Abstract
A series of N6-substituted adenine–ferrocene conjugates was prepared and the reaction mechanism underlying the synthesis was explored. The SN2-like reaction between ferrocenoyl chloride and adenine anions is a regioselective process in which the product ratio (N7/N9-ferrocenoyl isomers) is governed by the steric property of the substituent at the N6-position. Steric effects were evaluated by using Charton (empirical) and Sterimol (computational) parameters. The bulky substituents may shield the proximal N7 region of space, which prevents the approach of an electrophile towards the N7 atom. As a consequence, the formation of N7-isomer is a kinetically less feasible process, i.e., the corresponding transition state structure increases in relative energy (compared to the formation of the N9-isomer). In cases where the steric hindrance is negligible, the electronic effect of the N6-substituent is prevailing. That was supported by calculations of Fukui functions and molecular orbital coefficients. Both descriptors indicated that the N7 atom was more nucleophilic than its N9-counterpart in all adenine anion derivatives. We demonstrated that selected substituents may shift the acylation of purines from a regioselective to a regiospecific mode.
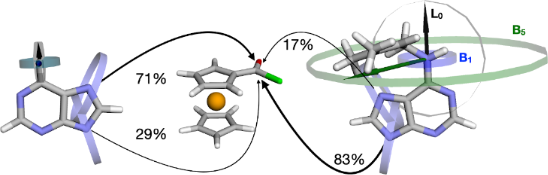
Graphical Abstract
Introduction
Nucleosides in which the sugar part is replaced with an organometallic moiety have attracted remarkable interest [1-3]. One important class are ferrocene–nucleobase conjugates [4], which are known to exhibit anticancer [5-7], antibacterial [8-10], or antitrypanosomal activity [11], but also may serve as electrochemical biosensors [12,13], self-assembled molecular materials [14,15], decorations of carbon tubes and nanomaterials [16,17], or structural motifs in xeno nucleic acids [18].
In continuation of our work on ferrocenoyl-substituted pyrimidine nucleobases [19], we report herewith a combined theoretical and experimental work on purine series. The novelty of these compounds is the carbonyl linker which connects the organometallic (metallocene) and heterocyclic (purine) parts. Specifically, adenine and its N6-derivatives, most of which are pharmaceutically attractive and/or biologically relevant [20-22], have been selected to study the mechanism underlying the synthesis of the ferrocene–nucleobase conjugates.
Several procedures for preparing N-ferocenoylated pyrimidines were tested earlier [19,23], and the reaction of nucleobase with (chlorocarbonyl)ferrocene (or ferrocenoyl chloride, FcCOCl) under basic conditions appeared as a simple and optimal method for the synthesis [24]. Herewith, we demonstrate that substituents at the exocyclic amino group of adenine affect the reactivity of the respective purine anion and govern the regioselectivity of the ferocenoylation reaction (N7- versus N9-product). By using an appropriate substituent at the C6 position in the purine ring, one can tune the isomeric product ratio, i.e. may influence the regioselectivity of the ferrocenoylation reaction. While the N9-position of the purine ring is a typical site of substitution, the N7-position may be preferred in some situations. In any case, the interplay between steric and electronic effects of selected substituents is crucial to kinetic and thermodynamic control of the acylation reaction.
Results and Discussion
It was shown that the reaction between the pyrimidine anion (uracil, thymine, or 5-fluorouracil) and FcCOCl in N,N-dimethylformamide (DMF) proceeded in a full regiospecific mode [19]. In the purine series, however, the analogous reaction is regioselective and resulted in the formation of two products, i.e., N7- and N9-regioisomers (Scheme 1). In no case, the N1-, N3-, or N6-products were formed, which is comparable to the results reported for the reaction between benzoyl chloride (BzCl) and purine anions [25].
Scheme 1: Regioselective ferrocenoylation of the adenine anion 1 and its derivatives 2–6 substituted at the N6-position. The respective NMR yields for regioisomers N9 and N7 are shown in parentheses.
Scheme 1: Regioselective ferrocenoylation of the adenine anion 1 and its derivatives 2–6 substituted at the N6...
The two different regioisomers, 1-N7 and 1-N9, were formed simultaneously in the course of the reaction between adenine anion 1 and FcCOCl in DMF (Scheme 1). According to the 1H NMR spectrum (Figure 1) of a reaction aliquot, the ratio of N9/N7 isomers is 1.5:1, i.e. 60% regioselectivity is reached. The formation of both isomers is, therefore, a competitive process. This confirms that the adenine anion behaves as an ambident nucleophile with two competing reaction centers at the N7- and N9-position [26].
![[1860-5397-18-133-1]](/bjoc/content/figures/1860-5397-18-133-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: 1H NMR spectrum (downfield region) of the reaction mixture (adenine anion 1 and FcCOCl) in DMF, taken after 10 minutes (see numbering in Scheme 1) at 25 °C. The DMF signal was suppressed by the presaturation option, and DMSO-d6 was used as a deuterated solvent in capillary.
Figure 1: 1H NMR spectrum (downfield region) of the reaction mixture (adenine anion 1 and FcCOCl) in DMF, tak...
It is known that acylation [25] or alkylation [27-29] of adenine is rarely regiospecific, and mixtures of N7- and N9-isomers are usually obtained. In some cases, the acylation of adenine may also occur at the exo-amino group (N6) [30,31]. In general, the literature on the regioselectivity of alkylation of adenines/purines is more abundant, includes an array of reaction conditions (base, solvent, temperature) [32], and introduces various effects of microwaves [33], cyclodextrines [34], or tetrabutylammonium fluoride on the N7/N9-product ratio [35]. It is therefore of interest to collect complementary data on analogous acylation reactions, which are required for a future comparative study.
