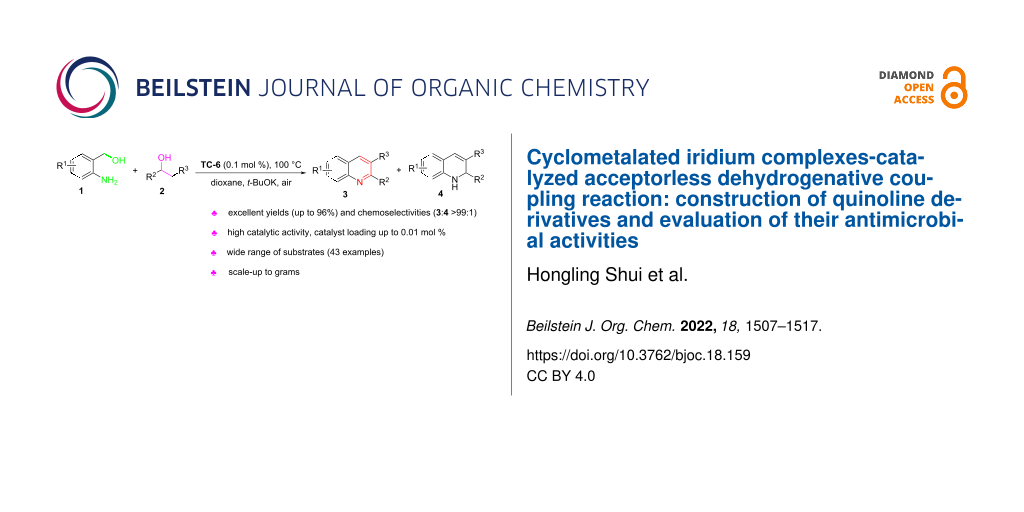
Abstract
The acceptorless dehydrogenative coupling (ADC) reaction is an efficient method for synthesizing quinoline and its derivatives. In this paper, various substituted quinolines were synthesized from 2-aminobenzyl alcohols and aryl/heteroaryl/alkyl secondary alcohols in one pot via a cyclometalated iridium-catalyzed ADC reaction. This method has some advantages, such as easy availability of raw materials, mild reaction conditions, wide range of substrates, and environmental friendliness which conforms to the principles of green chemistry. Furthermore, a gram-scale experiment with low catalyst loading offers the potential to access the aryl/heteroaryl quinolones in suitable amounts. In addition, the antibacterial and antifungal activities of the synthesized quinolines were evaluated in vitro, and the experimental results showed that the antibacterial activities of compounds 3ab, 3ad, and 3ah against Gram-positive bacteria and compound 3ck against C. albicans were better than the reference drug norfloxacin.
Graphical Abstract
Introduction
As an important class of heterocyclic compounds, quinoline and its derivatives widely exist in natural products. They have a wide range of biological activities, such as antibacterial [1], anti-inflammatory [2], antitumor [3], antihepatitis C (HCV) [4], antituberculosis (TB) [5], antimalarial [6], and anti-Alzheimer's disease (AD) [7]. Among these biological activities, their antibacterial effect is more prominent. As we know, antimicrobial agents are a significant source to overcome bacterial infections, but overuse will lead to drug resistance [8], so it is necessary to synthesize new antibacterial compounds to overcome this problem. Quinolines whose physical and chemical properties and pharmacological activities could be improved by structural modifications are used as important antibacterial agents. The compounds are characterized by high efficiency, low toxicity, and low residue, and play an important role in pharmacy and medicine. Therefore, it is still of great significance to develop new and broad-spectrum quinoline antibacterial agents, and the research on antibacterial quinolines is one of the most promising and dynamic research fields in contemporary anti-infective therapy. For example, Eswaran's group [9] synthesized some 1,2,4-triazoquinoline derivatives, and the biological activity evaluation showed that most of the compounds had a higher antibacterial activity (the optimal MIC value was 6.25 mg/mL) against Gram-positive bacteria, Gram-negative bacteria and all tested fungi than the standard ciprofloxacin (Figure 1a). Bodke's group [10] synthesized a series of carbohydrazide derivatives through reaction of 2-methylbenzofuran-2-quinoline-4-carboxylate with hydrazine hydrate in refluxing ethanol (Figure 1b). All compounds showed higher activity against Staphylococcus aureus than ampicillin and the optimal MIC value was 0.064 mg/mL. In addition, Aravinda's group [11] prepared 3-(1,3-dioxolan-2-yl)benzo[h]quinolines containing thiol and selenol groups in one pot by microwave irradiation, and tested the antibacterial activity of the compounds. The results showed that the antibacterial effect of some compounds was better than ciprofloxacin (Figure 1c).
Figure 1: Some new quinoline antibacterial drugs.
Figure 1: Some new quinoline antibacterial drugs.
In recent years, the synthesis of quinolines has received great attention and remarkable achievements have been made to produce quinolones by various methods: Skraup reaction [12], Doebner–Miller reaction [13], Combes synthesis method [14], Conrad–Limpach reaction [15], Pfitzinger reaction [16], and Friedländer reaction [17]. Among these syntheses, the Friedländer reaction [17] is one of the most commonly used methods for the synthesis of quinolines. However, it has the disadvantages of harsh reaction conditions and low yields owing to the reactivity of o-aminobenzaldehyde when used as raw material. In order to solve such problems, chemists have developed ADC reactions catalyzed by metal complexes (such as Ir [18,19], Ru [20-24], Re [25], Mn [26,27], Pd [28], Ni [29], Cu [30], etc.) to synthesize quinolines using o-aminobenzyl alcohol as starting material.
