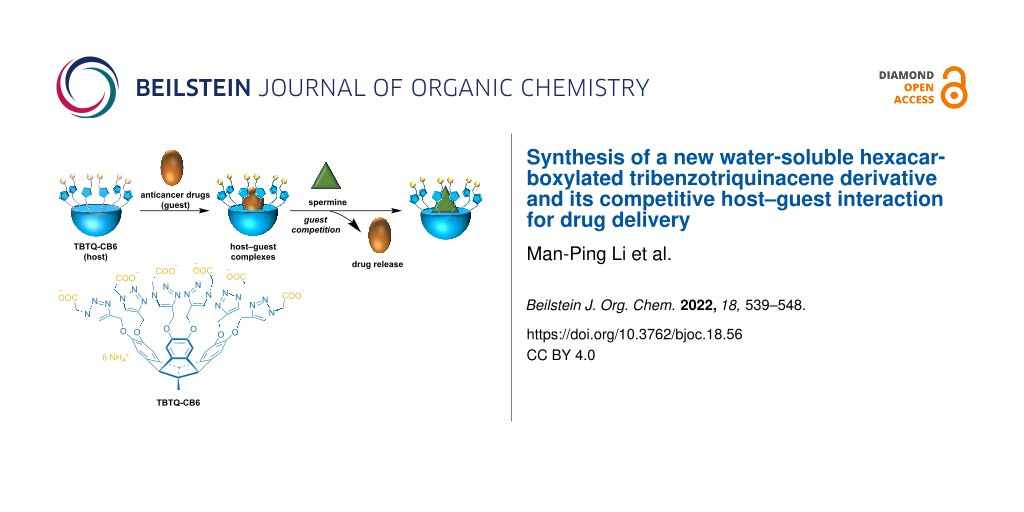
Abstract
A new water-soluble hexacarboxylated tribenzotriquinacene derivative (TBTQ-CB6) was synthesized and used as a supramolecular drug carrier to load the model anticancer drugs dimethyl viologen (MV) and doxorubicin (DOX) via host–guest interactions. The drugs could be effectively released by spermine (SM), a molecule overexpressed in cancer cells, through host–guest competitive substitution since TBTQ-CB6 has a stronger binding affinity toward SM than MV and DOX. The host–guest interactions of the complexes of TBTQ-CB6 with MV, DOX and SM were investigated by NMR spectroscopy and fluorescence spectroscopy. The association stoichiometry of the complexes of TBTQ-CB6 with MV, DOX, and SM was found to be 1:1 with association constants of Ka = (7.67 ± 0.34) × 104 M−1, Ka = (6.81 ± 0.33) × 104 M−1, and Ka = (5.09 ± 0.98) × 105 M−1, respectively. The competitive substitution process was visualized by NMR titration. This novel TBTQ-based host–guest drug delivery system may have potential use in supramolecular chemotherapy.
Graphical Abstract
Introduction
Chemotherapy is considered to be one of the most effective strategies in cancer treatment [1,2]. Many types of chemotherapeutic drugs have been commonly used in clinical practice, including doxorubicin (DOX) [3], chlorambucil [4], oxaliplatin [5], etc. However, the use of most chemotherapeutic agents is often challenged by their poor water solubility and non-selective targeting of cancer cells, resulting in low bioavailability and systemic side effects [6]. To address these drawbacks, various approaches have been developed to improve the bioavailability of these and other drugs and to enable their targeted delivery to cancer cells [7-10]. In recent years, supramolecular chemotherapy has received considerable attention by utilizing a supramolecular strategy to decrease the cytotoxicity of anticancer drugs to normal cells while preserving their cytotoxicity against cancer cells [11]. Supramolecular systems derived from macrocycles [12,13], such as calix[n]arenes (CXs), cyclodextrins (CDs), cucurbiturils (CBs), and pillararenes, are of particular interest because they can act as vehicles for anticancer drugs by either self-assembling into nanocarriers [14-16] or forming host–guest complexes with anticancer drugs [17-20].
Tribenzotriquinacene (TBTQ) derivatives are a class of molecules possessing a rigid bowl-shaped structure and a C3v-symmetric skeleton [21]. Bearing similarity with macrocycles in general, they can act as versatile hosts for encapsulating guest molecules. Over the past two decades, host–guest interactions and self-assembly based on TBTQ derivatives in organic media have been well established [22-27]. Recently, the host–guest chemistry of TBTQ derivatives in aqueous phase was also investigated by us [28,29]. In particular, a water-soluble TBTQ-based hexacarboxylate (TBTQ-C6) was prepared and bound with an azobenzene-containing amphiphile (trans-AZO) to produce the TBTQ-C6-trans-AZO supra-amphiphile by host–guest interactions in water. The supra-amphiphile was further self-assembled into photo and pH dual-responsive supramolecular vesicles that have a potential to serve as drug nanocarriers to enable controlled drug delivery [28]. These findings therefore opened up the possibility for supramolecular chemotherapy based on TBTQ derivatives. However, the use of TBTQ derivatives as hosts to directly encapsulate drugs by forming host–guest complexes has not been reported. In this paper, we report a new water-soluble derivative with an extended cavity that can associate with anticancer drugs through host–guest interactions. The resulting host–guest complexes can be considered as camouflaged anticancer drugs that may exhibit low or no cytotoxicity in normal cellular environment. Furthermore, it is hoped that the release of the drugs is regulated by competitive binding of the host to some overexpressed tumor biomarker molecules, thus restoring the antitumor bioactivity of the drugs in cancer cells.