Intrinsic nucleophilicity [36] is an important factor in governing competition between the various nucleophilic centers in adenine. To estimate the relative inherent reactivity of different nucleophilic sites in the adenine anion, conceptual DFT tools were employed [37]. Specifically, frontier molecular orbital (FMO) properties and Fukui indices [38] were calculated to explain the observed regioselectivity in the ferrocenoylation of adenine.
The visualization of the highest occupied molecular orbital (HOMO) of the adenine anion is useful in predicting the nucleophilic reactivity of different nitrogen atoms toward electrophilic substrates (Figure 2). The HOMO orbital is distributed over the purine ring with the largest amplitude on the N7 atom, which designates this position as the most nucleophilic in 1. The second most populated site is the N6 atom, which appears as more nucleophilic than N9 atom.
![[1860-5397-18-133-2]](/bjoc/content/figures/1860-5397-18-133-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: HOMO map of space distribution (left) of adenine anion (1) at the B3LYP/6-31+G(d) level of theory (MO = 35, isovalue = 0.08). Molecular orbital coefficients in italics. The isosurface plot of the condensed Fukui function f- (right) for the adenine anion (isovalue = 0.008) calculated with NPA charges.
Figure 2: HOMO map of space distribution (left) of adenine anion (1) at the B3LYP/6-31+G(d) level of theory (...
To facilitate a quantitative comparison between different sites, the condensed Fukui function based on atomic charges was calculated. We calculated total populations for all nitrogen atoms in the adenine anion in its N and N−1 electron states to obtain the condensed f− descriptor according to the equation for the nucleophilicity (for details, see Computational part in Supporting Information File 1).
In purines 2–5, the method for charge fitting suggests that the most positive part of the f– function is localized at the N6 atom, which means that this nitrogen is the most nucleophilic site in adenines (except 1 and 6). In no case, however, the ferrocenoylation reaction at the N6 position was observed in 1H NMR spectra. This is expected, as the nucleophilic addition pathway involving the quaternary ammonium intermediate is not viable.
In all purine anions, according to the calculated Fukui functions f–, the N7 atom is more nucleophilic than the N9 atom. It comes out that the N7-nitrogen in the adenine anion reacts more readily with electrophiles, i.e., nucleophilic reactions occur preferably at the N7-position. The same conclusion was made by Stachowicz–Kuśnierz and Korchowiec who have shown that the inherent nucleophilicity of the N7 atom is higher compared to that of the N9 atom [39].
This is, however, in discrepancy with the regioselectivity observed in the 1H NMR spectra (Figure 1) of the reaction mixture, where the formation of the N9-isomer (1-N9) is favored. This suggests that intrinsic nucleophilicity or Fukui functions are not sufficient to explain the regioselective reaction, but other factors are to be considered.
The disagreement between the experimentally observed N9/N7 regioselectivity and calculated N9/N7 nucleophilicity was found (Table 1) for all adenine derivatives in which the N6 atom was substituted with different groups (H, Me, Bz, isopentenyl, or Boc). In 1 and 6 the N7 nitrogen atom was calculated the most nucleophilic (Table 1, values in bold), suggesting this site should be acylated predominantly. On the contrary, according to 1H NMR analysis and isolated yields, the N9-isomer was the major product in each case. It comes out that electronic properties of the respective purine are not decisive in terms of regioselectivity. Instead, steric effects may govern the N9/N7 ratio in the reaction mixture.
Table 1: The condensed Fukui functions f− (based on NBO atomic charges) for nitrogen atoms in the purine anions calculated at the (U)B3LYP/6-31+G(d) method.a
|
Purine
anion |
Nitrogen atom | Exp. ratiob N9:N7 | ||||
| N1 | N3 | N6 | N7 | N9 | ||
| purinec | 0.0460 | 0.0691 | – | 0.2730 | 0.1611 | 1:2.4 |
| 1 | 0.0468 | 0.0805 | 0.1131 | 0.1705 | 0.0836 | 1.5:1 |
| 2 | 0.0411 | 0.0726 | 0.1351 | 0.1339 | 0.0727 | 2.8:1 |
| 3 | 0.0389 | 0.0771 | 0.1590 | 0.1223 | 0.0704 | 4.0:1 |
| 4 | 0.0418 | 0.0706 | 0.1403 | 0.1303 | 0.0696 | 4.9:1 |
| 5 | 0.0449 | 0.0721 | 0.1424 | 0.1260 | 0.0682 | 7.3:1 |
| 6 | 0.0364 | 0.0561 | 0.0120 | 0.2483 | 0.1338 | 9.0:1 |
aThe largest value of f− in the respective purine anion is in bold; bisomer ratio determined from the 1H NMR spectrum of the corresponding reaction mixture in DMF; cthe anion derived from the parent 9H-purine structure.
Attempts to find a correlation between the N9/N7 ratio and the steric bulk of the C6-substituent in the alkylation of purines were reported earlier [40]. Now, we demonstrate for the first time that similar effect is operative in the acylation of purines. It is evident from the results in Table 1 that the N9/N7 ratio increases with the increasing size of the substituent at the exocyclic amino group.
In case when the steric effect is negligible (e.g., H atom at the C6-position), the N9-isomer is a minor product (less than 30%), and the acylation of the N7 position is strongly favored. In the parent purine, therefore, the nucleophilic attack is mostly controlled by electronic effects, i.e., the regioselectivity is governed by the intrinsic nucleophilicity of the N7 position (Table 1).