ADC reactions have the advantages of high atom economy, simple operation, clean and green, and have become a research hotspot [31-35]. Cyclometalated iridium complexes with good catalytic efficiency and selectivity are very effective catalysts in ADC reactions. Moreover, these catalysts are easy to synthesize and stable to air [36], and have good operability and reproducibility [37,38]. In recent years, our research group has carried out relevant research on ADC reactions catalyzed by cyclometalated iridium complexes and obtained some interesting research results [39].
In previous studies [39-42], we found that cyclometalated iridium catalysts can effectively catalyze the dehydrogenation of alcohols to produce carbonyl compounds and hydrogen gas. Therefore, we used cyclometalated iridium complex (TC-6) to catalyze the ADC reaction of o-aminobenzyl alcohols 1 and aryl/heteroaryl/alkyl secondary alcohols 2 that allowed for the efficient synthesis of a series of quinolines 3 (up to 95% yield and >99:1 selectivity) (Figure 2). A preliminary evaluation of the compounds’ potential antibacterial activity was also performed.
Figure 2: Cyclometalated iridium-catalyzed ADC reaction of o-aminobenzyl alcohols and secondary alcohols.
Figure 2: Cyclometalated iridium-catalyzed ADC reaction of o-aminobenzyl alcohols and secondary alcohols.
Results and Discussion
We started our research with the ADC reaction of 2-aminobenzyl alcohol (1a) with 1-phenylethanol (2a) as model reaction in the presence of various cyclometalated iridium complexes TC-1–TC-6 (Table 1). Encouragingly, employing TC-1 as the catalyst, toluene as the solvent and t-BuOK as the base at 100 °C, quinoline 3aa was obtained in 73% yield accompanied by 27% yield of 1,2-dihydroquinoline 4aa (Table 1, entry 1). Then, several other cyclometalated iridium complexes were studied. The catalysts TC-2 and TC-4 containing electron-donating ligands provided quinoline 3aa in higher chemoselectivity and yield (Table 1, entries 2 and 4). On the contrary, the catalysts TC-3 and TC-5 containing electron-withdrawing ligands offered lower chemoselectivity and yield (Table 1, entries 3 and 5). Further catalyst screening revealed that TC-6 (6-methoxy) is the best catalyst for the ADC reaction affording the product in a yield of 95% (Table 1, entry 6). On the other hand, when no catalyst was added to the reaction system under the above conditions, the reaction also proceeded, but the chemical selectivity and yield were significantly lower (Table 1, entry 7).
Table 1: Optimization of catalyst for ADC reaction of 2-aminobenzyl alcohol and 1-phenylethanol.a
|
|
||||
| Entry | Tang’s catalyst | Time (h) | 3aa:4aab | Yield of 3aab (%) |
| 1 | TC-1 | 24 | 73:27 | 73 |
| 2 | TC-2 | 24 | 79:21 | 79 |
| 3 | TC-3 | 24 | 56:44 | 56 |
| 4 | TC-4 | 24 | 82:18 | 82 |
| 5 | TC-5 | 24 | 59:41 | 59 |
| 6 | TC-6 | 24 | 95:5 | 95 (93)c |
| 7d | – | 48 | 51:49 | 51 |
aReaction conditions: 1a (1.1 mmol), 2a (1.0 mmol), t-BuOK (1.0 mmol), dioxane (3 mL) and Tang’s catalyst (0.1 mol %) at 100 °C for 24 h. bDetermined by GC–MS. cYield of isolated product 3aa. dReaction performed without Tang’s catalyst.
In order to obtain optimal conditions, the bases, reaction medium, and temperature were also surveyed (Table 2). First, several bases were examined and the results showed that different bases have different effects on the chemoselectivity and yield of the reaction. The weak bases including HCO2Na, CH3CO2K, and Na2CO3, resulted in decreased yields of quinoline 3aa (Table 2, entries 1–3). Interestingly, the chemoselectivity of the reaction and product yield were significantly improved with strong bases, such as NaOH, KOH, or t-BuOK (Table 2, entries 4–6). To our excitement, a loading of 1.1 equiv of t-BuOK delivered the product 3aa in the yield of 96% with perfect selectivity (Table 2, entries 6–8).
Table 2: Studies of reaction parameters in the iridium-catalyzed ADC reaction.a
|
|
||||||
| Entry | Base | Solvent | Temperature (°C) | Time (h) | 3aa:4aab | Yield of 3aab (%) |
| 1 | CH3CO2K | 1,4-dioxane | 100 | 24 | 74:26 | 74 |
| 2 | HCO2Na | 1,4-dioxane | 100 | 24 | 69:31 | 69 |
| 3 | Na2CO3 | 1,4-dioxane | 100 | 24 | 76:24 | 76 |
| 4 | NaOH | 1,4-dioxane | 100 | 24 | 82:18 | 82 |
| 5 | KOH | 1,4-dioxane | 100 | 24 | 93:7 | 93 |
| 6 | t-BuOK | 1,4-dioxane | 100 | 24 | 95:5 | 95 |
| 7c | t-BuOK | 1,4-dioxane | 100 | 24 | >99:1 | >99 (96)d |
| 8e | t-BuOK | 1,4-dioxane | 100 | 24 | 94:6 | 94 |
| 9c | t-BuOK | toluene | 100 | 24 | 90:10 | 90 |
| 10c | t-BuOK | THF | 80 | 24 | 81:19 | 81 |
| 11c | t-BuOK | DMF | 100 | 24 | 69:31 | 69 |
| 12c | t-BuOK | H2O | 100 | 24 | 83:17 | 83 |
| 13c | t-BuOK | 1,4-dioxane | 80 | 36 | 87:13 | 87 |
| 14c,f | t-BuOK | 1,4-dioxane | 100 | 48 | >99:1 | >99 |
aReaction conditions: 1a (1.1 mmol), 2a (1.0 mmol), base, solvent (3 mL), and TC-6 (0.1 mol %) at 100 °C for 24 h. bDetermined by GC–MS. c1.1 mmol t-BuOK was used. dYield of isolated product 3aa. e0.8 mmol t-BuOK was used. f0.01 mol % TC-6 was used.
