Abstract
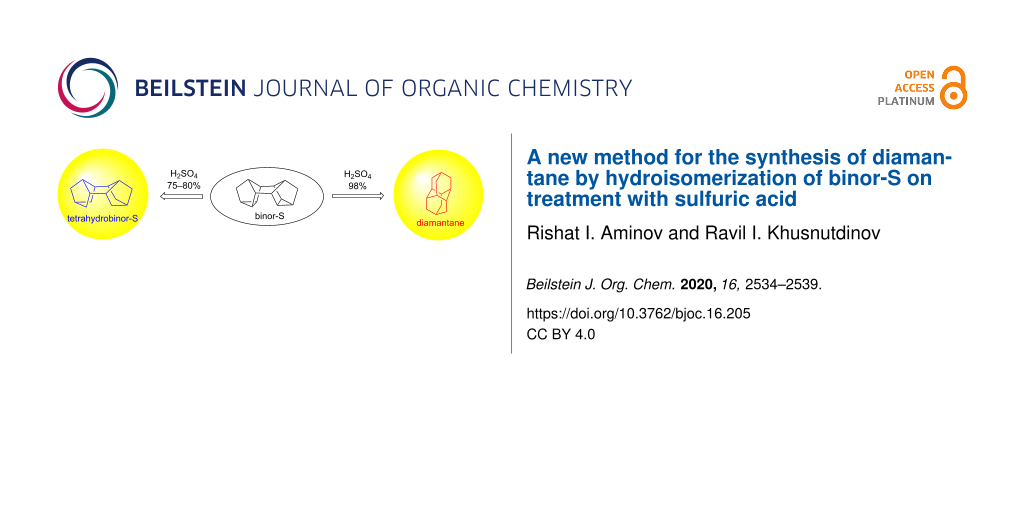
A new method was developed for the direct synthesis of the second representative of the homologous series of diamond-like hydrocarbons, diamantane, in 65% yield by hydroisomerization of the norbornadiene dimer, endo-endo-heptacyclo[8.4.0.02,12.03,8.04,6.05,9.011,13]tetradecane (binor-S) on treatment with concentrated sulfuric acid (98%). In the presence of H2SO4 of lower concentration (75–80%), the reaction stops after the hydrogenation step giving endo-endo-pentacyclo[7.3.1.12,5.18,10.03,7]tetradecane in 68% yield with excellent selectivity (100%).
Graphical Abstract
Introduction
Among the highly diverse polycyclic and cage compounds, an important place is occupied by diamond-like compounds called diamondoids, whose lower representatives belong to the homologous series C4n+6H4n+12. Owing to the rigid structure, diamondoids typically have high thermal stability and high reactivity compared with aliphatic and alicyclic saturated hydrocarbons and show peculiar chemical behavior.
Crude oil is known to be the main natural source of diamondoids. In the oil and gas field exploration, the presence of diamondoids is used to evaluate the field maturity. Whereas the synthesis and chemical reactivity of adamantane, the first member of the diamondoid homologous series, which is produced on an industrial scale (prepared by AlBr3 or AlCl3-induced skeletal isomerization of a petrochemical monomer, hydrogenated dicyclopentadiene) [1], have been studied rather extensively, the chemical behavior of diamantane, the second member of the diamandoid homologous series, has been poorly studied. The main cause of this situation is the lack of facile methods for its synthesis.
In the literature, diamantane (1) is prepared by skeletal isomerization of strained С14Н20 polycyclic hydrocarbons [2-7]. In particular, the most suitable initial compounds for the preparation of diamantane are three isomeric polycyclic hydrocarbons C14H20 3а–с, which are obtained by hydrogenation of the norbornadiene dimer, heptacyclo[8.4.0.02,12.03,8.04,6.05,9.011,13]tetradecane (binor-S, 2). Binor-S is hydrogenated in the presence of a platinum catalyst (Н2PtCl6, PtO2) in glacial acetic acid under high pressure conditions at 70 °С and 200 psi of H2 [8,9]. In the presence of superacid catalysts, such as B(OSO2CF3)3, CF3SO3H/SbF5 1:1, CF3SO3H/B(OSO2CF3)3 1:1 [10], NaBH4/CF3SO3H [11], or zeolite Y in the NaH form (NaY) [12], hydrocarbons 3a–c isomerize to diamantane in up to 99% yield (Scheme 1).