To correlate steric effects in ferrocenoylation reactions of purines to the measured N9/N7 ratio, the Charton (ν) [41,42] and Sterimol steric parameters [43-45] for selected substituents were introduced (Figure 3). The former parameter is empirical, and is not available for all functional groups, while the latter is a computational parameter, which constitutes a significant improvement in terms of overall utility and accuracy [43].
![[1860-5397-18-133-3]](/bjoc/content/figures/1860-5397-18-133-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Dependence of N9-isomer product ratio (%), in the reaction between FcCOCl and adenine anions 1–6, on the averaged perpendicular Sterimol parameter B = (B1 + B5)/2 of the C6-substituent (open circles; in the parent purine the C6-substituent is a H atom). The logarithmic trendline is for illustration purposes only. The inset shows the dependence of N9-isomer ratio (%) on the Charton value (closed circles; ν is not available for all C6-substituents). Purine (B = 1; ν = 0) is the anion derived from the parent 9H-purine structure.
Figure 3: Dependence of N9-isomer product ratio (%), in the reaction between FcCOCl and adenine anions 1–6, o...
An increasing trend of the N9/N7 ratio with steric bulk was observed in both cases (Figure 3). However, Charton parameters for isopentenyl (as in 4) and Boc group (as in 6) substituents (to name but a few) do not exist in the literature, thus limiting the number of points and the range of values in our diagram. Sterimol parameters are broadly applicable Boltzmann-weighted parameters, which are conformationally dependent, and thus may define the steric effect for any conceivable substituent. They are multidimensional parameters, that is, they describe steric bulk along different principal axes, hence, the effects of unsymmetrical substituents are better described with Sterimol parameters. B1, B5, and L0 subparameters comprise Sterimol parameters and are defined using Corey–Pauling–Koltun (CPK) molecular models, with B1 being the shortest perpendicular distance from the primary axis of the attachment, B5 being the maximum width form the same axis and L0 representing the total distance along the primary axis of the attachment. All Boltzmann-weighted subparameters for all purines (1–6) are deposited in Table S2 (Supporting Information File 1).
In addition, we calculated the percentage of buried volume (%VBur), another popular steric descriptor which may be applied to quantify the fraction of the defined sphere around a reaction center [46]. It was introduced for ligands on metals [47], but may be adapted to estimate the steric hindrance of substituents in different chemical environments (see Supporting Information File 1 for more details). As expected, within the group of N7-regioisomers, the calculated %VBur increases with more bulkier groups at the C6-position (as going from 1 to 6), whereas no significant effects are observed in the series of N9-isomers (Table S3 in Supporting Information File 1). These results nicely complement the trend measured with Sterimol parameters.
We assume that the steric effect of the C6-substituent is the most evident in the transition state structure leading to the formation of the N7-ferrocenoylated product. The bulky substituents at the C6 atom may shield the proximal N7 region of space, which prevents the approach of an electrophile (e.g., FcCOCl) towards the N7 atom. In the course of N9-isomer formation no similar steric hindrance is encountered.
This is supported by our quantum-chemical calculations which compared the two transition state structures for the ferrocenoylation of the N6,N6-di-tert-butyloxycarbonyladenine, i.e., the derivative with the bulkiest substituents at the C6-position. The calculated energy barrier for the formation of the N7-isomer (6-N7) is higher than the corresponding barrier for the formation of the N9-isomer (6-N9) (ΔΔG‡ = 11.3 kJ/mol). The respective transition state structures 6-TSN7 and 6-TSN9 (Figure 4) are characterized by one imaginary frequency (134i and 163i cm−1, respectively), which corresponds to the N–C bond formation concomitant with C–Cl bond breaking. Both structures support a concerted SN2-type mechanism in which a tetrahedral intermediate does not exist. Therefore, the one-step mechanism is operative in the reaction between adenine anion 6 and FcCOCl.
![[1860-5397-18-133-4]](/bjoc/content/figures/1860-5397-18-133-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: B3LYP/6-31+G(d)/SDD optimized transition state structures for N7- (6-TSN7) and N9-ferrocenoylation (6-TSN9) of the adenine anion 6. Two bulky tert-butyloxycarbonyl groups are displayed in the tube mode for clarity.
Figure 4: B3LYP/6-31+G(d)/SDD optimized transition state structures for N7- (6-TSN7) and N9-ferrocenoylation (...
The structure 6-TSN7 is characterized with an unfavorable steric repulsion between the tert-butyloxycarbonyl group at the C6-position and the acyl group approaching the N7 atom. This steric constraint is not present in the transition structure 6-TSN9, which is, as reported above, more stable (11.3 kJ/mol) than the structure 6-TSN7. It comes out that the regioselectivity of the ferrocenoylation of adenine anion 6 is kinetically controlled, mostly due to steric effects.
The same SN2-type mechanism is operative for the reaction between N6-substituted adenine anions 1–5 and FcCOCl. In no case the tetrahedral intermediate, typical of a nucleophilic addition–elimination pathway, was located as a genuine minimum on the potential energy landscape. Instead, the structure with tetrahedral geometry corresponds to the transition state, which directly (in a single step manner) connects respective reactants and acylated product. All optimized geometries are deposited in Supporting Information File 1.