Afterward, we further screened the solvent and catalyst loading (Table 2, entries 7, 9–12, and 14) and the results showed that 1,4-dioxane was the most favorable solvent for the outcome of product 3aa, even when the catalyst loading was decreased to 0.01 mol % (Table 2, entry 14). All other solvents screened resulted in lower product yield (Table 2, entries 9–12). Finally, we examined the effect of temperature on the reaction and found that decreasing the reaction temperature hindered the production of compound 3aa (Table 2, entry 13).
Based on the screening of above reaction conditions, we obtained the optimal catalytic system, with 0.1 mol % TC-6 as the catalyst, 1.1 equiv of t-BuOK as the base, and 1,4-dioxane as reaction solvent. Under the optimal reaction conditions, we investigated the universality of the cyclometalated iridium-catalyzed ADC reaction by expanding the range of substrates (Table 3). It can be seen that quinoline compounds 3 were obtained with excellent yield and chemoselectivity through the cyclometalated iridium-catalyzed ADC reaction of 2-aminobenzyl alcohol and different substituted aromatic secondary alcohols including electron-donating (Me, OMe) and electron-withdrawing substituents (F, Cl, Br) as the substrate (Table 3, entries 1–24). Aromatic secondary alcohols substituted with electron-donating groups led to higher chemoselectivities and yields of the products (Table 3, entries 2–5) than the aryl secondary alcohols and aminobenzyl alcohol with electron-withdrawing groups (Table 3, entries 8, 11, 12, 15, 16, 19, 20, 23, and 24). Meanwhile, the heteroaromatic secondary alcohols 2i–n could also be employed in the cyclometalated iridium-catalyzed system obtaining the products 3ai–an with excellent yield and chemoselectivity (Table 3, entries 26–42). The results showed that the yield and chemoselectivity was higher when the heteroaromatic secondary alcohols and aminobenzyl alcohols have electron-donating groups (Table 3, entries 27, 30, 31, 34, 35, 39, and 42). On the contrary, with the electron-withdrawing group, the yield and chemoselectivity of the reaction were relatively lower (Table 3, entries 28, 29, 32, 33, 36, 37, and 40). It is worth noting that high conversions were also accomplished when 1-cyclohexylethanol and pentan-1-ol were employed in this catalytic system (Table 3, entries 43 and 44).
Table 3: Cyclometalated iridium-catalyzed ADC reaction of various 2-aminobenzyl alcohols and secondary alcohols.a
|
|
|||||
| Entry | 1 | 2 | Time (h) | 3:4b | Yield of 3c (%) |
| 1 |
|
|
16 | >99:1 | (3aa) 96 |
| 2 |
|
|
14 | 97:3 | (3ab) 95 |
| 3 |
|
|
18 | 92:8 | (3bb) 92 |
| 4 |
|
|
20 | 93:7 | (3cb) 93 |
| 5 |
|
|
18 | 91:9 | (3db) 91 |
| 6 |
|
|
10 | 94:6 | (3ac) 94 |
| 7 |
|
|
8 | 95:5 | (3bc) 95 |
| 8 |
|
|
18 | 91:9 | (3cc) 91 |
| 9 |
|
|
16 | 93:7 | (3ad) 93 |
| 10 |
|
|
18 | 95:5 | (3bd) 95 |
| 11 |
|
|
20 | 91:9 | (3cd) 91 |
| 12 |
|
|
16 | 92:8 | (3dd) 92 |
| 13 |
|
|
10 | 93:7 | (3ae) 93 |
| 14 |
|
|
14 | 95:5 | (3be) 95 |
| 15 |
|
|
16 | 90:10 | (3ce) 90 |
| 16 |
|
|
20 | 91:9 | (3de) 91 |
| 17 |
|
|
8 | 88:12 | (3af) 88 |
| 18 |
|
|
17 | 93:7 | (3bf) 93 |
| 19 |
|
|
20 | 95:5 | (3cf) 95 |
| 20 |
|
|
18 | 88:12 | (3df) 88 |
| 21 |
|
|
18 | 90:10 | (3ag) 90 |
| 22 |
|
|
20 | 93:7 | (3bg) 93 |
| 23 |
|
|
22 | 89:11 | (3cg) 89 |
| 24 |
|
|
20 | 91:9 | (3dg) 91 |
| 25 |
|
|
16 | 89:11 | (3ah) 89 |
| 26 |
|
|
16 | 94:6 | (3ai) 94 |
| 27 |
|
|
18 | 94:6 | (3bi) 94 |
| 28 |
|
|
12 | 92:8 | (3ci) 92 |
| 29 |
|
|
20 | 90:10 | (3di) 90 |
| 30 |
|
|
8 | 96:4 | (3aj) 96 |
| 31 |
|
|
12 | 93:7 | (3bj) 93 |
| 32 |
|
|
18 | 92:8 | (3cj) 92 |
| 33 |
|
|
18 | 91:9 | (3dj) 91 |
| 34 |
|
|
16 | 97:3 | (3ak) 97 |
| 35 |
|
|
18 | 95:5 | (3bk) 95 |
| 36 |
|
|
16 | 93:7 | (3ck) 93 |
| 37 |
|
|
20 | 91:9 | (3dk) 91 |
| 38 |
|
|
18 | 92:8 | (3al) 92 |
| 39 |
|
|
16 | 95:5 | (3bl) 95 |
| 40 |
|
|
20 | 92:8 | (3cl) 92 |
| 41 |
|
|
22 | 94:6 | (3am) 94 |
| 42 |
|
|
22 | 96:4 | (3an) 96 |
| 43 |
|
|
20 | 97:3 | (3ao) 88 |
| 44 |
|
|
21 | 98:2 | (3ap) 86 |
aReaction conditions: a mixture of 1 (1.1 mmol), 2 (1.0 mmol), t-BuOK (1.0 mmol), dioxane (3 mL), and TC-6 (0.1 mol %) at 100 °C. bDetermined by GC–MS. cYield of isolated product 3.
