Abstract
Starting from substituted alkynones, α-pyrones and/or 1H-pyridines were generated in a Michael addition–cyclocondensation with ethyl cyanoacetate. The peculiar product formation depends on the reaction conditions as well as on the electronic substitution pattern of the alkynone. While electron-donating groups furnish α-pyrones as main products, electron-withdrawing groups predominantly give the corresponding 1H-pyridines. Both heterocycle classes fluoresce in solution and in the solid state. In particular, dimethylamino-substituted α-pyrones, as donor–acceptor systems, display remarkable photophysical properties, such as strongly red-shifted absorption and emission maxima with daylight fluorescence and fluorescence quantum yields up to 99% in solution and around 11% in the solid state, as well as pronounced emission solvatochromism. Also a donor-substituted α-pyrone shows pronounced aggregation-induced emission enhancement.
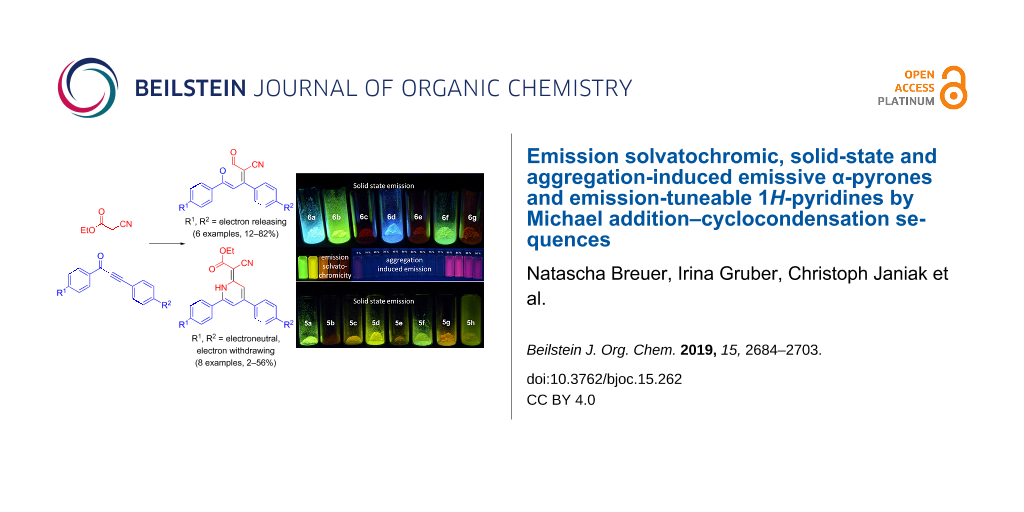
Graphical Abstract
Introduction
A high sensitivity and precise tuneability of fluorescence colors are prerequisites for the application of fluorescent substances in chemistry, medicine and materials science [1]. With this respect emissive small molecules [2], fluorescent proteins [3], and quantum dots have received considerable attention and remarkable progress in their synthesis and photophysics has been achieved [4]. Small molecule organic fluorophores are particularly advantageous due to the potential of a tailored fine-tuning of their photophysical properties through synthetic modifications [5]. Based on their structural features, functionalized organic chromophores, containing N-, O- or S-atoms, are increasingly used in OLEDs [6-10] and LCDs [11-13] of mobile phones [14]. Fluorescent compounds often intensively emit in solution but only weakly or not in the solid state [15]. Dyes which fluoresce both in the solid state and in solution are still relatively rare, due to the fact that often molecular aggregation in the solid state causes fluorescence quenching [16].
In recent years, we have coined diversity-oriented syntheses of functional chromophores by multicomponent strategies [17,18], opening accesses to substance libraries for systematic studies of structure–property relationships on fluorophores [19], in particular on aggregation-induced emissive polar dyes [20]. Conceptually, many of these consecutive multicomponent syntheses rely on transition-metal-catalyzed heterocyclic syntheses [21]. By virtue of catalytic generation of alkynones [22] we have recently disclosed consecutive alkynylation–Michael addition–cyclocondensation (AMAC) multicomponent syntheses of α-pyrones [23].
While most α-pyrones neither fluoresce in solution nor in the solid state specific substitution patterns have been identified for fluorophore design for this heterocyclic family. Tominaga and co-workers synthesized a series of α-pyrone derivatives with emission maxima between 400 and 675 nm in the solid state and between 486 and 542 nm in chloroform [16,24-26], including fluorescence quantum yields as high as 95% in solution and 58% in the solid state [16,24]. While these fluorophores were synthesized by cyclocondensation with ketene dithioacetals and substituted acetophenones other cyano-containing derivatives became accessible by desymmetrizing cyclocondensation of 1,2-diaroylacetylenes with ethyl cyanoacetate [27], similar to related studies with dialkyl malonates [28]. Here, we report on effects of base and temperature on Michael addition–cyclocondensation sequences in the formation of α-pyrones and/or 1H-pyridines starting from diversely substituted alkynones and cyanoethylacetate. This bifurcating domino process furnishes small chromophore libraries which were characterized by photophysical studies (absorption and emission spectroscopy) and the studies on the electronic structure were accompanied by TD-DFT calculations for assigning the dominant longest-wavelength absorption bands.
Results and Discussion
Synthesis and tentative mechanism
Recently, we reported a straightforward access to α-pyrones through a consecutive alkynylation–Michael addition–cyclocondensation (AMAC) multicomponent synthesis [23]. The reaction can be rationalized by a Sonogashira coupling between an acid chloride and a terminal alkyne furnishing an alkynone, which is transformed without isolation by addition of dialkyl malonates in a Michael addition–cyclocondensation to form α-pyrones (Scheme 1).
Scheme 1: Consecutive three-component alkynylation–Michael addition–cyclocondensation (AMAC) synthesis of α-pyrones from acid chlorides, terminal alkynes and dialkyl malonates.
Scheme 1: Consecutive three-component alkynylation–Michael addition–cyclocondensation (AMAC) synthesis of α-p...
With this sequence in hand, we envisioned the variation of CH-acidic esters to generate differently 3-substituted α-pyrones. For introducing a cyano substituent we employed benzoyl chloride (1a), phenylacetylene (2a), and ethyl cyanoacetate (4) within the AMAC sequence (Scheme 2). Surprisingly, the desired α-pyrone was not isolated, but two other compounds were detected. On the one hand a 1H-pyridine derivative 5a (2% yield) and on the other hand an aniline derivative with two ester groups (4% yield). Both compounds indicate that two molecules of ethyl cyanoacetate (4) were incorporated in the final structure.
Scheme 2: Consecutive pseudo-four-component alkynylation–Michael addition–cyclocondensation (AMAC) synthesis of 1H-pyridines 5a and an aniline derivative.
Scheme 2: Consecutive pseudo-four-component alkynylation–Michael addition–cyclocondensation (AMAC) synthesis ...
With an increased amount of ethyl cyanoacetate the yield of both products could be increased. By the addition of ethanol as a cosolvent in the second step of the sequence, 1H-pyridine 5a could be isolated in 30% yield, while the aniline derivative was not formed (Scheme 3).
Scheme 3: Consecutive pseudo-four-component alkynylation–Michael addition–cyclocondensation (AMAC) synthesis of 1H-pyridines 5a from acid chlorides 1, terminal alkynes 2 and ethyl cyanoacetate (4).
Scheme 3: Consecutive pseudo-four-component alkynylation–Michael addition–cyclocondensation (AMAC) synthesis ...
There are only a few known methods for the synthesis of this kind of 1H-pyridines. In a cyclocondensation, starting from 1,3-dicarbonyl compounds, Elnagdi and co-workers synthesized 1H-pyridines with an additional cyano substituent in the 3-position [29]. Most syntheses generating 1H-pyridines make use of ethyl cyanoactate as a starting material. It can react with itself and forms a dimer by selfcondensation, catalyzed by transition metals [30,31].
Intrigued by the unusual pseudo-four-component AMAC synthesis we investigated the reaction conditions of the terminal Michael addition–cyclocondensation step starting from alkynone 3a. By varying the amount of the base we could observe the formation of 1H-pyridine 5a, but also of α-pyrone 6a (Scheme 4, Table 1), similarly to the reaction of compound 4 with 1,2-diaroylacetylenes [28].
Scheme 4: Model system for the optimization of the Michael addition–cyclocondensation reaction step to 1H-pyridine 5a or/and α-pyrone 6a.
Scheme 4: Model system for the optimization of the Michael addition–cyclocondensation reaction step to 1H-pyr...
Table 1: Optimization of the cyclization step of 1,5-diacyl-5-hydroxypyrazoline 5b.a
| Entry | Base (equiv) | Compound 5a (yield)b | Compound 6a (yield)b |
|---|---|---|---|
| 1 | Na2CO3∙10H2O (0.80) | 20% | 50% |
| 2 | Na2CO3∙10H2O (1.0) | 8% | 64% |
| 3 | Na2CO3∙10H2O (1.5) | 32% | – |
| 4 | Na2CO3∙10H2O (2.0) | 26% | – |
| 5 | Na2CO3 (0.80) | 3% | 41% |
| 6c | Na2CO3 (0.80) | 22% | 52% |
| 7b | Na2CO3 (1.4) | 9% | 66% |
aAll reactions were carried out on a 0.500 mmol scale (c0(3a) = 0.50 M, c0(4) = 2.0 M; ball yields refer to isolated and purified products; cadditional water (5.6 equiv).
With either 0.8 or 1.0 equiv of Na2CO3·10H2O α-pyrone 6a is formed as the main product (50–64%), while 1H-pyridine 5a can also be isolated in around 15% yield (Table 1, entries 1 and 2). By increasing the amount of Na2CO3·10H2O, exclusively 1H-pyridine 5a can be isolated in low yield (Table 1, entries 3 and 4). Using anhydrous sodium carbonate α-pyrone 6a is again formed as the main product in 41% yield, but the yield of 1H-pyridine 5a drops to 3% (Table 1, entry 5). By the addition of water, the yield of 6a could be increased (Table 1, entries 6 and 7).
Next we evaluated the use of a mixture of two bases, sodium carbonate and sodium acetate, and water (Table 2). With 0.80 equiv of sodium carbonate, 0.60 equiv of sodium acetate and 5.6 equiv of water 1H-pyridine 5a could be isolated in 56% yield. Decreasing the amount of ethyl cyanoacetate (4) the yields drops (Table 2, entry 2), however, increasing the amount of substrate 4 does not improve the yield (Table 2, entry 3). The exclusion of water only causes a decrease in yield (Table 2, entry 4). With sodium acetate as the only base, both 1H-pyridine 5a and α-pyrone 6a are formed in ca. 25% yield each (Table 2, entry 5). Sodium acetate with additional water gives 1H-pyridine 5a in 23% yield (Table 2, entry 6). It seems to be important that both bases and water are present, but neither a reduction nor an increase of the amount of water increased the yields of 1H-pyridine 5a (Table 2, entries 7–9). The increase of neither sodium carbonate (Table 2, entry 10) nor sodium acetate (Table 2, entry 11) caused an increase in yields.
