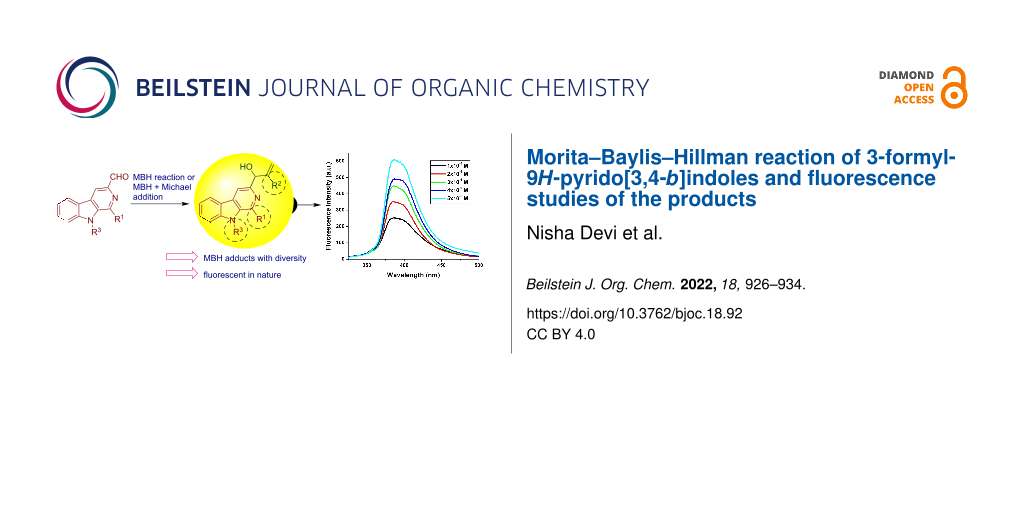
Abstract
β-Carboline is a privileged class of the alkaloid family and is associated with a broad spectrum of biological properties. 3-Formyl-9H-pyrido[3,4-b]indole is a such potent precursor belonging to this family which can be tailored for installing diversity at various positions of β-carboline to generate unique molecular hybrids of biological importance. The present work is a step towards this and assimilates the results related to the exploration of 3-formyl-9H-β-carbolines for the synthesis of β-carboline C-3 substituted MBH adducts followed by evaluation of their fluorescent characteristic. The effect of contact time, solvent system, concentration and substituents was also studied during investigation of fluorescence properties of these derivatives.
Graphical Abstract
Introduction
Among the polycyclic alkaloids based on indole, the tricyclic structure β-carboline represents a promising class of pyridoindole alkaloids with a variety of biological activities which make them interesting synthetic targets [1-8]. Alkaloids containing the β-carboline nucleus in their molecular architecture are present ubiquitously in nature and a large number of natural products are reported representing this scaffold [9-16]. The key precursor used in the biosynthesis of β-carboline is ʟ-tryptophan which forms the basis of great abundance of β-carboline-containing natural products [17]. A broad spectrum of biological activities is displayed by this pharmacologically rich nucleus which includes antibacterial, antifungal, anticancer, anxiolytic, antimalarial, antiviral, anti-HIV, anti-Alzheimer, and anticonvulsant activities etc. [18-26]. Potent anticancer activities are shown by the majority of β-carboline-containing compounds [27-30]. Figure 1 summarizes some examples of β-carboline-based drugs and bioactive natural products some of which have even been commercialized successfully showing the importance of this nucleus [31-33]. This pharmacological richness and colossal medicinal importance is the reason that the synthesis of β-carboline-containing derivatives has been an exciting area for researchers [34-40].
Figure 1: Few examples of β-carboline-based drugs and bioactive natural products.
Figure 1: Few examples of β-carboline-based drugs and bioactive natural products.
The Morita–Baylis–Hillman (MBH) reaction is an astonishing C–C bond forming reaction between a carbonyl electrophile and an activated alkene leading to the formation of allylic alcohol; a highly functionalized product [41-44]. The chemistry of the MBH reaction is decorated with several unique features viz. atom economy, complexity generation and generation of a chiral center from a pro-chiral electrophile. The chemistry of the MBH reaction has gained considerable attention from the past two decades as these MBH adducts are highly functionalized and offer various points of diversity. Due to these amazing features, these MBH adducts act as starting material on which various organic transformations can be performed leading to the synthesis of various natural and synthetic products. MBH adducts itself display diverse biological activities like antifungal, antibacterial, herbicide, antiparasitic and antitumor as reviewed by Lima-Junior et al. (2012) [45].
It was envisaged that in comparison to the traditional methods like Pictet–Spengler (P-S) or Bischler–Napieralski (B-N) cyclisation, introduction of a formyl group at C-1 or C-3 position of the β-carboline frameworks may provide a new route for generating unlimited diversity at C-1 as well as at the C-3 position of β-carbolines. As depicted in Figure 2, 1-formyl-β-carbolines and 3-formyl-β-carbolines are decorated with different sites for diversification which make these synthons a promising template for the construction of β-carboline-fused frameworks via C-1 N-9, C-1 N-2 and C-3 N-2 cyclisation. Similarly, β-carboline-substituted molecular frameworks can be generated at C-3 position.
Figure 2: 1/3-Formyl-9H-β-carboline: new synthons for the synthesis of β-carboline-fused and substituted frameworks.
Figure 2: 1/3-Formyl-9H-β-carboline: new synthons for the synthesis of β-carboline-fused and substituted fram...
