commun. 2011, 47, 12313. doi:10.1039/c1cc15671c
Return to citation in text: [1] -
Han, Y.; Hou, H.; Fu, Q.; Yan, C.-G. Tetrahedron 2011, 67, 2313–2322. doi:10.1016/j.tet.2011.01.046
Return to citation in text: [1] -
Dumitrascu, F.; Caira, M. R.; Georgescu, E.; Georgescu, F.; Draghici, C.; Popa, M. M. Heteroat. Chem. 2011, 22, 723–729. doi:10.1002/hc.20740
Return to citation in text: [1] -
Grigg, R.; Heaney, F. J. Chem. Soc., Perkin Trans. 1 1989, 198. doi:10.1039/p19890000198
Return to citation in text: [1] -
Nicolescu, A.; Deleanu, C.; Georgescu, E.; Georgescu, F.; Iurascu, A.-M.; Shova, S.; Filip, P. Tetrahedron Lett. 2013, 54, 1486–1488. doi:10.1016/j.tetlet.2013.01.036
Return to citation in text: [1] -
Caira, M. R.; Dumitrascu, F.; Georgescu, E.; Georgescu, F.; Popa, M. M. Rev. Roum. Chim. 2011, 56, 771.
Return to citation in text: [1] -
Jones, R. C. F.; Rafiq, S.; Elsegood, M. R. J.; McKee, V.; Slater, M. J. Chem. – Asian J. 2010, 5, 461–465. doi:10.1002/asia.200900547
Return to citation in text: [1] -
Hazra, A.; Mondal, S.; Maity, A.; Naskar, S.; Saha, P.; Paira, R.; Sahu, K. B.; Paira, P.; Ghosh, S.; Sinha, C.; Samanta, A.; Banerjee, S.; Mondal, N. B. Eur. J. Med. Chem. 2011, 46, 2132–2140. doi:10.1016/j.ejmech.2011.02.066
Return to citation in text: [1] -
Cappelli, A.; Giuliani, G.; Anzini, M.; Riitano, D.; Giorgi, G.; Vomero, S. Bioorg. Med. Chem. 2008, 16, 6850–6859. doi:10.1016/j.bmc.2008.05.067
Return to citation in text: [1] -
Jones, D. T.; Harris, A. L. Mol. Cancer Ther. 2006, 5, 2193–2202. doi:10.1158/1535-7163.mct-05-0443
Return to citation in text: [1] -
Gomha, S. M.; Dawood, K. M. J. Chem. Res. 2014, 38, 515–519. doi:10.3184/174751914x14067338307126
Return to citation in text: [1] -
Liu, J.; Yan, P.; Li, Y.; Zhou, Z.; Ye, W.; Yao, J.; Wang, C. Monatsh. Chem. 2014, 145, 617–625. doi:10.1007/s00706-013-1120-6
Return to citation in text: [1] -
Allgäuer, D. S.; Mayer, P.; Mayr, H. J. Am. Chem. Soc. 2013, 135, 15216–15224. doi:10.1021/ja407885h
Return to citation in text: [1] [2] [3] -
Wu, L.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2012, 10, 9452. doi:10.1039/c2ob26849c
Return to citation in text: [1] -
Liu, Y.; Zhang, Y.; Shen, Y.-M.; Hu, H.-W.; Xu, J.-H. Org. Biomol. Chem. 2010, 8, 2449. doi:10.1039/c000277a
Return to citation in text: [1] -
Georgescu, E.; Dumitrascu, F.; Georgescu, F.; Draghici, C.; Popa, M. M. Rev. Roum. Chim. 2010, 55, 217–221.
Return to citation in text: [1] -
Georgescu, E.; Georgescu, F.; Draghici, C.; Caproiu, M. T.; Dumitrascu, F. Rev. Roum. Chim. 2010, 55, 1027–1031.