Table 2: Optimization of the formation of 1H-pyridine 5a or/and α-pyrone 6a in the Michael addition–cyclocondensation reaction with Na2CO3, NaOAc and water.a
| Entry | Na2CO3 [equiv] | NaOAc [equiv] | H2O [equiv] | Compound 5a (yield)b | Compound 6a (yield)b |
|---|---|---|---|---|---|
| 1 | 0.80 | 0.60 | 5.6. | 56% | – |
| 2c | 0.80 | 0.60 | 5.6 | 26% | – |
| 3d | 0.80 | 0.60 | 5.6 | 56% | – |
| 4 | 0.80 | 0.60 | – | 44% | – |
| 5 | – | 0.60 | – | 26% | 28% |
| 6 | – | 0.60 | 5.6 | 23% | – |
| 7 | 0.80 | 0.60 | 2.8 | 40% | – |
| 8 | 0.80 | 0.60 | 8.4 | 40% | 20% |
| 9 | 0.80 | 0.60 | 11 | 38% | 28% |
| 10 | 1.0 | 0.60 | 5.6 | 45% | – |
| 11 | 0.80 | 0.80 | 5.6 | 43% | – |
aAll reactions were carried out on a 0.500 mmol scale (c0(3a) = 0.50 M, c0(4) = 2.0 M; ball yields refer to isolated and purified products; cc0(4) = 1.0 M; dadditional 4.0 equiv of ethyl cyanoacetate (4) after 2 h.
Only lowering the reaction temperature to 20 °C α-pyrone 6a was isolated as the main product in 78% yield and 1H-pyridine 5a was obtained in only 7% yield (Scheme 5).
Scheme 5: Formation of α-pyrone 6a and 1H-pyridine 5a at 20 °C.
Scheme 5: Formation of α-pyrone 6a and 1H-pyridine 5a at 20 °C.
Since base(s) and reaction temperature exert a significant impact on which heterocyclic compound is formed, we also tried to change the electronic nature of the starting material. Therefore, an electron-donating substituent was introduced in the alkynone 3b and the reaction was performed at 75 °C. To our surprise, we only could isolate α-pyrone 6b (Scheme 6).
Scheme 6: Formation of α-pyrone 6a starting from alkynone 3b having an electron-donating substituent.
Scheme 6: Formation of α-pyrone 6a starting from alkynone 3b having an electron-donating substituent.
However, when we introduced an electron-withdrawing group 1H-pyridine 5b was the only product (Scheme 7).
Scheme 7: Formation of 1H-pyridine 5b starting from alkynone 3d having an electron-withdrawing substituent.
Scheme 7: Formation of 1H-pyridine 5b starting from alkynone 3d having an electron-withdrawing substituent.
For elucidating whether 1H-pyridine 5a is formed from α-pyrone 6a and ethyl cyanoacetate (4) a reaction between α-pyrone 6a and ethyl cyanoacetate (4) under the same reaction conditions as for the 1H-pyridine from alkynone 3a was conducted, but only starting material could be isolated. Another option for the formation of the 1H-pyridine 5a was envisioned by an in situ generation of a dimer of ethyl cyanoacetate (4). The dimer 7 can be synthesized by iridium catalysis [30]. With dimer 7 in hand, we performed the reaction at 75 °C for 16 h, but we only could isolate 1H-pyridine 8a, which still contains an ester group (Scheme 8). Therefore, the in situ formation of the dimer starting from the alkynone 3a and ethyl cyanoacetate (4) was excluded for the formation of the 1H-pyridine 5a.
Scheme 8: Formation of 1H-pyridine 8a by Michael addition–cyclocondensation reaction.
Scheme 8: Formation of 1H-pyridine 8a by Michael addition–cyclocondensation reaction.
While the in situ generation of dimer 7 does not happen during the formation of 1H-pyridine 5a, we examined the reaction between ethyl cyanoacetate (4) and the optimized base system by adding alkynone 3a to the reaction after different times (Table 3).
Table 3: Influence of the reaction time on the self-condensation of ethyl cyanoacetate (4) in the presence of the optimized base system.a
|
|
||||
| Entry | t1 | t2 | Compound 5a (yield)b | Compound 6a (yield)b |
| 1 | 2 h | 16 h | 53% | – |
| 2 | 6 h | 16 h | 5% | 46% |
| 3 | 24 h | 16 h | 3% | 2% |
aAll reactions were carried out on a 0.500 mmol scale (c0(3a) = 0.50 M, c0(4) = 2.0 M; ball yields refer to isolated and purified products.
In the first attempt, alkynone 3a was added after 2 h. 1H-Pyridine 5a was isolated in 53% yield (Table 3, entry 1), indicating that the time of addition of the alkynone is not relevant within the first two hours of the reaction. However, if alkynone 3a was added after 6 h α-pyrone 6a was the main product and 1H-pyridine 5a could only be isolated in 5% yield (Table 3, entry 2). Upon the addition of alkynone 3a after 24 h, both 1H-pyridine 5a and α-pyrone 6a were isolated in only around 3% yield. This finding supports that within the first two hours ethyl cyanoacetate (4) is consumed and thereafter the ethyl cyanoacetate concentration is just too low for the formation of 1H–pyridine 5a, therefore α-pyrone 6a is formed. At longer initial reaction times (6 and 24 h) there is no ethyl cyanoacetate (4) left for the formation of any product. Also, ethyl cyanoacetate (4) probably does not form dimer 7 because in that case under these conditions 1H-pyridine 8a would have been detected.
Therefore, the tentative mechanistic rationale takes into account that the formation of 1H-pyridine 5a rather proceeds via stepwise condensation of alkynone 3 with two equivalents of ethyl cyanoacetate (4) than by reaction with dimer 7 (Scheme 9).
Scheme 9: Mechanistic rationale for the formation of the 1H-pyridine 5a.
Scheme 9: Mechanistic rationale for the formation of the 1H-pyridine 5a.
First, a molecule of ethyl cyanoacetate (4) attacks the alkynone 3a in a Michael addition. A second molecule 4 then attacks the cyano substituent and an imine is formed. The ester substituent of the initially reacted more electrophilic ethyl cyanoacetate (4) is presumably cleaved by a base-mediated acyl cleavage furnishing directly 1H-pyridine 5a after protonation.
For examining the influence of the electronic nature of the alkynone 3 on the product formation, a range of differently substituted alkynones 3 (for experimental details on their preparation, see chapters 2.1 and 2.2 in Supporting Information File 1) bearing electron-donating and/or electron-withdrawing substituents were synthesized and employed in the cyclocondensation step under the optimized reaction conditions [32-34]. Alkynones 3b–e with only one electron-donating substituent furnish the corresponding α-pyrones 6b–e, while the alkynone with a single electron-withdrawing substituent furnishes 1H-pyridines 5b–e. Interestingly, the position of substitution on the alkynone does not affect the outcome (Table 4, entries 2 and 6). Also, for alkynone 3j bearing an electron-donating substituent on either aryl ring, α-pyrone 6f is formed likewise (Table 4, entry 10). For electronically unsymmetrically substituted alkynones 3 the product formation depends rather on the strength of the employed electron-donating group. Whereas the p-anisyl substituent leads to the formation of 1H-pyridine (Table 4, entries 11 and 12), the N,N-dimethylaminophenyl substituent furnishes α-pyrone 6g (Table 4, entry 13).
Table 4: Michael addition–cyclocondensation synthesis of 1H-pyridine 5 or α-pyrone 6.
|
|
|||
| Entry | Alkynone 3 | 1H-Pyridine 5a | α-Pyrone 6a |
| 1 | 3a (R1 = Ph, R2 = Ph) | 5a (R1 = Ph, R2 = Ph, 56%) | – |
| 2 | 3b (R1 = p-MeOC6H4, R2 = Ph) | – | 6b (R1 = p-MeOC6H4, R2 = Ph; 70%) |
| 3 | 3c (R1 = p-Me2NC6H4, R2 = Ph) | – | 6c (R1 = p-Me2NC6H4, R2 = Ph, 12%) |
| 4 | 3d (R1 = p-F3CC6H4, R2 = Ph) | 5b (R1 = p-F3CC6H4, R2 = Ph, 22%) | – |
| 5 | 3e (R1 = p-NCC6H4, R2 = Ph) | 5c (R1 = p-NCC6H4, R2 = Ph, 20%) | – |
| 6 | 3f (R1 = Ph, R2 = p-MeOC6H4) | – | 6d (R1 = Ph, R2 = p-MeOC6H4, 82%) |
| 7 | 3g (R1 = Ph, R2 = p-Me2NC6H4) | – | 6e (R1 = Ph, R2 = p-Me2NC6H4, 62%) |
| 8 | 3h (R1 = Ph, R2 = p-F3CC6H4) | 5d (R1 = Ph, R2 = p-F3CC6H4, 25%) | – |
| 9 | 3i (R1 = Ph, R2 = p-NCC6H4) | 5e (R1 = Ph, R2 = p-NCC6H4, 2%) | – |
| 10 | 3j (R1 = p-MeOC6H4, R2 = p-MeOC6H4) | – | 6f (R1 = p-MeOC6H4, R2 = p-MeOC6H4, 45%) |
| 11 | 3k (R1 = p-MeOC6H4, R2 = p-F3CC6H4) | 5f (R1 = p-MeOC6H4, R2 = p-F3CC6H4, 37%) | – |
| 12 | 3l (R1 = p-F3CC6H4, R2 = p-MeOC6H4) | 5g (R1 = p-F3CC6H4, R2 = p-MeOC6H4,, 40%) | – |
| 13 | 3m (R1 = p-F3CC6H4, R2 = p-Me2NC6H4) | – | 6g (R1 = p-F3CC6H4, R2 = p-Me2NC6H4, 71%) |
| 14 | 3n (R1 = 2-thienyl, R2 = Ph) | 5h (R1 = 2-thienyl, R2 = Ph, 51%) | – |
aAll yields refer to isolated and purified products.
For synthesizing 1H-pyridine derivatives 8 with an electron-donating group we employed the isolated dimer 7 and were able to isolate 1H-pyridines 8 in 52 and 34% yield (Scheme 10).
Scheme 10: Formation of 1H-pyridine 8a from alkynone 3b and dimer 7.
Scheme 10: Formation of 1H-pyridine 8a from alkynone 3b and dimer 7.
Crystal structure of 1H-pyridine 5a
The structure of 1H-pyridines 5 was further corroborated by a single crystal X-ray structure determination of compound 5a (Figure 1) [35]. In the single crystal the carboxyl ester group is oriented to the N–H and via a hydrogen bond. Solid-state torsional/dihedral angles between the 4- and 6-positioned aryl rings differ especially for the 6-positioned phenyl ring with 27° in the X-ray structure and 38° from calculation (for comparison to the DFT calculated ground state structure of 1H-pyridine 5a, see chapter 12.3 in Supporting Information File 1). This is probably due to packing constraints from the involvement of the 6-phenyl ring in C-H···N [36-39] and C-H···π [40-49] interactions (Figure 2, for details, see Supporting Information File 1). It is noteworthy to mention that there are no significant π···π interactions in the solid-state structure of 5a (for details, see Supporting Information File 1) [50-57].
![[1860-5397-15-262-1]](/bjoc/content/figures/1860-5397-15-262-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Molecular structure of 1H-pyridine 5a (50% thermal ellipsoids), showing the intramolecular N–H···O bond as dashed orange line. H-bond details N1–H 0.90(2) Å, H···O1 1.87(2) Å, N1···O2 2.624(2) Å, O1–H···O2 140(1)°.
Figure 1: Molecular structure of 1H-pyridine 5a (50% thermal ellipsoids), showing the intramolecular N–H···O ...
![[1860-5397-15-262-2]](/bjoc/content/figures/1860-5397-15-262-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Supramolecular C–H···N [36-39] and C–H···π [40-49] interactions around the 6-positioned phenyl ring in 5a. Details of C–H···N bond (dashed orange line) C11–H 0.95 Å, H···N2 2.61 Å, C11···N2 3.263(2) Å, C11–H···N2 127°. Symmetry transformations are i = 1−x, 1−y, 1−z; ii = x, 3/2−y, −1/2+z, iii = 1−x, −1/2+y, −1/2−z.