Our group has previously explored 1-formyl-β-carbolines and 3-formyl-β-carbolines for the generation of β-carboline-imidazo[1,2-a]azine conjugates at C-1 as well as C-3 position by the application of the Groebke–Blackburn–Bienaymé (GBB) multicomponent approach [46,47]. Our research group has also investigated the scope of 1-formyl-β-carbolines for generating unique molecular hybrids by application of the Morita–Baylis–Hillman reaction [48-50]. It was also revealed from a detailed literature survey that only limited reports have been documented toward exploration of 3-formyl-9H-β-carbolines for generating diversity at the β-carboline skeleton as outlined in Figure 3 [51-56].
Figure 3: A summary of previous reports toward exploration of 3-formyl-9H-β-carbolines.
Figure 3: A summary of previous reports toward exploration of 3-formyl-9H-β-carbolines.
Therefore, we herein report the synthesis of C-3-substituted pyrido[3,4-b]indole MBH adducts from substituted 3-formyl-9H-β-carbolines by the application of the MBH reaction followed by evaluation of their fluorescence properties.
Results and Discussion
The current study began with the synthesis of substituted 3-formyl-9H-β-carbolines (6a–e), which was accomplished by modifying the previously disclosed process as presented in Scheme 1 [46,47,57]. Pictet–Spengler (P-S) condensation of ʟ-tryptophan (1) with different aldehydes (a–e) in dry DCM at room temperature yielded tetrahydro-β-carboline derivatives 2a–e, which were then oxidized with KMnO4 in anhydrous DMF for 45 minutes to yield β-carboline derivatives 3a–e. It was encouraging to observe that the P-S condensation with ʟ-tryptophan (1) was much faster than with the tryptophan ester, taking only 45 minutes to complete. Interestingly, KMnO4 oxidation was selective, with no decarboxylation seen. Within 15 minutes, further treatment of 3a–e with methyl iodide in the presence of K2CO3 provided the corresponding methyl ester 4a–e in high yield (83–87%) and ester functionality reduction with LiAlH4 in dry THF yielded the alcohols 5a–e in excellent yield (90–98%). The required 3-formyl-9H-β-carbolines 6a–e were obtained in 73–88% yield by oxidizing the alcohol derivatives 5a–e with MnO2 in dry DCM. The present methodology is decorated with several advantages like scalability and selectivity. Additionally, no column chromatographic purification was required at any stage and each step was high yielding.
Scheme 1: Synthesis of 3-formyl-9H-pyrido[3,4-b]indole derivatives.
Scheme 1: Synthesis of 3-formyl-9H-pyrido[3,4-b]indole derivatives.
After the synthesis of starting materials, the Morita–Baylis–Hillman reaction was explored for C-3 functionalization of the β-carboline framework. Accordingly, 3-formyl-9H-β-carbolines 6a–e were subjected to MBH reaction with acrylonitrile A and various acrylates B–E under neat conditions in the presence of DABCO as depicted in Scheme 2. All the products were furnished smoothly in 27–72% yield. During this study, it was observed that the MBH reaction of 6b with acrylonitrile A resulted in the formation of product 8bA which evidenced that 6b underwent Morita–Baylis–Hillman reaction at the electrophilic carbonyl center as well as Michael addition reaction at the nucleophilic nitrogen center (N-9). Similar results were obtained when 6e was subjected to MBH reaction with acrylonitrile A and methylacrylate B and products 8eA and 8eB were generated as outlined in Scheme 2.
Scheme 2: Synthesis of C-3 substituted pyrido[3,4-b]indole MBH derivatives (7 and 8).
Scheme 2: Synthesis of C-3 substituted pyrido[3,4-b]indole MBH derivatives (7 and 8).
The effect of a substituent at N-9 position on the reactivity of 3-formyl-9H-pyrido[3,4-b]indole was also investigated during this study. For this purpose, the N-ethyl derivative 9e of 6e was prepared and subjected to MBH reaction with acrylonitrile A and methylacrylate B under neat conditions to generate the corresponding MBH adducts (10eA and 10eB) (Scheme 3). Interestingly, 9e showed more affinity towards this C–C bond forming transformation than 6e. It is noteworthy here that all the products were purified by column chromatography.
Scheme 3: Synthesis of C-3 substituted pyrido[3,4-b]indole MBH derivatives 10.
Scheme 3: Synthesis of C-3 substituted pyrido[3,4-b]indole MBH derivatives 10.
A small library of C-3-substituted pyrido[3,4-b]indole derivatives was designed and synthesized which is presented in Figure 4. All the products were characterized using NMR, FTIR and mass spectrometry.
Figure 4: Library of C-3-substituted pyrido[3,4-b]indole MBH derivatives 7, 8 and 10.
Figure 4: Library of C-3-substituted pyrido[3,4-b]indole MBH derivatives 7, 8 and 10.
Fluorescence studies
Fluorescence studies of these C-3-substituted pyrido[3,4-b]indole derivatives were examined and various parameters (contact time, concentration and solvent) were optimized for obtaining the best results using 7dA as a model substrate. Fluorescence emission spectra for optimizing the contact time were recorded in chloroform at different intervals of time (5 min, 15 min, 1 h and 24 h) at 1 × 10−6 M concentration. 7dA displayed the highest fluorescence intensity after 15 minutes and its fluorescence activity lasted even after 24 h with a slight decrease in fluorescence intensity. Further, the fluorescence emission profile of 7dA was recorded in chloroform at different concentrations viz. 1 × 10−6 M, 2 × 10−6 M, 3 × 10−6 M, 4 × 10−6 M and 5 × 10−6 M which indicated that fluorescence intensity was found to increase with increase in concentration and fluorescence spectra above this concentration showed a fluorescence intensity >1000 a.u. After optimizing the time and concentration parameters, dilutions of 7dA in different organic solvents such as dichloromethane, DMF and ethyl acetate were prepared for optimizing the solvent for obtaining the best fluorescence results. Fluorescence spectra were recorded after 15 minutes of sample preparation in 5 × 10−6 M concentration and fluorescence intensity was observed to be in the following order: CHCl3 > EtOAc > CH2Cl2 > DMF. The results of the optimization studies are presented in Figure 5 and it was concluded from the studies that C-3-substituted pyrido[3,4-b]indole derivative 7dA displayed the maximum fluorescence intensity in chloroform at a concentration of 5 × 10−6 M after 15 minutes of sample preparation.