Return to citation in text: [1] -
Dawood, K. M.; Ragab, E. A.; Khedr, N. A. J. Chin. Chem. Soc. 2009, 56, 1180–1185. doi:10.1002/jccs.200900170
Return to citation in text: [1] -
Dawood, K. M.; Ragab, E. A.; Mohamed, S. N. Z. Naturforsch., B: J. Chem. Sci. 2009, 64, 434–438. doi:10.1515/znb-2009-0413
Return to citation in text: [1] -
Caira, M. R.; Georgescu, E.; Georgescu, F.; Popa, M. M.; Dumitraşcu, F. ARKIVOC 2009, No. xii, 242–253. doi:10.3998/ark.5550190.0010.c21
Return to citation in text: [1] -
Shestopalov, A. M.; Chunikhin, K. S.; Rodinovskaya, L. A. Chem. Heterocycl. Compd. 2002, 38, 310–313. doi:10.1023/a:1015687320793
Return to citation in text: [1] [2] -
Danac, R.; Al Matarneh, C. M.; Shova, S.; Daniloaia, T.; Balan, M.; Mangalagiu, I. I. Bioorg. Med. Chem. 2015, 23, 2318–2327. doi:10.1016/j.bmc.2015.03.077
Return to citation in text: [1] -
Singh, D.; Sinha, P.; Bansal, R. K. Curr. Green Chem. 2014, 1, 227–231. doi:10.2174/2213346101666140210194354
Return to citation in text: [1] -
Paira, R.; Mondal, S.; Chowdhury, A.; Banerjee, M.; Maity, A.; Hazra, A.; Mondal, N. B. Tetrahedron Lett. 2013, 54, 3046–3050. doi:10.1016/j.tetlet.2013.03.095
Return to citation in text: [1] -
Pospíšil, J.; Trávníček, M.; Potáček, M. ARKIVOC 2001, No. ii, 146–162. doi:10.3998/ark.5550190.0002.217
Return to citation in text: [1] -
Matusiak, G.; Śliwa, W. Monatsh. Chem. 1993, 124, 161–165. doi:10.1007/bf00808675
Return to citation in text: [1] -
Sun, J.; Zhang, Y.; Shen, G.-L.; Yan, C.-G. ChemistrySelect 2017, 2, 10835–10839. doi:10.1002/slct.201702161
Return to citation in text: [1] [2] -
Chen, R.; Zhao, Y.; Sun, H.; Shao, Y.; Xu, Y.; Ma, M.; Ma, L.; Wan, X. J. Org. Chem. 2017, 82, 9291–9304. doi:10.1021/acs.joc.7b01042
Return to citation in text: [1] -
Glushchenko, T. P.; Aksenov, A. V.; Goncharov, V. I. Chem. Heterocycl. Compd. 2009, 45, 351–356. doi:10.1007/s10593-009-0267-x
Return to citation in text: [1] -
Liu, Y.; Hu, H.-Y.; Liu, Q.-J.; Hu, H.-W.; Xu, J.-H. Tetrahedron 2007, 63, 2024–2033. doi:10.1016/j.tet.2006.12.050
Return to citation in text: [1] -
Serov, A. B.; Kartsev, V. G.; Aleksandrov, Y. A.; Dolgushin, F. M. Russ. Chem. Bull. 2005, 54, 2432–2436. doi:10.1007/s11172-006-0133-2
Return to citation in text: [1]
| 1. | Li, J.; Ye, Y.; Zhang, Y. Org. Chem. Front. 2018, 5, 864–892. doi:10.1039/c7qo01077j |
| 2. | Meyer, A. G.; Ryan, J. H. Molecules 2016, 21, 935. doi:10.3390/molecules21080935 |
| 3. | Moyano, A.; Rios, R. Chem. Rev. 2011, 111, 4703–4832. doi:10.1021/cr100348t |
| 4. | Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765–2810. doi:10.1021/cr040004c |
| 33. | Nicolescu, A.; Deleanu, C.; Georgescu, E.; Georgescu, F.; Iurascu, A.-M.; Shova, S.; Filip, P. Tetrahedron Lett. 2013, 54, 1486–1488. doi:10.1016/j.tetlet.2013.01.036 |