Figure 2: Supramolecular C–H···N [36-39] and C–H···π [40-49] interactions around the 6-positioned phenyl ring in 5a. Detail...
Photophysical properties
Photophysical properties of 1H-pyridines 5 and 8
1H-Pyridine derivatives 5 are yellow or orange compounds under daylight (Figure 3, top) and fluoresce in solution (Figure 3, center) and in the solid state (Figure 3, bottom). Therefore, the photophysical properties were studied by absorption and emission spectroscopy (Figure 4, Table 5).
![[1860-5397-15-262-3]](/bjoc/content/figures/1860-5397-15-262-3.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: 1H-Pyridine derivatives 5 as solids under daylight (top), under UV light (λexc = 365 nm, c(5) = 10−4 M) in dichloromethane solution (center), and under UV light (λexc = 365 nm) in the solid state (bottom).
Figure 3: 1H-Pyridine derivatives 5 as solids under daylight (top), under UV light (λexc = 365 nm, c(5) = 10−4...
![[1860-5397-15-262-4]](/bjoc/content/figures/1860-5397-15-262-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Selected normalized absorption (solid lines) and emission (dashed lines) spectra of 1H-pyridines 5a–e (recorded in dichloromethane at T = 298 K).
Figure 4: Selected normalized absorption (solid lines) and emission (dashed lines) spectra of 1H-pyridines 5a...
Table 5: Photophysical properties of 1H-pyridines 5.
| Entry | Compound | R1 | R2 | λmax,abs [nm]a (ε [L·mol−1·cm−1]) | λmax,em [nm]b (Φf)c | Stokes shift Δν̃ [cm−1] |
|---|---|---|---|---|---|---|
| 1 | 5a | Ph | Ph | 281 (26100), 319 (17400), 418 (9800) | 545 (0.02) | 5600 |
| 2 | 5b | p-F3CC6H4 | Ph | 272 (27000), 326 (17500), 424 (8900) | 565 (0.01) | 5600 |
| 3 | 5c | p-NCC6H4 | Ph | 280 (37600), 333 (17300), 431 (9500) | 585 (0.01) | 6100 |
| 4 | 5d | Ph | p-F3CC6H4 | 274 (32000), 321 (18100), 428 (10000) | 565 (0.01) | 5700 |
| 5 | 5e | Ph | p-NCC6H4 | 283 (36000), 322 (15600), 434 (8600) | 579 (0.01) | 5800 |
| 6 | 5f | p-MeOC6H4 | p-F3CC6H4 | 261 (26200), 306 (28400), 429 (10400) | 557 (0.02) | 5400 |
| 7 | 5g | p-F3CC6H4 | p-MeOC6H4 | 260 (20300), 324 (43800), 420 (10000) | 562 (0.02) | 6000 |
| 8 | 5h | 2-thienyl | Ph | 273 (21800), 308 (26700), 433 (9200) | 560 (0.03) | 5200 |
aRecorded in dichloromethane, T = 293 K, c(5) = 10−6 M; brecorded in dichloromethane, T = 293 K, c(5) = 10−7 M; cfluorescence quantum yields were determined relative to coumarin153 (Φf = 0.54) as a standard in ethanol [58].
All compounds show three absorption maxima at around 275, 320 and 430 nm, where the longest wavelength absorption maxima exhibit extinction coefficients of around 9500 L·mol−1·cm−1 (Table 5). Upon introducing electron-withdrawing substituents on the aryl rings the longest wavelength maxima shift bathochromically (Table 5, entries 2–5). The redshift qualitatively corresponds with the strength of the acceptor group (Table 5, entries 3 and 5). However, as can be seen from entries 2–5 (Table 5), the placement of the acceptor group at the 4 or 6-aryl substituent does not affect the absorption energies. This situation changes to a minor extent upon placing an additional donor substituent at the remaining phenyl substituent (Table 5, entries 6 and 7). A thienyl substituent instead of a phenyl substituent causes a redshift of the longest wavelength absorption maximum (Table 5, entries 1 and 8).
Upon excitation at the longest wavelength absorption band dichloromethane solutions of all compounds 5 fluoresce with emission maxima at around 565 nm (Table 5, entries 2–5). Upon the introduction of electron-withdrawing substituents the maxima are shifted bathochromically, similarly as the absorption maxima, and the shift is qualitatively correlated with the acceptor strength. In comparison, the introduction of another electron-donating substituent does not significantly change the luminescence characteristics (Table 5, entries 2, 4, 6 and 7). The Stokes shifts fall in a range between 5000 and 6100 cm−1 and the fluorescence quantum yields of the 1H-pyridines 5 account between 1 and 3%.
Besides solution fluorescence all 1H-pyridines 5 also luminesce in the solid state (Figure 3, bottom). The emission maxima of two selected 1H-pyridines 5 were determined (Figure 5, Table 6), showing a similar behavior in the solid state as in solution. The emission maximum of the unsubstituted 1H-pyridine 5a appears at 540 nm, while the CF3-substituted 1H-pyridine 5b emits bathochromically shifted at 604 nm.
![[1860-5397-15-262-5]](/bjoc/content/figures/1860-5397-15-262-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Selected normalized emission spectra of 1H-pyridine 5a and 5b in the solid state at T = 298 K.
Figure 5: Selected normalized emission spectra of 1H-pyridine 5a and 5b in the solid state at T = 298 K.
Table 6: Photophysical properties of 1H-pyridines 5a and 5b in the solid state.
| Compound | R1 | R2 | λmax,em [nm]a |
|---|---|---|---|
| 5a | H | H | 540 |
| 5b | CF3 | H | 604 |
aλexc = 420 nm.
In addition, both ester-substituted 1H-pyridines 8a and 8b also possess interesting photophysical properties (Figure 6, Table 7). Under daylight they are yellow and they fluoresce in solution and in the solid state. The three absorption maxima are found at around 270, 315 and 415 nm. The methoxy group in the spectrum of compound 8b only has a minor influence on the absorption maximum, however, slightly more on the emission maximum. The fluorescence quantum yields Φf of both compounds are lower than 1%.
![[1860-5397-15-262-6]](/bjoc/content/figures/1860-5397-15-262-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Selected normalized absorption (solid lines) and emission (dashed lines) spectra of 1H-pyridines 8a and 8b (recorded in dichloromethane at T = 298 K).
Figure 6: Selected normalized absorption (solid lines) and emission (dashed lines) spectra of 1H-pyridines 8a...
Table 7: Photophysical properties of 1H-pyridines 8.
| Compound | R1 | R2 | λmax,abs [nm]a (ε [L·mol−1·cm−1]) | λmax,em [nm]b (Φf)c | Stokes shift Δ ν̃ [cm−1] |
|---|---|---|---|---|---|
| 8a | Ph | Ph | 274 (20300), 324 (20100), 417 (7700) | 557 (<0.01) | 6000 |
| 8b | p-MeOC6H4 | Ph | 261 (16200), 307 (31300), 419 (11000) | 565 (<0.01) | 6200 |
aRecorded in dichloromethane, T = 293 K, c(8) = 10−6 M; brecorded in dichloromethane, T = 293 K, c(8) = 10−7 M (λexc = 420 nm); cfluorescence quantum yields were determined relative to coumarin153 (Φf = 0.54) as a standard in ethanol [58].
With the addition of the second ester group in the 3-position to 1H-pyridine 8a the fluorescence in the solid state appears to shift to blue. If the phenyl substituent in the 4-position bears an additional methoxy substituent the fluorescence of the 1H-pyridine 8b appears yellow again (Figure 7).
![[1860-5397-15-262-7]](/bjoc/content/figures/1860-5397-15-262-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: Solid-state luminescence of 1H-pyridines 5a, 8a and 8b (λexc = 365 nm).
Figure 7: Solid-state luminescence of 1H-pyridines 5a, 8a and 8b (λexc = 365 nm).
Photophysical properties of α-pyrones 6
All α-pyrone derivatives 6 are yellow or red under daylight (Figure 8, top) and some of them fluoresce in solution (Figure 8, center) and in the solid state (Figure 8, bottom). Therefore, the photophysical properties were studied by absorption and emission spectroscopy (Figure 9, Table 8).
![[1860-5397-15-262-8]](/bjoc/content/figures/1860-5397-15-262-8.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 8: α-Pyrones 6 as solids under daylight (top), selected derivatives under UV light (λexc = 365 nm, c(6) = 10−4 M) in dichloromethane solution (center), and under UV light (λexc = 365 nm) in the solid state (bottom).
Figure 8: α-Pyrones 6 as solids under daylight (top), selected derivatives under UV light (λexc = 365 nm, c(6...
![[1860-5397-15-262-9]](/bjoc/content/figures/1860-5397-15-262-9.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 9: Selected normalized absorption spectra of α-pyrones 6a, 6b, 6d, and 6e recorded in dichloromethane at T = 298 K.
Figure 9: Selected normalized absorption spectra of α-pyrones 6a, 6b, 6d, and 6e recorded in dichloromethane ...
Table 8: Photophysical properties of α-pyrones 6.
| Entry | Compound | R1 | R2 | λmax,abs [nm]a (ε [L·mol−1·cm−1]) | λmax,em [nm]b (Φf)c | Stokes shift Δν̃ [cm−1] |
|---|---|---|---|---|---|---|
| 1 | 6a | Ph | Ph | 258 (15300), 311 (12800), 381 (18000) | – | – |
| 2 | 6b | p-MeOC6H4 | Ph | 254 (10300), 271 (11500), 312 (9600), 404 (21900) | – | – |
| 3 | 6c | p-Me2NC6H4 | Ph | 294 (18800), 482 (47300) | 567 (0.99) | 3100 |
| 4 | 6d | Ph | p-MeOC6H4 | 258 (19700), 358 (30400) | – | – |
| 5 | 6e | Ph | p-Me2NC6H4 | 255 (25600), 289 (23300), 375 (39600), 453 (40600) | 634 (0.01) | 6300 |
| 6 | 6f | p-MeOC6H4 | p-MeOC6H4 | 254 (15000), 364 (25600), 400 (25100) | – | – |
| 7 | 6g | p-F3CC6H4 | p-Me2NC6H4 | 251 (16200), 309 (13900), 372 (20200), 465 (21600) | 673 (<0.01) | 6600 |
aRecorded in dichloromethane, T = 293 K, c(6) = 10−6 M; brecorded in dichloromethane, T = 293 K, c(6) = 10−7 M (λexc = 465 nm); cfluorescence quantum yields were determined relative to DCM (Φf = 0.435) as a standard in ethanol [58].
All compounds show 2–4 absorption maxima and the shortest wavelength maxima appear at around 255 nm. The unsubstituted α-pyrone 6a exhibits its longest wavelength maximum at 381 nm (Table 8, entry 1). A p-methoxyphenyl substituent in the 6-position causes a bathochromic shift (Table 8, entry 2), whereas the same substituent in 4-position leads to a hypsochromic shift (Table 8, entry 4). Interestingly, p-methoxyphenyl substituents at positions 4 and 6 split the longest absorption band into two maxima at 358 nm (arising from the p-methoxyphenyl substituent in the 4-position and at 400 nm arising from the p-methoxyphenyl substituent in the 6-position) (Table 8, entry 6). The introduction of a more strongly electron-donating substituent, such as N,N-dimethylaminophenyl, causes a significant bathochromic shift (Table 8, entries 3 and 5). Donor–acceptor substitution in positions 4 and 6 causes a further bathochromic shift (Table 8, entry 7).
