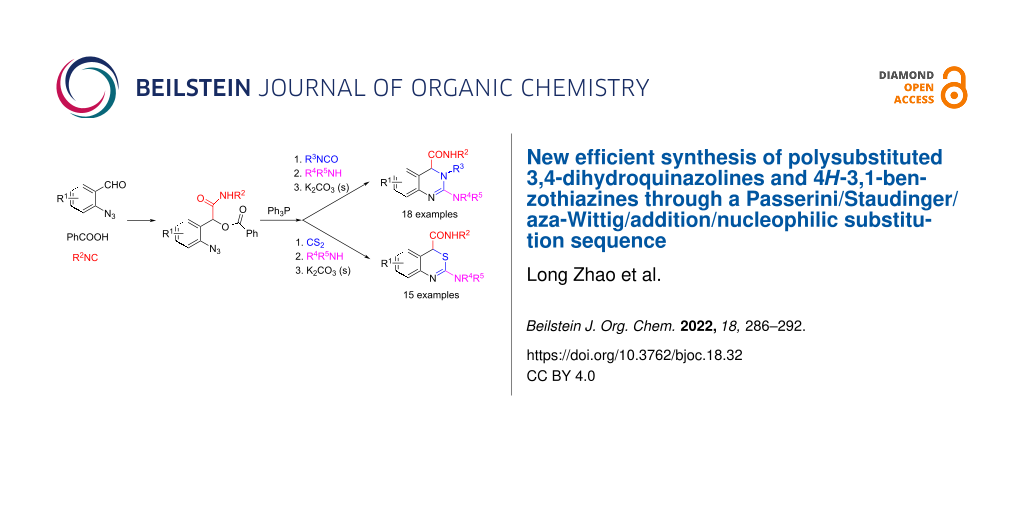
Abstract
A new efficient synthesis of polysubstituted 3,4-dihydroquinazolines and 4H-3,1-benzothiazines via sequential Passerini/Staudinger/aza-Wittig/addition/nucleophilic substitution reaction has been developed. The three-component Passerini reactions of 2-azidobenzaldehydes 1, benzoic acid (2), and isocyanides 3 produced the azide intermediates 4, which were treated sequentially with triphenylphosphine, isocyanates (or CS2), and secondary amines to give polysubstituted 3,4-dihydroquinazolines 8 and 4H-3,1-benzothiazines 11 in good overall yields through consecutive Passerini/Staudinger/aza-Wittig/addition/nucleophilic substitution reactions.
Graphical Abstract
Introduction
The chemistry of 3,4-dihydroquinazolines and 4H-3,1-benzothiazines is of constant interest owing to the occurrence of these ring systems in various biologically important compounds (Figure 1). A number of 3,4-dihydroquinazolines were found to show remarkable anticancer [1], antiviral [2], antidepressant [3], antifungal [4], selective somatostatin 2 (ss2) agonistical [5], β-site amyloid precursor protein cleaving enzyme 1 (BACE-1) inhibitive [6], and cholinesterase enzyme inhibitive activities [7]. The 3,4-dihydroquinazoline skeleton also exists in some natural products such as vasicine and vasicoline [8]. Some 4H-3,1-benzothiazine derivatives have also received attention due to their good biological activities, including anticancer [9], neuroprotective [10], antiproliferative and antifungal activities [11]. Due to the significant bioactive properties of the 3,4-dihydroquinazoline and 4H-3,1-benzothiazine moieties, many preparation procedures have appeared in the literature for the synthesis of their derivatives [12-22]. For example (Scheme 1), a one-pot Tf2O-mediated assembly of amides, amines, and ketones provided 3,4-dihydroquinazolines in good yields via successive triflic anhydride-mediated amide dehydration, ketimine addition, and Pictet–Spengler-like cyclization processes [12]. Some 4-substituted 3,4-dihydroquinazolines were prepared by copper-catalyzed oxidative cross coupling of hydroxy intermediates with various nucleophiles [13]. Other 3,4-dihydroquinazolines were also obtained efficiently by intramolecular aza-Wittig reactions [14]. Some 4H-3,1-benzothiazines were prepared by intramolecular thia-Michael addition with broad reaction scopes [19]. The rearrangement of 2-isothiocyano triarylmethanes in the presence of AlCl3 were also used for the synthesis 2,4-diaryl-4H-3,1-benzothiazines through aromatic ring transfer [20]. A facile protocol towards the synthesis of 4H-3,1-benzothiazines was established by using a P(NMe2)3-mediated C–N/C–S bond formation reaction of 2-aminobenzyl alcohol with isothiocyanates under aerobic conditions [21]. Despite of the above achievements, the development of new efficient methods for the synthesis of polysubstituted 3,4-dihydroquinazolines and 4H-3,1-benzothiazines under mild reaction conditions is still of high demand in the discovery of biologically active compounds.