We designed and synthesized a new water-soluble hexacarboxylated TBTQ derivative, TBTQ-CB6 (Scheme 1), which features a larger cavity as compared to TBTQ-C6 due to the introduction of triazole rings. Two anticancer drug molecules, dimethyl viologen (MV) with a smaller size and DOX with a larger size, were selected as model anticancer agents for encapsulation by TBTQ-CB6 to form the host–guest complexes of TBTQ-CB6MV and TBTQ-CB6
DOX. Spermine (SM), an aliphatic polyamine overexpressed in some cancer cells, was expected to exhibit a higher binding affinity to the negatively charged TBTQ-CB6 host, because it exists mainly in a four positively charged form at physiological pH (about 7) [30,31]. Thus, SM was chosen as a competitive guest to control the release of MV and DOX. The structures of the target molecule TBTQ-CB6 and its precursors were characterized by NMR spectroscopy and mass spectrometry. The host–guest chemistry of TBTQ-CB6
MV, TBTQ-CB6
DOX and TBTQ-CB6
SM was investigated by NMR spectroscopy and fluorescence spectroscopy. The competitive binding of TBTQ-CB6 to SM was revealed by NMR titration. In essence, this represents the first application of the convex-concave TBTQ motif for potential use in drug delivery systems.
Scheme 1:
(a) Synthesis route to TBTQ-CB6. Conditions: (i) ethyl azidoacetate, CuSO4, sodium ascorbate, THF/H2O, 24 h, 73%; (ii) NaOH, MeOH/H2O, 12 h; (iii) HCl, 85% for two steps; (iv) NH3∙H2O, 6 h, 97%. (b) Schematic diagram of the construction of TBTQ-CB6MV and TBTQ-CB6
DOX drug delivery systems and competitive release and replacement of MV and DOX by SM.
Scheme 1: (a) Synthesis route to TBTQ-CB6. Conditions: (i) ethyl azidoacetate, CuSO4, sodium ascorbate, THF/H2...
Results and Discussion
Synthesis and characterization of host TBTQ-CB6. The host TBTQ-CB6 was synthesized starting from the known TBTQ-based hexakis(propargyl ether) 1 [29] (Scheme 1a). Through the CuAAC reaction with ethyl azidoacetate under Cu(I) catalysis, the TBTQ-based hexakis(ethyl acetate) compound 2 was obtained in 73% yield. Subsequent hydrolysis with sodium hydroxide followed by acidification with hydrochloric acid gave the precursor hexakis(carboxylic acid) compound 3 in 85% yield in two steps. Finally, the desired hexacarboxylated TBTQ derivative TBTQ-CB6 was successfully obtained by reaction with ammonium hydroxide in 97% yield and found to have good water solubility. All synthesized compounds were fully characterized by 1H and 13C NMR spectroscopy and mass spectrometry (see Supporting Information File 1) and the data were found to be consistent with the proposed structures.
Host–guest complexation of TBTQ-CB6 with dimethyl viologen (MV). The host–guest complexation between TBTQ-CB6 and MV was first studied by 1H NMR spectroscopy. According to Figure 1a, all proton signals (Ha, Hb, and Hc) of MV showed significant upfield shifts (∆δ = −0.67, −0.42, and −0.70 ppm, respectively) in the presence of one equivalent amount of TBTQ-CB6 due to the shielding effect of the electron-rich cavity of TBTQ-CB6 [32]. In addition, the proton resonances became broadened caused by the complexation dynamics, indicating the successful host–guest complexation between TBTQ-CB6 and MV. Subsequently, the association stoichiometry of the complex was studied by the Job plot method using fluorescence spectroscopy (Figure S10 in Supporting Information File 1). The corresponding Job plot curve (Figure 1b) showed the maximum value at a molar fraction of 0.5 for MV, indicating that TBTQ-CB6 encapsulated MV in a 1:1 stoichiometry. To further evaluate the association affinity of TBTQ-CB6 and MV, fluorescence titration experiments were conducted. As presented in Figure 1c, the emission of TBTQ-CB6 was gradually reduced upon increasing the MV concentration, suggesting charge transfer from the electron-rich host to the electron-deficient guest. According to the fluorescence titration data, the association constant was determined to be Ka = (7.67 ± 0.34) × 104 M−1 by nonlinear curve fitting [33] (Figure 1d).
![[1860-5397-18-56-1]](/bjoc/content/figures/1860-5397-18-56-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (a) 1H NMR spectra (400 MHz, D2O, 25 °C) of (1) MV, (2) TBTQ-CB6 and MV, (3) TBTQ-CB6 with [TBTQ-CB6] = [MV] = 3 mM in each case. (b) Job plot curve of TBTQ-CB6 and MV by plotting fluorescence changes at 321 nm against the molar fraction of TBTQ-CB6 (XTBTQ-CB6). (c) Fluorescence titration spectra of TBTQ-CB6 (10 μM) with varying concentrations of MV in water (λex = 290 nm). (d) Plot of ΔF vs [MV] at 321 nm according to (c) (the solid line was obtained from the nonlinear curve fitting).
