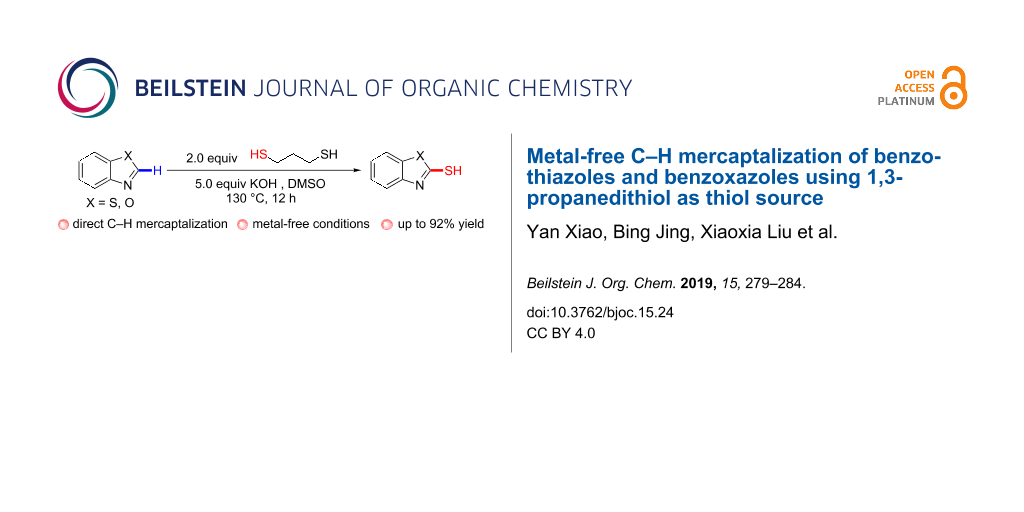
Abstract
A facile and effective C–H functionalization strategy for the synthesis of 2-mercaptobenzothiazoles and 2-mercaptobenzoxazoles is described. 1,3-Propanedithiol was employed to convert benzothiazoles and benzoxazoles to the corresponding heteroarylthiols in the presence of potassium hydroxide and DMSO. This novel protocol is featured by direct C–H mercaptalization of heteroarenes and a simple reaction system.
Graphical Abstract
Introduction
Both 2-mercaptobenzothiazoles and 2-mercaptobenzoxazoles are not only fundamental building blocks in organic synthesis, but also possess various biological activities (Figure 1) [1,2]. A complex of a transition metal (such as Ru, Pt, Bi, etc.) with either a 2-mercaptobenzoxazole or a 2-mercaptobenzothiazole often provides cytotoxic activity against cancer cells [3-5]. 2-Mercapto-N-(substituted arylidine)benzoxazole-5-carbohydrazide derivatives have promising anti-inflammatory activities [6]. 2-Mercapto-5-nitro-1,3-benzoxazole and its derivatives shows strong anthelmintic activity [7]. 2-Mercapto-5-chloro-1,3-benzothiazoles possess antifungal activity against Candida albicans and Candida tropicalis [8] and 2-mercapto-1,3-benzothiol and its derivatives exhibit inhibitory effects against thyroid peroxidase [9].
Figure 1: Representative examples of biologically active 2-mercaptobenzoxazoles and 2-mercaptobenzothiazoles.
Figure 1: Representative examples of biologically active 2-mercaptobenzoxazoles and 2-mercaptobenzothiazoles.
(Hetero)aryl thiols are often prepared from the corresponding halides through direct nucleophilic substitution [10-12] or metal-catalyzed C–S coupling reactions [13]. Conventional methods for the synthesis of 2-mercaptobenzoxazoles and 2-mercaptobenzothiazoles include the interaction of 2-aminophenol or 2-haloanilines with carbon disulfide [14-16], or potassium ethyl xanthate [17,18] (Scheme 1). In 2017, the Dong group reported a new method for the synthesis of 2-mercaptobenzoxazoles and 2-mercaptobenzothiazoles by cyclization of 2-aminothiophenols or 2-aminophenols with tetramethylthiuram disulfide in water [19]. Very recently, the Liu group developed a novel protocol for the synthesis of 2-mercaptobenzothiazoles via a three-component reaction of o-iodoanilines and K2S in DMSO [20]. Another way to prepare 2-mercaptobenzothiazoles and 2-mercaptobenzoxazoles is the nucleophilic substitution of 2-halo-substituted benzothiazoles and benzoxazoles with sulfur-containing reagents including sodium thiosulfate [21], thiourea [22] and 1,2-ethanedithiol [23].