| 34. | Caira, M. R.; Dumitrascu, F.; Georgescu, E.; Georgescu, F.; Popa, M. M. Rev. Roum. Chim. 2011, 56, 771. |
| 35. | Jones, R. C. F.; Rafiq, S.; Elsegood, M. R. J.; McKee, V.; Slater, M. J. Chem. – Asian J. 2010, 5, 461–465. doi:10.1002/asia.200900547 |
| 26. | Tsuge, O.; Kanemasa, S.; Takenaka, S. Bull. Chem. Soc. Jpn. 1985, 58, 3137–3157. doi:10.1246/bcsj.58.3137 |
| 27. | Tsuge, O.; Kanemasa, S.; Takenaka, S. Bull. Chem. Soc. Jpn. 1985, 58, 3320–3336. doi:10.1246/bcsj.58.3320 |
| 28. | An, J.; Yang, Q.-Q.; Wang, Q.; Xiao, W.-J. Tetrahedron Lett. 2013, 54, 3834–3837. doi:10.1016/j.tetlet.2013.05.053 |
| 29. | Fernández, N.; Carrillo, L.; Vicario, J. L.; Badía, D.; Reyes, E. Chem. Commun. 2011, 47, 12313. doi:10.1039/c1cc15671c |
| 30. | Han, Y.; Hou, H.; Fu, Q.; Yan, C.-G. Tetrahedron 2011, 67, 2313–2322. doi:10.1016/j.tet.2011.01.046 |
| 31. | Dumitrascu, F.; Caira, M. R.; Georgescu, E.; Georgescu, F.; Draghici, C.; Popa, M. M. Heteroat. Chem. 2011, 22, 723–729. doi:10.1002/hc.20740 |
| 32. | Grigg, R.; Heaney, F. J. Chem. Soc., Perkin Trans. 1 1989, 198. doi:10.1039/p19890000198 |
| 19. | Motornov, V. A.; Tabolin, A. A.; Nelyubina, Y. V.; Nenajdenko, V. G.; Ioffe, S. L. Org. Biomol. Chem. 2019, 17, 1442–1454. doi:10.1039/c8ob03126f |
| 20. | Zhang, D.; Lin, L.; Yang, J.; Liu, X.; Feng, X. Angew. Chem., Int. Ed. 2018, 57, 12323–12327. doi:10.1002/anie.201806630 |
| 21. | Day, J.; McKeever-Abbas, B.; Dowden, J. Angew. Chem., Int. Ed. 2016, 55, 5809–5813. doi:10.1002/anie.201511047 |
| 22. | Brioche, J.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 2800–2803. doi:10.1021/acs.orglett.5b01205 |
| 23. | Allgäuer, D. S.; Mayr, H. Eur. J. Org. Chem. 2013, 6379–6388. doi:10.1002/ejoc.201300784 |
| 24. | Kucukdisli, M.; Opatz, T. Eur. J. Org. Chem. 2012, 4555–4564. doi:10.1002/ejoc.201200424 |
| 25. | Jacobs, J.; Van Hende, E.; Claessens, S.; De Kimpe, N. Curr. Org. Chem. 2011, 15, 1340–1362. doi:10.2174/138527211795378209 |
| 26. | Tsuge, O.; Kanemasa, S.; Takenaka, S. Bull. Chem. Soc. Jpn. 1985, 58, 3137–3157. doi:10.1246/bcsj.58.3137 |
| 27. | Tsuge, O.; Kanemasa, S.; Takenaka, S. Bull. Chem. Soc. Jpn. 1985, 58, 3320–3336. doi:10.1246/bcsj.58.3320 |
| 41. | Allgäuer, D. S.; Mayer, P.; Mayr, H. J. Am. Chem. Soc. 2013, 135, 15216–15224. doi:10.1021/ja407885h |
| 5. | Xiong, Y.; Du, Z.; Chen, H.; Yang, Z.; Tan, Q.; Zhang, C.; Zhu, L.; Lan, Y.; Zhang, M. J. Am. Chem. Soc. 2019, 141, 961–971. doi:10.1021/jacs.8b10939 |
| 6. | Filatov, A. S.; Knyazev, N. A.; Shmakov, S. V.; Bogdanov, A. A.; Ryazantsev, M. N.; Shtyrov, A. A.; Starova, G. L.; Molchanov, A. P.; Larina, A. G.; Boitsov, V. M.; Stepakov, A. V. Synthesis 2019, 51, 713–729. doi:10.1055/s-0037-1611059 |