Figure 1: (a) 1H NMR spectra (400 MHz, D2O, 25 °C) of (1) MV, (2) TBTQ-CB6 and MV, (3) TBTQ-CB6 with [TBTQ-CB6...
Host–guest complexation of TBTQ-CB6 with doxorubicin (DOX). The host–guest complexation between TBTQ-CB6 and DOX was studied by methods similar to those used for TBTQ-CB6 and MV. The 1H NMR spectra (Figure 2a) reveal that the proton resonances of DOX display considerable upfield shifts upon the addition of equimolar amounts of TBTQ-CB6. In particular, the signals of Ha, Hbc, Hd, He, and Hf are shifted by ∆δ = −0.44, −0.18, −0.10, −0.12, and −0.07 ppm, respectively, which suggests that the electron-rich cavity of TBTQ-CB6 exerts a shielding effect on DOX, i.e., DOX is located in the internal cavity of TBTQ-CB6. Furthermore, the proton signals were all significantly broadened as a result of the complexation dynamics, indicating the formation of a host–guest complex between TBTQ-CB6 and DOX. Subsequently, the binding stoichiometric ratio of the complex was determined to be 1:1 by the Job plot method (Figure 2b and Figure S11 in Supporting Information File 1) through fluorescence spectrometry, and the host–guest binding constant between TBTQ-CB6 and DOX was calculated to be Ka = (6.81 ± 0.33) × 104 M−1 according to the fluorescence titration and nonlinear curve fitting (Figure 2c and 2d). The association affinity of TBTQ-CB6DOX was slightly smaller than that of TBTQ-CB6
MV, which might result from the fact that the two positive charges of MV could bind more effectively to the negatively charged TBTQ-CB6.
![[1860-5397-18-56-2]](/bjoc/content/figures/1860-5397-18-56-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: (a) 1H NMR spectra (400 MHz, D2O, 25 °C) of (1) DOX, (2) TBTQ-CB6 and DOX, (3) TBTQ-CB6 with [TBTQ-CB6] = [DOX] = 3 mM in each case. (b) Job plot curve of TBTQ-CB6 and DOX by plotting fluorescence changes at 321 nm against the molar fraction of TBTQ-CB6 (XTBTQ-CB6). (c) Fluorescence titration spectra of TBTQ-CB6 (10 μM) with varying concentrations of DOX in water (λex = 290 nm). (d) Plot of ΔF vs [DOX] at 321 nm according to (c) (the solid line was obtained from the nonlinear curve fitting).
Figure 2: (a) 1H NMR spectra (400 MHz, D2O, 25 °C) of (1) DOX, (2) TBTQ-CB6 and DOX, (3) TBTQ-CB6 with [TBTQ-...
Host−guest complexation of TBTQ-CB6 with spermine (SM). As a biomarker overexpressed in some tumor cells, SM was hypothesized to bind TBTQ-CB6 more strongly than MV and DOX because of its positive charge distribution. To verify this hypothesis, the host–guest complexation between TBTQ-CB6 and SM was investigated. The 1H NMR spectra (Figure 3) show that the proton signals (Hd and He) located in the middle of the SM structure display upfield shifts (∆δ = −0.04 and −0.10 ppm, respectively) upon the addition of equimolar amounts of TBTQ-CB6. Furthermore, the proton resonances (H1 and H2) of TBTQ-CB6 shifted downfield (∆δ = +0.11 and +0.12 ppm, respectively), indicating the successful binding of SM to TBTQ-CB6. The subsequent Job plot curve obtained from the fluorescence spectroscopy (Figure 4a and 4b) revealed a 1:1 stoichiometric ratio between TBTQ-CB6 and SM. According to the fluorescence titration experiments and the corresponding nonlinear fitting curve (Figure 4c and 4d), the binding constant Ka for TBTQ-CB6 and SM was determined to be (5.09 ± 0.98) × 105 M−1. Compared with the binding constants determined for TBTQ-CB6MV and TBTQ-CB6
DOX, the higher binding constant of TBTQ-CB6
SM should give SM a competitive binding advantage to TBTQ-CB6 and, therefore, enable the release of MV and DOX from TBTQ-CB6
MV and TBTQ-CB6
DOX complexes.
![[1860-5397-18-56-3]](/bjoc/content/figures/1860-5397-18-56-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) 1H NMR spectra (400 MHz, D2O, 25 °C) and (b) partial magnified 1H NMR spectra of (1) SM, (2) TBTQ-CB6 and SM, and (3) TBTQ-CB6 with [TBTQ-CB6] = [SM] = 3 mM in each case.
Figure 3: (a) 1H NMR spectra (400 MHz, D2O, 25 °C) and (b) partial magnified 1H NMR spectra of (1) SM, (2) TB...
![[1860-5397-18-56-4]](/bjoc/content/figures/1860-5397-18-56-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: (a) Fluorescence spectra of the mixture of TBTQ-CB6 and SM in different molar ratios at a constant total concentration of 10 μM. (b) Job plot of TBTQ-CB6 and SM by plotting the difference in fluorescence intensity at 321 nm against the molar fraction of TBTQ-CB6 (XTBTQ-CB6). (c) Fluorescence titration spectra of TBTQ-CB6 (10 μM) with varying concentrations of SM in water (λex = 290 nm). (d) Plot of ΔF vs [SM] at 321 nm according to (c) (the solid line was obtained from the nonlinear curve fitting).