Scheme 1: Isomerization of 3а–с to diamantane (1). Reaction conditions: (a) CoBr2·2PPh3–BF3·OEt2, 110 °C, 12 h; (b) Pt, H2 (200 psi), 70 °C, 3 h; (c) superacidic catalysts or NaY–NaH.
Scheme 1: Isomerization of 3а–с to diamantane (1). Reaction conditions: (a) CoBr2·2PPh3–BF3·OEt2, 110 °C, 12 ...
As can be seen from Scheme 1, the synthesis of diamantane (1) from binor-S (2) is a two-step process, in which the hydrogenation performed in the first step is most complex and has always been an obstacle to the generation of large amounts of diamantane. In view of the foregoing, we set ourselves the task to develop a one-pot method for the synthesis of diamantane (1) from binor-S (2).
Results and Discussion
In this study, we developed a new method for the synthesis of pentacyclo[7.3.1.14,12.02,7.06,11]tetradecane (diamantane, 1) by skeletal hydroisomerization of endo-endo-heptacyclo[8.4.0.02,12.03,8.04,6.05,9.011,13]tetradecane (binor-S, 2) on treatment with sulfuric acid (Scheme 2).
Scheme 2: Isomerization of binor-S (2) to diamantane (1).
Scheme 2: Isomerization of binor-S (2) to diamantane (1).
The reaction selectivity and the yield of diamantane (1) considerably depend on the reaction conditions and the solvent nature. Indeed, at 20–40 °C, hydroizomerization of binor-S (2) in cyclohexane in the presence of 98% sulfuric acid ([2]/[H2SO4] = 1:10–50) during 7–15 h affords a mixture of endo-endo-pentacyclo[7.3.1.12,5.18,10]tetradecane (tetrahydrobinor-S, 3c) and diamantane (1) (Table 1). An increase in the sulfuric acid ratio to binor-S (2) ([2]/[H2SO4] = 1:20–50) and rising the temperature to 40 °С lead to decreased product yield due to resinification. When the H2SO4 ratio to binor-S (2) is 1:5, the conversion of compound 2 decreases to 10%. On the other hand, when the reactions are carried out in CS2 or without any solvent, the selectivity to diamantane (1) increases to 100%, with the maximum yield being 65% (Table 1, entry 12). A portion of binor-S (2) is converted to resinous products. When the reaction was ultrasonically assisted, the reaction time decreased to 2 h with the yield of diamantane (1) being retained (62%).
Table 1: Hydroisomerization of binor-S (2) in the presence of sulfuric acid.