According to the calculated results, the energy barrier for the N7-ferrocenoylation reaction increases with the size of the group attached at the C6 position (Figure 5). A nearly linear relationship (r2 = 0.93) between the calculated barrier (ΔG‡) for the N7-ferrocenoylation and the steric parameter (B) is obtained. The only exception is the energy barrier for the N7-ferrocenoylation of the N6,N6-dimethyladenine (3). For some reason, the calculated barrier is prohibitively high (ΔG‡ = 144 kJ/mol), which suggests an inappropriate quality of the selected theoretical level, or indicates that an alternative ferrocenoylation mechanism in case of 3 is operative (e.g., the reaction which includes the quaternary nitrogen intermediate, see Scheme S1 in Supporting Information File 1). In any case, the N7-ferrocenoylation of adenine anion 3 is a viable process, as demonstrated by the 1H NMR spectroscopy evidence (see above), and by the isolation of the N7-ferrocenoylated product.
![[1860-5397-18-133-5]](/bjoc/content/figures/1860-5397-18-133-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Relation between the Gibbs free energy barrier (ΔG‡) for the N7-ferrocenoylation of C6-substituted adenines and the averaged perpendicular Sterimol parameter B = (B1 + B5)/2. The linear trendline is for illustration purposes only. Data point for 3 (N6,N6-dimethyladenine; open circle) appears as an outlier. Purine (B = 1) is the anion derived from the parent 9H-purine structure.
Figure 5: Relation between the Gibbs free energy barrier (ΔG‡) for the N7-ferrocenoylation of C6-substituted ...
We have continued our study with an extended set of C6- and C2-substituents in the purine ring, but preliminary results suggested that a simple correlation between the N9/N7 product ratio and steric parameters was lost. In order to relate the regioselectivity observed in the acylation of purines, additional descriptors, such as the Swain and Lupton resonance parameter, may be included in multiple regression analysis [40]. In some cases, e.g., C6-chloropurine and C6-bromopurine, only the N9-ferrocenoylated product was detected (see Figures S3 and S4 in Supporting Information File 1), which confirms that the regioselective reaction may be switched to the regiospecific mode by selecting suitable substituents on the purine ring.
Conclusion
In the reaction between N6-substituted adenine anions and ferrocenoyl chloride two regioisomeric products were formed: N7- and N9-ferrocenoylated adenines. The product ratio is strongly dependent on the steric parameter of the N6-substituent. The N9/N7 ratio is increasing with the larger substituent size. The bulkiness of substituents (H, Me, Bz, isopentenyl, and tert-butyloxycarbonyl) was defined by Charton and/or Sterimol parameters. The latter descriptor is a computational descriptor and may be assigned to any type of substituents. Specifically, the averaged perpendicular Sterimol parameter B linearly correlate with the calculated energy barrier (ΔG‡) for the N7-ferrocenoylation of adenine derivatives, which supports our claim that the observed N9/N7 regioselectivity is kinetically controlled.
When steric hindrance is negligible (e.g., the parent purine), the regioselectivity for a respective reaction is governed by electronic properties, such as nucleophilicity and/or electrophilicity. This may be predicted computationally using the conceptual DFT approach. We applied Fukui indices as descriptors for chemical reactivity, which revealed that the N7 atom is more nucleophilic than the N9 atom in all adenine derivatives. Both, steric and electronic properties are to be included when considering the regioselectivity of the acylation reaction of purines. In some cases, the regioselectivity was translated into a regiospecific process, i.e., only the N9-regioisomer appeared as a product in the ferocenoylation reaction.
Supporting Information
| Supporting Information File 1: Details on experimental procedures, DFT calculated energies and optimized coordinates for transition state structures and reactants, and the results of in situ 1H NMR monitoring. | ||