Figure 4: (a) Fluorescence spectra of the mixture of TBTQ-CB6 and SM in different molar ratios at a constant ...
Competitive replacement of MV from the TBTQ-CB6MV complex by SM. 1H NMR titrations [20] were performed to characterize the competitive binding process of TBTQ-CB6 to SM and the release of MV from the TBTQ-CB6
MV complex. Successive amounts of SM, ranging from 0.25 to 2.00 equivalents, were quantitatively dosed into aqueous solutions of TBTQ-CB6
MV (3.0 mM). As shown in Figure 5, the proton resonances associated with MV (Ha, Hb, and Hc highlighted in blue) in complex TBTQ-CB6
MV shifted gradually back downfield and became sharper with the increasing concentration of SM, suggesting a gradual destruction of the TBTQ-CB6
MV host–guest complex. It should be noted that the resonances of MV did not change significantly after the addition of 1.00 equivalent of SM, indicating that most of the MV had been released and gradually reached equilibrium. In addition, the proton signals (green Hd and He) of SM were located upfield in comparison with the free SM (see Figure 3) and the TBTQ-CB6
SM complex, which might be because SM was embedded in the cavity of the host. These results suggested that MV could be effectively released from the cavity of TBTQ-CB6 by the competitive host–guest interaction of SM, since the binding affinity between TBTQ-CB6 and SM was about seven times higher than that between TBTQ-CB6 and MV.
![[1860-5397-18-56-5]](/bjoc/content/figures/1860-5397-18-56-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: 1H NMR spectra (400 MHz, D2O, 25 °C) of (1) MV (3 mM), (2) TBTQ-CB6 and MV (3 mM each), (3–9) TBTQ-CB6 and MV (3 mM each) with gradual addition of SM, and (10) TBTQ-CB6 and SM (3.0 mM each).
Figure 5: 1H NMR spectra (400 MHz, D2O, 25 °C) of (1) MV (3 mM), (2) TBTQ-CB6 and MV (3 mM each), (3–9) TBTQ-...
Competitive replacement of DOX from the TBTQ-CB6DOX complex by SM. Similarly, 1H NMR titration experiments were performed to determine the competing effect of SM and the release of DOX from the TBTQ-CB6
DOX complex. SM was added to TBTQ-CB6
DOX (3.0 mM) in amounts ranging from 0.25 to 2.00 equivalents. As shown in Figure 6, the proton signals of DOX were strongly broadened in TBTQ-CB6
DOX and after the addition of SM. Nevertheless, it can be observed that the broadened Hf of DOX reappears as a small doublet after the addition of 1.00 equivalent of SM. Moreover, the proton signals (H1, H2, and H3) of the host TBTQ-CB6 first became sharpened and then broadened again with the addition of 0.25 to 1.00 equivalents of SM, and the signals remained essentially unchanged thereafter. These observations suggest that DOX was successfully released from the cavity of the host TBTQ-CB6. Furthermore, the resonances of the protons Hd and He of SM were located upfield as compared to those of the free SM (see Figure 3) and the TBTQ-CB6
SM complex, which suggests that SM occupied the cavity of TBTQ-CB6. These results indicate that DOX was effectively released from the TBTQ-CB6
DOX complex by competitive binding of SM to TBTQ-CB6 due to the approximately 7.5-fold higher binding affinity of TBTQ-CB6
SM than TBTQ-CB6
DOX.
![[1860-5397-18-56-6]](/bjoc/content/figures/1860-5397-18-56-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: 1H NMR spectra (400 MHz, D2O, 25 °C) of (1) DOX (3 mM), (2) TBTQ-CB6 and DOX (3 mM each), (3–9) TBTQ-CB6 and DOX (3 mM each) with gradual addition of SM, (10) TBTQ-CB6 and SM (3.0 mM each).
Figure 6: 1H NMR spectra (400 MHz, D2O, 25 °C) of (1) DOX (3 mM), (2) TBTQ-CB6 and DOX (3 mM each), (3–9) TBT...
Conclusion
In conclusion, we designed and synthesized a novel water-soluble tribenzotriquinacene receptor, TBTQ-CB6, as a molecular-scale drug carrier for encapsulating the anticancer drugs dimethyl viologen (MV) and doxorubicin (DOX) via host–guest interactions. The target compound TBTQ-CB6 was characterized in detail by NMR spectroscopy and mass spectrometry. The host–guest complexes of TBTQ-CB6 with MV, DOX, and the overexpressed molecule spermine (SM) found within cancer cells are formed in a 1:1 stoichiometry in all cases and with association constants of Ka = (7.67 ± 0.34) × 104 M−1, Ka = (6.81 ± 0.33) × 104 M−1, and Ka = (5.09 ± 0.98) × 105 M−1, respectively. As a result of the higher binding affinity between TBTQ-CB6 and SM, MV and DOX are released from their corresponding host–guest complexes by the competitive replacement effect of SM, as confirmed by 1H NMR titration experiments. This host–guest competitive substitution system may have potential applications in controlled drug delivery.