Scheme 1: Strategies for the synthesis of 2-mercaptobenzothiazole and 2-mercaptobenzoxazole (X = O, S).
Scheme 1: Strategies for the synthesis of 2-mercaptobenzothiazole and 2-mercaptobenzoxazole (X = O, S).
In the past decades, C–H functionalization has become an effective strategy for constructing different molecules directly from simple arenes and alkanes. C–H functionalization is an important method for C–S coupling reactions [24,25]. For example, transition metal-catalyzed C–H thiolation of benzothiazole or benzoxazole with a disulfide and a thiol provides easy access to the corresponding sulfides [26-34]. However, the examples using C–H functionalization for preparing 2-mercaptobenzoxazoles or 2-mercaptobenzothiazoles are still rare. In 2009, the Daugulis group reported that benzoxazole was converted to 2-mercaptobenzoxazoles in the presence of sulfur and potassium tert-butoxide, but only one example was shown [35]. In 2017, the Lei group reported a copper catalyzed C–H mercaptalization strategy using elementary sulfur as thiol source [36]. This conversion proceeds under very mild conditions, however, a metal catalyst and an additional ligand are required (Scheme 1).
Although some protocols have been developed, these methods still suffer from some drawbacks, such as limited substrate scope, low yield, and/or a complicated reaction system. Accordingly, developing a new and simple method for the synthesis of 2-mercaptobenzothiazoles and 2-mercaptobenzoxazoles is still desirable. As a continuous study on C–S coupling reactions using aliphatic dithiols, herein we reported a simple and effective method for converting benzothiazoles and benzoxazoles to the corresponding thiols through direct C–H mercaptalization using 1,3-propanedithiol as thiol source.
Results and Discussion
Previous studies in our group revealed that small aliphatic diols and dithiols are promising reagents for the synthesis of phenols and arylthiols, respectively [23,37]. Therefore, we envisioned that aliphatic dithiol may be able to work as thiol source in the C–H mercaptalization of benzothiazole and benzoxazole as well, leading to the formation of 2-mercaptobenzothiazole and 2-mercaptobenzoxazole, respectively. We tested our hypothesis using benzothiazole (1a) as model substrate together with several aliphatic dithiols 2. Initially, benzothiazole was treated with 3.0 equiv of each aliphatic dithiol and 5.0 equiv of KOH in DMSO at 130 °C. After 12 h, we are delighted to find that 1a was predominantly converted to 2-mercaptobenzothiazole (3a). The investigation of the reaction mixture by proton nuclear magnetic resonance spectroscopy showed that no byproduct was formed. It should be noted that the length of aliphatic dithiols had a significant effect on the reaction performance. The reaction with 1,3-propanedithiol (2b) showed the best reaction performance, providing 3a with an isolated yield of 88% while the reaction with 1,2-ethanedithiol (2a) and 1,4-butanedithol (2c) gave yields of 36% and 45%, respectively (Table 1, entries 1–3). In the control reaction without aliphatic dithiol, 3a was not observed (Table 1, entry 4).