The excellent developed methodology prompted us to further extend the practicality of the catalytic system. Firstly, we carried out a gram-scale reaction with the template reaction under the optimal catalytic system, which delivered quinoline 3aa in 94% isolated yield (Figure 3a). Additionally, the 2-furanquoline product 3ai was also obtained up to a gram-scale with excellent yield (92%) by iridium-catalyzed ADC reaction of 2-aminobenzyl alcohol 1a and 2-furanol 2i (Figure 3b).
To further stretch out the process of this cyclometalated iridium-catalyzed ADC reaction, comparative experiments were carried out. Quinoline 3aa was obtained in 91% yield by ADC reaction between 2-aminobenzaldehyde (5) and 1-phenylethanol (2a) catalyzed by cyclometalated iridium TC-6 (Figure 4a). In the same way, quinoline 3aa could also be synthesized from 2-aminobenzyl alcohol (1a) and acetophenone (6) with TC-6 as the catalyst (Figure 4b). Further study found that quinoline 3aa could be obtained by the condensation reaction of 2-aminobenzaldehyde (5) with acetophenone (6) in the absence of cyclometalated iridium (Figure 4c).
According to experimental results and literature findings [19,28,29,43,44], a possible mechanism of cyclometalated iridium-catalyzed ADC reaction was proposed (Figure 5). Firstly, by the interaction of TC-6 with 1a/2a under the “dehydrogenative” process, the Int-I/Int-II were formed [28,29]. Then, Int-III and 2-aminobenzaldehyde (5)/acetophenone (6) were formed by β-H elimination of Int-I/Int-II. In this process, an amount of liberated H2 would be released from the dehydrogenation of 2-aminobenzyl alcohol/1-phenylethanol according to the previous literature [28]. Lastly, the desired product 3aa was obtained by the condensation and cyclization of the aldehyde 5 with acetophenone (6) under base conditions.
Figure 5: A speculated possible mechanism.
Figure 5: A speculated possible mechanism.
The potential antimicrobial activity of the compounds was evaluated against Staphylococcus aureus (Gram-positive), Escherichia coli (Gram-negative), and Candida albicans (fungi) mainly by examining the minimum inhibitory concentration (MIC) (Table 4). As shown in Table 4, the compounds 3ab, 3ah, and 3ad showed high antibacterial activities against Gram-positive bacteria. In particular, the antibacterial activity of compound 3ad against Staphylococcus aureus (MIC = 2 μg/mL) was much higher than that of the positive control norfloxacin. Meanwhile, the antifungal activity of compound 3ck (MIC = 64 μg/mL) was stronger than norfloxacin. However, 3an and other compounds showed similar or lower antifungal activity than norfloxacin. Unfortunately, all compounds were less effective against Gram-negative bacteria (MIC > 128 μg/mL) than the parent drug norfloxacin. To sum up, the synthesized compounds exhibited enhanced antibacterial activity against Gram-positive bacteria and Candida albicans.
Table 4: Results of antimicrobial activity of synthetic quinoline compounds.
| Compounds | Minimum inhibitory concentration (μg/mL) | |||||
| C. albicans | S. aureus | E. coli | ||||
| predicted | experimental | predicted | experimental | predicted | experimental | |
| 3ab | >128 | >128 | 128 | 16 | 128 | >128 |
| 3ad | >128 | >128 | 128 | 2 | 128 | >128 |
| 3ah | >128 | >128 | 128 | 64 | 128 | >128 |
| 3ai | >128 | 128 | 128 | >128 | 128 | >128 |
| 3aj | >128 | 128 | 128 | >128 | 128 | >128 |
| 3bj | >128 | 128 | 128 | >128 | 128 | >128 |
| 3ak | >128 | 128 | 128 | >128 | 128 | >128 |
| 3ck | >128 | 64 | 128 | >128 | 128 | >128 |
| 3an | >128 | 128 | 128 | >128 | 128 | >128 |
| norfloxacin | 128 | 128 | 128 | |||
Conclusion
In summary, we have developed a new route for the efficient synthesis of quinoxaline and its derivatives with high yield and good chemoselectivity via the ADC reaction of 2-aminobenzyl alcohol and aryl aryl/heteroaryl/alkyl secondary alcohols including electron-donating (Me, OMe) and electron-withdrawing substituents (F, Cl, Br) catalyzed by cyclometalated iridium complexes. Besides, this reaction could also be used on a gram-scale, by which the aryl/heteroaryl quinolines were synthesized. In the evaluation of antimicrobial activity, the antimicrobial effects of compounds 3ab, 3ad, 3ah, 3ck, 3an and other compounds were better than the parent drug norfloxacin. This method could be used to further synthesis of quinoline derivatives and provide theoretical support for the synthesis of new antibacterial drugs.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, copies of 1H and 13C NMR spectra, HRMS of new compounds. | ||
| Format: PDF | Size: 4.4 MB | Download |
Funding
This research was financially supported by the National Natural Science Foundation of China (21962004), Jiangxi provincial department of science and technology (20161BAB213059), Jiangxi Education Hall Science and Technology Foundation (GJJ211541, GJJ180801), Gannan Medical University (QD201816) and the innovation and entrepreneurship training program for college students in Jiangxi Province (S202210413034).