![[1860-5397-18-92-5]](/bjoc/content/figures/1860-5397-18-92-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Results of optimization for fluorescence studies: a) contact time; b) concentration; c) solvent.
Figure 5: Results of optimization for fluorescence studies: a) contact time; b) concentration; c) solvent.
Accordingly, fluorescence studies of all the other derivatives were conducted following these optimized parameters, i.e., time: 15 min; concentration: 5 × 10−6 M; solvent: CHCl3. The results of the fluorescence studies of all the C-3 substituted pyrido[3,4-b]indole derivatives are presented in Table 1.
Table 1: Results of fluorescence studies of C-3-substituted pyrido[3,4-b]indole derivatives 7–8 and 10.
| sample | compound | R1 | R2 | λEx (nm) | λEm (nm) | flourescence intensity (a.u.) |
| 1 | 7aA | CH(OMe)2 | CN | 266 | 384 | 669.99 |
| 2 | 7aB | CH(OMe)2 | CO2Me | 260 | 369 | 624.79 |
| 3 | 7aC | CH(OMe)2 | CO2Et | 374 | 407 | 223.68 |
| 4 | 7aD | CH(OMe)2 | CO2n-Bu | 258 | 378 | 486.49 |
| 5 | 7aE | CH(OMe)2 | CO2t-Bu | 278 | 388 | 669.30 |
| 6 | 8bA | Ph | CN | 278 | 391 | 592.48 |
| 7 | 7bB | Ph | CO2Me | 278 | 384 | 768.75 |
| 8 | 7bC | Ph | CO2Et | 278 | 383 | 766.89 |
| 9 | 7cB | 2-Br-C6H4 | CO2Me | 278 | 375 | >1000 |
| 10 | 7cE | 2-Br-C6H4 | CO2t-Bu | 278 | 383 | 834.06 |
| 11 | 7dA | 4-Br-C6H4 | CN | 278 | 386 | 609.70 |
| 12 | 7dB | 4-Br-C6H4 | CO2Me | 294 | 386 | 768.78 |
| 13 | 8eA | 4-Cl-C6H4 | CN | 270 | 395 | 385.38 |
| 14 | 8eB | 4-Cl-C6H4 | CO2Me | 270 | 401 | 353.34 |
| 15 | 7eD | 4-Cl-C6H4 | CO2n-Bu | 292 | 385 | 829.04 |
| 16 | 10eA | 4-Cl-C6H4 | CN | 278 | 403 | 556.46 |
| 17 | 10eB | 4-Cl-C6H4 | CO2Me | 270 | 428 | 415.32 |
Structure–fluorescence activity relationships
From the results presented in Table 1, some structure–fluorescence activity relationships were concluded which are outlined in Figure 6. It was concluded from the structure–fluorescence activity relationships of C-3-substituted pyrido[3,4-b]indole derivatives that the products with o-bromophenyl substituent at R1 position (7cB and 7cE) were the most fluorescent derivatives among all. Also, substituted phenyl derivatives were more fluorescent than the dimethoxymethyl substituted derivatives 7aA–7aE. Further, it was observed that an ethyl substituent at N-9 position of β-carboline decreased the fluorescence intensity in 10eA–10eB than 7eD which is a N-unsubstituted derivative while the λemission was red shifted in N-ethyl-substituted derivatives as is clearly indicated from the data presented in Table 1. CO2n-Bu and CO2t-Bu substituents enhanced the fluorescence intensity more than the other substituents (7aE, 7cE and 7eD). It is noteworthy here that in the derivatives prepared from the same aldehydes, a noticeable decrease in fluorescence intensity of product 8 (Morita–Baylis–Hilman + Michael adducts 8bA, 8eA and 8eB) was observed than in case of product 7 (Morita–Baylis–Hilman adducts 7bB, 7bC and 7eD) while their λemission was red-shifted in comparison to type 7 compounds. This difference in the fluorescence intensity values of compound 7 and 8 may be attributed to the addition of a substituent at N-9 position of the β-carboline ring after Michael addition reaction (CH2CH2CN or CH2CH2CO2Me).
Figure 6: Pictorial representation of structure–fluorescence activity relationship of C-3 substituted pyrido[3,4-b]indole derivatives 7, 8 and 10.
Figure 6: Pictorial representation of structure–fluorescence activity relationship of C-3 substituted pyrido[...
Conclusion
In conclusion, we have successfully explored 3-formyl-1-aryl-9H-pyrido[3,4-b]indole derivatives for the C-3 functionalization by application of the MBH reaction to generate C-3-substituted β-carboline MBH adducts. It was revealed from spectroscopic analysis that few derivatives underwent MBH reaction as well as Michael addition reaction to form type 8 compounds. Additionally, the scope of the reaction was further extended and the effect of substituents at the N-9 position on the reactivity of 3-formyl-1-aryl-9H-pyrido[3,4-b]indoles was also investigated. Furthermore, fluorescence properties of these β-carboline conjugates were also studied and they were found to exhibit excellent fluorescence characteristics. Different parameters like contact time, concentration, solvent effects and substituent effects were examined for obtaining the optimal results. It was observed that the MBH derivatives exhibited excellent fluorescence characteristics at a concentration of 5 × 10−6 M in chloroform solvent after 15 minutes of sample preparation. Derivatives 7cB and 7cE bearing an o-bromophenyl substituent at R1 position emerged as two most fluorescent compounds in the present series. Furthermore, products of type 7 (Morita–Baylis–Hilman adducts) were more fluorescent than products 8 (Morita–Baylis–Hilman + Michael adducts). Antimicrobial evaluation of the title compounds is underway and will be reported in due course.