Figure 1: Some bioactive 3,4-dihydroquinazolines and 4H-3,1-benzothiazines.
Figure 1: Some bioactive 3,4-dihydroquinazolines and 4H-3,1-benzothiazines.
Scheme 1: Representative preperation of 3,4-dihydroquinazolines and 4H-3,1-benzothiazines.
Scheme 1: Representative preperation of 3,4-dihydroquinazolines and 4H-3,1-benzothiazines.
The Passerini reaction is an isocyanide-based multicomponent reaction, which has been used in preparing various α-acyloxy adducts starting from aldehydes, a carboxylic acid, and a isonitrile as the three components [23]. The sequences of Passerini reactions, followed by post-condensation reactions, constitute useful synthetic methods in the preparation of structurally diverse heterocyclic compounds [24-29]. The aza-Wittig reaction has also been utilized widely in preparation of various heterocycles under mild neutral conditions [30-32]. Recently we have reported the synthesis of 3H-2-benzoxepin-1-ones, 4H-3,1-benzoxazines and oxazoles by combination of a Passerini with an intramolecular aza-Wittig reaction [33-35]. Continuing our interest in the synthesis of N-heterocycles via the aza-Wittig reaction and multicomponent reactions [36-38], we wish to report herein a facile synthesis of polysubstituted 3,4-dihydroquinazolines and 4H-3,1-benzothiazines via sequential Passerini/Staudinger/aza-Wittig/addition/nucleophilic substitution reactions. Compared with the synthetic method to 4H-3,1-benzothiazines in Scheme 1f, we provide another new sequential synthetic route to 4H-3,1-benzothiazines, especially for N,N-disubstituted 2-amino-4H-3,1-benzothiazines.
Results and Discussion
We initially selected 2-azidobenzaldehyde (1a), benzoic acid (2a) and tert-butyl isocyanide (3a) as the reactants (Scheme 2). When a mixture of 1a, 2a, and 3a in CH2Cl2 was stirred at room temperature for 48 h, the three-component Passerini reaction was carried out smoothly and the azide 4a (R = Ph) was finally obtained in 87% yield. Compound 4a was then allowed to react with triphenylphosphine in CH2Cl2 at room temperature for 2 h to produce the iminophosphorane 5a by Staudinger reaction. Aza-Wittig reaction of 5a with phenyl isocyanate generated carbodiimide 6a, which was then treated with diethylamine to form the guanidine intermediate 7a. In the presence of K2CO3 in CH3CN at refluxing temperature, the 3,4-dihydroquinazoline 8a was finally obtained in 84% yield (Table 1, entry 1, the overall yield is 73%) by intramolecular nucleophilic substitution. The reaction conditions for the transformation of guanidine intermediate 7a into 3,4-dihydroquinazoline 8a was then optimized (Table 1). As K2CO3 in different solvents (DMF, CH2Cl2 and toluene) were used, 0–72% yields of the product 8a were obtained (Table 1, entries 2–4). Utilizing a stronger base (NaOH and EtONa) resulted in a dark solution and no product was received (entries 5 and 6) owning to side reactions under the stronger base conditions. No product 8a was obtained when NEt3 in CH3CN was used (Table 1, entry 7) probably due to the weaker basic conditions. The effect of different R groups on the reaction yield was also investigated. With R = methyl, no product 8a was obtained in the presence of K2CO3/CH3CN probably due to the lower reactivity of the -OAc leaving group. In case when R was a 4-NO2C6H4 group, 86% yield of the product 8a was obtained, however, in this case the Passerini product 4a (R = 4-NO2C6H4) was obtained only in 62% yield and the overall yield of product 8a was 53%. Therefore, the reaction conditions of entry 1 in Table 1 were optimal for the above transformation.