Table 1: Screening of the conditions for C–H mercaptalization of benzothiazole.a
|
|
||||
| Entry | Base (equiv) | Thiol surrogate (equiv) | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|
| 1 | KOH (5) | 2a (3) | 130 | 36 |
| 2 | KOH (5) | 2b (3) | 130 | 88, 79c |
| 3 | KOH (5) | 2c (3) | 130 | 45 |
| 4 | KOH (5) | – | 130 | 0 |
| 5 | KOH (5) | 2b (3) | 120 | 20 |
| 6 | KOH (5) | 2b (3) | 110 | 0 |
| 7 | NaOt-Bu (5) | 2b (3) | 130 | 80 |
| 8 | K2CO3(5) | 2b (3) | 130 | 8 |
| 9 | Cs2CO3 (5) | 2b (3) | 130 | 67d |
| 10 | KOH (5) | 2b (2) | 130 | 92 |
| 11 | KOH (5) | 2b (1) | 130 | 46 |
| 12 | KOH (3) | 2b (2) | 130 | 78 |
| 13e | KOH (5) | 2b (3) | 130 | 8 |
| 14 | KOH (5) | 1-butanethiol (2) | 130 | 15 |
| 15 | KOH (5) | S (2) | 130 | 21 |
| 16 | KOH (5) | Na2S2O3 (2) | 130 | 6 |
| 17 | KOH (5) | Na2S·9H2O (2) | 130 | 14 |
| 18 | KOH (5) | K2S (2) | 130 | 12 |
|
|
||||
aReaction conditions: benzothiazole (1a, 1 mmol), thiol surrogate, base, DMSO (3 mL), 12 h. bIsolated yield. c6 h. d5 mL of DMSO. eDMF as solvent.
Shortening the reaction time to 6 h provided 79% yield of 3a (Table 1, entry 2). Lowering of the reaction temperature led to lower yields. No product was observed at 110 °C while 20% yield of 3a was obtained at 120 °C (Table 1, entries 5 and 6). The investigation of different bases revealed that KOH was the most effective in this reaction in comparison to other bases such as NaOt-Bu, K2CO3 and Cs2CO3 (Table 1, entries 7–9). Using 2.0 equiv of 1,3-propanedithiol did not lead to the loss of yield, however, only 46% yield was obtained when the amount of 1,3-propanedithiol was further lowered to 1.0 equiv. Five equivalents of KOH were required for this transformation as only 78% yield was obtained when 3.0 equiv of KOH was used (Table 1, entries 10–12). DMSO was essential for this reaction because replacing DMSO with another organic solvent such as DMF significantly decreased the reaction yield to 8% (Table 1, entry 13). Therefore, the optimized reaction conditions were obtained as follows: benzothiazole (1.0 mmol), 1,3-propanedithiol (2.0 equiv), KOH (5.0 equiv), DMSO (3 mL), 130 °C, 12 h.
We further investigated several common thiol surrogates, which are often used in the C–H mercaptalization of aryl halides. Under the optimized conditions, 1-butanethiol gave only 15% yield of 3a and many byproducts were formed (Table 1, entry 14). Other thiol surrogates including elementary sulfur, Na2S2O3, Na2S·9H2O and K2S also provided very low yields (Table 1, entries 15–18). These results show that 1,3-propanedithiol is a promising thiol source for C–H mercaptalization of benzothiazole.
With the optimized conditions in hand, we studied the substrate scope for this novel C–H mercaptalization strategy (Figure 2). Generally, benzothiazoles were converted to the corresponding heteroarylthiols in moderate to good yields. Functional groups including methyl and ethoxy groups as well as halogens are well tolerated under the developed reaction conditions. Benzoxazoles were also successfully converted to the corresponding thiols. The relatively lower yields can be attributed to the partial decomposition of benzoxazoles caused by KOH at high temperature.
Figure 2: Substrate scope of the developed C–H mercaptalization strategy. Reaction conditions: benzothiazole or benzoxazole 1 (1.0 mmol), 1,3-propanedithiol (2b, 2.0 equiv), KOH (5.0 equiv), DMSO (3 mL), 130 °C, 12 h.
Figure 2: Substrate scope of the developed C–H mercaptalization strategy. Reaction conditions: benzothiazole ...
In order to get more understanding of this novel C–H mercaptalization strategy, several control experiments were carried out (Scheme 2). 2-Mercaptobenzothiazole was not observed when the reaction was carried out in the absence of either 1,3-propanedithiol or DMSO. This result indicate that 1,3-propanedithiol may react with DMSO and give an active intermediate, which can further convert benzothiazole to 2-mercaptobenzothiazole. Indeed, several articles have reported that thiols can be oxidized by DMSO to the corresponding disulfides [38,39].