| Format: PDF | Size: 844.6 KB | Download |
References
-
Kowalski, K. Coord. Chem. Rev. 2021, 432, 213705. doi:10.1016/j.ccr.2020.213705
Return to citation in text: [1] -
Ismail, M. K.; Armstrong, K. A.; Hodder, S. L.; Horswell, S. L.; Male, L.; Nguyen, H. V.; Wilkinson, E. A.; Hodges, N. J.; Tucker, J. H. R. Dalton Trans. 2020, 49, 1181–1190. doi:10.1039/c9dt04174e
Return to citation in text: [1] -
Kaczmarek, R.; Korczyński, D.; Green, J. R.; Dembinski, R. Beilstein J. Org. Chem. 2020, 16, 1–8. doi:10.3762/bjoc.16.1
Return to citation in text: [1] -
Kowalski, K. Coord. Chem. Rev. 2016, 317, 132–156. doi:10.1016/j.ccr.2016.02.008
Return to citation in text: [1] -
Hocek, M.; Štěpnička, P.; Ludvík, J.; Císařová, I.; Votruba, I.; Řeha, D.; Hobza, P. Chem. – Eur. J. 2004, 10, 2058–2066. doi:10.1002/chem.200305621
Return to citation in text: [1] -
Ismail, M. K.; Khan, Z.; Rana, M.; Horswell, S. L.; Male, L.; Nguyen, H. V.; Perotti, A.; Romero‐Canelón, I.; Wilkinson, E. A.; Hodges, N. J.; Tucker, J. H. R. ChemBioChem 2020, 21, 2487–2494. doi:10.1002/cbic.202000124
Return to citation in text: [1] -
Skiba, J.; Kowalczyk, A.; Trzybiński, D.; Woźniak, K.; Vrček, V.; Gapińska, M.; Kowalski, K. Eur. J. Inorg. Chem. 2021, 2171–2181. doi:10.1002/ejic.202100193
Return to citation in text: [1] -
Skiba, J.; Schmidt, C.; Lippmann, P.; Ensslen, P.; Wagenknecht, H.-A.; Czerwieniec, R.; Brandl, F.; Ott, I.; Bernaś, T.; Krawczyk, B.; Szczukocki, D.; Kowalski, K. Eur. J. Inorg. Chem. 2017, 297–305. doi:10.1002/ejic.201600281
Return to citation in text: [1] -
Anisimov, I.; Saloman, S.; Hildebrandt, A.; Lang, H.; Trzybiński, D.; Woźniak, K.; Šakić, D.; Vrček, V.; Kowalski, K. ChemPlusChem 2017, 82, 859–866. doi:10.1002/cplu.201700215
Return to citation in text: [1] -
Lewandowski, E. M.; Szczupak, Ł.; Wong, S.; Skiba, J.; Guśpiel, A.; Solecka, J.; Vrček, V.; Kowalski, K.; Chen, Y. Organometallics 2017, 36, 1673–1676. doi:10.1021/acs.organomet.6b00888
Return to citation in text: [1] -
Kowalski, K.; Szczupak, Ł.; Saloman, S.; Steverding, D.; Jabłoński, A.; Vrček, V.; Hildebrandt, A.; Lang, H.; Rybarczyk-Pirek, A. ChemPlusChem 2017, 82, 303–314. doi:10.1002/cplu.201600462
Return to citation in text: [1] -
Ortiz, M.; Jauset-Rubio, M.; Skouridou, V.; Machado, D.; Viveiros, M.; Clark, T. G.; Simonova, A.; Kodr, D.; Hocek, M.; O’Sullivan, C. K. ACS Sens. 2021, 6, 4398–4407. doi:10.1021/acssensors.1c01710
Return to citation in text: [1] -
Simonova, A.; Magriñá, I.; Sýkorová, V.; Pohl, R.; Ortiz, M.; Havran, L.; Fojta, M.; O'Sullivan, C. K.; Hocek, M. Chem. – Eur. J. 2020, 26, 1286–1291. doi:10.1002/chem.201904700
Return to citation in text: [1] -
Fabre, B.; Ababou-Girard, S.; Singh, P.; Kumar, J.; Verma, S.; Bianco, A. Electrochem. Commun. 2010, 12, 831–834. doi:10.1016/j.elecom.2010.03.045
Return to citation in text: [1] -
Patwa, A. N.; Gonnade, R. G.; Kumar, V. A.; Bhadbhade, M. M.; Ganesh, K. N. J. Org. Chem. 2010, 75, 8705–8708. doi:10.1021/jo101813z
Return to citation in text: [1] -
Singh, P.; Ménard-Moyon, C.; Kumar, J.; Fabre, B.; Verma, S.; Bianco, A. Carbon 2012, 50, 3170–3177. doi:10.1016/j.carbon.2011.10.037
Return to citation in text: [1] -
Ji, C.; Li, H.; Zhang, L.; Wang, P.; Lv, Y.; Sun, Z.; Tan, J.; Yuan, Q.; Tan, W. Angew. Chem., Int. Ed. 2022, 61, e202200237. doi:10.1002/anie.202200237
Return to citation in text: [1] -
Skiba, J.; Yuan, Q.; Hildebrandt, A.; Lang, H.; Trzybiński, D.; Woźniak, K.; Balogh, R. K.; Gyurcsik, B.; Vrček, V.; Kowalski, K. ChemPlusChem 2018, 83, 77–86. doi:10.1002/cplu.201700551
Return to citation in text: [1] -
Lapić, J.; Havaić, V.; Šakić, D.; Sanković, K.; Djaković, S.; Vrček, V. Eur. J. Org. Chem. 2015, 5424–5431. doi:10.1002/ejoc.201500647
Return to citation in text: [1] [2] [3] -
Trávníček, Z.; Novotná, R.; Marek, J.; Popa, I.; Šipl, M. Org. Biomol. Chem. 2011, 9, 5703–5713. doi:10.1039/c1ob05649b
Return to citation in text: [1] -
Fernandes, S. B.; Grova, N.; Roth, S.; Duca, R. C.; Godderis, L.; Guebels, P.; Mériaux, S. B.; Lumley, A. I.; Bouillaud-Kremarik, P.; Ernens, I.; Devaux, Y.; Schroeder, H.; Turner, J. D. Front. Genet. 2021, 12, 657171. doi:10.3389/fgene.2021.657171