Scheme 2: Preparation of 3,4-dihydroquinazoline 8a.
Scheme 2: Preparation of 3,4-dihydroquinazoline 8a.
The optimal reaction conditions were then utilized for the sequential reactions of different 2-azidobenzaldehydes 1, benzoic acid (2a), isocyanides 3, isocyanates and secondary amines. Most of the reactions took place smoothly to give the corresponding 3,4-dihydroquinazolines 8 in good yields (Scheme 3 and Table 2). Various isocyanates and secondary amines can be used in the above one-pot cyclization to prepare 3,4-dihydroquinazolines 8. As indicated in Table 2, when aromatic isocyanates (Table 2, compounds 8a–l, R3 = Ph, 4-ClC6H4, 3-MeC6H4, 4-MeC6H4 and 4-CF3OC6H4) were used, good yields (69–86%) of the products were obtained, whereas moderate yields (54–57%) were obtained when the more steric secondary amines were utilized (Table 2, compound 8m and 8n, NR4R5 = N(Cy)2, N(iPr)2). In cases when aliphatic isocyanates (compounds 8o–q, R3 = n-Bu, cyclohexyl and PhCH2) were used, 65–74% yields of the products were obtained. Even as the steric tert-butyl isocyanate was applied, the 3,4-dihydroquinazoline 8r was obtained in 42% yield, but when diphenylamine was used, no product was obtained (compounds 8s, NR4R5 = NPh2).
Scheme 3: Preparation of 3,4-dihydroquinazolines 8.
Scheme 3: Preparation of 3,4-dihydroquinazolines 8.
Table 2: Yields of 3,4-dihydroquinazolines 8.
| R1 | R2 | R3 | NR4R5 | Yielda (%) | |
| 8a | H | t-Bu | Ph | NEt2 | 84 |
| 8b | H | t-Bu | 4-ClC6H4 | NEt2 | 80 |
| 8c | H | t-Bu | 3-MeC6H4 | NEt2 | 76 |
| 8d | H | t-Bu | 4-MeC6H4 | NEt2 | 79 |
| 8e | H | t-Bu | Ph | morpholin-4-yl | 72 |
| 8f | H | t-Bu | 4-MeC6H4 | NPr2 | 85 |
| 8g | H | t-Bu | 4-MeC6H4 | NBu2 | 69 |
| 8h | H | Cyb | 4-MeC6H4 | NEt2 | 71 |
| 8i | H | Cyb | Ph | NEt2 | 86 |
| 8j | H | Cyb | 4-ClC6H4 | NEt2 | 78 |
| 8k | H | Cyb | 4-CF3OC6H4 | NEt2 | 80 |
| 8l | H | t-Bu | 4-MeC6H4 | morpholin-4-yl | 70 |
| 8m | H | t-Bu | 4-MeC6H4 | NCy2b | 57 |
| 8n | 4-Cl | Cyb | 4-CH3OC6H4 | N(iPr)2 | 54 |
| 8o | 4-Cl | n-Bu | n-Bu | N(Ph)Me | 65 |
| 8p | 5-Me | t-Bu | Cyb | N(CH2Ph)Me | 74 |
| 8q | 4-Cl | Cyb | PhCH2 | N(CH2Ph)2 | 67 |
| 8r | 5-Me | Cyb | t-Bu | NEt2 | 42 |
| 8s | H | n-Bu | Ph | NPh2 | 0 |
aIsolated yields based on the azides 4. bCyclohexyl.
The aza-Wittig reaction of iminophosphoranes 5 with an excess of CS2 took place smoothly at 40 °C to produce isothiocyanates 9, which were allowed to react with secondary amines to generate thiourea intermediates 10. In the presence of K2CO3 in CH3CN at refluxing temperature, thioureas 10 were also successfully transformed into 4H-3,1-benzothiazines 11 via intramolecular nucleophilic substitution (Scheme 4). The results were listed in Table 3. Various secondary amines can be used in this one-pot cyclization to prepare 4H-3,1-benzothiazines 11. As indicated in Table 3, when dialkylamines including cyclic dialkylamines (Table 3, compounds 11a–k, NR4R5 = NEt2, NPr2, N(CH2Ph)Me, N(CH2Ph)2, piperidin-1-yl, morpholin-4-yl and pyrrolidin-1-yl) were used, good yields (72–84%) of the products were obtained, whereas mederate yield (48–54%) was obtained when the more steric dialkylamines were utilized (Table 3, compounds 11l and 11m, NR4R5 = N(Cy)2, N(iPr)2). In cases when phenylmethylamine (compounds 11n and 11o, NR4R5 = N(Ph)Me) was used, 51–56% yields of the products were obtained, but when diphenylamine was used, no product was obtained (compound 11p, NR4R5 = NPh2).