Supporting Information
Supporting information contains detailed experimental procedure for the synthesis of compounds 6–9 and 10 followed by detailed characterization data and copies of 1H NMR and 13C NMR spectra of newly synthesized compounds 6–10.
| Supporting Information File 1: Experimental procedures and characterization data. | ||
| Format: PDF | Size: 3.5 MB | Download |
References
-
Kubota, T.; Kurimoto, S.-i.; Kobayashi, J. The manzamine alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: London, UK, 2020; Vol. 84, pp 1–124. doi:10.1016/bs.alkal.2020.03.001
Return to citation in text: [1] -
Magnier, E.; Langlois, Y. Tetrahedron 1998, 54, 6201–6258. doi:10.1016/s0040-4020(98)00357-3
Return to citation in text: [1] -
Lavrado, J.; Moreira, R.; Paulo, A. Curr. Med. Chem. 2010, 17, 2348–2370. doi:10.2174/092986710791698521
Return to citation in text: [1] -
Cao, R.; Peng, W.; Wang, Z.; Xu, A. Curr. Med. Chem. 2007, 14, 479–500. doi:10.2174/092986707779940998
Return to citation in text: [1] -
Van Phuc, B.; Do, H. N.; Quan, N. M.; Tuan, N. N.; An, N. Q.; Van Tuyen, N.; Anh, H. L. T.; Hung, T. Q.; Dang, T. T.; Langer, P. Synlett 2021, 32, 1004–1008. doi:10.1055/s-0040-1720461
Return to citation in text: [1] -
Hung, T. Q.; Hieu, D. T.; Van Tinh, D.; Do, H. N.; Nguyen Tien, T. A.; Van Do, D.; Son, L. T.; Tran, N. H.; Van Tuyen, N.; Tan, V. M.; Ehlers, P.; Dang, T. T.; Langer, P. Tetrahedron 2019, 75, 130569. doi:10.1016/j.tet.2019.130569
Return to citation in text: [1] -
Hung, T. Q.; Dang, T. T.; Janke, J.; Villinger, A.; Langer, P. Org. Biomol. Chem. 2015, 13, 1375–1386. doi:10.1039/c4ob02226b
Return to citation in text: [1] -
Langer, P.; Anders, J. T.; Weisz, K.; Jähnchen, J. Chem. – Eur. J. 2003, 9, 3951–3964. doi:10.1002/chem.200204566
Return to citation in text: [1] -
Barnes, E. C.; Kumar, R.; Davis, R. A. Nat. Prod. Rep. 2016, 33, 372–381. doi:10.1039/c5np00121h
Return to citation in text: [1] -
Ishikura, M.; Abe, T.; Choshi, T.; Hibino, S. Nat. Prod. Rep. 2015, 32, 1389–1471. doi:10.1039/c5np00032g
Return to citation in text: [1] -
Abdelmohsen, U. R.; Bayer, K.; Hentschel, U. Nat. Prod. Rep. 2014, 31, 381–399. doi:10.1039/c3np70111e
Return to citation in text: [1] -
Ishikura, M.; Abe, T.; Choshi, T.; Hibino, S. Nat. Prod. Rep. 2013, 30, 694–752. doi:10.1039/c3np20118j
Return to citation in text: [1] -
Ishikura, M.; Yamada, K.; Abe, T. Nat. Prod. Rep. 2010, 27, 1630–1680. doi:10.1039/c005345g
Return to citation in text: [1] -
Mansoor, T. A.; Ramalhete, C.; Molnár, J.; Mulhovo, S.; Ferreira, M. J. U. J. Nat. Prod. 2009, 72, 1147–1150. doi:10.1021/np9001477
Return to citation in text: [1] -
Higuchi, K.; Kawasaki, T. Nat. Prod. Rep. 2007, 24, 843–868. doi:10.1039/b516351j
Return to citation in text: [1] -
Kawasaki, T.; Higuchi, K. Nat. Prod. Rep. 2005, 22, 761–793. doi:10.1039/b502162f
Return to citation in text: [1] -
Rommelspacher, H.; Wernicke, C.; Lehmann, J. β-Carbolines: Occurrence, Biosynthesis, and Biodegradation. In Isoquinolines And Beta-Carbolines As Neurotoxins And Neuroprotectants; Antkiewicz-Michaluk, L.; Rommelspacher, H., Eds.; Springer: Boston, MA, 2011; Vol. 1, pp 105–113. doi:10.1007/978-1-4614-1542-8_6
Return to citation in text: [1] -
Chen, H.; Gao, P.; Zhang, M.; Liao, W.; Zhang, J. New J. Chem. 2014, 38, 4155–4166. doi:10.1039/c4nj00262h
Return to citation in text: [1] -
Kuo, P.-C.; Shi, L.-S.; Damu, A. G.; Su, C.-R.; Huang, C.-H.; Ke, C.-H.; Wu, J.-B.; Lin, A.-J.; Bastow, K. F.; Lee, K.-H.; Wu, T.-S. J. Nat. Prod. 2003, 66, 1324–1327. doi:10.1021/np030277n
Return to citation in text: [1] -
Nenaah, G. Fitoterapia 2010, 81, 779–782. doi:10.1016/j.fitote.2010.04.004
Return to citation in text: [1] -