References
-
Eswaran, S.; Adhikari, A. V.; Ajay Kumar, R. Eur. J. Med. Chem. 2010, 45, 957–966. doi:10.1016/j.ejmech.2009.11.036
Return to citation in text: [1] -
Khalifa, N. M.; Al-Oma, M. A.; El-Galil, A.; El-Reheem, M. A. Biomed. Res. 2017, 28, 869–874.
Return to citation in text: [1] -
Liu, D.; Xue, A.; Liu, Z.; Zhang, Y.; Peng, P.; Wang, H. Lett. Drug Des. Discovery 2019, 16, 663–669. doi:10.2174/1570180815666180820131036
Return to citation in text: [1] -
Ruiz, I.; Nevers, Q.; Hernández, E.; Ahnou, N.; Brillet, R.; Softic, L.; Donati, F.; Berry, F.; Hamadat, S.; Fourati, S.; Pawlotsky, J.-M.; Ahmed-Belkacem, A. Antimicrob. Agents Chemother. 2020, 64, e02078-19. doi:10.1128/aac.02078-19
Return to citation in text: [1] -
Patel, S. R.; Gangwal, R.; Sangamwar, A. T.; Jain, R. Eur. J. Med. Chem. 2014, 85, 255–267. doi:10.1016/j.ejmech.2014.07.100
Return to citation in text: [1] -
Shah, R. B.; Valand, N. N.; Sutariya, P. G.; Menon, S. K. J. Inclusion Phenom. Macrocyclic Chem. 2016, 84, 173–178. doi:10.1007/s10847-015-0581-0
Return to citation in text: [1] -
Najafi, Z.; Saeedi, M.; Mahdavi, M.; Sabourian, R.; Khanavi, M.; Tehrani, M. B.; Moghadam, F. H.; Edraki, N.; Karimpor-Razkenari, E.; Sharifzadeh, M.; Foroumadi, A.; Shafiee, A.; Akbarzadeh, T. Bioorg. Chem. 2016, 67, 84–94. doi:10.1016/j.bioorg.2016.06.001
Return to citation in text: [1] -
Khan, A.; Miller, W. R.; Arias, C. A. Expert Rev. Anti-Infect. Ther. 2018, 16, 269–287. doi:10.1080/14787210.2018.1456919
Return to citation in text: [1] -
Eswaran, S.; Adhikari, A. V.; Shetty, N. S. Eur. J. Med. Chem. 2009, 44, 4637–4647. doi:10.1016/j.ejmech.2009.06.031
Return to citation in text: [1] -
Bodke, Y. D.; Shankerrao, S.; Kenchappa, R.; Telkar, S. Russ. J. Gen. Chem. 2017, 87, 1843–1849. doi:10.1134/s1070363217080321
Return to citation in text: [1] -
Naik, H. R. P.; Naik, H. S. B.; Naik, T. R. R.; Lamani, D. S.; Aravinda, T. Phosphorus, Sulfur Silicon Relat. Elem. 2010, 185, 355–360. doi:10.1080/10426500902797095
Return to citation in text: [1] -
Grigor´eva, N. G.; Bayburtli, A. V.; Kuvatova, R. Z.; Semenova, T. V.; Bubennov, S. V.; Raskildina, G. Z.; Zlotsky, S. S.; Kutepov, B. I. Russ. Chem. Bull. 2020, 69, 525–528. doi:10.1007/s11172-020-2793-8
Return to citation in text: [1] -
Reynoldsa, K. A.; Young, D. J.; Loughlin, W. A. Synthesis 2010, 3645–3648. doi:10.1055/s-0030-1258258
Return to citation in text: [1] -
Aribi, F.; Schmitt, E.; Panossian, A.; Vors, J.-P.; Pazenok, S.; Leroux, F. R. Org. Chem. Front. 2016, 3, 1392–1415. doi:10.1039/c6qo00319b
Return to citation in text: [1] -
Patel, A.; Patel, S.; Mehta, M.; Patel, Y.; Patel, R.; Shah, D.; Patel, D.; Shah, U.; Patel, M.; Patel, S.; Solanki, N.; Bambharoliya, T.; Patel, S.; Nagani, A.; Patel, H.; Vaghasiya, J.; Shah, H.; Prajapati, B.; Rathod, M.; Bhimani, B.; Patel, R.; Bhavsar, V.; Rakholiya, B.; Patel, M.; Patel, P. Green Chem. Lett. Rev. 2022, 15, 337–372. doi:10.1080/17518253.2022.2064194
Return to citation in text: [1] -
Mijangos, M. V.; Amador‐Sánchez, Y. A.; Miranda, L. D. Eur. J. Org. Chem. 2021, 637–647. doi:10.1002/ejoc.202001455
Return to citation in text: [1] -
Diedrich, C. L.; Haase, D.; Saak, W.; Christoffers, J. Eur. J. Org. Chem. 2008, 1811–1816. doi:10.1002/ejoc.200701179
Return to citation in text: [1] [2] -
Ruch, S.; Irrgang, T.; Kempe, R. Chem. – Eur. J. 2014, 20, 13279–13285. doi:10.1002/chem.201402952
Return to citation in text: [1] -
Maji, M.; Chakrabarti, K.; Panja, D.; Kundu, S. J. Catal. 2019, 373, 93–102. doi:10.1016/j.jcat.2019.03.028
Return to citation in text: [1] [2] -
Xie, F.; Zhang, M.; Chen, M.; Lv, W.; Jiang, H. ChemCatChem 2015, 7, 349–353. doi:10.1002/cctc.201402832