In solution only N,N-dimethylaminophenyl-substituted derivatives fluoresce (Figure 10). While the 6-substituted α-pyrone 6c has a fluorescence maximum at 567 nm, the one for the regioisomer 6e is shifted bathochromically to 634 nm (Table 8, entries 3 and 5). Donor–acceptor substitution in positions 4 and 6 causes a further bathochromic shift to 673 nm (Table 8, entry 7). Most remarkably, the regioisomers 6c and 6e differ quite significantly with respect to their fluorescence quantum yields Φf. While chromophore 6e only emits with an efficiency of 1%, the regioisomer 6c accounts for an extraordinarily high relative quantum yield of 99%.
![[1860-5397-15-262-10]](/bjoc/content/figures/1860-5397-15-262-10.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 10: Selected normalized absorption (solid lines) and emission (dashed lines) spectra of α-pyrones 6c, 6e, and 6g recorded in dichloromethane at T = 298 K.
Figure 10: Selected normalized absorption (solid lines) and emission (dashed lines) spectra of α-pyrones 6c, 6e...
Furthermore, the N,N-dimethylaminophenyl-derivative 6c shows a pronounced emission solvatochromism (Figure 11, Table 9). While the polarity effect on the absorption maximum is only minor within a range of the longest wavelength maximum between 469 and 490 nm, the emission maximum is shifted bathochromically with increasing solvent polarity in a range from green fluorescence (529 nm) in toluene to red fluorescence in DMSO (638 nm) (Figure 12). The observed positive emission solvatochromism is a consequence of a significant change in the dipole moment from the electronic ground to the vibrationally relaxed excited state [59]. Plotting Stokes shifts Δν̃ against the orientation polarizabilities Δƒ of the respective solvents (Lippert plot) [60] gives a reasonable linear correlation with a moderate fit of r2 = 0.970 (Figure 13). The orientation polarizabilities Δƒ were calculated according to Equation 1
where εr is the relative permittivity and n the optical refractive index of the respective solvent.
![[1860-5397-15-262-11]](/bjoc/content/figures/1860-5397-15-262-11.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 11: Absorption (top) and fluorescence (bottom) of compound 6c with variable solvent polarity (left to the right: toluene, ethyl acetate, acetone, DMF and DMSO, c(6c) = 10−4 M; λexc = 365 nm, handheld UV lamp).
Figure 11: Absorption (top) and fluorescence (bottom) of compound 6c with variable solvent polarity (left to t...
![[1860-5397-15-262-12]](/bjoc/content/figures/1860-5397-15-262-12.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 12: Absorption (solid lines) and emission (dashed lines) spectra of α-pyrone 6c in five solvents of different polarity (recorded at T = 298 K).
Figure 12: Absorption (solid lines) and emission (dashed lines) spectra of α-pyrone 6c in five solvents of dif...
![[1860-5397-15-262-13]](/bjoc/content/figures/1860-5397-15-262-13.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 13: Lippert plot for α-pyrone 6c (n = x, r2 = 0.970).
Figure 13: Lippert plot for α-pyrone 6c (n = x, r2 = 0.970).
The change in dipole from the ground to the excited state can be calculated according to the Lippert–Mataga equation (Equation 2)
where ν̃abs represents the absorption and ν̃em the emission maxima (in m−1), µE and µG are the dipole moments in the excited and ground state (in C·m), ε0 (8.8542·10−12 A·s/V·m) is the vacuum permittivity constant, h (6.6256·10−34 J·s) is the Planck’s constant, c (2.9979·1010 cm/s) is the speed of light and a is the radius of the solvent cavity occupied by the molecules (in m). The Onsager radius a, assuming a spherical dipole to approximate the molecular volume of the molecule in solution, was estimated from the optimized ground-state structure of compound 6c obtained by DFT calculations. With an a value of 5.46 Å, the change in dipole moment was calculated to 11.6 D (3.87·10−29 C·m).
All α-pyrones 6 fluoresce in the solid state (Figure 8, bottom) and for five selected α-pyrones 6 the emission maxima were determined (Figure 14, Table 9). The fluorescence maximum of unsubstituted α-pyrone 6a lies at 499 nm and the maxima of the monomethoxy-substituted regioisomers 6b (540 nm) and 6d (489 nm) appear at quite different energies, similar to their corresponding absorption maxima in solution. In comparison to α-pyrone 6a the introduction of two methoxy substituents in derivative 6f results in a bathochromic shift to 526 nm. The solid-state emission of N,N-dimethylaminophenyl derivative 6c shows an enormous redshift to 694 nm. The solid-state fluorescence quantum yield Φf of compound 6c was determined to 11%.
![[1860-5397-15-262-14]](/bjoc/content/figures/1860-5397-15-262-14.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 14: Normalized emission spectra of selected α-pyrones 6a–d,f in the solid state at T = 298 K.
Figure 14: Normalized emission spectra of selected α-pyrones 6a–d,f in the solid state at T = 298 K.
Table 9: Photophysical properties of selected α-pyrones 6 in the solid state.
| Compound | R1 | R2 | λmax,em [nm]a |
|---|---|---|---|
| 6a | Ph | Ph | 499 |
| 6b | p-MeOC6H4 | Ph | 540 |
| 6c | p-Me2NC6H4 | Ph | 694b |
| 6d | Ph | p-MeOC6H4 | 489 |
| 6f | p-MeOC6H4 | p-MeOC6H4 | 526 |
aλexc = 380 nm; bλexc = 480 nm.
Interestingly, the α-pyrone 6e with the N,N-dimethylaminophenyl substituent in 4-position only fluoresces weakly in solution but shows a strong fluorescence in the solid state. This finding suggests that by restricting intramolecular motion and vibration, which enables radiation-less deactivation of the excited state [61], an AIE (aggregation‐induced emission) or AIEE (aggregation-induced enhanced emission), might become operative [62-64].
The AIE or AIEE effect was assessed by measuring the emission spectra of α-pyrone 6e in THF/water at variable ratios (Figure 15). In pure THF α-pyrone 6e displays an emission maximum at 644 nm with a relative intensity of 54. The addition of water first quenches the fluorescence and at a water/THF ratio of 80% aggregates are formed and the emission maximum is shifted to 632 nm. The maximal relative intensity of 130 is reached for a ratio of 85%, which is more intense than in pure THF, therefore, an AIEE effect occurs. Further increasing the water content slightly quenches the emission.
![[1860-5397-15-262-15]](/bjoc/content/figures/1860-5397-15-262-15.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 15: Fluorescence of compound 6e in different THF/water fractions (top, λexc = 365 nm, handheld UV lamp) and I/I0 vs %H2O of α-pyrone 6e in THF/water mixtures containing different water fractions (bottom, recorded at T = 298 K).
Figure 15: Fluorescence of compound 6e in different THF/water fractions (top, λexc = 365 nm, handheld UV lamp)...
Computational studies
Computational studies on 1H-pyridines 5 and 8
For a further elucidation of the electronic structure the geometries of the electronical ground-state structures of the 1H-pyridines 5 and 8 were optimized using Gaussian 09 with the B3LYP functional [65-68] and the Pople 6-311G** basis set [69], applying vacuum calculations as well as the polarizable continuum model (PCM) with dichloromethane as a solvent [70] (for details of the DFT calculations, see Supporting Information File 1). The optimized geometries were verified by frequency analyses of the local minima. The electronic absorptions of the 1H-pyridines 5 and 8 were calculated on the level of TDDFT theory employing the B3LYP functional and the Pople 6-311G** basis set. The calculated absorption maxima are in accordance with the experimentally determined maxima (for details, see Tables S7 and S8 in Supporting Information File 1). Most characteristically, for all 1H-pyridines 5 and 8 the longest wavelength maxima representing the Franck–Condon S1 states are characterized by HOMO–LUMO transitions and S2 states are represented by HOMO–LUMO+1 transitions.
The computed Kohn–Sham frontier molecular orbitals show that the coefficient density of the HOMO of the 1H-pyridines 5f and 5g with an electron-withdrawing and an electron-donating substituent is located on the 1H-pyridine core, the ester and cyano substituents and also on the electron-rich aryl substituent. For the LUMO, the coefficient density is spread over the whole scaffold (Figure 16).
![[1860-5397-15-262-16]](/bjoc/content/figures/1860-5397-15-262-16.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 16: Selected DFT-computed (B3LYP 6-311G**) Kohn–Sham FMOs for 1H-pyridines 5f and 5g representing contributions of the longest wavelength Franck–Condon absorption bands.
Figure 16: Selected DFT-computed (B3LYP 6-311G**) Kohn–Sham FMOs for 1H-pyridines 5f and 5g representing contr...
Computational studies on α-pyrones 6
For further elucidation of the electronic structure the geometries of the electronical ground-state structures of the α-pyrones 6 were optimized using Gaussian 09 with the B3LYP functional [65-68] and the Pople 6-311G** basis set [69], applying vacuum calculations as well as the polarizable continuum model (PCM) with dichloromethane as a solvent [70] (for details on the DFT calculations, see Supporting Information File 1). The optimized geometries were verified by frequency analyses of the local minima. The electronic absorptions of the α-pyrones 6 were calculated on the level of TDDFT theory employing the B3LYP functional and the Pople 6-311G** basis set. The calculated absorption maxima are in accordance with the experimentally determined maxima (for details, see Table S10 in Supporting Information File 1). For all α-pyrones 6 the longest wavelength maxima are characterized by Franck–Condon S1 states representing HOMO–LUMO transitions (Figure 17).
![[1860-5397-15-262-17]](/bjoc/content/figures/1860-5397-15-262-17.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 17: Selected DFT-computed (B3LYP 6-311G**) Kohn–Sham FMOs for 1H-pyridines 6a, 6c, 6e, 6f, and 6g and representing contributions of the longest wavelength Franck-Condon absorption bands.
Figure 17: Selected DFT-computed (B3LYP 6-311G**) Kohn–Sham FMOs for 1H-pyridines 6a, 6c, 6e, 6f, and 6g and r...
The computed Kohn–Sham frontier molecular orbitals show that the coefficient density of the HOMO in the parent α-pyrone 6a is localized on the α-pyrone core and on the phenyl substituent in the 6-position. For an N,N-dimethylaminophenyl substituent, there is no difference, but for an N,N-dimethylaminophenyl substituent in the 4-position the coefficient density is shifted towards this substituent. With electron-donating substituents in the 4- and 6-position, the coefficient density is again located on the core and the phenyl substituent in 6-position. Donor substituents in 4-position and acceptor substituents in 6-position cause a coefficient density shift towards the 4-substituent. The coefficient density in the LUMO in all compounds is spread over the whole scaffold.
Conclusion
The cyclocondensation of alkynones and ethyl cyanoacetate, depending on the reaction conditions, the type of base, and the reaction temperature, as well as the electronic nature of the alkynone 3 furnishes either 1H-pyridines or α-pyrones. Optimized reaction conditions finally give rise to 8 examples of 1H-pyridines and 6 examples of α-pyrones. While the presence of electron-withdrawing substituents mainly furnish 1H-pyridines and electron-donating groups lead to the formation of α-pyrones. The strongly electron-donating p-N,N-dimethylaminophenyl group furnishes α-pyrones.