Return to citation in text: [1] -
McHugh, C.; Erxleben, A. Cryst. Growth Des. 2011, 11, 5096–5104. doi:10.1021/cg201007m
Return to citation in text: [1] -
Zherebker, K. Ya.; Rodionov, A. N.; Pilipenko, E. S.; Kachala, V. V.; Nikitin, O. M.; Belousov, Yu. A.; Simenel, A. A. Russ. J. Org. Chem. 2014, 50, 1150–1154. doi:10.1134/s1070428014080132
Return to citation in text: [1] -
Toma, M.; Božičević, L.; Lapić, J.; Djaković, S.; Šakić, D.; Tandarić, T.; Vianello, R.; Vrček, V. J. Org. Chem. 2019, 84, 12471–12480. doi:10.1021/acs.joc.9b01944
Return to citation in text: [1] -
Soltani Rad, M. N.; Behrouz, S.; Asrari, Z.; Khalafi-Nezhad, A. Monatsh. Chem. 2014, 145, 1933–1940. doi:10.1007/s00706-014-1270-1
Return to citation in text: [1] [2] -
Breugst, M.; Corral Bautista, F.; Mayr, H. Chem. – Eur. J. 2012, 18, 127–137. doi:10.1002/chem.201102411
Return to citation in text: [1] -
Joshi, R. V.; Zemlicka, J. Tetrahedron 1993, 49, 2353–2360. doi:10.1016/s0040-4020(01)86315-8
Return to citation in text: [1] -
Rasmussen, M.; Hope, J. M. Aust. J. Chem. 1982, 35, 525–534. doi:10.1071/ch9820525
Return to citation in text: [1] -
Zhong, M.; Robins, M. J. J. Org. Chem. 2006, 71, 8901–8906. doi:10.1021/jo061759h
Return to citation in text: [1] -
Ried, W.; Woithe, H.; Müller, A. Helv. Chim. Acta 1989, 72, 1597–1607. doi:10.1002/hlca.19890720720
Return to citation in text: [1] -
Dutta, S. P.; Hong, C. I.; Tritsch, G. L.; Cox, C.; Parthasarthy, R.; Chheda, G. B. J. Med. Chem. 1977, 20, 1598–1607. doi:10.1021/jm00222a013
Return to citation in text: [1] -
Petrov, V.; Dooley, R. J.; Marchione, A. A.; Diaz, E. L.; Clem, B. S.; Marshall, W. Beilstein J. Org. Chem. 2020, 16, 2739–2748. doi:10.3762/bjoc.16.224
Return to citation in text: [1] -
Zhang, Q.; Cheng, G.; Huang, Y.-Z.; Qu, G.-R.; Niu, H.-Y.; Guo, H.-M. Tetrahedron 2012, 68, 7822–7826. doi:10.1016/j.tet.2012.07.025
Return to citation in text: [1] -
Vinuesa, A.; Viñas, M.; Jahani, D.; Ginard, J.; Mur, N.; Pujol, M. D. J. Heterocycl. Chem. 2022, 59, 597–602. doi:10.1002/jhet.4407
Return to citation in text: [1] -
Brik, A.; Wu, C.-Y.; Best, M. D.; Wong, C.-H. Bioorg. Med. Chem. 2005, 13, 4622–4626. doi:10.1016/j.bmc.2005.02.066
Return to citation in text: [1] -
Freccero, M.; Gandolfi, R.; Sarzi-Amadè, M. J. Org. Chem. 2003, 68, 6411–6423. doi:10.1021/jo0346252
Return to citation in text: [1] -
Parr, R. G.; Yang, W. Annu. Rev. Phys. Chem. 1995, 46, 701–728. doi:10.1146/annurev.pc.46.100195.003413
Return to citation in text: [1] -
Parr, R. G.; Yang, W. J. Am. Chem. Soc. 1984, 106, 4049–4050. doi:10.1021/ja00326a036
Return to citation in text: [1] -
Stachowicz-Kuśnierz, A.; Korchowiec, J. Struct. Chem. 2016, 27, 543–555. doi:10.1007/s11224-015-0583-y
Return to citation in text: [1] -
Geen, G. R.; Grinter, T. J.; Kincey, P. M.; Jarvest, R. L. Tetrahedron 1990, 46, 6903–6914. doi:10.1016/s0040-4020(01)87878-9
Return to citation in text: [1] [2] -
Charton, M. J. Am. Chem. Soc. 1975, 97, 1552–1556. doi:10.1021/ja00839a047
Return to citation in text: [1] -
Charton, M. The upsilon steric parameter - definition and determination. In Steric Effects in Drug Design; Austel, V.; Balaban, A. T.; Bonchev, D.; Charton, M.; Fujita, T.; Iwamura, H.; Mekenyan, O.; Motoc, I., Eds.; Topics in Current Chemistry, Vol. 114; Springer: Berlin, Heidelberg, 1983; pp 57–91. doi:10.1007/bfb0111213
Return to citation in text: [1] -
Harper, K. C.; Bess, E. N.; Sigman, M. S. Nat. Chem. 2012, 4, 366–374. doi:10.1038/nchem.1297
Return to citation in text: [1] [2] -
Weighted Sterimol, August 2019. https://github.com/bobbypaton/wSterimol (accessed June 15, 2022).
Return to citation in text: [1] -
Brethomé, A. V.; Fletcher, S. P.; Paton, R. S. ACS Catal. 2019, 9, 2313–2323. doi:10.1021/acscatal.8b04043
Return to citation in text: [1] -
Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Nat. Chem. 2019, 11, 872–879. doi:10.1038/s41557-019-0319-5
Return to citation in text: [1] -
SambVca 2.1 A web application to characterize catalytic pockets. https://www.molnac.unisa.it/OMtools/sambvca2.1/index.html (accessed Aug 15, 2022).