| entry | ratio | solvent | temp. [°C] | time [h] | product ratio [%]a | ||
| [2]/[H2SO4] | 2 | 3 | 1 | ||||
| 1 | 1:50 | cyclohexane | 40 | 7 | 2 | 10 | 23 |
| 2 | 1:50 | cyclohexane | 20 | 7 | 3 | 26 | 10 |
| 3 | 1:20 | cyclohexane | 40 | 7 | 3 | 52 | 28 |
| 4 | 1:20 | cyclohexane | 20 | 7 | 12 | 46 | 22 |
| 5 | 1:20 | cyclohexane | 20 | 15 | – | 55 | 31 |
| 6 | 1:10 | cyclohexane | 20 | 7 | 22 | 41 | 36 |
| 7 | 1:10 | cyclohexane | 20 | 15 | 16 | 47 | 34 |
| 8 | 1:5 | cyclohexane | 40 | 15 | 56 | 31 | 2 |
| 9 | 1:20 | carbon disulfide | 20 | 7 | 21 | – | 36 |
| 10 | 1:20 | carbon disulfide | 20 | 15 | 15 | – | 44 |
| 11 | 1:10 | carbon disulfide | 20 | 7 | 24 | – | 52 |
| 12 | 1:10 | carbon disulfide | 20 | 15 | – | – | 65 |
| 13 | 1:5 | carbon disulfide | 40 | 7 | 78 | – | 10 |
| 14 | 1:5 | carbon disulfide | 20 | 7 | 90 | – | – |
| 15 | 1:10 | – | 20 | 7 | – | – | 8 |
| 16b | 1:10 | cyclohexane | 20 | 2 | 9 | 64 | 26 |
| 17b | 1:10 | carbon disulfide | 20 | 2 | – | 18 | 62 |
| 18b | 1:10 | – | 20 | 2 | – | – | 6 |
aDetermined by GC using C12H26 as the internal standard. bThe reaction was conducted under ultrasonic irradiation.
In order to answer the question of what is the hydrogen source in the hydroisomerization of binor-S (С14H16, 2) containing 4 hydrogen atoms less than diamantane (С14H20, 1), we carried out a series of control experiments using deuterated sulfuric acid (98%) in cyclohexane (С6H12, experiment A), in deuterated cyclohexane (C6D12, experiment B), or in carbon disulfide (CS2, experiment C).
In experiment А, the major isomer 1-D2, which is formed upon hydroisomerization of binor-S (2), contains two deuterium atoms. Two more hydrogen atoms are probably provided by cyclohexane. Unexpectedly, the reaction also gave undeuterated diamantane (1), which may be due to deuterium exchange with hydrogen of cyclohexane under the action of D2SO4.
The major product 1-D3, which is formed in experiment B with D2SO4 in C6D12 contains three deuterium atoms. The expected isomer with four deuterium atoms is formed in a minor amount. Evidently, binor-S (2) acts as the hydrogen source for the isomer C14H17D3, 1-D3. Our attempt to carry out the deuteration of diamantane (1) with D2SO4 in carbon disulfide for 7 h at 20 °C was unsuccessful. Evidently, the deuterium exchange, resulting in the formation of diamantanes 1-D7 and 1-D8 containing 7 and 8 deuterium atoms, occurs at the hydroisomerization step (experiment C).
As shown by further studies, when the sulfuric acid concentration decreases to 75–80%, the reaction stops at the intermediate step giving endo-endo-pentacyclo[7.3.1.12,5.18,10.03,7]tetradecane (tetrahydrobinor-S, 3с; Scheme 3). It should be emphasized that the reaction selectively gives only one of the possible isomers, hydrocarbon 3с, which is confirmed by 1H and 13C NMR spectral data. The 13C NMR spectrum of compound 3с shows five characteristic carbon signals at 33.44, 35.64, 37.84, 38.30, and 40.49 ppm, coinciding with the reported values [13]. Since 75–80% H2SO4 contains 20–25% water, the participation of water as a hydrogen source in the reaction cannot be ruled out either.
Scheme 3: Selective synthesis of tetrahydrobinor-S (3c) from binor-S (2).
Scheme 3: Selective synthesis of tetrahydrobinor-S (3c) from binor-S (2).
Attempts to perform hydroisomerization of binor-S (2) to diamantane (1) on treatment with nitric or orthophosphoric acid were unsuccessful, with the starting binor-S (2) being recovered unchanged. The reaction of hydrocarbon 2 with hydrochloric acid proceeds with the addition of HCl to the cyclopropane ring and results in the formation of a mixture of mono- and dichloro derivatives, the synthesis of which has been reported [13,14]. When sulfuric acid is replaced by an ionic liquid prepared from triethylamine and sulfuric acid [15], the reaction follows a different route: Starting binor-S (2) is converted to two isomeric hexacyclic hydrocarbons, hexacyclo[8.4.0.02,7.03,14.04,8.09,13]tetradec-5-ene (4а) and hexacyclo[6.6.0.0.2,6.05,14.07,12.09,13]tetradec-3-ene (4b), which are important precursors for the synthesis of triamantane [10,11,16-24] (Scheme 4).