Quintana, V. M.; Piccini, L. E.; Panozzo Zénere, J. D.; Damonte, E. B.; Ponce, M. A.; Castilla, V. Antiviral Res. 2016, 134, 26–33. doi:10.1016/j.antiviral.2016.08.018
Return to citation in text: [1] -
Wang, Y.-H.; Tang, J.-G.; Wang, R.-R.; Yang, L.-M.; Dong, Z.-J.; Du, L.; Shen, X.; Liu, J.-K.; Zheng, Y.-T. Biochem. Biophys. Res. Commun. 2007, 355, 1091–1095. doi:10.1016/j.bbrc.2007.02.081
Return to citation in text: [1] -
Rook, Y.; Schmidtke, K.-U.; Gaube, F.; Schepmann, D.; Wünsch, B.; Heilmann, J.; Lehmann, J.; Winckler, T. J. Med. Chem. 2010, 53, 3611–3617. doi:10.1021/jm1000024
Return to citation in text: [1] -
Maksay, G.; Simonyi, M. Eur. J. Pharmacol. 1985, 117, 275–278. doi:10.1016/0014-2999(85)90614-4
Return to citation in text: [1] -
Wang, S.; Fang, K.; Dong, G.; Chen, S.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; Sheng, C. J. Med. Chem. 2015, 58, 6678–6696. doi:10.1021/acs.jmedchem.5b00910
Return to citation in text: [1] -
Du, H.; Gu, H.; Li, N.; Wang, J. Med. Chem. Commun. 2016, 7, 636–645. doi:10.1039/c5md00581g
Return to citation in text: [1] -
Funayama, Y.; Nishio, K.; Wakabayashi, K.; Nagao, M.; Shimoi, K.; Ohira, T.; Hasegawa, S.; Saijo, N. Mutat. Res., Fundam. Mol. Mech. Mutagen. 1996, 349, 183–191. doi:10.1016/0027-5107(95)00176-x
Return to citation in text: [1] -
Castro, A. C.; Dang, L. C.; Soucy, F.; Grenier, L.; Mazdiyasni, H.; Hottelet, M.; Parent, L.; Pien, C.; Palombella, V.; Adams, J. Bioorg. Med. Chem. Lett. 2003, 13, 2419–2422. doi:10.1016/s0960-894x(03)00408-6
Return to citation in text: [1] -
Song, Y.; Kesuma, D.; Wang, J.; Deng, Y.; Duan, J.; Wang, J. H.; Qi, R. Z. Biochem. Biophys. Res. Commun. 2004, 317, 128–132. doi:10.1016/j.bbrc.2004.03.019
Return to citation in text: [1] -
Guan, H.; Chen, H.; Peng, W.; Ma, Y.; Cao, R.; Liu, X.; Xu, A. Eur. J. Med. Chem. 2006, 41, 1167–1179. doi:10.1016/j.ejmech.2006.05.004
Return to citation in text: [1] -
Sorbera, L. A.; Martin, L.; Leeson, P. A.; Castaner, J. Drugs Future 2001, 26, 15. doi:10.1358/dof.2001.026.01.606182
Return to citation in text: [1] -
Daugan, A.; Grondin, P.; Ruault, C.; Le Monnier de Gouville, A.-C.; Coste, H.; Kirilovsky, J.; Hyafil, F.; Labaudinière, R. J. Med. Chem. 2003, 46, 4525–4532. doi:10.1021/jm030056e
Return to citation in text: [1] -
Maw, G. N.; Allerton, C. M. N.; Gbekor, E.; Million, W. A. Bioorg. Med. Chem. Lett. 2003, 13, 1425–1428. doi:10.1016/s0960-894x(03)00159-8
Return to citation in text: [1] -
Cox, E. D.; Cook, J. M. Chem. Rev. 1995, 95, 1797–1842. doi:10.1021/cr00038a004
Return to citation in text: [1] -
Royer, J.; Bonin, M.; Micouin, L. Chem. Rev. 2004, 104, 2311–2352. doi:10.1021/cr020083x
Return to citation in text: [1] -
Chandrasekhar, D.; Borra, S.; Nanubolu, J. B.; Maurya, R. A. Org. Lett. 2016, 18, 2974–2977. doi:10.1021/acs.orglett.6b01321
Return to citation in text: [1] -
Zhu, Y.-P.; Liu, M.-C.; Cai, Q.; Jia, F.-C.; Wu, A.-X. Chem. – Eur. J. 2013, 19, 10132–10137. doi:10.1002/chem.201301734
Return to citation in text: [1] -
Pumphrey, A. L.; Dong, H.; Driver, T. G. Angew. Chem., Int. Ed. 2012, 51, 5920–5923. doi:10.1002/anie.201201788
Return to citation in text: [1] -
Ding, S.; Shi, Z.; Jiao, N. Org. Lett. 2010, 12, 1540–1543. doi:10.1021/ol100272s
Return to citation in text: [1] -
England, D. B.; Padwa, A. Org. Lett. 2008, 10, 3631–3634. doi:10.1021/ol801385h
Return to citation in text: [1] -
Basavaiah, D.; Rao, A. J.; Satyanarayana, T. Chem. Rev. 2003, 103, 811–892. doi:10.1021/cr010043d
Return to citation in text: [1] -
Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447–5674. doi:10.1021/cr900291g
Return to citation in text: [1] -
Bhowmik, S.; Batra, S. Curr. Org. Chem. 2014, 18, 3078–3119. doi:10.2174/1385272819666141125003114