Return to citation in text: [1] -
Dayan, O.; Tercan, M.; Özdemir, N. J. Mol. Struct. 2016, 1123, 35–43. doi:10.1016/j.molstruc.2016.06.017
Return to citation in text: [1] -
Martínez, R.; Ramón, D. J.; Yus, M. Tetrahedron 2006, 62, 8982–8987. doi:10.1016/j.tet.2006.07.012
Return to citation in text: [1] -
Premkumar, P.; Manikandan, R.; Nirmala, M.; Viswanathamurthi, P.; Malecki, J. G. J. Coord. Chem. 2017, 70, 3065–3079. doi:10.1080/00958972.2017.1381692
Return to citation in text: [1] -
Yun, X.-J.; Zhu, J.-W.; Jin, Y.; Deng, W.; Yao, Z.-J. Inorg. Chem. 2020, 59, 7841–7851. doi:10.1021/acs.inorgchem.0c00955
Return to citation in text: [1] -
Gaire, S.; Ortiz, R. J.; Schrage, B. R.; Lozada, I. B.; Mandapati, P.; Osinski, A. J.; Herbert, D. E.; Ziegler, C. J. J. Organomet. Chem. 2020, 921, 121338. doi:10.1016/j.jorganchem.2020.121338
Return to citation in text: [1] -
Zhang, D.; Zhang, H.; Chen, X. Russ. J. Gen. Chem. 2016, 86, 686–690. doi:10.1134/s1070363216030282
Return to citation in text: [1] -
Hofmann, N.; Homberg, L.; Hultzsch, K. C. Org. Lett. 2020, 22, 7964–7970. doi:10.1021/acs.orglett.0c02905
Return to citation in text: [1] -
Hu, X.; Chen, Y.; Huang, B.; Liu, Y.; Huang, H.; Xie, Z. ACS Sustainable Chem. Eng. 2019, 7, 11369–11376. doi:10.1021/acssuschemeng.9b01015
Return to citation in text: [1] [2] [3] [4] -
Bains, A. K.; Singh, V.; Adhikari, D. J. Org. Chem. 2020, 85, 14971–14979. doi:10.1021/acs.joc.0c01819
Return to citation in text: [1] [2] [3] -
Hu, W.; Zhang, Y.; Zhu, H.; Ye, D.; Wang, D. Green Chem. 2019, 21, 5345–5351. doi:10.1039/c9gc02086a
Return to citation in text: [1] -
Maji, M.; Panja, D.; Borthakur, I.; Kundu, S. Org. Chem. Front. 2021, 8, 2673–2709. doi:10.1039/d0qo01577f
Return to citation in text: [1] -
Tan, Z.; Fu, Z.; Yang, J.; Wu, Y.; Cao, L.; Jiang, H.; Li, J.; Zhang, M. iScience 2020, 23, 101003. doi:10.1016/j.isci.2020.101003
Return to citation in text: [1] -
Irrgang, T.; Kempe, R. Chem. Rev. 2019, 119, 2524–2549. doi:10.1021/acs.chemrev.8b00306
Return to citation in text: [1] -
Zell, T.; Milstein, D. Acc. Chem. Res. 2015, 48, 1979–1994. doi:10.1021/acs.accounts.5b00027
Return to citation in text: [1] -
Borthakur, I.; Sau, A.; Kundu, S. Coord. Chem. Rev. 2022, 451, 214257. doi:10.1016/j.ccr.2021.214257
Return to citation in text: [1] -
Vázquez-Villa, H.; Reber, S.; Ariger, M. A.; Carreira, E. M. Angew. Chem., Int. Ed. 2011, 50, 8979–8981. doi:10.1002/anie.201102732
Return to citation in text: [1] -
He, Y.-M.; Fan, Q.-H. ChemCatChem 2015, 7, 398–400. doi:10.1002/cctc.201402883
Return to citation in text: [1] -
Yang, Z.; Zhu, Z.; Luo, R.; Qiu, X.; Liu, J.-t.; Yang, J.-K.; Tang, W. Green Chem. 2017, 19, 3296–3301. doi:10.1039/c7gc01289f
Return to citation in text: [1] -
Shui, H.; Zhong, Y.; Ouyang, L.; Luo, N.; Luo, R. Synthesis 2022, 54, 2876–2884. doi:10.1055/a-1755-4700
Return to citation in text: [1] [2] -
Luo, N.; Shui, H.; Zhong, Y.; Huang, J.; Luo, R. Synthesis 2021, 53, 4516–4524. doi:10.1055/a-1545-7563
Return to citation in text: [1] -
Luo, N.; Zhong, Y.; Wen, H.; Luo, R. ACS Omega 2020, 5, 27723–27732. doi:10.1021/acsomega.0c04192
Return to citation in text: [1] -
Luo, N.; Zhong, Y.; Wen, H.; Shui, H.; Luo, R. Eur. J. Org. Chem. 2021, 1355–1364. doi:10.1002/ejoc.202001550
Return to citation in text: [1] -
Chen, B. W. J.; Chng, L. L.; Yang, J.; Wei, Y.; Yang, J.; Ying, J. Y. ChemCatChem 2013, 5, 277–283. doi:10.1002/cctc.201200496
Return to citation in text: [1] -
Guo, B.; Yu, T.-Q.; Li, H.-X.; Zhang, S.-Q.; Braunstein, P.; Young, D. J.; Li, H.-Y.; Lang, J.-P. ChemCatChem 2019, 11, 2500–2510. doi:10.1002/cctc.201900435
Return to citation in text: [1]