Scheme 4: Preparation of 4H-3,1-benzothiazines 11.
Scheme 4: Preparation of 4H-3,1-benzothiazines 11.
Table 3: Yields of 4H-3,1-benzothiazines 11.
| R1 | R2 | NR4R5 | Yielda (%) | |
| 11a | H | t-Bu | NEt2 | 82 |
| 11b | H | t-Bu | piperidin-1-yl | 83 |
| 11c | H | t-Bu | morpholin-4-yl | 84 |
| 11d | H | n-Bu | morpholin-4-yl | 78 |
| 11e | H | Cyb | pyrrolidin-1-yl | 77 |
| 11f | H | Cyb | N(CH2Ph)Me | 79 |
| 11g | 5-Me | Cyb | NEt2 | 72 |
| 11h | 5-Me | n-Bu | piperidin-1-yl | 81 |
| 11i | 5-Me | Cyb | N(CH2Ph)2 | 78 |
| 11j | 5-Me | t-Bu | NPr2 | 75 |
| 11k | 4-Cl | Cyb | NEt2 | 83 |
| 11l | 4-Cl | t-Bu | NCy2b | 54 |
| 11m | 5-Me | Cyb | N(iPr)2 | 48 |
| 11n | H | Cyb | N(Ph)Me | 56 |
| 11o | 5-Me | Cyb | N(Ph)Me | 51 |
| 11p | H | n-Bu | NPh2 | 0 |
aIsolated yields based on the azides 4. bCyclohexyl.
Conclusion
In conclusion, we have developed a new Passerini/Staudinger/aza-Wittig/addition/nucleophilic substitution sequence for the synthesis of polysubstituted 3,4-dihydroquinazolines and 4H-3,1-benzothiazines. By this method, 3,4-dihydroquinazolines and 4H-3,1-benzothiazines were prepared in good overall yields with the advantages of mild one-pot operation conditions and easily accessible starting materials containing various common substituents.
Supporting Information
| Supporting Information File 1: Experimental section and copies of NMR spectra. | ||
| Format: PDF | Size: 6.4 MB | Download |
References
-
Kim, J. H.; Jeong, H. R.; Jung, D. W.; Yoon, H. B.; Kim, S. Y.; Kim, H. J.; Lee, K.-T.; Gadotti, V. M.; Huang, J.; Zhang, F.-X.; Zamponi, G. W.; Lee, J. Y. Bioorg. Med. Chem. 2017, 25, 4656–4664. doi:10.1016/j.bmc.2017.07.010
Return to citation in text: [1] -
Jin, K.; Sang, Y.; Han, S.; De Clercq, E.; Pannecouque, C.; Meng, G.; Chen, F. Eur. J. Med. Chem. 2019, 176, 11–20. doi:10.1016/j.ejmech.2019.05.011
Return to citation in text: [1] -
Dukat, M.; Alix, K.; Worsham, J.; Khatri, S.; Schulte, M. K. Bioorg. Med. Chem. Lett. 2013, 23, 5945–5948. doi:10.1016/j.bmcl.2013.08.072
Return to citation in text: [1] -
Li, W.-J.; Li, Q.; Liu, D.-L.; Ding, M.-W. J. Agric. Food Chem. 2013, 61, 1419–1426. doi:10.1021/jf305355u
Return to citation in text: [1] -
Zhao, J.; Wang, S.; Han, S.; Kim, S. H.; Kusnetzow, A. K.; Nguyen, J.; Rico-Bautista, E.; Tan, H.; Betz, S. F.; Struthers, R. S.; Zhu, Y. Bioorg. Med. Chem. Lett. 2020, 30, 127391. doi:10.1016/j.bmcl.2020.127391
Return to citation in text: [1] -
Jagtap, A. D.; Kondekar, N. B.; Hung, P.-Y.; Hsieh, C.-E.; Yang, C.-R.; Chen, G. S.; Chern, J.-W. Bioorg. Chem. 2020, 95, 103135. doi:10.1016/j.bioorg.2019.103135
Return to citation in text: [1] -