| 7. | Jia, Z.-J.; Shan, G.; Daniliuc, C. G.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2018, 57, 14493–14497. doi:10.1002/anie.201712882 |
| 8. | Feng, B.; Lu, L.-Q.; Chen, J.-R.; Feng, G.; He, B.-Q.; Lu, B.; Xiao, W.-J. Angew. Chem., Int. Ed. 2018, 57, 5888–5892. doi:10.1002/anie.201802492 |
| 9. | Xu, S.; Zhang, Z.-M.; Xu, B.; Liu, B.; Liu, Y.; Zhang, J. J. Am. Chem. Soc. 2018, 140, 2272–2283. doi:10.1021/jacs.7b12137 |
| 10. | Williams, B. M.; Trauner, D. J. Org. Chem. 2018, 83, 3061–3068. doi:10.1021/acs.joc.8b00192 |
| 11. | Zhang, Q.; Zhang, Z.; Huang, Z.; Zhang, C.; Xi, S.; Zhang, M. Angew. Chem., Int. Ed. 2018, 57, 937–941. doi:10.1002/anie.201711414 |
| 12. | Otero-Fraga, J.; Suárez-Pantiga, S.; Montesinos-Magraner, M.; Rhein, D.; Mendoza, A. Angew. Chem., Int. Ed. 2017, 56, 12962–12966. doi:10.1002/anie.201706682 |
| 13. | Erguven, H.; Leitch, D. C.; Keyzer, E. N.; Arndtsen, B. A. Angew. Chem., Int. Ed. 2017, 56, 6078–6082. doi:10.1002/anie.201609726 |
| 14. | Hauduc, C.; Bélanger, G. J. Org. Chem. 2017, 82, 4703–4712. doi:10.1021/acs.joc.7b00345 |
| 15. | Boissarie, P.; Bélanger, G. Org. Lett. 2017, 19, 3739–3742. doi:10.1021/acs.orglett.7b01566 |
| 16. | Liu, Y.; Hu, H.; Wang, X.; Zhi, S.; Kan, Y.; Wang, C. J. Org. Chem. 2017, 82, 4194–4202. doi:10.1021/acs.joc.7b00180 |
| 17. | Wang, H.; Regan, C. J.; Codelli, J. A.; Romanato, P.; Puchlopek-Dermenci, A. L. A.; Reisman, S. E. Org. Lett. 2017, 19, 1698–1701. doi:10.1021/acs.orglett.7b00418 |
| 18. | Dong, Z.; Zhu, Y.; Li, B.; Wang, C.; Yan, W.; Wang, K.; Wang, R. J. Org. Chem. 2017, 82, 3482–3490. doi:10.1021/acs.joc.6b02949 |
| 41. | Allgäuer, D. S.; Mayer, P.; Mayr, H. J. Am. Chem. Soc. 2013, 135, 15216–15224. doi:10.1021/ja407885h |
| 49. | Shestopalov, A. M.; Chunikhin, K. S.; Rodinovskaya, L. A. Chem. Heterocycl. Compd. 2002, 38, 310–313. doi:10.1023/a:1015687320793 |
| 55. | Sun, J.; Zhang, Y.; Shen, G.-L.; Yan, C.-G. ChemistrySelect 2017, 2, 10835–10839. doi:10.1002/slct.201702161 |
| 56. | Chen, R.; Zhao, Y.; Sun, H.; Shao, Y.; Xu, Y.; Ma, M.; Ma, L.; Wan, X. J. Org. Chem. 2017, 82, 9291–9304. doi:10.1021/acs.joc.7b01042 |
| 57. | Glushchenko, T. P.; Aksenov, A. V.; Goncharov, V. I. Chem. Heterocycl. Compd. 2009, 45, 351–356. doi:10.1007/s10593-009-0267-x |
| 58. | Liu, Y.; Hu, H.-Y.; Liu, Q.-J.; Hu, H.-W.; Xu, J.-H. Tetrahedron 2007, 63, 2024–2033. doi:10.1016/j.tet.2006.12.050 |
| 59. | Serov, A. B.; Kartsev, V. G.; Aleksandrov, Y. A.; Dolgushin, F. M. Russ. Chem. Bull. 2005, 54, 2432–2436. doi:10.1007/s11172-006-0133-2 |
| 39. | Gomha, S. M.; Dawood, K. M. J. Chem. Res. 2014, 38, 515–519. doi:10.3184/174751914x14067338307126 |