1H-Pyridines absorb and emit intensively in solution and in the solid state. While the absorption behavior is not affected by the substitution pattern the emission maxima are shifted bathochromically with increasing acceptor strength. The same trend manifests for the solid-state emission.
For α-pyrones the photophysical properties are considerably depending on the substituent pattern. The absorption and emission maxima are shifted bathochromically with increasing donor strength. α-Pyrones are only weakly fluorescent in solution. However, with distinct p-N,N-dimethylaminophenyl substitution in 6-position, an extraordinarily high fluorescence quantum yield of 99% in solution and 11% in the solid state was achieved. Interestingly, the isomeric p-N,N-dimethylaminophenyl substitution in 6-position represents a system with aggregation-induced emission enhancement. These design principles of luminescent 1H-pyridines and α-pyrones as polarity sensitive tunable luminophores and the observed aggregation-induced emission enhancement are currently under further investigation.
Experimental
Typical procedure for the cyclocondensation synthesis of compound 5d
1-Phenyl-3-[4-(trifluoromethyl)phenyl]prop-2-yn-1-one (3h, 1.40 g, 5.00 mmol), was placed in a dry Schlenk tube and ethanol (10 mL) was added. Sodium carbonate (430 mg, 4.00 mmol), sodium acetate (250 mg, 3.00 mmol), water (5 mL), and ethyl cyanoacetate (4, 2.31 g, 20.0 mmol) were added and the mixture was stirred at 75 °C for 16 h. After the addition of CH2Cl2 (5.00 mL) and NaOH/FeSO4 solution (5.00 mL), the solution was extracted with CH2Cl2 (3 × 50.0 mL). The combined organic layers were dried (anhydrous MgSO4) and the solvent was removed in vacuo. The residue was purified by flash chromatography on silica gel (n-hexane/EtOAc 12:1 to 5:1 to 0:1) and washed with hot ethanol (5 mL) to give compound 5d (513 mg, 25%) as orange solid. Mp 223–233 °C; 1H NMR (300 MHz, CDCl3) δ 1.38 (t, J = 7.1 Hz, 3H), 4.30 (q, J = 7.1 Hz, 2H), 7.12 (dd, J = 1.5, 1.6 Hz, 1H), 7.44 (dd, J = 1.5, 1.6 Hz, 1H), 7.56–7.63 (m, 3H), 7.78–7.84 (m, 6H), 14.57 (s, 1H); 13C NMR (150 MHz, CDCl3) δ 14.7 (CH3), 60.6 (CH2), 63.5 (Cquat), 109.2 (CH), 116.0 (CH), 119.5 (Cquat), 123.9 (q, JC–F = 273 Hz, Cquat), 126.1 (CH), 126.2–126.7 (m, CH), 127.8 (CH), 130.1 (CH), 131.6 (CH), 132.3 (Cquat), 132.4 (q, JC–F = 32.9 Hz, Cquat), 140.6 (Cquat), 146.6 (Cquat), 151.2 (Cquat), 156.0 (Cquat), 171.0 (Cquat); EIMS (70 eV, m/z (%)): 410 ([M]+, 24), 366 (24), 365 ([M − C2H5O]+, 100), 339 (12), 338 ([M − C3H5O2]+, 53), 337 (38), 308 (8), 240 (23), 149 ([M − C16H12F3]+, 11); IR (ATR) ν̃ [cm−1]: 3092 (w), 2992 (w), 2963 (w), 2943 (w), 2876 (w), 2806 (w), 2193 (m), 1625 (m), 1620 (m), 1597 (m), 1577 (m), 1506 (w), 1466 (w), 1413 (w), 1396 (w), 1369 (w), 1308 (m), 1300 (m), 1283 (s), 1258 (m), 1206 (w), 1165 (m), 1115 (s), 1092 (m), 1082 (m), 1071 (m), 1045 (s), 1030 (m), 1015 (m), 980 (m), 976 (m), 968 (w), 920 (w), 885 (w), 874 (w), 837 (s), 829 (m), 764 (s), 745 (w), 727 (w), 685 (m), 662 (w), 655 (w), 650 (w); UV–vis (CH2Cl2) λmax [nm] (ε [L·mol−1·cm−1]): 274 (32000), 321 (18100), 428 (10000); emission (CH2Cl2) λmax [nm] (Stokes shift [cm−1]): 565 (5700); quantum yield (CH2Cl2) Φf: 0.01; Anal. calcd for C23H17F3N2O2 (410.1): C, 67.31; H, 4.18; N, 6.83; found: C, 67.50; H, 4.32; N, 6.70.
Typical procedure for the cyclocondensation synthesis of compound 6c
1-[4-(Dimethylamino)phenyl]-3-phenylprop-2-yn-1-one (3c, 249 mg, 1.00 mmol) was placed in a dry Schlenk tube and ethanol (2 mL) was added. Sodium carbonate (86.0 mg, 0.80 mmol), sodium acetate (50.0 mg, 0.60 mmol), water (1 mL), and ethyl cyanoacetate (4, 462 mg, 4.00 mmol) were added and the mixture was stirred at 75 °C for 16 h. After the addition of CH2Cl2 (5.00 mL) and NaOH/FeSO4 solution (5.00 mL), the solution was extracted with CH2Cl2 (3 × 50.0 mL). The combined organic layers were dried (anhydrous MgSO4) and the solvent was removed in vacuo. The residue was purified by flash chromatography on silica gel (n-hexane/EtOAc 5:1 to 1:1 to 0:1) and washed with hot ethanol (2.00 mL) and compound 6c (37.0 mg, 12%) was obtained as deep purple solid. Mp 224–253 °C; 1H NMR (300 MHz, CDCl3) δ 3.10 (s, 6H), 6.69 (s, 1H), 6.69–6.75 (m, 2H), 7.51–7.58 (m, 3H), 7.67–7.73 (m, 2H), 7.78–7.85 (m, 2 H); 13C NMR (75 MHz, CDCl3) δ 40.2 (CH3), 91.1 (Cquat), 100.1 (CH), 111.8 (CH), 115.7 (Cquat), 116.5 (Cquat), 128.0 (CH), 128.8 (CH), 129.3 (CH), 131.6 (CH), 135.3 (Cquat), 153.4 (Cquat), 160.3 (Cquat), 164.1 (Cquat), 164.9 (Cquat). EIMS (70 eV, m/z (%)): 317 (15), 316 ([M]+, 66), 293 (11), 289 ([M − CN]+, 11), 288 ([M − CO]+, 51), 287 (19), 167 ([M − C9H11NO]+, 18), 150 (11), 149 ([M − C11H5NO]+, 100), 148 (20), 144 (13), 127 ([M − C11H11NO2]+, 12), 85 (13), 71 (22), 57 (18), 43 ([M − C18H11NO2]+, 13); IR (ATR) ν̃ [cm−1]: 3092 (w), 3048 (w), 2901 (w), 2864 (w), 2812 (w), 2739 (w), 2212 (w), 1708 (m), 1706 (m), 1609 (m), 1589 (m), 1570 (m), 1530 (m), 1497 (m), 1491 (m), 1482 (m), 1478 (m), 1473 (m), 1467 (m), 1458 (m), 1433 (m), 1375 (m), 1360 (m), 1333 (m), 1252 (m), 1209 (m), 1171 (m), 1159 (m), 1125 (m), 1111 (m), 1082 (m), 1059 (m), 1020 (m), 995 (m), 953 (m), 945 (m), 924 (w), 853 (m), 818 (s), 795 (m), 750 (m), 748 (m), 692 (s), 669 (m), 640 (m); UV–vis (CH2Cl2) λmax [nm] (ε [L·mol−1·cm−1]): 294 (18800), 482 (47300); emission (CH2Cl2) λmax [nm] (Stokes-shift [cm−1]): 567 (3100); quantum yield (CH2Cl2) Φf: 0.99; emission (solid) λmax [nm]: 694; quantum yield (solid) Φf: 0.11; Anal. calcd for C20H16N2O2 (316.1): C, 75.93; H, 5.10; N, 8.86; found: C, 75.74; H, 5.19; N, 8.56.
Typical procedure for the cyclocondensation synthesis of compound 8a
1,3-Diphenylprop-2-yn-1-one (3a, 103 mg, 0.50 mmol) was placed in a dry Schlenk tube and ethanol (1.00 mL) was added. Sodium carbonate (43.0 mg, 0.40 mmol), sodium acetate (25.0 mg, 0.30 mmol), water (50.0 µL) and diethyl (Z)-3-amino-2-cyanopent-2-endioate (7, 226 mg, 1.00 mmol) were added and the mixture was stirred at 75 °C for 16 h. After the addition of CH2Cl2 (5.00 mL) and NaOH/FeSO4 solution (5.00 mL), the solution was extracted with CH2Cl2 (3 × 50.0 mL). The combined organic layers were dried (anhydrous MgSO4) and the solvent was removed in vacuo. The residue was purified by flash chromatography on silica gel (n-hexane/EtOAc 5:1 to 1:1 to 0:1) and washed with hot ethanol (5.00 mL) to furnish compound 8a (108 mg, 52%) as yellow solid. Mp 140–146 °C; 1H NMR (300 MHz, CDCl3) δ 1.08 (t, J = 7.2 Hz, 3H), 1.37 (t, J = 7.1 Hz, 3H), 4.22 (q, J = 7.2 Hz, 2H), 4.31 (q, J = 7.1 Hz, 2H), 6.94 (d, J = 1.9 Hz, 1H), 7.40–7.48 (m, 5H), 7.55–7.62 (m, 3H), 7.75–7.84 (m, 2H), 15.60 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 13.7 (CH3), 14.7 (CH3), 60.9 (CH2), 62.2 (CH2), 62.7 (Cquat), 112.2 (CH), 118.2 (Cquat), 122.8 (Cquat), 126.3 (CH), 127.9 (CH), 128.8 (CH), 129.6 (CH), 130.1 (CH), 131.77 (CH), 131.84 (Cquat), 137.4 (Cquat), 146.1 (Cquat), 151.9 (Cquat), 153.0 (Cquat), 165.1 (Cquat), 172.1 (Cquat); EIMS (70 eV, m/z (%)): 415 ([M + H]+, 26), 414 ([M]+, 97), 369 ([M − C2H5O]+, 18), 342 (28), 341 ([M − C3H5O]+, 27), 340 ([M − C3H6O]+, 12), 314 (20), 313 ([M − C8H5]+, 57), 298 (24), 297 ([M − C5H9O3]+, 27), 296 ([M − C5H10O3]+, 33), 287 (10), 286 (25), 271 (24), 270 ([M − C6H8O4]+, 100), 269 (23), 268 ([M − C6H10O4]+, 21), 266 (12), 258 ([M − C7H10NO3]+, 14), 245 (18), 241 (13), 240 (26), 231 (15), 230 ([M − C8H10NO4]+, 34), 203 (19), 202 (31), 164 (13); IR (ATR) ν̃ [cm−1]: 2978 (w), 2895 (w), 2197 (m), 1722 (m), 1636 (m), 1593 (s), 1578 (m), 1501 (m), 1489 (w), 1462 (w), 1441 (w), 1420 (w), 1364 (w), 1308 (m), 1288 (m), 1248 (s), 1188 (w), 1169 (m), 1134 (m), 1113 (s), 1092 (m), 1067 (m), 1047 (m), 1028 (w), 1001 (w), 885 (m), 854 (m), 847 (w), 775 (w), 758 (s), 746 (m), 694 (m), 658 (w); UV–vis (CH2Cl2) λmax [nm] (ε [L·mol−1·cm−1]): 274 (20300), 324 (20100), 417 (7700); emission (CH2Cl2) λmax [nm] (Stokes shift [cm−1]): 557 (6000); quantum yield (CH2Cl2) Φf = < 0.01; Anal. calcd for C25H22N2O4 (414.2): C, 72.45; H, 5.35; N, 6.76; found: C, 71.97; H, 5.45; N, 6.52.