Park, B.; Nam, J. H.; Kim, J. H.; Kim, H. J.; Onnis, V.; Balboni, G.; Lee, K.-T.; Park, J. H.; Catto, M.; Carotti, A.; Lee, J. Y. Bioorg. Med. Chem. Lett. 2017, 27, 1179–1185. doi:10.1016/j.bmcl.2017.01.068
Return to citation in text: [1] -
Wiedemann, S. H.; Ellman, J. A.; Bergman, R. G. J. Org. Chem. 2006, 71, 1969–1976. doi:10.1021/jo052345b
Return to citation in text: [1] -
Niewiadomy, A.; Matysiak, J.; Karpińska, M. M. Arch. Pharm. (Weinheim, Ger.) 2011, 344, 224–230. doi:10.1002/ardp.201000228
Return to citation in text: [1] -
Mancini, A.; Chelini, A.; Di Capua, A.; Castelli, L.; Brogi, S.; Paolino, M.; Giuliani, G.; Cappelli, A.; Frosini, M.; Ricci, L.; Leonelli, E.; Giorgi, G.; Giordani, A.; Magistretti, J.; Anzini, M. Eur. J. Med. Chem. 2017, 126, 614–630. doi:10.1016/j.ejmech.2016.11.053
Return to citation in text: [1] -
Matysiak, J. Bioorg. Med. Chem. 2006, 14, 2613–2619. doi:10.1016/j.bmc.2005.11.053
Return to citation in text: [1] -
Campbell, M. V.; Iretskii, A. V.; Mosey, R. A. J. Org. Chem. 2020, 85, 11211–11225. doi:10.1021/acs.joc.0c01308
Return to citation in text: [1] [2] -
Kumar, R. A.; Saidulu, G.; Sridhar, B.; Liu, S. T.; Reddy, K. R. J. Org. Chem. 2013, 78, 10240–10250. doi:10.1021/jo401622r
Return to citation in text: [1] [2] -
Kobayashi, K.; Matsumoto, N.; Nagashima, M.; Inouchi, H. Helv. Chim. Acta 2015, 98, 184–189. doi:10.1002/hlca.201400316
Return to citation in text: [1] [2] -
Ren, J.; Pi, C.; Wu, Y.; Cui, X. Org. Lett. 2019, 21, 4067–4071. doi:10.1021/acs.orglett.9b01246
Return to citation in text: [1] -
Meng, X.-H.; Yang, M.; Peng, J.-Y.; Zhao, Y.-L. Adv. Synth. Catal. 2021, 363, 244–250. doi:10.1002/adsc.202000957
Return to citation in text: [1] -
Mishra, A.; Batra, S. Synthesis 2009, 3077–3088. doi:10.1055/s-0029-1217603
Return to citation in text: [1] -
Gruber, N.; Díaz, J. E.; Orelli, L. R. Beilstein J. Org. Chem. 2018, 14, 2510–2519. doi:10.3762/bjoc.14.227
Return to citation in text: [1] -
Gimbert, C.; Vallribera, A. Org. Lett. 2009, 11, 269–271. doi:10.1021/ol802346r
Return to citation in text: [1] [2] -
Abaev, V. T.; Tsiunchik, F. A.; Gutnov, A. V.; Butin, A. V. Tetrahedron Lett. 2006, 47, 4029–4032. doi:10.1016/j.tetlet.2006.04.010
Return to citation in text: [1] [2] -
Polina, S.; Putta, V. P. R. K.; Gujjarappa, R.; Singh, V.; Pujar, P. P.; Malakar, C. C. Adv. Synth. Catal. 2021, 363, 431–445. doi:10.1002/adsc.202001149
Return to citation in text: [1] [2] -
Sashida, H.; Kaname, M.; Minoura, M. Tetrahedron 2013, 69, 6478–6487. doi:10.1016/j.tet.2013.05.069
Return to citation in text: [1] -
Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323–8359. doi:10.1021/cr400615v
Return to citation in text: [1] -