| 37. | He, Y.-M.; Fan, Q.-H. ChemCatChem 2015, 7, 398–400. doi:10.1002/cctc.201402883 |
| 38. | Yang, Z.; Zhu, Z.; Luo, R.; Qiu, X.; Liu, J.-t.; Yang, J.-K.; Tang, W. Green Chem. 2017, 19, 3296–3301. doi:10.1039/c7gc01289f |
| 39. | Shui, H.; Zhong, Y.; Ouyang, L.; Luo, N.; Luo, R. Synthesis 2022, 54, 2876–2884. doi:10.1055/a-1755-4700 |
| 39. | Shui, H.; Zhong, Y.; Ouyang, L.; Luo, N.; Luo, R. Synthesis 2022, 54, 2876–2884. doi:10.1055/a-1755-4700 |
| 40. | Luo, N.; Shui, H.; Zhong, Y.; Huang, J.; Luo, R. Synthesis 2021, 53, 4516–4524. doi:10.1055/a-1545-7563 |
| 41. | Luo, N.; Zhong, Y.; Wen, H.; Luo, R. ACS Omega 2020, 5, 27723–27732. doi:10.1021/acsomega.0c04192 |
| 42. | Luo, N.; Zhong, Y.; Wen, H.; Shui, H.; Luo, R. Eur. J. Org. Chem. 2021, 1355–1364. doi:10.1002/ejoc.202001550 |
| 1. | Eswaran, S.; Adhikari, A. V.; Ajay Kumar, R. Eur. J. Med. Chem. 2010, 45, 957–966. doi:10.1016/j.ejmech.2009.11.036 |
| 5. | Patel, S. R.; Gangwal, R.; Sangamwar, A. T.; Jain, R. Eur. J. Med. Chem. 2014, 85, 255–267. doi:10.1016/j.ejmech.2014.07.100 |
| 15. | Patel, A.; Patel, S.; Mehta, M.; Patel, Y.; Patel, R.; Shah, D.; Patel, D.; Shah, U.; Patel, M.; Patel, S.; Solanki, N.; Bambharoliya, T.; Patel, S.; Nagani, A.; Patel, H.; Vaghasiya, J.; Shah, H.; Prajapati, B.; Rathod, M.; Bhimani, B.; Patel, R.; Bhavsar, V.; Rakholiya, B.; Patel, M.; Patel, P. Green Chem. Lett. Rev. 2022, 15, 337–372. doi:10.1080/17518253.2022.2064194 |
| 4. | Ruiz, I.; Nevers, Q.; Hernández, E.; Ahnou, N.; Brillet, R.; Softic, L.; Donati, F.; Berry, F.; Hamadat, S.; Fourati, S.; Pawlotsky, J.-M.; Ahmed-Belkacem, A. Antimicrob. Agents Chemother. 2020, 64, e02078-19. doi:10.1128/aac.02078-19 |
| 16. | Mijangos, M. V.; Amador‐Sánchez, Y. A.; Miranda, L. D. Eur. J. Org. Chem. 2021, 637–647. doi:10.1002/ejoc.202001455 |
| 3. | Liu, D.; Xue, A.; Liu, Z.; Zhang, Y.; Peng, P.; Wang, H. Lett. Drug Des. Discovery 2019, 16, 663–669. doi:10.2174/1570180815666180820131036 |
| 13. | Reynoldsa, K. A.; Young, D. J.; Loughlin, W. A. Synthesis 2010, 3645–3648. doi:10.1055/s-0030-1258258 |
| 2. | Khalifa, N. M.; Al-Oma, M. A.; El-Galil, A.; El-Reheem, M. A. Biomed. Res. 2017, 28, 869–874. |
| 14. | Aribi, F.; Schmitt, E.; Panossian, A.; Vors, J.-P.; Pazenok, S.; Leroux, F. R. Org. Chem. Front. 2016, 3, 1392–1415. doi:10.1039/c6qo00319b |
| 9. | Eswaran, S.; Adhikari, A. V.; Shetty, N. S. Eur. J. Med. Chem. 2009, 44, 4637–4647. doi:10.1016/j.ejmech.2009.06.031 |
| 11. | Naik, H. R. P.; Naik, H. S. B.; Naik, T. R. R.; Lamani, D. S.; Aravinda, T. Phosphorus, Sulfur Silicon Relat. Elem. 2010, 185, 355–360. doi:10.1080/10426500902797095 |
| 28. | Hu, X.; Chen, Y.; Huang, B.; Liu, Y.; Huang, H.; Xie, Z. ACS Sustainable Chem. Eng. 2019, 7, 11369–11376. doi:10.1021/acssuschemeng.9b01015 |
| 8. | Khan, A.; Miller, W. R.; Arias, C. A. Expert Rev. Anti-Infect. Ther. 2018, 16, 269–287. doi:10.1080/14787210.2018.1456919 |
| 12. | Grigor´eva, N. G.; Bayburtli, A. V.; Kuvatova, R. Z.; Semenova, T. V.; Bubennov, S. V.; Raskildina, G. Z.; Zlotsky, S. S.; Kutepov, B. I. Russ. Chem. Bull. 2020, 69, 525–528. doi:10.1007/s11172-020-2793-8 |
| 7. | Najafi, Z.; Saeedi, M.; Mahdavi, M.; Sabourian, R.; Khanavi, M.; Tehrani, M. B.; Moghadam, F. H.; Edraki, N.; Karimpor-Razkenari, E.; Sharifzadeh, M.; Foroumadi, A.; Shafiee, A.; Akbarzadeh, T. Bioorg. Chem. 2016, 67, 84–94. doi:10.1016/j.bioorg.2016.06.001 |
| 19. | Maji, M.; Chakrabarti, K.; Panja, D.; Kundu, S. J. Catal. 2019, 373, 93–102. doi:10.1016/j.jcat.2019.03.028 |
| 28. | Hu, X.; Chen, Y.; Huang, B.; Liu, Y.; Huang, H.; Xie, Z. ACS Sustainable Chem. Eng. 2019, 7, 11369–11376. doi:10.1021/acssuschemeng.9b01015 |