Scheme 4: Isomerization of binor-S (2) to hydrocarbons 4а and b.
Scheme 4: Isomerization of binor-S (2) to hydrocarbons 4а and b.
Conclusion
Thus, we developed a new one-pot method for the synthesis of diamantane (1) by hydroisomerization of binor-S (2) on treatment with concentrated sulfuric acid (98%) in carbon disulfide or cyclohexane. It was found that both, sulfuric acid and cyclohexane can serve as the main hydrogen sources. In the presence of H2SO4 with a lower concentration (75–80%), the reaction stops at the step of formation of endo-endo-pentacyclo[7.3.1.12,5.18,10.03,7]tetradecane (3c) in 68% yield.
Experimental
General procedures and materials: 1H and 13С NMR spectra were measured on a Bruker Avance-III 400 Ascend instrument (400 MHz for 1Н and 100 MHz for 13С in CDCl3). Mass spectra were run on a Shimadzu GCMS-QP2010Plus mass spectrometer (SPB-5 capillary column, 30 m × 0.25 mm, helium as the carrier gas, temperature programming from 40 to 300 °С at 8 °C/min, evaporation temperature of 280 °С, ion source temperature of 200 °С, and ionization energy of 70 eV). The elemental composition of the samples was determined on a Carlo Erba 1106 elemental analyzer. The course of the reaction and the purity of the products were monitored by gas liquid chromatography on a Shimadzu GC-9A, GC-2014 instrument [2 m × 3 mm column, SE-30 silicone (5%) on Chromaton N-AW-HMDS as the stationary phase, temperature programming from 50 to 270 °С at 8 °C/min, helium as the carrier gas (47 mL/min)].
The sonication was carried out with an ultrasound generator IL10–0.63 (INLAB LTD) for 180 min at a frequency of 22 kHz with a submerged 15 mm diameter titanium horn, with output power 150 W. The reactions were carried out in a 100 × 35 mm glass reactor equipped with a jacket to maintain the required temperature (20 °C).
Preparation of diamantane: Heptacyclo[8.4.0.02,12.03,8.04,6.05,9. 011,13]tetradecane (2, 0.368 g, 2 mmol) and the solvent were charged into a glass reactor (V = 100 mL). Then, concentrated (98%) sulfuric acid (1.96 g, 20 mmol) was added in portions with vigorous stirring. When the whole amount of H2SO4 has been added, the reaction mixture was stirred at 20 °С for 15 h. After completion of the reaction, 10% NaOH was added to the reaction mixture, the organic phase was separated, and filtered through a silica gel layer (with petroleum ether as the eluent). The solvent was distilled off and the residue was recrystallized from a 1:1 ethyl acetate/cyclohexane mixture. The characteristic data and graphical spectra of diamantane are almost identical with the literature data [25].