Experimental
General information. All commercially available reagents were used as received unless otherwise specified. Anhydrous solvents were collected from a Mikrouna Solv Purer G3 solvent purification system. The 1H NMR and 13C NMR spectra were recorded on a 400 MHz Bruker NMR spectrometer and chemical shifts are reported in ppm (δ). The fluorescence spectra were measured on a HORIBA Fluorolog-3 spectrofluorometer. Mass spectra were recorded using either the ultrafleXtreme MALDI-TOF mass spectrometer (Bruker) with α-cyano-4-hydroxycinnamic acid (HCCA) as a matrix, or by electrospray ionization (ESI) on a Waters G2-XS QTOF instrument connected to a Waters H-class UPLC equipped with a Waters BEH C18 column using an eluent consisting of 90% acetonitrile with 0.1% formic acid and 10% water with 0.1% formic acid at a flow rate of 0.4 mL/min. Freeze-drying was conducted on a Scientz-18N freeze-dryer.
Synthesis of compound 2. A mixture of compound 1 (2.30 g, 3.7 mmol), ethyl azidoacetate (5.76 g, 44.7 mmol), copper(II) sulfate pentahydrate (0.93 g, 3.7 mmol) and sodium ascorbate (1.48 g, 7.4 mmol) in a cosolvent of tetrahydrofuran/water (60 mL/30 mL) was stirred vigorously under nitrogen atmosphere at 60 °C for 24 h. After the solvent was removed by rotary evaporation, a 0.1 M aqueous solution of EDTA (20 mL) was added and the mixture was further stirred for 1 h. Following the addition of water (100 mL), the mixture was extracted with dichloromethane (3 × 50 mL). A substantial amount of solvent was removed from the combined organic layers to produce a concentrated solution. Afterward, diethyl ether was added and the precipitate was collected by suction filtration, washed, and dried to obtain compound 2 as a yellow solid (3.76 g, 73%). Mp 104.8–106.6 °C; 1H NMR (400 MHz, DMSO-d6, 25 °C) δ 8.18 (s, 6H), 7.36 (s, 6H), 5.36 (s, 12H), 5.21, 5.17 (ABq, J = 12.4 Hz, 12H), 4.21 (s, 3H), 4.15 (q, J = 7.1 Hz, 12H), 1.58 (s, 3H), 1.19 (t, J = 7.1 Hz, 18H); 13C NMR (100 MHz, DMSO-d6, 25 °C) δ 167.20, 147.89, 143.15, 137.57, 126.07, 110.42, 62.61, 62.55, 62.15, 61.50, 50.38, 27.48, 13.93; (+)-ESI-HRMS (m/z): [M + H]+ calcd for C65H73N18O18, 1393.5345; found, 1393.5354 (Δ = +0.7 ppm).
Synthesis of compound 3. To a methanol solution (10 mL) of compound 2 (0.50 g, 0.36 mmol), 40% aqueous sodium hydroxide was added. The mixture was heated under reflux for 12 h. The solvent was removed by distillation and the residue was dissolved in water and the pH of the solution was adjusted to 2 with hydrochloric acid. The resulting precipitate was collected by suction filtration, and washed sequentially with water, dichloromethane, and acetone to give compound 3 as a colorless solid (0.37 g, 85%). Mp 59.6–60.7 °C; 1H NMR (400 MHz, DMSO-d6, 25 °C) δ 8.19 (br s, 6H), 7.38 (br s, 6H), 5.26 (br s, 12H), 5.21 (br s, 12H), 4.23 (s, 3H), 1.60 (s, 3H); 13C NMR (100 MHz, DMSO-d6, 25 °C) δ 168.55, 148.01, 143.23, 137.64, 126.25, 110.44, 62.71, 62.63, 62.26, 50.89, 27.58; MALDI-TOF MS (m/z) [M + H]+ calcd for C53H49N18O18, 1225.3467; found, 1225.3449 (Δ = −1.5 ppm).
Synthesis of compound TBTQ-CB6. Compound 3 (0.27 g, 0.22 mmol) was dissolved in a 40% ammonium hydroxide solution (30 mL) and stirred at room temperature for 5 h. The solvent was removed by distillation, and the residue was dissolved in water and washed several times with dichloromethane. The aqueous layer was collected and freeze-dried to afford compound TBTQ-CB6 as a colorless solid (0.28 g, 97%). Mp 198.2–199.4 °C; 1H NMR (400 MHz, D2O, 25 °C) δ 7.89 (s, 6H), 7.21 (s, 6H), 5.25 (s, 12H), 4.97, 4.91 (ABq, J = 17.2 Hz, 12H), 4.31 (s, 3H), 1.57 (s, 3H); 13C NMR (100 MHz, D2O, 25 °C) δ 172.58, 147.37, 143.07, 138.51, 126.22, 110.74, 63.11, 62.15, 61.82, 52.77, 24.69; MALDI-TOF MS (m/z) [M – 6NH4 + 6H + Na]+ calcd for C53H48N18O18Na, 1247.3286; found, 1247.3211 (Δ = −6.0 ppm); [M – 6NH4 + 7H]+ calcd for C53H49N18O18, 1225.3467; found, 1225.3252 (Δ = −17.6 ppm), and [M – 6NH4 + 5H + 2 Na]+ calcd for C53H47N18Na2O18, 1269.3106; found, 1269.3199 (Δ = + 7.3 ppm).