Youcef, S. D.; Kerim, M. D.; Ilitki, H.; El Kaïm, L. Tetrahedron Lett. 2019, 60, 102–105. doi:10.1016/j.tetlet.2018.11.068
Return to citation in text: [1] -
Singh, A.; Kumar, R. Chem. Commun. 2021, 57, 9708–9711. doi:10.1039/d1cc03256a
Return to citation in text: [1] -
Jia, S.; El Kaïm, L. Eur. J. Org. Chem. 2018, 6457–6464. doi:10.1002/ejoc.201800958
Return to citation in text: [1] -
Liu, N.; Chao, F.; Liu, M.-G.; Huang, N.-Y.; Zou, K.; Wang, L. J. Org. Chem. 2019, 84, 2366–2371. doi:10.1021/acs.joc.8b03242
Return to citation in text: [1] -
De Moliner, F.; Bigatti, M.; Banfi, L.; Riva, R.; Basso, A. Org. Lett. 2014, 16, 2280–2283. doi:10.1021/ol500813p
Return to citation in text: [1] -
Martinand-Lurin, E.; Dos Santos, A.; El Kaim, L.; Grimaud, L.; Retailleau, P. Chem. Commun. 2014, 50, 2214–2217. doi:10.1039/c3cc49022j
Return to citation in text: [1] -
Pedrood, K.; Montazer, M. N.; Larijani, B.; Mahdavi, M. Synthesis 2021, 53, 2342–2366. doi:10.1055/a-1394-7511
Return to citation in text: [1] -
Polychronidou, V.; Krupp, A.; Strohmann, C.; Antonchick, A. P. Org. Lett. 2021, 23, 6024–6029. doi:10.1021/acs.orglett.1c02099
Return to citation in text: [1] -
Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Muriph, R. E.; Zhang, W. Tetrahedron Lett. 2020, 61, 151392. doi:10.1016/j.tetlet.2019.151392
Return to citation in text: [1] -
Ren, Z.-L.; Liu, J.-C.; Ding, M.-W. Synthesis 2017, 49, 745–754. doi:10.1055/s-0036-1588333
Return to citation in text: [1] -
Wang, L.; Ren, Z.-L.; Ding, M.-W. J. Org. Chem. 2015, 80, 641–646. doi:10.1021/jo502275f
Return to citation in text: [1] -
Wang, L.; Ren, Z.-L.; Chen, M.; Ding, M.-W. Synlett 2014, 25, 721–723. doi:10.1055/s-0033-1340596
Return to citation in text: [1] -
Wu, J.; Zhao, L.; Yang, M.-L.; Ding, M.-W. J. Org. Chem. 2021, 86, 10755–10761. doi:10.1021/acs.joc.1c00735
Return to citation in text: [1] -
Sun, M.; Yu, Y.-L.; Zhao, L.; Ding, M.-W. Tetrahedron 2021, 96, 132368. doi:10.1016/j.tet.2021.132368
Return to citation in text: [1] -
Sun, M.; Yu, Y.-L.; Zhao, L.; Ding, M.-W. Tetrahedron 2021, 80, 131868. doi:10.1016/j.tet.2020.131868
Return to citation in text: [1]
| 23. | Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323–8359. doi:10.1021/cr400615v |
| 20. | Abaev, V. T.; Tsiunchik, F. A.; Gutnov, A. V.; Butin, A. V. Tetrahedron Lett. 2006, 47, 4029–4032. doi:10.1016/j.tetlet.2006.04.010 |
| 21. | Polina, S.; Putta, V. P. R. K.; Gujjarappa, R.; Singh, V.; Pujar, P. P.; Malakar, C. C. Adv. Synth. Catal. 2021, 363, 431–445. doi:10.1002/adsc.202001149 |
| 1. | Kim, J. H.; Jeong, H. R.; Jung, D. W.; Yoon, H. B.; Kim, S. Y.; Kim, H. J.; Lee, K.-T.; Gadotti, V. M.; Huang, J.; Zhang, F.-X.; Zamponi, G. W.; Lee, J. Y. Bioorg. Med. Chem. 2017, 25, 4656–4664. doi:10.1016/j.bmc.2017.07.010 |