| 40. | Liu, J.; Yan, P.; Li, Y.; Zhou, Z.; Ye, W.; Yao, J.; Wang, C. Monatsh. Chem. 2014, 145, 617–625. doi:10.1007/s00706-013-1120-6 |
| 41. | Allgäuer, D. S.; Mayer, P.; Mayr, H. J. Am. Chem. Soc. 2013, 135, 15216–15224. doi:10.1021/ja407885h |
| 42. | Wu, L.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2012, 10, 9452. doi:10.1039/c2ob26849c |
| 43. | Liu, Y.; Zhang, Y.; Shen, Y.-M.; Hu, H.-W.; Xu, J.-H. Org. Biomol. Chem. 2010, 8, 2449. doi:10.1039/c000277a |
| 44. | Georgescu, E.; Dumitrascu, F.; Georgescu, F.; Draghici, C.; Popa, M. M. Rev. Roum. Chim. 2010, 55, 217–221. |
| 45. | Georgescu, E.; Georgescu, F.; Draghici, C.; Caproiu, M. T.; Dumitrascu, F. Rev. Roum. Chim. 2010, 55, 1027–1031. |
| 46. | Dawood, K. M.; Ragab, E. A.; Khedr, N. A. J. Chin. Chem. Soc. 2009, 56, 1180–1185. doi:10.1002/jccs.200900170 |
| 47. | Dawood, K. M.; Ragab, E. A.; Mohamed, S. N. Z. Naturforsch., B: J. Chem. Sci. 2009, 64, 434–438. doi:10.1515/znb-2009-0413 |
| 48. | Caira, M. R.; Georgescu, E.; Georgescu, F.; Popa, M. M.; Dumitraşcu, F. ARKIVOC 2009, No. xii, 242–253. doi:10.3998/ark.5550190.0010.c21 |
| 49. | Shestopalov, A. M.; Chunikhin, K. S.; Rodinovskaya, L. A. Chem. Heterocycl. Compd. 2002, 38, 310–313. doi:10.1023/a:1015687320793 |
| 55. | Sun, J.; Zhang, Y.; Shen, G.-L.; Yan, C.-G. ChemistrySelect 2017, 2, 10835–10839. doi:10.1002/slct.201702161 |
| 36. | Hazra, A.; Mondal, S.; Maity, A.; Naskar, S.; Saha, P.; Paira, R.; Sahu, K. B.; Paira, P.; Ghosh, S.; Sinha, C.; Samanta, A.; Banerjee, S.; Mondal, N. B. Eur. J. Med. Chem. 2011, 46, 2132–2140. doi:10.1016/j.ejmech.2011.02.066 |
| 37. | Cappelli, A.; Giuliani, G.; Anzini, M.; Riitano, D.; Giorgi, G.; Vomero, S. Bioorg. Med. Chem. 2008, 16, 6850–6859. doi:10.1016/j.bmc.2008.05.067 |
| 38. | Jones, D. T.; Harris, A. L. Mol. Cancer Ther. 2006, 5, 2193–2202. doi:10.1158/1535-7163.mct-05-0443 |
| 23. | Allgäuer, D. S.; Mayr, H. Eur. J. Org. Chem. 2013, 6379–6388. doi:10.1002/ejoc.201300784 |
| 50. | Danac, R.; Al Matarneh, C. M.; Shova, S.; Daniloaia, T.; Balan, M.; Mangalagiu, I. I. Bioorg. Med. Chem. 2015, 23, 2318–2327. doi:10.1016/j.bmc.2015.03.077 |
| 51. | Singh, D.; Sinha, P.; Bansal, R. K. Curr. Green Chem. 2014, 1, 227–231. doi:10.2174/2213346101666140210194354 |
| 52. | Paira, R.; Mondal, S.; Chowdhury, A.; Banerjee, M.; Maity, A.; Hazra, A.; Mondal, N. B. Tetrahedron Lett. 2013, 54, 3046–3050. doi:10.1016/j.tetlet.2013.03.095 |
| 53. | Pospíšil, J.; Trávníček, M.; Potáček, M. ARKIVOC 2001, No. ii, 146–162. doi:10.3998/ark.5550190.0002.217 |
| 54. | Matusiak, G.; Śliwa, W. Monatsh. Chem. 1993, 124, 161–165. doi:10.1007/bf00808675 |
© 2019 Choi et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)