Supporting Information
| Supporting Information File 1: Copies of NMR and mass spectra and fluorescence spectra of TBTQ–CB6 with MV and DOX, respectively, at different ratios. | ||
| Format: PDF | Size: 899.7 KB | Download |
References
-
Bukowski, K.; Kciuk, M.; Kontek, R. Int. J. Mol. Sci. 2020, 21, 3233. doi:10.3390/ijms21093233
Return to citation in text: [1] -
Chabner, B. A.; Roberts, T. G., Jr. Nat. Rev. Cancer 2005, 5, 65–72. doi:10.1038/nrc1529
Return to citation in text: [1] -
Rivankar, S. J. Cancer Res. Ther. (Mumbai, India) 2014, 10, 853–858. doi:10.4103/0973-1482.139267
Return to citation in text: [1] -
Cannon, G. W.; Jackson, C. G.; Samuelson, C. O., Jr.; Ward, J. R.; Williams, H. J.; Clegg, D. O. Semin. Arthritis Rheum. 1985, 15, 106–118. doi:10.1016/0049-0172(85)90028-9
Return to citation in text: [1] -
Kang, L.; Tian, Y.; Xu, S.; Chen, H. J. Neurol. 2021, 268, 3269–3282. doi:10.1007/s00415-020-09942-w
Return to citation in text: [1] -
Jones, R. Medicine 2016, 44, 25–29. doi:10.1016/j.mpmed.2015.10.007
Return to citation in text: [1] -
Senapati, S.; Mahanta, A. K.; Kumar, S.; Maiti, P. Signal Transduction Targeted Ther. 2018, 3, 7. doi:10.1038/s41392-017-0004-3
Return to citation in text: [1] -
Parveen, S.; Sahoo, S. K. J. Drug Targeting 2008, 16, 108–123. doi:10.1080/10611860701794353
Return to citation in text: [1] -
Pugazhendhi, A.; Edison, T. N. J. I.; Karuppusamy, I.; Kathirvel, B. Int. J. Pharm. 2018, 539, 104–111. doi:10.1016/j.ijpharm.2018.01.034
Return to citation in text: [1] -
Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H. M. N. J. Mater. Res. Technol. 2019, 8, 1497–1509. doi:10.1016/j.jmrt.2018.03.007
Return to citation in text: [1] -
Chen, Y.; Sun, Z. ACS Appl. Bio Mater. 2020, 3, 2449–2454. doi:10.1021/acsabm.0c00172
Return to citation in text: [1] -
Webber, M. J.; Langer, R. Chem. Soc. Rev. 2017, 46, 6600–6620. doi:10.1039/c7cs00391a
Return to citation in text: [1] -
Zheng, Z.; Geng, W.-C.; Xu, Z.; Guo, D.-S. Isr. J. Chem. 2019, 59, 913–927. doi:10.1002/ijch.201900032
Return to citation in text: [1] -
Chen, J.; Zhang, Y.; Zhao, L.; Zhang, Y.; Chen, L.; Ma, M.; Du, X.; Meng, Z.; Li, C.; Meng, Q. ACS Appl. Mater. Interfaces 2021, 13, 53564–53573. doi:10.1021/acsami.1c14385
Return to citation in text: [1] -
Zhang, Z.; Yue, Y.-X.; Xu, L.; Wang, Y.; Geng, W.-C.; Li, J.-J.; Kong, X.-l.; Zhao, X.; Zheng, Y.; Zhao, Y.; Shi, L.; Guo, D.-S.; Liu, Y. Adv. Mater. (Weinheim, Ger.) 2021, 33, 2007719. doi:10.1002/adma.202007719
Return to citation in text: [1] -
Xing, P.; Zhao, Y. Small Methods 2018, 2, 1700364. doi:10.1002/smtd.201700364
Return to citation in text: [1] -
Chen, Y.; Huang, Z.; Zhao, H.; Xu, J.-F.; Sun, Z.; Zhang, X. ACS Appl. Mater. Interfaces 2017, 9, 8602–8608. doi:10.1021/acsami.7b01157
Return to citation in text: [1] -
Chen, Y.; Huang, Z.; Xu, J.-F.; Sun, Z.; Zhang, X. ACS Appl. Mater. Interfaces 2016, 8, 22780–22784. doi:10.1021/acsami.6b08295
Return to citation in text: [1] -
Yang, J.; Dai, D.; Ma, L.; Yang, Y.-W. Chin. Chem. Lett. 2021, 32, 729–734. doi:10.1016/j.cclet.2020.08.035
Return to citation in text: [1] -
Hao, Q.; Chen, Y.; Huang, Z.; Xu, J.-F.; Sun, Z.; Zhang, X. ACS Appl. Mater. Interfaces 2018, 10, 5365–5372. doi:10.1021/acsami.7b19784
Return to citation in text: [1] [2] -
Kuck, D. Chem. Rev. 2006, 106, 4885–4925. doi:10.1021/cr050546+
Return to citation in text: [1] -
Wang, T.; Li, Z.-Y.; Xie, A.-L.; Yao, X.-J.; Cao, X.-P.; Kuck, D. J. Org. Chem. 2011, 76, 3231–3238. doi:10.1021/jo2000918