| 5. | Zhao, J.; Wang, S.; Han, S.; Kim, S. H.; Kusnetzow, A. K.; Nguyen, J.; Rico-Bautista, E.; Tan, H.; Betz, S. F.; Struthers, R. S.; Zhu, Y. Bioorg. Med. Chem. Lett. 2020, 30, 127391. doi:10.1016/j.bmcl.2020.127391 |
| 14. | Kobayashi, K.; Matsumoto, N.; Nagashima, M.; Inouchi, H. Helv. Chim. Acta 2015, 98, 184–189. doi:10.1002/hlca.201400316 |
| 4. | Li, W.-J.; Li, Q.; Liu, D.-L.; Ding, M.-W. J. Agric. Food Chem. 2013, 61, 1419–1426. doi:10.1021/jf305355u |
| 19. | Gimbert, C.; Vallribera, A. Org. Lett. 2009, 11, 269–271. doi:10.1021/ol802346r |
| 3. | Dukat, M.; Alix, K.; Worsham, J.; Khatri, S.; Schulte, M. K. Bioorg. Med. Chem. Lett. 2013, 23, 5945–5948. doi:10.1016/j.bmcl.2013.08.072 |
| 12. | Campbell, M. V.; Iretskii, A. V.; Mosey, R. A. J. Org. Chem. 2020, 85, 11211–11225. doi:10.1021/acs.joc.0c01308 |
| 2. | Jin, K.; Sang, Y.; Han, S.; De Clercq, E.; Pannecouque, C.; Meng, G.; Chen, F. Eur. J. Med. Chem. 2019, 176, 11–20. doi:10.1016/j.ejmech.2019.05.011 |
| 13. | Kumar, R. A.; Saidulu, G.; Sridhar, B.; Liu, S. T.; Reddy, K. R. J. Org. Chem. 2013, 78, 10240–10250. doi:10.1021/jo401622r |
| 9. | Niewiadomy, A.; Matysiak, J.; Karpińska, M. M. Arch. Pharm. (Weinheim, Ger.) 2011, 344, 224–230. doi:10.1002/ardp.201000228 |
| 11. | Matysiak, J. Bioorg. Med. Chem. 2006, 14, 2613–2619. doi:10.1016/j.bmc.2005.11.053 |
| 33. | Ren, Z.-L.; Liu, J.-C.; Ding, M.-W. Synthesis 2017, 49, 745–754. doi:10.1055/s-0036-1588333 |
| 34. | Wang, L.; Ren, Z.-L.; Ding, M.-W. J. Org. Chem. 2015, 80, 641–646. doi:10.1021/jo502275f |
| 35. | Wang, L.; Ren, Z.-L.; Chen, M.; Ding, M.-W. Synlett 2014, 25, 721–723. doi:10.1055/s-0033-1340596 |
| 8. | Wiedemann, S. H.; Ellman, J. A.; Bergman, R. G. J. Org. Chem. 2006, 71, 1969–1976. doi:10.1021/jo052345b |
| 12. | Campbell, M. V.; Iretskii, A. V.; Mosey, R. A. J. Org. Chem. 2020, 85, 11211–11225. doi:10.1021/acs.joc.0c01308 |
| 13. | Kumar, R. A.; Saidulu, G.; Sridhar, B.; Liu, S. T.; Reddy, K. R. J. Org. Chem. 2013, 78, 10240–10250. doi:10.1021/jo401622r |
| 14. | Kobayashi, K.; Matsumoto, N.; Nagashima, M.; Inouchi, H. Helv. Chim. Acta 2015, 98, 184–189. doi:10.1002/hlca.201400316 |
| 15. | Ren, J.; Pi, C.; Wu, Y.; Cui, X. Org. Lett. 2019, 21, 4067–4071. doi:10.1021/acs.orglett.9b01246 |
| 16. | Meng, X.-H.; Yang, M.; Peng, J.-Y.; Zhao, Y.-L. Adv. Synth. Catal. 2021, 363, 244–250. doi:10.1002/adsc.202000957 |
| 17. | Mishra, A.; Batra, S. Synthesis 2009, 3077–3088. doi:10.1055/s-0029-1217603 |
| 18. | Gruber, N.; Díaz, J. E.; Orelli, L. R. Beilstein J. Org. Chem. 2018, 14, 2510–2519. doi:10.3762/bjoc.14.227 |