Return to citation in text: [1] -
Langer, P. Angew. Chem., Int. Ed. 2000, 39, 3049–3052. doi:10.1002/1521-3773(20000901)39:17<3049::aid-anie3049>3.0.co;2-5
Return to citation in text: [1] -
Lima–Junior, C. G.; Vasconcellos, M. L. A. A. Bioorg. Med. Chem. 2012, 20, 3954–3971. doi:10.1016/j.bmc.2012.04.061
Return to citation in text: [1] -
Devi, N.; Singh, D.; Honey, H.; Mor, S.; Chaudhary, S.; Rawal, R. K.; Kumar, V.; Chowdhury, A. K.; Singh, V. RSC Adv. 2016, 6, 43881–43891. doi:10.1039/c6ra04841b
Return to citation in text: [1] [2] -
Devi, N.; Singh, D.; Kaur, G.; Mor, S.; Putta, V. P. R. K.; Polina, S.; Malakar, C. C.; Singh, V. New J. Chem. 2017, 41, 1082–1093. doi:10.1039/c6nj03210a
Return to citation in text: [1] [2] -
Singh, V.; Hutait, S.; Batra, S. Eur. J. Org. Chem. 2009, 6211–6216. doi:10.1002/ejoc.200900962
Return to citation in text: [1] -
Singh, V.; Hutait, S.; Batra, S. Eur. J. Org. Chem. 2010, 3684–3691. doi:10.1002/ejoc.201000322
Return to citation in text: [1] -
Singh, V.; Hutait, S.; Biswas, S.; Batra, S. Eur. J. Org. Chem. 2010, 531–539. doi:10.1002/ejoc.200901166
Return to citation in text: [1] -
Kamal, A.; Srinivasulu, V.; Nayak, V. L.; Sathish, M.; Shankaraiah, N.; Bagul, C.; Reddy, N. V. S.; Rangaraj, N.; Nagesh, N. ChemMedChem 2014, 9, 2084–2098. doi:10.1002/cmdc.201300406
Return to citation in text: [1] -
Ábrányi-Balogh, P.; Dancsó, A.; Frigyes, D.; Volk, B.; Keglevich, G.; Milen, M. Tetrahedron 2014, 70, 5711–5719. doi:10.1016/j.tet.2014.06.073
Return to citation in text: [1] -
Liu, L.; Yang, Q.; Yu, H.; Li, J.-L.; Pei, Y.-N.; Zhu, H.-J.; Li, Z.-Q.; Wang, X.-K. Tetrahedron 2015, 71, 3296–3302. doi:10.1016/j.tet.2015.03.109
Return to citation in text: [1] -
Ling, Y.; Xu, C.; Luo, L.; Cao, J.; Feng, J.; Xue, Y.; Zhu, Q.; Ju, C.; Li, F.; Zhang, Y.; Zhang, Y.; Ling, X. J. Med. Chem. 2015, 58, 9214–9227. doi:10.1021/acs.jmedchem.5b01052
Return to citation in text: [1] -
Shankaraiah, N.; Sharma, P.; Pedapati, S.; Nekkanti, S.; Srinivasulu, V.; Praveen Kumar, N.; Kamal, A. Lett. Drug Des. Discovery 2016, 13, 335–342. doi:10.2174/157018081304160303180050
Return to citation in text: [1] -
Zhang, M.; Sun, D. Anti-Cancer Agents Med. Chem. 2015, 15, 537–547. doi:10.2174/1871520614666141128121812
Return to citation in text: [1] -
Kamal, A.; Narasimha Rao, M. P.; Swapna, P.; Srinivasulu, V.; Bagul, C.; Shaik, A. B.; Mullagiri, K.; Kovvuri, J.; Reddy, V. S.; Vidyasagar, K.; Nagesh, N. Org. Biomol. Chem. 2014, 12, 2370–2387. doi:10.1039/c3ob42236d
Return to citation in text: [1]
| 1. | Kubota, T.; Kurimoto, S.-i.; Kobayashi, J. The manzamine alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: London, UK, 2020; Vol. 84, pp 1–124. doi:10.1016/bs.alkal.2020.03.001 |
| 2. | Magnier, E.; Langlois, Y. Tetrahedron 1998, 54, 6201–6258. doi:10.1016/s0040-4020(98)00357-3 |
| 3. | Lavrado, J.; Moreira, R.; Paulo, A. Curr. Med. Chem. 2010, 17, 2348–2370. doi:10.2174/092986710791698521 |
| 4. | Cao, R.; Peng, W.; Wang, Z.; Xu, A. Curr. Med. Chem. 2007, 14, 479–500. doi:10.2174/092986707779940998 |
| 5. | Van Phuc, B.; Do, H. N.; Quan, N. M.; Tuan, N. N.; An, N. Q.; Van Tuyen, N.; Anh, H. L. T.; Hung, T. Q.; Dang, T. T.; Langer, P. Synlett 2021, 32, 1004–1008. doi:10.1055/s-0040-1720461 |
| 6. | Hung, T. Q.; Hieu, D. T.; Van Tinh, D.; Do, H. N.; Nguyen Tien, T. A.; Van Do, D.; Son, L. T.; Tran, N. H.; Van Tuyen, N.; Tan, V. M.; Ehlers, P.; Dang, T. T.; Langer, P. Tetrahedron 2019, 75, 130569. doi:10.1016/j.tet.2019.130569 |
| 7. | Hung, T. Q.; Dang, T. T.; Janke, J.; Villinger, A.; Langer, P. Org. Biomol. Chem. 2015, 13, 1375–1386. doi:10.1039/c4ob02226b |
| 8. | Langer, P.; Anders, J. T.; Weisz, K.; Jähnchen, J. Chem. – Eur. J. 2003, 9, 3951–3964. doi:10.1002/chem.200204566 |