Supporting Information
For experimental details of the synthesis and analytical data of compounds 3, 5, 6, and 8, 1H and 13C NMR, and absorption and emission spectra of compounds 5, 6, and 8, solid state emission spectra of compounds 5 and 6, X-ray structural data of compound 5a, and DFT/TDDFT calculations of compounds 5 and 6, see below.
| Supporting Information File 1: Additional experimental and calculated data. | ||
| Format: PDF | Size: 4.7 MB | Download |
Acknowledgements
The authors gratefully acknowledge the Deutsche Forschungsgemeinschaft (DFG, Mu 1088-9/1) and the Fonds der Chemischen Industrie and also cordially thank Arno Schneeweis for measuring the quantum yield of compound 6c in the solid state in cooperation with Hamamatsu Photonics Deutschland GmbH, Herrsching am Ammersee, Germany.
References
-
Yao, J.; Yang, M.; Duan, Y. Chem. Rev. 2014, 114, 6130–6178. doi:10.1021/cr200359p
Return to citation in text: [1] -
Wysocki, L. M.; Lavis, L. D. Curr. Opin. Chem. Biol. 2011, 15, 752–759. doi:10.1016/j.cbpa.2011.10.013
Return to citation in text: [1] -
Day, R. N.; Davidson, M. W. Chem. Soc. Rev. 2009, 38, 2887–2921. doi:10.1039/b901966a
Return to citation in text: [1] -
Wegner, K. D.; Hildebrandt, N. Chem. Soc. Rev. 2015, 44, 4792–4834. doi:10.1039/c4cs00532e
Return to citation in text: [1] -
Zhu, M.; Yang, C. Chem. Soc. Rev. 2013, 42, 4963–4976. doi:10.1039/c3cs35440g
Return to citation in text: [1] -
Figueira-Duarte, T. M.; Müllen, K. Chem. Rev. 2011, 111, 7260–7314. doi:10.1021/cr100428a
Return to citation in text: [1] -
Forrest, S. R.; Thompson, M. E. Chem. Rev. 2007, 107, 923–925. doi:10.1021/cr0501590
Return to citation in text: [1] -
Grimsdale, A. C.; Leok Chan, K.; Martin, R. E.; Jokisz, P. G.; Holmes, A. B. Chem. Rev. 2009, 109, 897–1091. doi:10.1021/cr000013v
Return to citation in text: [1] -
Kanibolotsky, A. L.; Perepichka, I. F.; Skabara, P. J. Chem. Soc. Rev. 2010, 39, 2695–2728. doi:10.1039/b918154g
Return to citation in text: [1] -
Wang, X.; Zhang, F.; Liu, J.; Tang, R.; Fu, Y.; Wu, D.; Xu, Q.; Zhuang, X.; He, G.; Feng, X. Org. Lett. 2013, 15, 5714–5717. doi:10.1021/ol402745r
Return to citation in text: [1] -
de Halleux, V.; Calbert, J.-P.; Brocorens, P.; Cornil, J.; Declercq, J.-P.; Brédas, J.-L.; Geerts, Y. Adv. Funct. Mater. 2004, 14, 649–659. doi:10.1002/adfm.200400006
Return to citation in text: [1] -
Hassheider, T.; Benning, S. A.; Kitzerow, H.-S.; Achard, M.-F.; Bock, H. Angew. Chem., Int. Ed. 2001, 40, 2060–2063. doi:10.1002/1521-3773(20010601)40:11<2060::aid-anie2060>3.3.co;2-8
Return to citation in text: [1] -
Kartha, K. K.; Babu, S. S.; Srinivasan, S.; Ajayaghosh, A. J. Am. Chem. Soc. 2012, 134, 4834–4841. doi:10.1021/ja210728c
Return to citation in text: [1] -
Thimmarayaperumal, S.; Shanmugam, S. ACS Omega 2017, 2, 4900–4910. doi:10.1021/acsomega.7b00627
Return to citation in text: [1] -
Park, S.-Y.; Ebihara, M.; Kubota, Y.; Funabiki, K.; Matsui, M. Dyes Pigm. 2009, 82, 258–267. doi:10.1016/j.dyepig.2009.01.014
Return to citation in text: [1] -
Hagimori, M.; Mizuyama, N.; Yokota, K.; Nishimura, Y.; Suzuta, M.; Tai, C.-K.; Wang, B.-C.; Wang, S.-L.; Shih, T.-L.; Wu, K.-D.; Huang, Z.-S.; Tseng, S.-C.; Chen, C.-Y.; Lu, J.-W.; Wei, H.-H.; Kawashima, K.; Kawashima, S.; Tominaga, Y. Dyes Pigm. 2012, 92, 1069–1074. doi:10.1016/j.dyepig.2011.05.014
Return to citation in text: [1] [2] [3] -
Levi, L.; Müller, T. J. J. Chem. Soc. Rev. 2016, 45, 2825–2846. doi:10.1039/c5cs00805k
Return to citation in text: [1] -
Levi, L.; Müller, T. J. J. Eur. J. Org. Chem. 2016, 2902–2918. doi:10.1002/ejoc.201600409
Return to citation in text: [1] -
Müller, T. J. J. Drug Discovery Today: Technol. 2018, 29, 19–26. doi:10.1016/j.ddtec.2018.06.003
Return to citation in text: [1] -
Merkt, F. K.; Müller, T. J. J. Isr. J. Chem. 2018, 58, 889–900. doi:10.1002/ijch.201800058
Return to citation in text: [1] -
D'Souza, D. M.; Müller, T. J. J. Chem. Soc. Rev. 2007, 36, 1095–1108. doi:10.1039/b608235c
Return to citation in text: [1] -
Gers-Panther, C. F.; Müller, T. J. J. Adv. Heterocycl. Chem. 2016, 120, 67–98. doi:10.1016/bs.aihch.2016.04.007
Return to citation in text: [1] -
Breuer, N.; Müller, T. J. J. Synthesis 2018, 50, 2741–2752. doi:10.1055/s-0037-1610129
Return to citation in text: [1] [2] -
Tominaga, Y.; Mizuyama, N.; Murakami, Y.; Nagaoka, J.; Kohra, S.; Ueda, K.; Hiraoka, K.; Shigemitsu, Y. Heterocycles 2006, 68, 1105–1108. doi:10.3987/com-06-10741
Return to citation in text: [1] [2] -
Mizuyama, N.; Murakami, Y.; Nakatani, T.; Kuronita, K.; Kohra, S.; Ueda, K.; Hiraoka, K.; Tominaga, Y. J. Heterocycl. Chem. 2008, 45, 265–277. doi:10.1002/jhet.5570450133
Return to citation in text: [1] -
Tominaga, Y.; Mizuyama, N.; Shigemitsu, Y.; Wang, B.-C. Heterocycles 2009, 78, 555–570. doi:10.3987/rev-08-642
Return to citation in text: [1] -
Shankar, R.; Shukla, H.; Singh, U. S.; Thakur, V.; Hajela, K. Synth. Commun. 2011, 41, 2738–2746. doi:10.1080/00397911.2010.515350
Return to citation in text: [1] -
Fouli, F. A.; Basyouni, M. N. Acta Chim. Acad. Sci. Hung. 1981, 106, 297–302.
Return to citation in text: [1] [2] -
Sadek, K. U.; Fahmy, S. M.; Mohareb, R. M.; Elnagdi, M. H. J. Chem. Eng. Data 1984, 29, 101–103. doi:10.1021/je00035a033
Return to citation in text: [1] -
Takaya, H.; Naota, T.; Murahashi, S.-I. J. Am. Chem. Soc. 1998, 120, 4244–4245. doi:10.1021/ja974106e
Return to citation in text: [1] [2] -
Hammond, G. B.; Plevey, R. G.; Sampson, P.; Tatlow, J. C. J. Fluorine Chem. 1988, 40, 81–98. doi:10.1016/s0022-1139(00)83057-2
Return to citation in text: [1] -
D'Souza, D. M.; Müller, T. J. J. Nat. Protoc. 2008, 3, 1660–1665. doi:10.1038/nprot.2008.152
Return to citation in text: [1] -
Götzinger, A. C.; Müller, T. J. J. Org. Biomol. Chem. 2016, 14, 3498–3500. doi:10.1039/c6ob00483k
Return to citation in text: [1] -
Karpov, A. S.; Müller, T. J. J. Org. Lett. 2003, 5, 3451–3454. doi:10.1021/ol035212q
Return to citation in text: [1] -
CCDC 1944699 (5a) contains the supplementary crystallographic data (excluding structure factors) for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.
Return to citation in text: [1] -
Desiraju, G. R.; Steiner, T. The weak hydrogen bond. IUCr Monograph on Crystallography; Oxford Science: Oxford, United KIngdom, 1999; Vol. 9.
Return to citation in text: [1] [2] -
Desiraju, G. R. Acc. Chem. Res. 2002, 35, 565–573. doi:10.1021/ar010054t
Return to citation in text: [1] [2] -
Janiak, C.; Scharmann, T. G. Polyhedron 2003, 22, 1123–1133. doi:10.1016/s0277-5387(03)00098-6
Return to citation in text: [1] [2] -
Shivakumar, K.; Vidyasagar, A.; Naidu, A.; Gonnade, R. G.; Sureshan, K. M. CrystEngComm 2012, 14, 519–524. doi:10.1039/c1ce05997a
Return to citation in text: [1] [2] -
Anelli, P. L.; Ashton, P. R.; Ballardini, R.; Balzani, V.; Delgado, M.; Gandolfi, M. T.; Goodnow, T. T.; Kaifer, A. E.; Philp, D. J. Am. Chem. Soc. 1992, 114, 193–218. doi:10.1021/ja00027a027
Return to citation in text: [1] [2] -
Janiak, C.; Temizdemir, S.; Dechert, S.; Deck, W.; Girgsdies, F.; Heinze, J.; Kolm, M.; Scharmann, T.; Zipffel, O. Eur. J. Inorg. Chem. 2000, 1229–1241. doi:10.1002/(sici)1099-0682(200006)2000:6<1229::aid-ejic1229>3.3.co;2-g
Return to citation in text: [1] [2] -
N. Laxmi Madhavi, N.; R. Desiraju, G.; K. Katz, A.; L. Carrell, H.; Nangia, A. Chem. Commun. 1997, 1953–1954. doi:10.1039/a705836e
Return to citation in text: [1] [2] -
Nishio, M. CrystEngComm 2004, 6, 130–158. doi:10.1039/b313104a
Return to citation in text: [1] [2] -
Nishio, M. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. doi:10.1039/c1cp20404a
Return to citation in text: [1] [2] -
Nishio, M.; Hirota, M.; Umezawa, Y. The CH/π interaction (evidence, nature and consequences); Wiley-VCH: New York, NY, U.S.A., 1998.