Based on the above results and related references, a plausible reaction pathway is proposed (Scheme 3). Initially, 1,3-propanedithiol is possibly oxidized to disulfides 4 and 5 by DMSO. We failed to isolate and determinate these two sulfides, possibly because they are very active in the following coupling reactions. Both disulfides coupled with 1a to give the same C–S coupling product 6 [40]. As our previous work shows [23], (hetero)arylthioalkylthiols are easily converted to the corresponding (hetero)arylthiols in the presence of KOH and DMSO through an intramolecular nucleophilic substitution.
Conclusion
In this work, we developed a facile protocol for the direct synthesis of 2-mercaptobenzothiazoles and 2-mercaptobenzoxazoles from benzothiazoles and benzoxazoles. 1,3-Propanedithiol served as a thiol source and was superior to other common thiol surrogates under our developed conditions. DMSO was indispensable for this conversion and a preliminary mechanism study showed it served not only as a solvent but also as an oxidant. The developed reaction system required neither a metal catalyst nor a ligand. This simple method is expected to have potential application in both laboratory and industry.
Supporting Information
| Supporting Information File 1: General experimental information, synthetic procedures, analytical data and NMR spectra for the reported compounds. | ||
| Format: PDF | Size: 1.5 MB | Download |
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (Grant No. DUT17LK23; DUT17RC(4)31; DUT17LK41), the Natural Science Foundation of the Liaoning Province of China (Grant No. 20170540148), and the National Natural Science Foundation of China (Grant No. 31400307).
References
-
Gill, R. K.; Rawal, R. K.; Bariwal, J. Arch. Pharm. (Weinheim, Ger.) 2015, 348, 155–178. doi:10.1002/ardp.201400340
Return to citation in text: [1] -
Ranjit, S.; Lee, R.; Heryadi, D.; Shen, C.; Wu, J.; Zhang, P.; Huang, K.-W.; Liu, X. J. Org. Chem. 2011, 76, 8999–9007. doi:10.1021/jo2017444
Return to citation in text: [1] -
Yarar, S.; Ozturk, I. I.; Banti, C. N.; Panagiotou, N.; Papatriantafyllopoulou, C.; Manoli, M.; Manos, M. J.; Tasiopoulos, A. J.; Hadjikakou, S. K. Inorg. Chim. Acta 2018, 471, 23–33. doi:10.1016/j.ica.2017.10.026
Return to citation in text: [1] -
El-Asmy, H. A.; Butler, I. S.; Mouhri, Z. S.; Jean-Claude, B. J.; Emmam, M.; Mostafa, S. I. Inorg. Chim. Acta 2016, 441, 20–33. doi:10.1016/j.ica.2015.10.041
Return to citation in text: [1] -
Mitra, R.; Samuelson, A. G. Eur. J. Inorg. Chem. 2014, 3536–3546. doi:10.1002/ejic.201402205
Return to citation in text: [1] -
Prasad, A. V. G. S.; Rao, P. V.; Prasad, P. S. S. Int. J. Pharm. Res. Scholars 2014, 3, 63–68.