Preparation of endo-endo-pentacyclo[7.3.1.12,5.18,10.03,7]tetradecane (tetrahydrobinor-S, 3c): Heptacyclo[8.4.0.02,12.03,8.04,6.05,9. 011,13]tetradecane (2, 0.368 g, 2 mmol) was charged into a glass reactor (V = 100 mL) and dissolved in cyclohexane (10 mL). Then, 75–80% sulfuric acid (1.96 g, 20 mmol) was added in portions with vigorous stirring. When the whole amount of H2SO4 has been added, the reaction mixture was stirred at 20 °С for 7 h. After completion of the reaction, 10% NaOH was added to the reaction mixture, the organic part was separated, and filtered through a silica gel layer (with petroleum ether as the eluent). The solvent was distilled off and the residue was recrystallized from a 1:1 ethyl acetate/cyclohexane mixture. Colorless crystals; 68% yield; mp 104–106 °C; 1H NMR (400 MHz, CDCl3) δ 0.95–0.98 (m, 4H), 1.38 (s, 8H), 1.66–1.71 (m, 4H), 1.99–2.01 (m, 2H), 2.12–2.16 (m, 2H); 13С NMR (100 MHz, CDCl3) δ 33.42 (С6, С9, C13, C14), 35.63 (С1, С2, C7, C8), 37.82 (С5, С10), 38.27(С3, С12), 40.47 (С4, С11); EIMS (70 eV, m/z): 188 [M]+ (100), 187 (35), 159 (24), 145 (23), 131 (38), 117 (25), 105 (39), 91(82), 79 (57), 67 (29), 41 (47) %; Anal. calcd for C14H20: С, 89.29; H, 10.71; found: С, 89.14; H, 10.86.
Preparation of hexacyclo[8.4.0.02,7.03,14.04,8.09,13]tetradec-5-ene (4a) and hexacyclo[6.6.0.0.2,6.05,14.07,12.09,13]tetradec-3-ene (4b): Heptacyclo[8.4.0.02,12.03,8.04,6.05,9. 011,13]tetradecane (2, 0.368 g, 2 mmol) was charged into a glass reactor (V = 100 mL) and dissolved in cyclohexane. Then, [Et3NH]+[HSO4]− (1.99 g, 10 mmol) was added and the reaction mixture was stirred at 40 °С for 8 h. Then the reactor was cooled to room temperature, the reaction mixture extracted with petroleum ether, and filtered through a silica gel layer (with petroleum ether as the eluent). Hexacyclo[8.4.0.02,7.03,14.04,8.09,13]tetradec-5-ene (4а) and hexacyclo[6.6.0.0.2,6.05,14.07,12.09,13]tetradec-3-ene (4b) (45:55). Colorless oil; 78% yield; 4a: 1H NMR (400 MHz, CDCl3) δ 1.04 (d, J = 7.2 Hz, 2H), 1.41 (d, J = 7.6 Hz, 2H), 1.95 (s, 2H), 2.09 (d, J = 7.2 Hz, 4H), 2.21 (d, J = 7.2 Hz, 2H) 2.56 (s, 2H), 5.87 (s, 2H); 13С NMR (100 MHz, CDCl3) δ 26.27 (C11, C12), 34.62 (C10, C13), 36.34 (C1, C14), 37.27 (C2, C3), 40.68 (C4, C7), 44.68 (C8), 52.88 (C9), 134.82 (C5, C6); EIMS (70 eV, m/z): 184 [M]+ (44), 169 (14), 155 (16), 142 (34), 117 (100), 115 (37), 105 (22), 91 (73), 80 (38), 65 (17), 41 (21) %; 4b: 1H NMR (400 MHz, CDCl3) δ 1.19–1.24 (m, 1H), 1.31–1.36 (m, 1H), 1.48 (s, 1H), 1.56–1.59 (m, 2H), 1.71 (t, J = 6 Hz, 1H) 2.03–2.06 (m, 3H), 2.15–2.17 (m, 2H), 2.22 (s, 1H), 2.52 (s, 2H), 2.59 (s, 1H), 5.96–5.98 (m, 1H); 13С NMR (100 MHz, CDCl3) δ 24.08 (C10), 27.16 (C11), 40.52 (C1), 40.93 (C12), 42.30 (C14), 45.66 (C9), 47.38 (C2), 47.94 (C13), 48.61 (C7), 50.20 (C8), 54.09 (C5), 60.05 (C6), 133.69 (C4), 133.75 (C3); EIMS (70 eV, m/z): 184 [M]+ (40), 169 (21), 155 (45), 141 (45), 129 (51), 117 (100), 115 (53), 91 (88), 78 (43), 65 (21), 41 (20) %.