Return to citation in text: [1] [2] -
Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawa, H. CrystEngComm 2009, 11, 1757–1788. doi:10.1039/b902318f
Return to citation in text: [1] [2] -
Steiner, T.; Tamm, M.; Lutz, B.; Van Der Maas, J. Chem. Commun. 1996, 1127–1128. doi:10.1039/cc9960001127
Return to citation in text: [1] [2] -
Umezawa, Y.; Tsuboyama, S.; Honda, K.; Uzawa, J.; Nishio, M. Bull. Chem. Soc. Jpn. 1998, 71, 1207–1213. doi:10.1246/bcsj.71.1207
Return to citation in text: [1] [2] -
Weiss, H.-C.; Bläser, D.; Boese, R.; Doughan, B. M.; Haley, M. M. Chem. Commun. 1997, 1703–1704. doi:10.1039/a704070i
Return to citation in text: [1] [2] -
Janiak, C. J. Chem. Soc., Dalton Trans. 2000, 3885–3896. doi:10.1039/b003010o
Return to citation in text: [1] -
Janiak, C.; Uehlin, L.; Wu, H.-P.; Klüfers, P.; Piotrowski, H.; Scharmann, T. G. J. Chem. Soc., Dalton Trans. 1999, 3121–3131. doi:10.1039/a904829d
Return to citation in text: [1] -
Lozana, V.; Lassahn, P.-G.; Zhang, C.; Wu, B.; Janiak, C.; Rheinwald, G.; Lang, H. Z. Naturforsch., B: J. Chem. Sci. 2003, 58, 1152–1164. doi:10.1515/znb-2003-1202
Return to citation in text: [1] -
Wu, H.-P.; Janiak, C.; Rheinwald, G.; Lang, H. J. Chem. Soc., Dalton Trans. 1999, 183–190. doi:10.1039/a807450j
Return to citation in text: [1] -
Wu, H.-P.; Janiak, C.; Uehlin, L.; Klüfers, P.; Mayer, P. Chem. Commun. 1998, 2637–2638. doi:10.1039/a807522k
Return to citation in text: [1] -
Yang, X.-J.; Drepper, F.; Wu, B.; Sun, W.-H.; Haehnel, W.; Janiak, C. Dalton Trans. 2005, 256–267. doi:10.1039/b414999h
Return to citation in text: [1] -
Zhang, C.; Janiak, C. Z. Anorg. Allg. Chem. 2001, 627, 1972–1975. doi:10.1002/1521-3749(200108)627:8<1972::aid-zaac1972>3.0.co;2-k
Return to citation in text: [1] -
Zhang, C.; Janiak, C. J. Chem. Crystallogr. 2001, 31, 29–35. doi:10.1023/a:1013774502147
Return to citation in text: [1] -
Rurack, K.; Spieles, M. Anal. Chem. (Washington, DC, U. S.) 2011, 83, 1232–1242. doi:10.1021/ac101329h
Return to citation in text: [1] [2] [3] -
Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, U.S.A., 2006. doi:10.1007/978-0-387-46312-4
Return to citation in text: [1] -
Lippert, E. Z. Elektrochem. 1957, 962–975.
Return to citation in text: [1] -
Luo, J.; Song, K.; Gu, F. I.; Miao, Q. Chem. Sci. 2011, 2, 2029–2034. doi:10.1039/c1sc00340b
Return to citation in text: [1] -
Mei, J.; Hong, Y.; Lam, J. W. Y.; Qin, A.; Tang, Y.; Tang, B. Z. Adv. Mater. (Weinheim, Ger.) 2014, 26, 5429–5479. doi:10.1002/adma.201401356
Return to citation in text: [1] -
Müller, T. J. J. Multicomponent and Domino Syntheses of AIE Chromophores. In Aggregation Induced Emission: Materials and Applications, Fujiki, M.; Liu, B.; Tang, B. Z., Eds.; American Chemical Society: Washington, DC, U.S.A., 2016; pp 85–112. doi:10.1021/bk-2016-1226.ch006
Return to citation in text: [1] -
Liu, B.; Tang, B. Z. Chem. – Asian J. 2019, 14, 672–673. doi:10.1002/asia.201900185
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 1372–1377. doi:10.1063/1.464304
Return to citation in text: [1] [2] -
Kim, K.; Jordan, K. D. J. Phys. Chem. 1994, 98, 10089–10094. doi:10.1021/j100091a024
Return to citation in text: [1] [2] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785
Return to citation in text: [1] [2] -
Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001
Return to citation in text: [1] [2] -
Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650–654. doi:10.1063/1.438955
Return to citation in text: [1] [2] -
Scalmani, G.; Frisch, M. J. J. Chem. Phys. 2010, 132, 114110. doi:10.1063/1.3359469
Return to citation in text: [1] [2]
| 36. | Desiraju, G. R.; Steiner, T. The weak hydrogen bond. IUCr Monograph on Crystallography; Oxford Science: Oxford, United KIngdom, 1999; Vol. 9. |
| 37. | Desiraju, G. R. Acc. Chem. Res. 2002, 35, 565–573. doi:10.1021/ar010054t |
| 38. | Janiak, C.; Scharmann, T. G. Polyhedron 2003, 22, 1123–1133. doi:10.1016/s0277-5387(03)00098-6 |
| 39. | Shivakumar, K.; Vidyasagar, A.; Naidu, A.; Gonnade, R. G.; Sureshan, K. M. CrystEngComm 2012, 14, 519–524. doi:10.1039/c1ce05997a |
| 40. | Anelli, P. L.; Ashton, P. R.; Ballardini, R.; Balzani, V.; Delgado, M.; Gandolfi, M. T.; Goodnow, T. T.; Kaifer, A. E.; Philp, D. J. Am. Chem. Soc. 1992, 114, 193–218. doi:10.1021/ja00027a027 |
| 41. | Janiak, C.; Temizdemir, S.; Dechert, S.; Deck, W.; Girgsdies, F.; Heinze, J.; Kolm, M.; Scharmann, T.; Zipffel, O. Eur. J. Inorg. Chem. 2000, 1229–1241. doi:10.1002/(sici)1099-0682(200006)2000:6<1229::aid-ejic1229>3.3.co;2-g |
| 42. | N. Laxmi Madhavi, N.; R. Desiraju, G.; K. Katz, A.; L. Carrell, H.; Nangia, A. Chem. Commun. 1997, 1953–1954. doi:10.1039/a705836e |
| 43. | Nishio, M. CrystEngComm 2004, 6, 130–158. doi:10.1039/b313104a |
| 44. | Nishio, M. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. doi:10.1039/c1cp20404a |
| 45. | Nishio, M.; Hirota, M.; Umezawa, Y. The CH/π interaction (evidence, nature and consequences); Wiley-VCH: New York, NY, U.S.A., 1998. |
| 46. | Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawa, H. CrystEngComm 2009, 11, 1757–1788. doi:10.1039/b902318f |
| 47. | Steiner, T.; Tamm, M.; Lutz, B.; Van Der Maas, J. Chem. Commun. 1996, 1127–1128. doi:10.1039/cc9960001127 |
| 48. | Umezawa, Y.; Tsuboyama, S.; Honda, K.; Uzawa, J.; Nishio, M. Bull. Chem. Soc. Jpn. 1998, 71, 1207–1213. doi:10.1246/bcsj.71.1207 |
| 49. | Weiss, H.-C.; Bläser, D.; Boese, R.; Doughan, B. M.; Haley, M. M. Chem. Commun. 1997, 1703–1704. doi:10.1039/a704070i |
| 50. | Janiak, C. J. Chem. Soc., Dalton Trans. 2000, 3885–3896. doi:10.1039/b003010o |
| 51. | Janiak, C.; Uehlin, L.; Wu, H.-P.; Klüfers, P.; Piotrowski, H.; Scharmann, T. G. J. Chem. Soc., Dalton Trans. 1999, 3121–3131. doi:10.1039/a904829d |
| 52. | Lozana, V.; Lassahn, P.-G.; Zhang, C.; Wu, B.; Janiak, C.; Rheinwald, G.; Lang, H. Z. Naturforsch., B: J. Chem. Sci. 2003, 58, 1152–1164. doi:10.1515/znb-2003-1202 |
| 53. | Wu, H.-P.; Janiak, C.; Rheinwald, G.; Lang, H. J. Chem. Soc., Dalton Trans. 1999, 183–190. doi:10.1039/a807450j |
| 54. | Wu, H.-P.; Janiak, C.; Uehlin, L.; Klüfers, P.; Mayer, P. Chem. Commun. 1998, 2637–2638. doi:10.1039/a807522k |
| 55. | Yang, X.-J.; Drepper, F.; Wu, B.; Sun, W.-H.; Haehnel, W.; Janiak, C. Dalton Trans. 2005, 256–267. doi:10.1039/b414999h |
| 56. | Zhang, C.; Janiak, C. Z. Anorg. Allg. Chem. 2001, 627, 1972–1975. doi:10.1002/1521-3749(200108)627:8<1972::aid-zaac1972>3.0.co;2-k |
| 57. | Zhang, C.; Janiak, C. J. Chem. Crystallogr. 2001, 31, 29–35. doi:10.1023/a:1013774502147 |
| 1. | Yao, J.; Yang, M.; Duan, Y. Chem. Rev. 2014, 114, 6130–6178. doi:10.1021/cr200359p |
| 22. | Gers-Panther, C. F.; Müller, T. J. J. Adv. Heterocycl. Chem. 2016, 120, 67–98. doi:10.1016/bs.aihch.2016.04.007 |
| 4. | Wegner, K. D.; Hildebrandt, N. Chem. Soc. Rev. 2015, 44, 4792–4834. doi:10.1039/c4cs00532e |
| 23. | Breuer, N.; Müller, T. J. J. Synthesis 2018, 50, 2741–2752. doi:10.1055/s-0037-1610129 |
| 61. | Luo, J.; Song, K.; Gu, F. I.; Miao, Q. Chem. Sci. 2011, 2, 2029–2034. doi:10.1039/c1sc00340b |
| 3. | Day, R. N.; Davidson, M. W. Chem. Soc. Rev. 2009, 38, 2887–2921. doi:10.1039/b901966a |
| 20. | Merkt, F. K.; Müller, T. J. J. Isr. J. Chem. 2018, 58, 889–900. doi:10.1002/ijch.201800058 |
| 58. | Rurack, K.; Spieles, M. Anal. Chem. (Washington, DC, U. S.) 2011, 83, 1232–1242. doi:10.1021/ac101329h |
| 2. | Wysocki, L. M.; Lavis, L. D. Curr. Opin. Chem. Biol. 2011, 15, 752–759. doi:10.1016/j.cbpa.2011.10.013 |
| 21. | D'Souza, D. M.; Müller, T. J. J. Chem. Soc. Rev. 2007, 36, 1095–1108. doi:10.1039/b608235c |
| 59. | Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, U.S.A., 2006. doi:10.1007/978-0-387-46312-4 |
| 15. | Park, S.-Y.; Ebihara, M.; Kubota, Y.; Funabiki, K.; Matsui, M. Dyes Pigm. 2009, 82, 258–267. doi:10.1016/j.dyepig.2009.01.014 |
| 17. | Levi, L.; Müller, T. J. J. Chem. Soc. Rev. 2016, 45, 2825–2846. doi:10.1039/c5cs00805k |
| 18. | Levi, L.; Müller, T. J. J. Eur. J. Org. Chem. 2016, 2902–2918. doi:10.1002/ejoc.201600409 |