Return to citation in text: [1] -
Satyendra, R. V.; Vishnumurthy, K. A.; Vagdevi, H. M.; Dhananjaya, B. L.; Shruthi, A. Med. Chem. Res. 2015, 24, 1342–1350. doi:10.1007/s00044-014-1207-6
Return to citation in text: [1] -
Defrenza, I.; Catalano, A.; Carocci, A.; Carrieri, A.; Muraglia, M.; Rosato, A.; Corbo, F.; Franchini, C. J. Heterocycl. Chem. 2015, 52, 1705–1712. doi:10.1002/jhet.2222
Return to citation in text: [1] -
Hornung, M. W.; Kosian, P. A.; Haselman, J. T.; Korte, J. J.; Challis, K.; Macherla, C.; Nevalainen, E.; Degitz, S. J. Toxicol. Sci. 2015, 146, 254–264. doi:10.1093/toxsci/kfv090
Return to citation in text: [1] -
Testaferri, L.; Tingoli, M.; Tiecco, M. Tetrahedron Lett. 1980, 21, 3099–3100. doi:10.1016/s0040-4039(00)77418-1
Return to citation in text: [1] -
Testaferri, L.; Tiecco, M.; Tingoli, M.; Chianelli, D.; Montanucci, M. Synthesis 1983, 751–755. doi:10.1055/s-1983-30501
Return to citation in text: [1] -
Shaw, J. E. J. Org. Chem. 1991, 56, 3728–3729. doi:10.1021/jo00011a057
Return to citation in text: [1] -
Liu, Y.; Liu, S.; Xiao, Y. Beilstein J. Org. Chem. 2017, 13, 589–611. doi:10.3762/bjoc.13.58
Return to citation in text: [1] -
Wang, F.; Cai, S.; Wang, Z.; Xi, C. Org. Lett. 2011, 13, 3202–3205. doi:10.1021/ol2011105
Return to citation in text: [1] -
Varun, B. V.; Prabhu, K. R. J. Org. Chem. 2014, 79, 9655–9668. doi:10.1021/jo501793q
Return to citation in text: [1] -
Lou, C.; Zhu, N.; Fan, R.; Hong, H.; Han, L.; Zhang, J.; Suo, Q. Green Chem. 2017, 19, 1102–1108. doi:10.1039/c6gc03053j
Return to citation in text: [1] -
Deligeorgiev, T. G.; Kaloyanova, S. S.; Lesev, N. Y.; Vaquero, J. J. Monatsh. Chem. 2011, 142, 895–899. doi:10.1007/s00706-011-0551-1
Return to citation in text: [1] -
Liu, L.; Zhu, N.; Gao, M.; Zhao, X.; Han, L.; Hong, H. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 699–701. doi:10.1080/10426507.2015.1067208
Return to citation in text: [1] -
Liu, X.; Liu, M.; Xu, W.; Zeng, M.-T.; Zhu, H.; Chang, C.-Z.; Dong, Z.-B. Green Chem. 2017, 19, 5591–5598. doi:10.1039/c7gc02311a
Return to citation in text: [1] -
Zhu, X.; Li, W.; Luo, X.; Deng, G.; Liang, Y.; Liu, J. Green Chem. 2018, 20, 1970–1974. doi:10.1039/c8gc00477c
Return to citation in text: [1] -
Foye, W. O.; Abood, N.; Kauffman, J. M.; Kim, Y.-H.; Patel, B. R. Phosphorus Sulfur Relat. Elem. 1980, 8, 205–207. doi:10.1080/03086648008078190
Return to citation in text: [1] -
Watt, G. W. J. Org. Chem. 1939, 4, 436–441. doi:10.1021/jo01216a010
Return to citation in text: [1] -
Liu, Y.; Kim, J.; Seo, H.; Park, S.; Chae, J. Adv. Synth. Catal. 2015, 357, 2205–2212. doi:10.1002/adsc.201400941
Return to citation in text: [1] [2] [3] -
Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T. S. A.; Liu, X. Chem. Soc. Rev. 2015, 44, 291–314. doi:10.1039/c4cs00239c
Return to citation in text: [1] -
Lee, C.-F.; Basha, R. S.; Badsara, S. S. Top. Curr. Chem. 2018, 376, 25. doi:10.1007/s41061-018-0203-6
Return to citation in text: [1] -
Dai, C.; Xu, Z.; Huang, F.; Yu, Z.; Gao, Y.-F. J. Org. Chem. 2012, 77, 4414–4419. doi:10.1021/jo202624s
Return to citation in text: [1] -
Rosario, A. R.; Casola, K. K.; Oliveira, C. E. S.; Zeni, G. Adv. Synth. Catal. 2013, 355, 2960–2966. doi:10.1002/adsc.201300497
Return to citation in text: [1] -
Rafique, J.; Saba, S.; Frizon, T. E. A.; Braga, A. L. ChemistrySelect 2018, 3, 328–334. doi:10.1002/slct.201702623
Return to citation in text: [1] -
He, Z.; Luo, F.; Li, Y.; Zhu, G. Tetrahedron Lett. 2013, 54, 5907–5910. doi:10.1016/j.tetlet.2013.08.097
Return to citation in text: [1] -
Gandeepan, P.; Mo, J.; Ackermann, L. Chem. Commun. 2017, 53, 5906–5909. doi:10.1039/c7cc03107f
Return to citation in text: [1] -
Liu, Y.; Wang, H.; Wang, C.; Wan, J.-P.; Wen, C. RSC Adv. 2013, 3, 21369–21372. doi:10.1039/c3ra42915f
Return to citation in text: [1] -
Zhou, A.-X.; Liu, X.-Y.; Yang, K.; Zhao, S.-C.; Liang, Y.-M. Org. Biomol. Chem. 2011, 9, 5456–5462. doi:10.1039/c1ob05395g
Return to citation in text: [1] -
Inomata, H.; Toh, A.; Mitsui, T.; Fukuzawa, S.-i. Tetrahedron Lett. 2013, 54, 4729–4731. doi:10.1016/j.tetlet.2013.06.104
Return to citation in text: [1] -
Fukuzawa, S.-i.; Shimizu, E.; Atsuumi, Y.; Haga, M.; Ogata, K. Tetrahedron Lett. 2009, 50, 2374–2376. doi:10.1016/j.tetlet.2009.02.214
Return to citation in text: [1] -
Popov, I.; Do, H.-Q.; Daugulis, O. J. Org. Chem. 2009, 74, 8309–8313. doi:10.1021/jo9015369
Return to citation in text: [1] -
Yan, H.; Huang, Z.; Chen, M.; Li, C.; Chen, Y.; Gao, M.; Lei, A. Org. Biomol. Chem. 2017, 15, 8276–8279. doi:10.1039/c7ob02036h
Return to citation in text: [1] -
Liu, Y.; Park, S. K.; Xiao, Y.; Chae, J. Org. Biomol. Chem. 2014, 12, 4747–4753. doi:10.1039/c4ob00649f
Return to citation in text: [1] -
Le Quéméner, F.; Subervie, D.; Morlet-Savary, F.; Lalevée, J.; Lansalot, M.; Bourgeat-Lami, E.; Lacôte, E. Angew. Chem., Int. Ed. 2018, 57, 957–961. doi:10.1002/anie.201710488
Return to citation in text: [1] -
Liu, X. G.; Wu, J. P.; Liang, X. M.; Wang, D. Q. Chin. J. Org. Chem. 2001, 21, 549–556.
Return to citation in text: [1] -
Zou, L.-H.; Reball, J.; Mottweiler, J.; Bolm, C. Chem. Commun. 2012, 48, 11307–11309. doi:10.1039/c2cc36711d
Return to citation in text: [1]
| 36. | Yan, H.; Huang, Z.; Chen, M.; Li, C.; Chen, Y.; Gao, M.; Lei, A. Org. Biomol. Chem. 2017, 15, 8276–8279. doi:10.1039/c7ob02036h |