Return to citation in text: [1] -
Bredenkötter, B.; Henne, S.; Volkmer, D. Chem. – Eur. J. 2007, 13, 9931–9938. doi:10.1002/chem.200700915
Return to citation in text: [1] -
Bredenkötter, B.; Grzywa, M.; Alaghemandi, M.; Schmid, R.; Herrebout, W.; Bultinck, P.; Volkmer, D. Chem. – Eur. J. 2014, 20, 9100–9110. doi:10.1002/chem.201304980
Return to citation in text: [1] -
Henne, S.; Bredenkötter, B.; Alaghemandi, M.; Bureekaew, S.; Schmid, R.; Volkmer, D. ChemPhysChem 2014, 15, 3855–3863. doi:10.1002/cphc.201402475
Return to citation in text: [1] -
Henne, S.; Bredenkötter, B.; Dehghan Baghi, A. A.; Schmid, R.; Volkmer, D. Dalton Trans. 2012, 41, 5995–6002. doi:10.1039/c2dt12435a
Return to citation in text: [1] -
Georghiou, P. E.; Dawe, L. N.; Tran, H.-A.; Strübe, J.; Neumann, B.; Stammler, H.-G.; Kuck, D. J. Org. Chem. 2008, 73, 9040–9047. doi:10.1021/jo801782z
Return to citation in text: [1] -
Wang, X.-R.; Li, M.-P.; Xu, W.-R.; Kuck, D. Asian J. Org. Chem. 2021, 10, 567–570. doi:10.1002/ajoc.202000683
Return to citation in text: [1] [2] -
Liu, S.-Y.; Wang, X.-R.; Li, M.-P.; Xu, W.-R.; Kuck, D. Beilstein J. Org. Chem. 2020, 16, 2551–2561. doi:10.3762/bjoc.16.207
Return to citation in text: [1] [2] -
Frassineti, C.; Alderighi, L.; Gans, P.; Sabatini, A.; Vacca, A.; Ghelli, S. Anal. Bioanal. Chem. 2003, 376, 1041–1052. doi:10.1007/s00216-003-2020-0
Return to citation in text: [1] -
Weisell, J.; Hyvönen, M. T.; Häkkinen, M. R.; Grigorenko, N. A.; Pietilä, M.; Lampinen, A.; Kochetkov, S. N.; Alhonen, L.; Vepsäläinen, J.; Keinänen, T. A.; Khomutov, A. R. J. Med. Chem. 2010, 53, 5738–5748. doi:10.1021/jm100439p
Return to citation in text: [1] -
Kamieth, M.; Klärner, F.-G.; Diederich, F. Angew. Chem., Int. Ed. 1998, 37, 3303–3306. doi:10.1002/(sici)1521-3773(19981217)37:23<3303::aid-anie3303>3.0.co;2-t
Return to citation in text: [1] -
Yu, G.; Zhao, R.; Wu, D.; Zhang, F.; Shao, L.; Zhou, J.; Yang, J.; Tang, G.; Chen, X.; Huang, F. Polym. Chem. 2016, 7, 6178–6188. doi:10.1039/c6py01402j
Return to citation in text: [1]
| 20. | Hao, Q.; Chen, Y.; Huang, Z.; Xu, J.-F.; Sun, Z.; Zhang, X. ACS Appl. Mater. Interfaces 2018, 10, 5365–5372. doi:10.1021/acsami.7b19784 |
| 32. | Kamieth, M.; Klärner, F.-G.; Diederich, F. Angew. Chem., Int. Ed. 1998, 37, 3303–3306. doi:10.1002/(sici)1521-3773(19981217)37:23<3303::aid-anie3303>3.0.co;2-t |
| 33. | Yu, G.; Zhao, R.; Wu, D.; Zhang, F.; Shao, L.; Zhou, J.; Yang, J.; Tang, G.; Chen, X.; Huang, F. Polym. Chem. 2016, 7, 6178–6188. doi:10.1039/c6py01402j |
| 1. | Bukowski, K.; Kciuk, M.; Kontek, R. Int. J. Mol. Sci. 2020, 21, 3233. doi:10.3390/ijms21093233 |
| 2. | Chabner, B. A.; Roberts, T. G., Jr. Nat. Rev. Cancer 2005, 5, 65–72. doi:10.1038/nrc1529 |
| 30. | Frassineti, C.; Alderighi, L.; Gans, P.; Sabatini, A.; Vacca, A.; Ghelli, S. Anal. Bioanal. Chem. 2003, 376, 1041–1052. doi:10.1007/s00216-003-2020-0 |
| 31. | Weisell, J.; Hyvönen, M. T.; Häkkinen, M. R.; Grigorenko, N. A.; Pietilä, M.; Lampinen, A.; Kochetkov, S. N.; Alhonen, L.; Vepsäläinen, J.; Keinänen, T. A.; Khomutov, A. R. J. Med. Chem. 2010, 53, 5738–5748. doi:10.1021/jm100439p |
| 5. | Kang, L.; Tian, Y.; Xu, S.; Chen, H. J. Neurol. 2021, 268, 3269–3282. doi:10.1007/s00415-020-09942-w |
| 29. | Liu, S.-Y.; Wang, X.-R.; Li, M.-P.; Xu, W.-R.; Kuck, D. Beilstein J. Org. Chem. 2020, 16, 2551–2561. doi:10.3762/bjoc.16.207 |