Return to citation in text: [1]
| 40. | Geen, G. R.; Grinter, T. J.; Kincey, P. M.; Jarvest, R. L. Tetrahedron 1990, 46, 6903–6914. doi:10.1016/s0040-4020(01)87878-9 |
| 41. | Charton, M. J. Am. Chem. Soc. 1975, 97, 1552–1556. doi:10.1021/ja00839a047 |
| 42. | Charton, M. The upsilon steric parameter - definition and determination. In Steric Effects in Drug Design; Austel, V.; Balaban, A. T.; Bonchev, D.; Charton, M.; Fujita, T.; Iwamura, H.; Mekenyan, O.; Motoc, I., Eds.; Topics in Current Chemistry, Vol. 114; Springer: Berlin, Heidelberg, 1983; pp 57–91. doi:10.1007/bfb0111213 |
| 43. | Harper, K. C.; Bess, E. N.; Sigman, M. S. Nat. Chem. 2012, 4, 366–374. doi:10.1038/nchem.1297 |
| 44. | Weighted Sterimol, August 2019. https://github.com/bobbypaton/wSterimol (accessed June 15, 2022). |
| 45. | Brethomé, A. V.; Fletcher, S. P.; Paton, R. S. ACS Catal. 2019, 9, 2313–2323. doi:10.1021/acscatal.8b04043 |
| 1. | Kowalski, K. Coord. Chem. Rev. 2021, 432, 213705. doi:10.1016/j.ccr.2020.213705 |
| 2. | Ismail, M. K.; Armstrong, K. A.; Hodder, S. L.; Horswell, S. L.; Male, L.; Nguyen, H. V.; Wilkinson, E. A.; Hodges, N. J.; Tucker, J. H. R. Dalton Trans. 2020, 49, 1181–1190. doi:10.1039/c9dt04174e |
| 3. | Kaczmarek, R.; Korczyński, D.; Green, J. R.; Dembinski, R. Beilstein J. Org. Chem. 2020, 16, 1–8. doi:10.3762/bjoc.16.1 |
| 11. | Kowalski, K.; Szczupak, Ł.; Saloman, S.; Steverding, D.; Jabłoński, A.; Vrček, V.; Hildebrandt, A.; Lang, H.; Rybarczyk-Pirek, A. ChemPlusChem 2017, 82, 303–314. doi:10.1002/cplu.201600462 |
| 25. | Soltani Rad, M. N.; Behrouz, S.; Asrari, Z.; Khalafi-Nezhad, A. Monatsh. Chem. 2014, 145, 1933–1940. doi:10.1007/s00706-014-1270-1 |
| 8. | Skiba, J.; Schmidt, C.; Lippmann, P.; Ensslen, P.; Wagenknecht, H.-A.; Czerwieniec, R.; Brandl, F.; Ott, I.; Bernaś, T.; Krawczyk, B.; Szczukocki, D.; Kowalski, K. Eur. J. Inorg. Chem. 2017, 297–305. doi:10.1002/ejic.201600281 |
| 9. | Anisimov, I.; Saloman, S.; Hildebrandt, A.; Lang, H.; Trzybiński, D.; Woźniak, K.; Šakić, D.; Vrček, V.; Kowalski, K. ChemPlusChem 2017, 82, 859–866. doi:10.1002/cplu.201700215 |
| 10. | Lewandowski, E. M.; Szczupak, Ł.; Wong, S.; Skiba, J.; Guśpiel, A.; Solecka, J.; Vrček, V.; Kowalski, K.; Chen, Y. Organometallics 2017, 36, 1673–1676. doi:10.1021/acs.organomet.6b00888 |
| 26. | Breugst, M.; Corral Bautista, F.; Mayr, H. Chem. – Eur. J. 2012, 18, 127–137. doi:10.1002/chem.201102411 |
| 5. | Hocek, M.; Štěpnička, P.; Ludvík, J.; Císařová, I.; Votruba, I.; Řeha, D.; Hobza, P. Chem. – Eur. J. 2004, 10, 2058–2066. doi:10.1002/chem.200305621 |
| 6. | Ismail, M. K.; Khan, Z.; Rana, M.; Horswell, S. L.; Male, L.; Nguyen, H. V.; Perotti, A.; Romero‐Canelón, I.; Wilkinson, E. A.; Hodges, N. J.; Tucker, J. H. R. ChemBioChem 2020, 21, 2487–2494. doi:10.1002/cbic.202000124 |
| 7. | Skiba, J.; Kowalczyk, A.; Trzybiński, D.; Woźniak, K.; Vrček, V.; Gapińska, M.; Kowalski, K. Eur. J. Inorg. Chem. 2021, 2171–2181. doi:10.1002/ejic.202100193 |
| 24. | Toma, M.; Božičević, L.; Lapić, J.; Djaković, S.; Šakić, D.; Tandarić, T.; Vianello, R.; Vrček, V. J. Org. Chem. 2019, 84, 12471–12480. doi:10.1021/acs.joc.9b01944 |
| 4. | Kowalski, K. Coord. Chem. Rev. 2016, 317, 132–156. doi:10.1016/j.ccr.2016.02.008 |
| 19. | Lapić, J.; Havaić, V.; Šakić, D.; Sanković, K.; Djaković, S.; Vrček, V. Eur. J. Org. Chem. 2015, 5424–5431. doi:10.1002/ejoc.201500647 |
| 18. | Skiba, J.; Yuan, Q.; Hildebrandt, A.; Lang, H.; Trzybiński, D.; Woźniak, K.; Balogh, R. K.; Gyurcsik, B.; Vrček, V.; Kowalski, K. ChemPlusChem 2018, 83, 77–86. doi:10.1002/cplu.201700551 |
| 20. | Trávníček, Z.; Novotná, R.; Marek, J.; Popa, I.; Šipl, M. Org. Biomol. Chem. 2011, 9, 5703–5713. doi:10.1039/c1ob05649b |
| 21. | Fernandes, S. B.; Grova, N.; Roth, S.; Duca, R. C.; Godderis, L.; Guebels, P.; Mériaux, S. B.; Lumley, A. I.; Bouillaud-Kremarik, P.; Ernens, I.; Devaux, Y.; Schroeder, H.; Turner, J. D. Front. Genet. 2021, 12, 657171. doi:10.3389/fgene.2021.657171 |