| 26. | Dai, C.; Xu, Z.; Huang, F.; Yu, Z.; Gao, Y.-F. J. Org. Chem. 2012, 77, 4414–4419. doi:10.1021/jo202624s |
| 27. | Rosario, A. R.; Casola, K. K.; Oliveira, C. E. S.; Zeni, G. Adv. Synth. Catal. 2013, 355, 2960–2966. doi:10.1002/adsc.201300497 |
| 28. | Rafique, J.; Saba, S.; Frizon, T. E. A.; Braga, A. L. ChemistrySelect 2018, 3, 328–334. doi:10.1002/slct.201702623 |
| 29. | He, Z.; Luo, F.; Li, Y.; Zhu, G. Tetrahedron Lett. 2013, 54, 5907–5910. doi:10.1016/j.tetlet.2013.08.097 |
| 30. | Gandeepan, P.; Mo, J.; Ackermann, L. Chem. Commun. 2017, 53, 5906–5909. doi:10.1039/c7cc03107f |
| 31. | Liu, Y.; Wang, H.; Wang, C.; Wan, J.-P.; Wen, C. RSC Adv. 2013, 3, 21369–21372. doi:10.1039/c3ra42915f |
| 32. | Zhou, A.-X.; Liu, X.-Y.; Yang, K.; Zhao, S.-C.; Liang, Y.-M. Org. Biomol. Chem. 2011, 9, 5456–5462. doi:10.1039/c1ob05395g |
| 33. | Inomata, H.; Toh, A.; Mitsui, T.; Fukuzawa, S.-i. Tetrahedron Lett. 2013, 54, 4729–4731. doi:10.1016/j.tetlet.2013.06.104 |
| 34. | Fukuzawa, S.-i.; Shimizu, E.; Atsuumi, Y.; Haga, M.; Ogata, K. Tetrahedron Lett. 2009, 50, 2374–2376. doi:10.1016/j.tetlet.2009.02.214 |
| 35. | Popov, I.; Do, H.-Q.; Daugulis, O. J. Org. Chem. 2009, 74, 8309–8313. doi:10.1021/jo9015369 |
| 1. | Gill, R. K.; Rawal, R. K.; Bariwal, J. Arch. Pharm. (Weinheim, Ger.) 2015, 348, 155–178. doi:10.1002/ardp.201400340 |
| 2. | Ranjit, S.; Lee, R.; Heryadi, D.; Shen, C.; Wu, J.; Zhang, P.; Huang, K.-W.; Liu, X. J. Org. Chem. 2011, 76, 8999–9007. doi:10.1021/jo2017444 |
| 8. | Defrenza, I.; Catalano, A.; Carocci, A.; Carrieri, A.; Muraglia, M.; Rosato, A.; Corbo, F.; Franchini, C. J. Heterocycl. Chem. 2015, 52, 1705–1712. doi:10.1002/jhet.2222 |
| 23. | Liu, Y.; Kim, J.; Seo, H.; Park, S.; Chae, J. Adv. Synth. Catal. 2015, 357, 2205–2212. doi:10.1002/adsc.201400941 |
| 7. | Satyendra, R. V.; Vishnumurthy, K. A.; Vagdevi, H. M.; Dhananjaya, B. L.; Shruthi, A. Med. Chem. Res. 2015, 24, 1342–1350. doi:10.1007/s00044-014-1207-6 |
| 24. | Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T. S. A.; Liu, X. Chem. Soc. Rev. 2015, 44, 291–314. doi:10.1039/c4cs00239c |
| 25. | Lee, C.-F.; Basha, R. S.; Badsara, S. S. Top. Curr. Chem. 2018, 376, 25. doi:10.1007/s41061-018-0203-6 |
| 6. | Prasad, A. V. G. S.; Rao, P. V.; Prasad, P. S. S. Int. J. Pharm. Res. Scholars 2014, 3, 63–68. |
| 21. | Foye, W. O.; Abood, N.; Kauffman, J. M.; Kim, Y.-H.; Patel, B. R. Phosphorus Sulfur Relat. Elem. 1980, 8, 205–207. doi:10.1080/03086648008078190 |
| 3. | Yarar, S.; Ozturk, I. I.; Banti, C. N.; Panagiotou, N.; Papatriantafyllopoulou, C.; Manoli, M.; Manos, M. J.; Tasiopoulos, A. J.; Hadjikakou, S. K. Inorg. Chim. Acta 2018, 471, 23–33. doi:10.1016/j.ica.2017.10.026 |