| 29. | Bains, A. K.; Singh, V.; Adhikari, D. J. Org. Chem. 2020, 85, 14971–14979. doi:10.1021/acs.joc.0c01819 |
| 43. | Chen, B. W. J.; Chng, L. L.; Yang, J.; Wei, Y.; Yang, J.; Ying, J. Y. ChemCatChem 2013, 5, 277–283. doi:10.1002/cctc.201200496 |
| 44. | Guo, B.; Yu, T.-Q.; Li, H.-X.; Zhang, S.-Q.; Braunstein, P.; Young, D. J.; Li, H.-Y.; Lang, J.-P. ChemCatChem 2019, 11, 2500–2510. doi:10.1002/cctc.201900435 |
| 6. | Shah, R. B.; Valand, N. N.; Sutariya, P. G.; Menon, S. K. J. Inclusion Phenom. Macrocyclic Chem. 2016, 84, 173–178. doi:10.1007/s10847-015-0581-0 |
| 10. | Bodke, Y. D.; Shankerrao, S.; Kenchappa, R.; Telkar, S. Russ. J. Gen. Chem. 2017, 87, 1843–1849. doi:10.1134/s1070363217080321 |
| 28. | Hu, X.; Chen, Y.; Huang, B.; Liu, Y.; Huang, H.; Xie, Z. ACS Sustainable Chem. Eng. 2019, 7, 11369–11376. doi:10.1021/acssuschemeng.9b01015 |
| 29. | Bains, A. K.; Singh, V.; Adhikari, D. J. Org. Chem. 2020, 85, 14971–14979. doi:10.1021/acs.joc.0c01819 |
| 18. | Ruch, S.; Irrgang, T.; Kempe, R. Chem. – Eur. J. 2014, 20, 13279–13285. doi:10.1002/chem.201402952 |
| 19. | Maji, M.; Chakrabarti, K.; Panja, D.; Kundu, S. J. Catal. 2019, 373, 93–102. doi:10.1016/j.jcat.2019.03.028 |
| 17. | Diedrich, C. L.; Haase, D.; Saak, W.; Christoffers, J. Eur. J. Org. Chem. 2008, 1811–1816. doi:10.1002/ejoc.200701179 |
| 17. | Diedrich, C. L.; Haase, D.; Saak, W.; Christoffers, J. Eur. J. Org. Chem. 2008, 1811–1816. doi:10.1002/ejoc.200701179 |
| 31. | Maji, M.; Panja, D.; Borthakur, I.; Kundu, S. Org. Chem. Front. 2021, 8, 2673–2709. doi:10.1039/d0qo01577f |
| 32. | Tan, Z.; Fu, Z.; Yang, J.; Wu, Y.; Cao, L.; Jiang, H.; Li, J.; Zhang, M. iScience 2020, 23, 101003. doi:10.1016/j.isci.2020.101003 |
| 33. | Irrgang, T.; Kempe, R. Chem. Rev. 2019, 119, 2524–2549. doi:10.1021/acs.chemrev.8b00306 |
| 34. | Zell, T.; Milstein, D. Acc. Chem. Res. 2015, 48, 1979–1994. doi:10.1021/acs.accounts.5b00027 |
| 35. | Borthakur, I.; Sau, A.; Kundu, S. Coord. Chem. Rev. 2022, 451, 214257. doi:10.1016/j.ccr.2021.214257 |
| 36. | Vázquez-Villa, H.; Reber, S.; Ariger, M. A.; Carreira, E. M. Angew. Chem., Int. Ed. 2011, 50, 8979–8981. doi:10.1002/anie.201102732 |
| 29. | Bains, A. K.; Singh, V.; Adhikari, D. J. Org. Chem. 2020, 85, 14971–14979. doi:10.1021/acs.joc.0c01819 |
| 30. | Hu, W.; Zhang, Y.; Zhu, H.; Ye, D.; Wang, D. Green Chem. 2019, 21, 5345–5351. doi:10.1039/c9gc02086a |
| 26. | Zhang, D.; Zhang, H.; Chen, X. Russ. J. Gen. Chem. 2016, 86, 686–690. doi:10.1134/s1070363216030282 |
| 27. | Hofmann, N.; Homberg, L.; Hultzsch, K. C. Org. Lett. 2020, 22, 7964–7970. doi:10.1021/acs.orglett.0c02905 |
| 28. | Hu, X.; Chen, Y.; Huang, B.; Liu, Y.; Huang, H.; Xie, Z. ACS Sustainable Chem. Eng. 2019, 7, 11369–11376. doi:10.1021/acssuschemeng.9b01015 |
| 20. | Xie, F.; Zhang, M.; Chen, M.; Lv, W.; Jiang, H. ChemCatChem 2015, 7, 349–353. doi:10.1002/cctc.201402832 |
| 21. | Dayan, O.; Tercan, M.; Özdemir, N. J. Mol. Struct. 2016, 1123, 35–43. doi:10.1016/j.molstruc.2016.06.017 |
| 22. | Martínez, R.; Ramón, D. J.; Yus, M. Tetrahedron 2006, 62, 8982–8987. doi:10.1016/j.tet.2006.07.012 |
| 23. | Premkumar, P.; Manikandan, R.; Nirmala, M.; Viswanathamurthi, P.; Malecki, J. G. J. Coord. Chem. 2017, 70, 3065–3079. doi:10.1080/00958972.2017.1381692 |
| 24. | Yun, X.-J.; Zhu, J.-W.; Jin, Y.; Deng, W.; Yao, Z.-J. Inorg. Chem. 2020, 59, 7841–7851. doi:10.1021/acs.inorgchem.0c00955 |
| 25. | Gaire, S.; Ortiz, R. J.; Schrage, B. R.; Lozada, I. B.; Mandapati, P.; Osinski, A. J.; Herbert, D. E.; Ziegler, C. J. J. Organomet. Chem. 2020, 921, 121338. doi:10.1016/j.jorganchem.2020.121338 |
© 2022 Shui et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.