Supporting Information
| Supporting Information File 1: Experimental procedures, NMR, and mass spectral data. | ||
| Format: PDF | Size: 604.8 KB | Download |
Funding
The results were obtained with the financial support of the Russian Ministry of Education and Science (project no. 2019-05-595-000-058) on unique equipment at the 'Agidel' Collective Usage Center (Ufa Federal Research Center, Russian Academy of Sciences), by the Scholarship of the President of the Russian Federation to young scientists and postgraduates (SP-1601.2018.1) and carried out within the RF state assignment, reg. no. АААА-А19-119022290009-3.
References
-
Murray, R. K.; Morgan, T. K.; Babiak, K. A. J. Org. Chem. 1975, 40, 1079–1083. doi:10.1021/jo00896a019
Return to citation in text: [1] -
Williams, V. Z., Jr.; von Ragué Schleyer, P.; Gleicher, G. J.; Rodewald, L. B. J. Am. Chem. Soc. 1966, 88, 3862–3863. doi:10.1021/ja00968a036
Return to citation in text: [1] -
Mrowca, J. J.; Katz, T. J. J. Am. Chem. Soc. 1966, 88, 4012–4015. doi:10.1021/ja00969a021
Return to citation in text: [1] -
Gund, T. M.; Osawa, E.; Williams, V. Z.; Schleyer, P. v. R. J. Org. Chem. 1974, 39, 2979–2987. doi:10.1021/jo00934a009
Return to citation in text: [1] -
Aminov, R. I.; Khusnutdinov, R. I. Russ. J. Org. Chem. 2017, 53, 1881–1883. doi:10.1134/s107042801712017x
Return to citation in text: [1] -
Gunchenko, P. A.; Novikovskii, A. A.; Byk, M. V.; Fokin, A. A. Russ. J. Org. Chem. 2014, 50, 1749–1754. doi:10.1134/s1070428014120057
Return to citation in text: [1] -
Tureček, F.; Hanuš, V.; Sedmera, P.; Antropiusová, H.; Mach, K. Collect. Czech. Chem. Commun. 1981, 46, 1474–1485. doi:10.1135/cccc19811474
Return to citation in text: [1] -
Gund, T. M.; Thielecke, W.; Schleyer, P. v. R. Org. Synth. 1973, 53, 30–34. doi:10.15227/orgsyn.053.0030
Return to citation in text: [1] -
Dzhemilev, U. M.; Khusnutdinov, R. I.; Muslimov, Z. S.; Mazitov, M. F. Pet. Chem. 1996, 36, 507–512.
Return to citation in text: [1] -
Farooq, O.; Farnia, S. M. F.; Stephenson, M.; Olah, G. A. J. Org. Chem. 1988, 53, 2840–2843. doi:10.1021/jo00247a035
Return to citation in text: [1] [2] -
Olah, G. A.; Wu, A.-h.; Farooq, O.; Prakash, G. K. S. J. Org. Chem. 1989, 54, 1450–1451. doi:10.1021/jo00267a042
Return to citation in text: [1] [2] -
Dzhemilev, U. M.; Khusnutdinov, R. I.; Kislitsina, K. S.; Kutepov, B. I.; Khazipova, A. N.; Travkina, O. S. Method of producing diamantane (pentacyclo[7,3,1,14,12,02,7,06,11]tetradecane). Russian Patent RU2459794C1, Aug 27, 2012.