| 4. | Cannon, G. W.; Jackson, C. G.; Samuelson, C. O., Jr.; Ward, J. R.; Williams, H. J.; Clegg, D. O. Semin. Arthritis Rheum. 1985, 15, 106–118. doi:10.1016/0049-0172(85)90028-9 |
| 28. | Wang, X.-R.; Li, M.-P.; Xu, W.-R.; Kuck, D. Asian J. Org. Chem. 2021, 10, 567–570. doi:10.1002/ajoc.202000683 |
| 29. | Liu, S.-Y.; Wang, X.-R.; Li, M.-P.; Xu, W.-R.; Kuck, D. Beilstein J. Org. Chem. 2020, 16, 2551–2561. doi:10.3762/bjoc.16.207 |
| 3. | Rivankar, S. J. Cancer Res. Ther. (Mumbai, India) 2014, 10, 853–858. doi:10.4103/0973-1482.139267 |
| 28. | Wang, X.-R.; Li, M.-P.; Xu, W.-R.; Kuck, D. Asian J. Org. Chem. 2021, 10, 567–570. doi:10.1002/ajoc.202000683 |
| 14. | Chen, J.; Zhang, Y.; Zhao, L.; Zhang, Y.; Chen, L.; Ma, M.; Du, X.; Meng, Z.; Li, C.; Meng, Q. ACS Appl. Mater. Interfaces 2021, 13, 53564–53573. doi:10.1021/acsami.1c14385 |
| 15. | Zhang, Z.; Yue, Y.-X.; Xu, L.; Wang, Y.; Geng, W.-C.; Li, J.-J.; Kong, X.-l.; Zhao, X.; Zheng, Y.; Zhao, Y.; Shi, L.; Guo, D.-S.; Liu, Y. Adv. Mater. (Weinheim, Ger.) 2021, 33, 2007719. doi:10.1002/adma.202007719 |
| 16. | Xing, P.; Zhao, Y. Small Methods 2018, 2, 1700364. doi:10.1002/smtd.201700364 |
| 12. | Webber, M. J.; Langer, R. Chem. Soc. Rev. 2017, 46, 6600–6620. doi:10.1039/c7cs00391a |
| 13. | Zheng, Z.; Geng, W.-C.; Xu, Z.; Guo, D.-S. Isr. J. Chem. 2019, 59, 913–927. doi:10.1002/ijch.201900032 |
| 22. | Wang, T.; Li, Z.-Y.; Xie, A.-L.; Yao, X.-J.; Cao, X.-P.; Kuck, D. J. Org. Chem. 2011, 76, 3231–3238. doi:10.1021/jo2000918 |
| 23. | Bredenkötter, B.; Henne, S.; Volkmer, D. Chem. – Eur. J. 2007, 13, 9931–9938. doi:10.1002/chem.200700915 |
| 24. | Bredenkötter, B.; Grzywa, M.; Alaghemandi, M.; Schmid, R.; Herrebout, W.; Bultinck, P.; Volkmer, D. Chem. – Eur. J. 2014, 20, 9100–9110. doi:10.1002/chem.201304980 |
| 25. | Henne, S.; Bredenkötter, B.; Alaghemandi, M.; Bureekaew, S.; Schmid, R.; Volkmer, D. ChemPhysChem 2014, 15, 3855–3863. doi:10.1002/cphc.201402475 |
| 26. | Henne, S.; Bredenkötter, B.; Dehghan Baghi, A. A.; Schmid, R.; Volkmer, D. Dalton Trans. 2012, 41, 5995–6002. doi:10.1039/c2dt12435a |
| 27. | Georghiou, P. E.; Dawe, L. N.; Tran, H.-A.; Strübe, J.; Neumann, B.; Stammler, H.-G.; Kuck, D. J. Org. Chem. 2008, 73, 9040–9047. doi:10.1021/jo801782z |
| 11. | Chen, Y.; Sun, Z. ACS Appl. Bio Mater. 2020, 3, 2449–2454. doi:10.1021/acsabm.0c00172 |
| 7. | Senapati, S.; Mahanta, A. K.; Kumar, S.; Maiti, P. Signal Transduction Targeted Ther. 2018, 3, 7. doi:10.1038/s41392-017-0004-3 |
| 8. | Parveen, S.; Sahoo, S. K. J. Drug Targeting 2008, 16, 108–123. doi:10.1080/10611860701794353 |
| 9. | Pugazhendhi, A.; Edison, T. N. J. I.; Karuppusamy, I.; Kathirvel, B. Int. J. Pharm. 2018, 539, 104–111. doi:10.1016/j.ijpharm.2018.01.034 |
| 10. | Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H. M. N. J. Mater. Res. Technol. 2019, 8, 1497–1509. doi:10.1016/j.jmrt.2018.03.007 |
| 17. | Chen, Y.; Huang, Z.; Zhao, H.; Xu, J.-F.; Sun, Z.; Zhang, X. ACS Appl. Mater. Interfaces 2017, 9, 8602–8608. doi:10.1021/acsami.7b01157 |
| 18. | Chen, Y.; Huang, Z.; Xu, J.-F.; Sun, Z.; Zhang, X. ACS Appl. Mater. Interfaces 2016, 8, 22780–22784. doi:10.1021/acsami.6b08295 |
| 19. | Yang, J.; Dai, D.; Ma, L.; Yang, Y.-W. Chin. Chem. Lett. 2021, 32, 729–734. doi:10.1016/j.cclet.2020.08.035 |
| 20. | Hao, Q.; Chen, Y.; Huang, Z.; Xu, J.-F.; Sun, Z.; Zhang, X. ACS Appl. Mater. Interfaces 2018, 10, 5365–5372. doi:10.1021/acsami.7b19784 |
© 2022 Li et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.