| 19. | Gimbert, C.; Vallribera, A. Org. Lett. 2009, 11, 269–271. doi:10.1021/ol802346r |
| 20. | Abaev, V. T.; Tsiunchik, F. A.; Gutnov, A. V.; Butin, A. V. Tetrahedron Lett. 2006, 47, 4029–4032. doi:10.1016/j.tetlet.2006.04.010 |
| 21. | Polina, S.; Putta, V. P. R. K.; Gujjarappa, R.; Singh, V.; Pujar, P. P.; Malakar, C. C. Adv. Synth. Catal. 2021, 363, 431–445. doi:10.1002/adsc.202001149 |
| 22. | Sashida, H.; Kaname, M.; Minoura, M. Tetrahedron 2013, 69, 6478–6487. doi:10.1016/j.tet.2013.05.069 |
| 36. | Wu, J.; Zhao, L.; Yang, M.-L.; Ding, M.-W. J. Org. Chem. 2021, 86, 10755–10761. doi:10.1021/acs.joc.1c00735 |
| 37. | Sun, M.; Yu, Y.-L.; Zhao, L.; Ding, M.-W. Tetrahedron 2021, 96, 132368. doi:10.1016/j.tet.2021.132368 |
| 38. | Sun, M.; Yu, Y.-L.; Zhao, L.; Ding, M.-W. Tetrahedron 2021, 80, 131868. doi:10.1016/j.tet.2020.131868 |
| 7. | Park, B.; Nam, J. H.; Kim, J. H.; Kim, H. J.; Onnis, V.; Balboni, G.; Lee, K.-T.; Park, J. H.; Catto, M.; Carotti, A.; Lee, J. Y. Bioorg. Med. Chem. Lett. 2017, 27, 1179–1185. doi:10.1016/j.bmcl.2017.01.068 |
| 24. | Youcef, S. D.; Kerim, M. D.; Ilitki, H.; El Kaïm, L. Tetrahedron Lett. 2019, 60, 102–105. doi:10.1016/j.tetlet.2018.11.068 |
| 25. | Singh, A.; Kumar, R. Chem. Commun. 2021, 57, 9708–9711. doi:10.1039/d1cc03256a |
| 26. | Jia, S.; El Kaïm, L. Eur. J. Org. Chem. 2018, 6457–6464. doi:10.1002/ejoc.201800958 |
| 27. | Liu, N.; Chao, F.; Liu, M.-G.; Huang, N.-Y.; Zou, K.; Wang, L. J. Org. Chem. 2019, 84, 2366–2371. doi:10.1021/acs.joc.8b03242 |
| 28. | De Moliner, F.; Bigatti, M.; Banfi, L.; Riva, R.; Basso, A. Org. Lett. 2014, 16, 2280–2283. doi:10.1021/ol500813p |
| 29. | Martinand-Lurin, E.; Dos Santos, A.; El Kaim, L.; Grimaud, L.; Retailleau, P. Chem. Commun. 2014, 50, 2214–2217. doi:10.1039/c3cc49022j |
| 6. | Jagtap, A. D.; Kondekar, N. B.; Hung, P.-Y.; Hsieh, C.-E.; Yang, C.-R.; Chen, G. S.; Chern, J.-W. Bioorg. Chem. 2020, 95, 103135. doi:10.1016/j.bioorg.2019.103135 |
| 10. | Mancini, A.; Chelini, A.; Di Capua, A.; Castelli, L.; Brogi, S.; Paolino, M.; Giuliani, G.; Cappelli, A.; Frosini, M.; Ricci, L.; Leonelli, E.; Giorgi, G.; Giordani, A.; Magistretti, J.; Anzini, M. Eur. J. Med. Chem. 2017, 126, 614–630. doi:10.1016/j.ejmech.2016.11.053 |
| 30. | Pedrood, K.; Montazer, M. N.; Larijani, B.; Mahdavi, M. Synthesis 2021, 53, 2342–2366. doi:10.1055/a-1394-7511 |
| 31. | Polychronidou, V.; Krupp, A.; Strohmann, C.; Antonchick, A. P. Org. Lett. 2021, 23, 6024–6029. doi:10.1021/acs.orglett.1c02099 |
| 32. | Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Muriph, R. E.; Zhang, W. Tetrahedron Lett. 2020, 61, 151392. doi:10.1016/j.tetlet.2019.151392 |
© 2022 Zhao et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.