Return to citation in text: [1] -
Khusnutdinov, R. I.; Muslimov, Z. S.; Dzhemilev, U. M.; Nefedov, O. M. Russ. Chem. Bull. 1993, 42, 692–697. doi:10.1007/bf00704004
Return to citation in text: [1] [2] -
Dzhemilev, U. M.; Khusnutdinov, R. I.; Muslimov, Z. S.; Tolstikov, G. A.; Nefedov, O. M. Russ. Chem. Bull. 1991, 40, 236. doi:10.1007/bf00959680
Return to citation in text: [1] -
Karimi-Jaberi, Z.; Masoudi, B.; Rahmani, A.; Alborzi, K. Polycyclic Aromat. Compd. 2020, 40, 99–107. doi:10.1080/10406638.2017.1363061
Return to citation in text: [1] -
Schwertfeger, H.; Fokin, A. A.; Schreiner, P. R. Angew. Chem., Int. Ed. 2008, 47, 1022–1036. doi:10.1002/anie.200701684
Return to citation in text: [1] -
Hollowood, F. S.; McKervey, M. A.; Hamilton, R.; Rooney, J. J. J. Org. Chem. 1980, 45, 4954–4958. doi:10.1021/jo01312a026
Return to citation in text: [1] -
Kafka, Z.; Vodička, L.; Galík, V. Collect. Czech. Chem. Commun. 1982, 47, 286–289. doi:10.1135/cccc19820286
Return to citation in text: [1] -
Kafka, Z.; Vodicka, L. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1985, 51, 247–255.
Return to citation in text: [1] -
Kafka, Z. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1991, 59, 79–90.
Return to citation in text: [1] -
Kafka, Z.; Vodicka, L. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1984, 49, 125–137.
Return to citation in text: [1] -
Kafka, Z.; Nahunek, M. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1986, 55, 71–99.
Return to citation in text: [1] -
Kafka, Z.; Vodicka, L. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1986, 54, 65–74.
Return to citation in text: [1] -
Khusnutdinov, R. I.; Mukminov, R. R.; Aminov, R. I.; Khalilov, L. M.; Mesсheryakova, E. S.; Dzhemilev, U. M. Tetrahedron Lett. 2015, 56, 536–538. doi:10.1016/j.tetlet.2014.12.006
Return to citation in text: [1] -
Aminov, R. I.; Akshieva, A. N.; Khusnutdinov, R. I. Catal. Commun. 2019, 130, 105756. doi:10.1016/j.catcom.2019.105756
Return to citation in text: [1]
| 1. | Murray, R. K.; Morgan, T. K.; Babiak, K. A. J. Org. Chem. 1975, 40, 1079–1083. doi:10.1021/jo00896a019 |
| 11. | Olah, G. A.; Wu, A.-h.; Farooq, O.; Prakash, G. K. S. J. Org. Chem. 1989, 54, 1450–1451. doi:10.1021/jo00267a042 |
| 10. | Farooq, O.; Farnia, S. M. F.; Stephenson, M.; Olah, G. A. J. Org. Chem. 1988, 53, 2840–2843. doi:10.1021/jo00247a035 |
| 8. | Gund, T. M.; Thielecke, W.; Schleyer, P. v. R. Org. Synth. 1973, 53, 30–34. doi:10.15227/orgsyn.053.0030 |
| 9. | Dzhemilev, U. M.; Khusnutdinov, R. I.; Muslimov, Z. S.; Mazitov, M. F. Pet. Chem. 1996, 36, 507–512. |
| 2. | Williams, V. Z., Jr.; von Ragué Schleyer, P.; Gleicher, G. J.; Rodewald, L. B. J. Am. Chem. Soc. 1966, 88, 3862–3863. doi:10.1021/ja00968a036 |
| 3. | Mrowca, J. J.; Katz, T. J. J. Am. Chem. Soc. 1966, 88, 4012–4015. doi:10.1021/ja00969a021 |