| 27. | Funayama, Y.; Nishio, K.; Wakabayashi, K.; Nagao, M.; Shimoi, K.; Ohira, T.; Hasegawa, S.; Saijo, N. Mutat. Res., Fundam. Mol. Mech. Mutagen. 1996, 349, 183–191. doi:10.1016/0027-5107(95)00176-x |
| 28. | Castro, A. C.; Dang, L. C.; Soucy, F.; Grenier, L.; Mazdiyasni, H.; Hottelet, M.; Parent, L.; Pien, C.; Palombella, V.; Adams, J. Bioorg. Med. Chem. Lett. 2003, 13, 2419–2422. doi:10.1016/s0960-894x(03)00408-6 |
| 29. | Song, Y.; Kesuma, D.; Wang, J.; Deng, Y.; Duan, J.; Wang, J. H.; Qi, R. Z. Biochem. Biophys. Res. Commun. 2004, 317, 128–132. doi:10.1016/j.bbrc.2004.03.019 |
| 30. | Guan, H.; Chen, H.; Peng, W.; Ma, Y.; Cao, R.; Liu, X.; Xu, A. Eur. J. Med. Chem. 2006, 41, 1167–1179. doi:10.1016/j.ejmech.2006.05.004 |
| 18. | Chen, H.; Gao, P.; Zhang, M.; Liao, W.; Zhang, J. New J. Chem. 2014, 38, 4155–4166. doi:10.1039/c4nj00262h |
| 19. | Kuo, P.-C.; Shi, L.-S.; Damu, A. G.; Su, C.-R.; Huang, C.-H.; Ke, C.-H.; Wu, J.-B.; Lin, A.-J.; Bastow, K. F.; Lee, K.-H.; Wu, T.-S. J. Nat. Prod. 2003, 66, 1324–1327. doi:10.1021/np030277n |
| 20. | Nenaah, G. Fitoterapia 2010, 81, 779–782. doi:10.1016/j.fitote.2010.04.004 |
| 21. | Quintana, V. M.; Piccini, L. E.; Panozzo Zénere, J. D.; Damonte, E. B.; Ponce, M. A.; Castilla, V. Antiviral Res. 2016, 134, 26–33. doi:10.1016/j.antiviral.2016.08.018 |
| 22. | Wang, Y.-H.; Tang, J.-G.; Wang, R.-R.; Yang, L.-M.; Dong, Z.-J.; Du, L.; Shen, X.; Liu, J.-K.; Zheng, Y.-T. Biochem. Biophys. Res. Commun. 2007, 355, 1091–1095. doi:10.1016/j.bbrc.2007.02.081 |
| 23. | Rook, Y.; Schmidtke, K.-U.; Gaube, F.; Schepmann, D.; Wünsch, B.; Heilmann, J.; Lehmann, J.; Winckler, T. J. Med. Chem. 2010, 53, 3611–3617. doi:10.1021/jm1000024 |
| 24. | Maksay, G.; Simonyi, M. Eur. J. Pharmacol. 1985, 117, 275–278. doi:10.1016/0014-2999(85)90614-4 |
| 25. | Wang, S.; Fang, K.; Dong, G.; Chen, S.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; Sheng, C. J. Med. Chem. 2015, 58, 6678–6696. doi:10.1021/acs.jmedchem.5b00910 |
| 26. | Du, H.; Gu, H.; Li, N.; Wang, J. Med. Chem. Commun. 2016, 7, 636–645. doi:10.1039/c5md00581g |
| 17. | Rommelspacher, H.; Wernicke, C.; Lehmann, J. β-Carbolines: Occurrence, Biosynthesis, and Biodegradation. In Isoquinolines And Beta-Carbolines As Neurotoxins And Neuroprotectants; Antkiewicz-Michaluk, L.; Rommelspacher, H., Eds.; Springer: Boston, MA, 2011; Vol. 1, pp 105–113. doi:10.1007/978-1-4614-1542-8_6 |
| 46. | Devi, N.; Singh, D.; Honey, H.; Mor, S.; Chaudhary, S.; Rawal, R. K.; Kumar, V.; Chowdhury, A. K.; Singh, V. RSC Adv. 2016, 6, 43881–43891. doi:10.1039/c6ra04841b |
| 47. | Devi, N.; Singh, D.; Kaur, G.; Mor, S.; Putta, V. P. R. K.; Polina, S.; Malakar, C. C.; Singh, V. New J. Chem. 2017, 41, 1082–1093. doi:10.1039/c6nj03210a |
| 57. | Kamal, A.; Narasimha Rao, M. P.; Swapna, P.; Srinivasulu, V.; Bagul, C.; Shaik, A. B.; Mullagiri, K.; Kovvuri, J.; Reddy, V. S.; Vidyasagar, K.; Nagesh, N. Org. Biomol. Chem. 2014, 12, 2370–2387. doi:10.1039/c3ob42236d |
| 9. | Barnes, E. C.; Kumar, R.; Davis, R. A. Nat. Prod. Rep. 2016, 33, 372–381. doi:10.1039/c5np00121h |
| 10. | Ishikura, M.; Abe, T.; Choshi, T.; Hibino, S. Nat. Prod. Rep. 2015, 32, 1389–1471. doi:10.1039/c5np00032g |
| 11. | Abdelmohsen, U. R.; Bayer, K.; Hentschel, U. Nat. Prod. Rep. 2014, 31, 381–399. doi:10.1039/c3np70111e |
| 12. | Ishikura, M.; Abe, T.; Choshi, T.; Hibino, S. Nat. Prod. Rep. 2013, 30, 694–752. doi:10.1039/c3np20118j |
| 13. | Ishikura, M.; Yamada, K.; Abe, T. Nat. Prod. Rep. 2010, 27, 1630–1680. doi:10.1039/c005345g |
| 14. | Mansoor, T. A.; Ramalhete, C.; Molnár, J.; Mulhovo, S.; Ferreira, M. J. U. J. Nat. Prod. 2009, 72, 1147–1150. doi:10.1021/np9001477 |