| 22. | McHugh, C.; Erxleben, A. Cryst. Growth Des. 2011, 11, 5096–5104. doi:10.1021/cg201007m |
| 47. | SambVca 2.1 A web application to characterize catalytic pockets. https://www.molnac.unisa.it/OMtools/sambvca2.1/index.html (accessed Aug 15, 2022). |
| 16. | Singh, P.; Ménard-Moyon, C.; Kumar, J.; Fabre, B.; Verma, S.; Bianco, A. Carbon 2012, 50, 3170–3177. doi:10.1016/j.carbon.2011.10.037 |
| 17. | Ji, C.; Li, H.; Zhang, L.; Wang, P.; Lv, Y.; Sun, Z.; Tan, J.; Yuan, Q.; Tan, W. Angew. Chem., Int. Ed. 2022, 61, e202200237. doi:10.1002/anie.202200237 |
| 19. | Lapić, J.; Havaić, V.; Šakić, D.; Sanković, K.; Djaković, S.; Vrček, V. Eur. J. Org. Chem. 2015, 5424–5431. doi:10.1002/ejoc.201500647 |
| 23. | Zherebker, K. Ya.; Rodionov, A. N.; Pilipenko, E. S.; Kachala, V. V.; Nikitin, O. M.; Belousov, Yu. A.; Simenel, A. A. Russ. J. Org. Chem. 2014, 50, 1150–1154. doi:10.1134/s1070428014080132 |
| 40. | Geen, G. R.; Grinter, T. J.; Kincey, P. M.; Jarvest, R. L. Tetrahedron 1990, 46, 6903–6914. doi:10.1016/s0040-4020(01)87878-9 |
| 14. | Fabre, B.; Ababou-Girard, S.; Singh, P.; Kumar, J.; Verma, S.; Bianco, A. Electrochem. Commun. 2010, 12, 831–834. doi:10.1016/j.elecom.2010.03.045 |
| 15. | Patwa, A. N.; Gonnade, R. G.; Kumar, V. A.; Bhadbhade, M. M.; Ganesh, K. N. J. Org. Chem. 2010, 75, 8705–8708. doi:10.1021/jo101813z |
| 43. | Harper, K. C.; Bess, E. N.; Sigman, M. S. Nat. Chem. 2012, 4, 366–374. doi:10.1038/nchem.1297 |
| 12. | Ortiz, M.; Jauset-Rubio, M.; Skouridou, V.; Machado, D.; Viveiros, M.; Clark, T. G.; Simonova, A.; Kodr, D.; Hocek, M.; O’Sullivan, C. K. ACS Sens. 2021, 6, 4398–4407. doi:10.1021/acssensors.1c01710 |
| 13. | Simonova, A.; Magriñá, I.; Sýkorová, V.; Pohl, R.; Ortiz, M.; Havran, L.; Fojta, M.; O'Sullivan, C. K.; Hocek, M. Chem. – Eur. J. 2020, 26, 1286–1291. doi:10.1002/chem.201904700 |
| 19. | Lapić, J.; Havaić, V.; Šakić, D.; Sanković, K.; Djaković, S.; Vrček, V. Eur. J. Org. Chem. 2015, 5424–5431. doi:10.1002/ejoc.201500647 |
| 46. | Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Nat. Chem. 2019, 11, 872–879. doi:10.1038/s41557-019-0319-5 |
| 30. | Ried, W.; Woithe, H.; Müller, A. Helv. Chim. Acta 1989, 72, 1597–1607. doi:10.1002/hlca.19890720720 |
| 31. | Dutta, S. P.; Hong, C. I.; Tritsch, G. L.; Cox, C.; Parthasarthy, R.; Chheda, G. B. J. Med. Chem. 1977, 20, 1598–1607. doi:10.1021/jm00222a013 |
| 25. | Soltani Rad, M. N.; Behrouz, S.; Asrari, Z.; Khalafi-Nezhad, A. Monatsh. Chem. 2014, 145, 1933–1940. doi:10.1007/s00706-014-1270-1 |
| 27. | Joshi, R. V.; Zemlicka, J. Tetrahedron 1993, 49, 2353–2360. doi:10.1016/s0040-4020(01)86315-8 |
| 28. | Rasmussen, M.; Hope, J. M. Aust. J. Chem. 1982, 35, 525–534. doi:10.1071/ch9820525 |
| 29. | Zhong, M.; Robins, M. J. J. Org. Chem. 2006, 71, 8901–8906. doi:10.1021/jo061759h |
| 38. | Parr, R. G.; Yang, W. J. Am. Chem. Soc. 1984, 106, 4049–4050. doi:10.1021/ja00326a036 |
| 39. | Stachowicz-Kuśnierz, A.; Korchowiec, J. Struct. Chem. 2016, 27, 543–555. doi:10.1007/s11224-015-0583-y |
| 36. | Freccero, M.; Gandolfi, R.; Sarzi-Amadè, M. J. Org. Chem. 2003, 68, 6411–6423. doi:10.1021/jo0346252 |
| 37. | Parr, R. G.; Yang, W. Annu. Rev. Phys. Chem. 1995, 46, 701–728. doi:10.1146/annurev.pc.46.100195.003413 |
| 34. | Vinuesa, A.; Viñas, M.; Jahani, D.; Ginard, J.; Mur, N.; Pujol, M. D. J. Heterocycl. Chem. 2022, 59, 597–602. doi:10.1002/jhet.4407 |
| 35. | Brik, A.; Wu, C.-Y.; Best, M. D.; Wong, C.-H. Bioorg. Med. Chem. 2005, 13, 4622–4626. doi:10.1016/j.bmc.2005.02.066 |
| 32. | Petrov, V.; Dooley, R. J.; Marchione, A. A.; Diaz, E. L.; Clem, B. S.; Marshall, W. Beilstein J. Org. Chem. 2020, 16, 2739–2748. doi:10.3762/bjoc.16.224 |
| 33. | Zhang, Q.; Cheng, G.; Huang, Y.-Z.; Qu, G.-R.; Niu, H.-Y.; Guo, H.-M. Tetrahedron 2012, 68, 7822–7826. doi:10.1016/j.tet.2012.07.025 |
© 2022 Toma et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.