| 4. | Gund, T. M.; Osawa, E.; Williams, V. Z.; Schleyer, P. v. R. J. Org. Chem. 1974, 39, 2979–2987. doi:10.1021/jo00934a009 |
| 5. | Aminov, R. I.; Khusnutdinov, R. I. Russ. J. Org. Chem. 2017, 53, 1881–1883. doi:10.1134/s107042801712017x |
| 6. | Gunchenko, P. A.; Novikovskii, A. A.; Byk, M. V.; Fokin, A. A. Russ. J. Org. Chem. 2014, 50, 1749–1754. doi:10.1134/s1070428014120057 |
| 7. | Tureček, F.; Hanuš, V.; Sedmera, P.; Antropiusová, H.; Mach, K. Collect. Czech. Chem. Commun. 1981, 46, 1474–1485. doi:10.1135/cccc19811474 |
| 15. | Karimi-Jaberi, Z.; Masoudi, B.; Rahmani, A.; Alborzi, K. Polycyclic Aromat. Compd. 2020, 40, 99–107. doi:10.1080/10406638.2017.1363061 |
| 25. | Aminov, R. I.; Akshieva, A. N.; Khusnutdinov, R. I. Catal. Commun. 2019, 130, 105756. doi:10.1016/j.catcom.2019.105756 |
| 13. | Khusnutdinov, R. I.; Muslimov, Z. S.; Dzhemilev, U. M.; Nefedov, O. M. Russ. Chem. Bull. 1993, 42, 692–697. doi:10.1007/bf00704004 |
| 14. | Dzhemilev, U. M.; Khusnutdinov, R. I.; Muslimov, Z. S.; Tolstikov, G. A.; Nefedov, O. M. Russ. Chem. Bull. 1991, 40, 236. doi:10.1007/bf00959680 |
| 13. | Khusnutdinov, R. I.; Muslimov, Z. S.; Dzhemilev, U. M.; Nefedov, O. M. Russ. Chem. Bull. 1993, 42, 692–697. doi:10.1007/bf00704004 |
| 12. | Dzhemilev, U. M.; Khusnutdinov, R. I.; Kislitsina, K. S.; Kutepov, B. I.; Khazipova, A. N.; Travkina, O. S. Method of producing diamantane (pentacyclo[7,3,1,14,12,02,7,06,11]tetradecane). Russian Patent RU2459794C1, Aug 27, 2012. |
| 10. | Farooq, O.; Farnia, S. M. F.; Stephenson, M.; Olah, G. A. J. Org. Chem. 1988, 53, 2840–2843. doi:10.1021/jo00247a035 |
| 11. | Olah, G. A.; Wu, A.-h.; Farooq, O.; Prakash, G. K. S. J. Org. Chem. 1989, 54, 1450–1451. doi:10.1021/jo00267a042 |
| 16. | Schwertfeger, H.; Fokin, A. A.; Schreiner, P. R. Angew. Chem., Int. Ed. 2008, 47, 1022–1036. doi:10.1002/anie.200701684 |
| 17. | Hollowood, F. S.; McKervey, M. A.; Hamilton, R.; Rooney, J. J. J. Org. Chem. 1980, 45, 4954–4958. doi:10.1021/jo01312a026 |
| 18. | Kafka, Z.; Vodička, L.; Galík, V. Collect. Czech. Chem. Commun. 1982, 47, 286–289. doi:10.1135/cccc19820286 |
| 19. | Kafka, Z.; Vodicka, L. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1985, 51, 247–255. |
| 20. | Kafka, Z. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1991, 59, 79–90. |
| 21. | Kafka, Z.; Vodicka, L. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1984, 49, 125–137. |
| 22. | Kafka, Z.; Nahunek, M. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1986, 55, 71–99. |
| 23. | Kafka, Z.; Vodicka, L. Sb. Vys. Sk. Chem.-Technol. Praze, D: Technol. Paliv 1986, 54, 65–74. |
| 24. | Khusnutdinov, R. I.; Mukminov, R. R.; Aminov, R. I.; Khalilov, L. M.; Mesсheryakova, E. S.; Dzhemilev, U. M. Tetrahedron Lett. 2015, 56, 536–538. doi:10.1016/j.tetlet.2014.12.006 |
© 2020 Aminov and Khusnutdinov; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)