| 15. | Higuchi, K.; Kawasaki, T. Nat. Prod. Rep. 2007, 24, 843–868. doi:10.1039/b516351j |
| 16. | Kawasaki, T.; Higuchi, K. Nat. Prod. Rep. 2005, 22, 761–793. doi:10.1039/b502162f |
| 45. | Lima–Junior, C. G.; Vasconcellos, M. L. A. A. Bioorg. Med. Chem. 2012, 20, 3954–3971. doi:10.1016/j.bmc.2012.04.061 |
| 48. | Singh, V.; Hutait, S.; Batra, S. Eur. J. Org. Chem. 2009, 6211–6216. doi:10.1002/ejoc.200900962 |
| 49. | Singh, V.; Hutait, S.; Batra, S. Eur. J. Org. Chem. 2010, 3684–3691. doi:10.1002/ejoc.201000322 |
| 50. | Singh, V.; Hutait, S.; Biswas, S.; Batra, S. Eur. J. Org. Chem. 2010, 531–539. doi:10.1002/ejoc.200901166 |
| 41. | Basavaiah, D.; Rao, A. J.; Satyanarayana, T. Chem. Rev. 2003, 103, 811–892. doi:10.1021/cr010043d |
| 42. | Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447–5674. doi:10.1021/cr900291g |
| 43. | Bhowmik, S.; Batra, S. Curr. Org. Chem. 2014, 18, 3078–3119. doi:10.2174/1385272819666141125003114 |
| 44. | Langer, P. Angew. Chem., Int. Ed. 2000, 39, 3049–3052. doi:10.1002/1521-3773(20000901)39:17<3049::aid-anie3049>3.0.co;2-5 |
| 51. | Kamal, A.; Srinivasulu, V.; Nayak, V. L.; Sathish, M.; Shankaraiah, N.; Bagul, C.; Reddy, N. V. S.; Rangaraj, N.; Nagesh, N. ChemMedChem 2014, 9, 2084–2098. doi:10.1002/cmdc.201300406 |
| 52. | Ábrányi-Balogh, P.; Dancsó, A.; Frigyes, D.; Volk, B.; Keglevich, G.; Milen, M. Tetrahedron 2014, 70, 5711–5719. doi:10.1016/j.tet.2014.06.073 |
| 53. | Liu, L.; Yang, Q.; Yu, H.; Li, J.-L.; Pei, Y.-N.; Zhu, H.-J.; Li, Z.-Q.; Wang, X.-K. Tetrahedron 2015, 71, 3296–3302. doi:10.1016/j.tet.2015.03.109 |
| 54. | Ling, Y.; Xu, C.; Luo, L.; Cao, J.; Feng, J.; Xue, Y.; Zhu, Q.; Ju, C.; Li, F.; Zhang, Y.; Zhang, Y.; Ling, X. J. Med. Chem. 2015, 58, 9214–9227. doi:10.1021/acs.jmedchem.5b01052 |
| 55. | Shankaraiah, N.; Sharma, P.; Pedapati, S.; Nekkanti, S.; Srinivasulu, V.; Praveen Kumar, N.; Kamal, A. Lett. Drug Des. Discovery 2016, 13, 335–342. doi:10.2174/157018081304160303180050 |
| 56. | Zhang, M.; Sun, D. Anti-Cancer Agents Med. Chem. 2015, 15, 537–547. doi:10.2174/1871520614666141128121812 |
| 34. | Cox, E. D.; Cook, J. M. Chem. Rev. 1995, 95, 1797–1842. doi:10.1021/cr00038a004 |
| 35. | Royer, J.; Bonin, M.; Micouin, L. Chem. Rev. 2004, 104, 2311–2352. doi:10.1021/cr020083x |
| 36. | Chandrasekhar, D.; Borra, S.; Nanubolu, J. B.; Maurya, R. A. Org. Lett. 2016, 18, 2974–2977. doi:10.1021/acs.orglett.6b01321 |
| 37. | Zhu, Y.-P.; Liu, M.-C.; Cai, Q.; Jia, F.-C.; Wu, A.-X. Chem. – Eur. J. 2013, 19, 10132–10137. doi:10.1002/chem.201301734 |
| 38. | Pumphrey, A. L.; Dong, H.; Driver, T. G. Angew. Chem., Int. Ed. 2012, 51, 5920–5923. doi:10.1002/anie.201201788 |
| 39. | Ding, S.; Shi, Z.; Jiao, N. Org. Lett. 2010, 12, 1540–1543. doi:10.1021/ol100272s |
| 40. | England, D. B.; Padwa, A. Org. Lett. 2008, 10, 3631–3634. doi:10.1021/ol801385h |
| 31. | Sorbera, L. A.; Martin, L.; Leeson, P. A.; Castaner, J. Drugs Future 2001, 26, 15. doi:10.1358/dof.2001.026.01.606182 |
| 32. | Daugan, A.; Grondin, P.; Ruault, C.; Le Monnier de Gouville, A.-C.; Coste, H.; Kirilovsky, J.; Hyafil, F.; Labaudinière, R. J. Med. Chem. 2003, 46, 4525–4532. doi:10.1021/jm030056e |
| 33. | Maw, G. N.; Allerton, C. M. N.; Gbekor, E.; Million, W. A. Bioorg. Med. Chem. Lett. 2003, 13, 1425–1428. doi:10.1016/s0960-894x(03)00159-8 |
| 46. | Devi, N.; Singh, D.; Honey, H.; Mor, S.; Chaudhary, S.; Rawal, R. K.; Kumar, V.; Chowdhury, A. K.; Singh, V. RSC Adv. 2016, 6, 43881–43891. doi:10.1039/c6ra04841b |
| 47. | Devi, N.; Singh, D.; Kaur, G.; Mor, S.; Putta, V. P. R. K.; Polina, S.; Malakar, C. C.; Singh, V. New J. Chem. 2017, 41, 1082–1093. doi:10.1039/c6nj03210a |
© 2022 Devi and Singh; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.