| 58. | Rurack, K.; Spieles, M. Anal. Chem. (Washington, DC, U. S.) 2011, 83, 1232–1242. doi:10.1021/ac101329h |
| 14. | Thimmarayaperumal, S.; Shanmugam, S. ACS Omega 2017, 2, 4900–4910. doi:10.1021/acsomega.7b00627 |
| 19. | Müller, T. J. J. Drug Discovery Today: Technol. 2018, 29, 19–26. doi:10.1016/j.ddtec.2018.06.003 |
| 58. | Rurack, K.; Spieles, M. Anal. Chem. (Washington, DC, U. S.) 2011, 83, 1232–1242. doi:10.1021/ac101329h |
| 11. | de Halleux, V.; Calbert, J.-P.; Brocorens, P.; Cornil, J.; Declercq, J.-P.; Brédas, J.-L.; Geerts, Y. Adv. Funct. Mater. 2004, 14, 649–659. doi:10.1002/adfm.200400006 |
| 12. | Hassheider, T.; Benning, S. A.; Kitzerow, H.-S.; Achard, M.-F.; Bock, H. Angew. Chem., Int. Ed. 2001, 40, 2060–2063. doi:10.1002/1521-3773(20010601)40:11<2060::aid-anie2060>3.3.co;2-8 |
| 13. | Kartha, K. K.; Babu, S. S.; Srinivasan, S.; Ajayaghosh, A. J. Am. Chem. Soc. 2012, 134, 4834–4841. doi:10.1021/ja210728c |
| 36. | Desiraju, G. R.; Steiner, T. The weak hydrogen bond. IUCr Monograph on Crystallography; Oxford Science: Oxford, United KIngdom, 1999; Vol. 9. |
| 37. | Desiraju, G. R. Acc. Chem. Res. 2002, 35, 565–573. doi:10.1021/ar010054t |
| 38. | Janiak, C.; Scharmann, T. G. Polyhedron 2003, 22, 1123–1133. doi:10.1016/s0277-5387(03)00098-6 |
| 39. | Shivakumar, K.; Vidyasagar, A.; Naidu, A.; Gonnade, R. G.; Sureshan, K. M. CrystEngComm 2012, 14, 519–524. doi:10.1039/c1ce05997a |
| 6. | Figueira-Duarte, T. M.; Müllen, K. Chem. Rev. 2011, 111, 7260–7314. doi:10.1021/cr100428a |
| 7. | Forrest, S. R.; Thompson, M. E. Chem. Rev. 2007, 107, 923–925. doi:10.1021/cr0501590 |
| 8. | Grimsdale, A. C.; Leok Chan, K.; Martin, R. E.; Jokisz, P. G.; Holmes, A. B. Chem. Rev. 2009, 109, 897–1091. doi:10.1021/cr000013v |
| 9. | Kanibolotsky, A. L.; Perepichka, I. F.; Skabara, P. J. Chem. Soc. Rev. 2010, 39, 2695–2728. doi:10.1039/b918154g |
| 10. | Wang, X.; Zhang, F.; Liu, J.; Tang, R.; Fu, Y.; Wu, D.; Xu, Q.; Zhuang, X.; He, G.; Feng, X. Org. Lett. 2013, 15, 5714–5717. doi:10.1021/ol402745r |
| 16. | Hagimori, M.; Mizuyama, N.; Yokota, K.; Nishimura, Y.; Suzuta, M.; Tai, C.-K.; Wang, B.-C.; Wang, S.-L.; Shih, T.-L.; Wu, K.-D.; Huang, Z.-S.; Tseng, S.-C.; Chen, C.-Y.; Lu, J.-W.; Wei, H.-H.; Kawashima, K.; Kawashima, S.; Tominaga, Y. Dyes Pigm. 2012, 92, 1069–1074. doi:10.1016/j.dyepig.2011.05.014 |
| 40. | Anelli, P. L.; Ashton, P. R.; Ballardini, R.; Balzani, V.; Delgado, M.; Gandolfi, M. T.; Goodnow, T. T.; Kaifer, A. E.; Philp, D. J. Am. Chem. Soc. 1992, 114, 193–218. doi:10.1021/ja00027a027 |
| 41. | Janiak, C.; Temizdemir, S.; Dechert, S.; Deck, W.; Girgsdies, F.; Heinze, J.; Kolm, M.; Scharmann, T.; Zipffel, O. Eur. J. Inorg. Chem. 2000, 1229–1241. doi:10.1002/(sici)1099-0682(200006)2000:6<1229::aid-ejic1229>3.3.co;2-g |
| 42. | N. Laxmi Madhavi, N.; R. Desiraju, G.; K. Katz, A.; L. Carrell, H.; Nangia, A. Chem. Commun. 1997, 1953–1954. doi:10.1039/a705836e |
| 43. | Nishio, M. CrystEngComm 2004, 6, 130–158. doi:10.1039/b313104a |
| 44. | Nishio, M. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. doi:10.1039/c1cp20404a |
| 45. | Nishio, M.; Hirota, M.; Umezawa, Y. The CH/π interaction (evidence, nature and consequences); Wiley-VCH: New York, NY, U.S.A., 1998. |
| 46. | Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawa, H. CrystEngComm 2009, 11, 1757–1788. doi:10.1039/b902318f |
| 47. | Steiner, T.; Tamm, M.; Lutz, B.; Van Der Maas, J. Chem. Commun. 1996, 1127–1128. doi:10.1039/cc9960001127 |
| 48. | Umezawa, Y.; Tsuboyama, S.; Honda, K.; Uzawa, J.; Nishio, M. Bull. Chem. Soc. Jpn. 1998, 71, 1207–1213. doi:10.1246/bcsj.71.1207 |
| 49. | Weiss, H.-C.; Bläser, D.; Boese, R.; Doughan, B. M.; Haley, M. M. Chem. Commun. 1997, 1703–1704. doi:10.1039/a704070i |
| 27. | Shankar, R.; Shukla, H.; Singh, U. S.; Thakur, V.; Hajela, K. Synth. Commun. 2011, 41, 2738–2746. doi:10.1080/00397911.2010.515350 |
| 16. | Hagimori, M.; Mizuyama, N.; Yokota, K.; Nishimura, Y.; Suzuta, M.; Tai, C.-K.; Wang, B.-C.; Wang, S.-L.; Shih, T.-L.; Wu, K.-D.; Huang, Z.-S.; Tseng, S.-C.; Chen, C.-Y.; Lu, J.-W.; Wei, H.-H.; Kawashima, K.; Kawashima, S.; Tominaga, Y. Dyes Pigm. 2012, 92, 1069–1074. doi:10.1016/j.dyepig.2011.05.014 |
| 24. | Tominaga, Y.; Mizuyama, N.; Murakami, Y.; Nagaoka, J.; Kohra, S.; Ueda, K.; Hiraoka, K.; Shigemitsu, Y. Heterocycles 2006, 68, 1105–1108. doi:10.3987/com-06-10741 |
| 25. | Mizuyama, N.; Murakami, Y.; Nakatani, T.; Kuronita, K.; Kohra, S.; Ueda, K.; Hiraoka, K.; Tominaga, Y. J. Heterocycl. Chem. 2008, 45, 265–277. doi:10.1002/jhet.5570450133 |
| 26. | Tominaga, Y.; Mizuyama, N.; Shigemitsu, Y.; Wang, B.-C. Heterocycles 2009, 78, 555–570. doi:10.3987/rev-08-642 |
| 62. | Mei, J.; Hong, Y.; Lam, J. W. Y.; Qin, A.; Tang, Y.; Tang, B. Z. Adv. Mater. (Weinheim, Ger.) 2014, 26, 5429–5479. doi:10.1002/adma.201401356 |
| 63. | Müller, T. J. J. Multicomponent and Domino Syntheses of AIE Chromophores. In Aggregation Induced Emission: Materials and Applications, Fujiki, M.; Liu, B.; Tang, B. Z., Eds.; American Chemical Society: Washington, DC, U.S.A., 2016; pp 85–112. doi:10.1021/bk-2016-1226.ch006 |
| 64. | Liu, B.; Tang, B. Z. Chem. – Asian J. 2019, 14, 672–673. doi:10.1002/asia.201900185 |
| 16. | Hagimori, M.; Mizuyama, N.; Yokota, K.; Nishimura, Y.; Suzuta, M.; Tai, C.-K.; Wang, B.-C.; Wang, S.-L.; Shih, T.-L.; Wu, K.-D.; Huang, Z.-S.; Tseng, S.-C.; Chen, C.-Y.; Lu, J.-W.; Wei, H.-H.; Kawashima, K.; Kawashima, S.; Tominaga, Y. Dyes Pigm. 2012, 92, 1069–1074. doi:10.1016/j.dyepig.2011.05.014 |
| 24. | Tominaga, Y.; Mizuyama, N.; Murakami, Y.; Nagaoka, J.; Kohra, S.; Ueda, K.; Hiraoka, K.; Shigemitsu, Y. Heterocycles 2006, 68, 1105–1108. doi:10.3987/com-06-10741 |
| 65. | Becke, A. D. J. Chem. Phys. 1993, 98, 1372–1377. doi:10.1063/1.464304 |
| 66. | Kim, K.; Jordan, K. D. J. Phys. Chem. 1994, 98, 10089–10094. doi:10.1021/j100091a024 |
| 67. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785 |
| 68. | Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001 |
| 69. | Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650–654. doi:10.1063/1.438955 |
| 32. | D'Souza, D. M.; Müller, T. J. J. Nat. Protoc. 2008, 3, 1660–1665. doi:10.1038/nprot.2008.152 |
| 33. | Götzinger, A. C.; Müller, T. J. J. Org. Biomol. Chem. 2016, 14, 3498–3500. doi:10.1039/c6ob00483k |
| 34. | Karpov, A. S.; Müller, T. J. J. Org. Lett. 2003, 5, 3451–3454. doi:10.1021/ol035212q |
| 35. | CCDC 1944699 (5a) contains the supplementary crystallographic data (excluding structure factors) for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre. |
| 28. | Fouli, F. A.; Basyouni, M. N. Acta Chim. Acad. Sci. Hung. 1981, 106, 297–302. |
| 30. | Takaya, H.; Naota, T.; Murahashi, S.-I. J. Am. Chem. Soc. 1998, 120, 4244–4245. doi:10.1021/ja974106e |
| 29. | Sadek, K. U.; Fahmy, S. M.; Mohareb, R. M.; Elnagdi, M. H. J. Chem. Eng. Data 1984, 29, 101–103. doi:10.1021/je00035a033 |
| 69. | Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650–654. doi:10.1063/1.438955 |
| 30. | Takaya, H.; Naota, T.; Murahashi, S.-I. J. Am. Chem. Soc. 1998, 120, 4244–4245. doi:10.1021/ja974106e |
| 31. | Hammond, G. B.; Plevey, R. G.; Sampson, P.; Tatlow, J. C. J. Fluorine Chem. 1988, 40, 81–98. doi:10.1016/s0022-1139(00)83057-2 |
| 70. | Scalmani, G.; Frisch, M. J. J. Chem. Phys. 2010, 132, 114110. doi:10.1063/1.3359469 |
| 28. | Fouli, F. A.; Basyouni, M. N. Acta Chim. Acad. Sci. Hung. 1981, 106, 297–302. |
| 70. | Scalmani, G.; Frisch, M. J. J. Chem. Phys. 2010, 132, 114110. doi:10.1063/1.3359469 |
| 23. | Breuer, N.; Müller, T. J. J. Synthesis 2018, 50, 2741–2752. doi:10.1055/s-0037-1610129 |
| 65. | Becke, A. D. J. Chem. Phys. 1993, 98, 1372–1377. doi:10.1063/1.464304 |
| 66. | Kim, K.; Jordan, K. D. J. Phys. Chem. 1994, 98, 10089–10094. doi:10.1021/j100091a024 |
| 67. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785 |
| 68. | Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001 |
© 2019 Breuer et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)