| 4. | El-Asmy, H. A.; Butler, I. S.; Mouhri, Z. S.; Jean-Claude, B. J.; Emmam, M.; Mostafa, S. I. Inorg. Chim. Acta 2016, 441, 20–33. doi:10.1016/j.ica.2015.10.041 |
| 5. | Mitra, R.; Samuelson, A. G. Eur. J. Inorg. Chem. 2014, 3536–3546. doi:10.1002/ejic.201402205 |
| 14. | Wang, F.; Cai, S.; Wang, Z.; Xi, C. Org. Lett. 2011, 13, 3202–3205. doi:10.1021/ol2011105 |
| 15. | Varun, B. V.; Prabhu, K. R. J. Org. Chem. 2014, 79, 9655–9668. doi:10.1021/jo501793q |
| 16. | Lou, C.; Zhu, N.; Fan, R.; Hong, H.; Han, L.; Zhang, J.; Suo, Q. Green Chem. 2017, 19, 1102–1108. doi:10.1039/c6gc03053j |
| 19. | Liu, X.; Liu, M.; Xu, W.; Zeng, M.-T.; Zhu, H.; Chang, C.-Z.; Dong, Z.-B. Green Chem. 2017, 19, 5591–5598. doi:10.1039/c7gc02311a |
| 40. | Zou, L.-H.; Reball, J.; Mottweiler, J.; Bolm, C. Chem. Commun. 2012, 48, 11307–11309. doi:10.1039/c2cc36711d |
| 13. | Liu, Y.; Liu, S.; Xiao, Y. Beilstein J. Org. Chem. 2017, 13, 589–611. doi:10.3762/bjoc.13.58 |
| 20. | Zhu, X.; Li, W.; Luo, X.; Deng, G.; Liang, Y.; Liu, J. Green Chem. 2018, 20, 1970–1974. doi:10.1039/c8gc00477c |
| 23. | Liu, Y.; Kim, J.; Seo, H.; Park, S.; Chae, J. Adv. Synth. Catal. 2015, 357, 2205–2212. doi:10.1002/adsc.201400941 |
| 10. | Testaferri, L.; Tingoli, M.; Tiecco, M. Tetrahedron Lett. 1980, 21, 3099–3100. doi:10.1016/s0040-4039(00)77418-1 |
| 11. | Testaferri, L.; Tiecco, M.; Tingoli, M.; Chianelli, D.; Montanucci, M. Synthesis 1983, 751–755. doi:10.1055/s-1983-30501 |
| 12. | Shaw, J. E. J. Org. Chem. 1991, 56, 3728–3729. doi:10.1021/jo00011a057 |
| 23. | Liu, Y.; Kim, J.; Seo, H.; Park, S.; Chae, J. Adv. Synth. Catal. 2015, 357, 2205–2212. doi:10.1002/adsc.201400941 |
| 37. | Liu, Y.; Park, S. K.; Xiao, Y.; Chae, J. Org. Biomol. Chem. 2014, 12, 4747–4753. doi:10.1039/c4ob00649f |
| 9. | Hornung, M. W.; Kosian, P. A.; Haselman, J. T.; Korte, J. J.; Challis, K.; Macherla, C.; Nevalainen, E.; Degitz, S. J. Toxicol. Sci. 2015, 146, 254–264. doi:10.1093/toxsci/kfv090 |
| 17. | Deligeorgiev, T. G.; Kaloyanova, S. S.; Lesev, N. Y.; Vaquero, J. J. Monatsh. Chem. 2011, 142, 895–899. doi:10.1007/s00706-011-0551-1 |
| 18. | Liu, L.; Zhu, N.; Gao, M.; Zhao, X.; Han, L.; Hong, H. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 699–701. doi:10.1080/10426507.2015.1067208 |
| 38. | Le Quéméner, F.; Subervie, D.; Morlet-Savary, F.; Lalevée, J.; Lansalot, M.; Bourgeat-Lami, E.; Lacôte, E. Angew. Chem., Int. Ed. 2018, 57, 957–961. doi:10.1002/anie.201710488 |
| 39. | Liu, X. G.; Wu, J. P.; Liang, X. M.; Wang, D. Q. Chin. J. Org. Chem. 2001, 21, 549–556. |
© 2019 Xiao et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)