Abstract
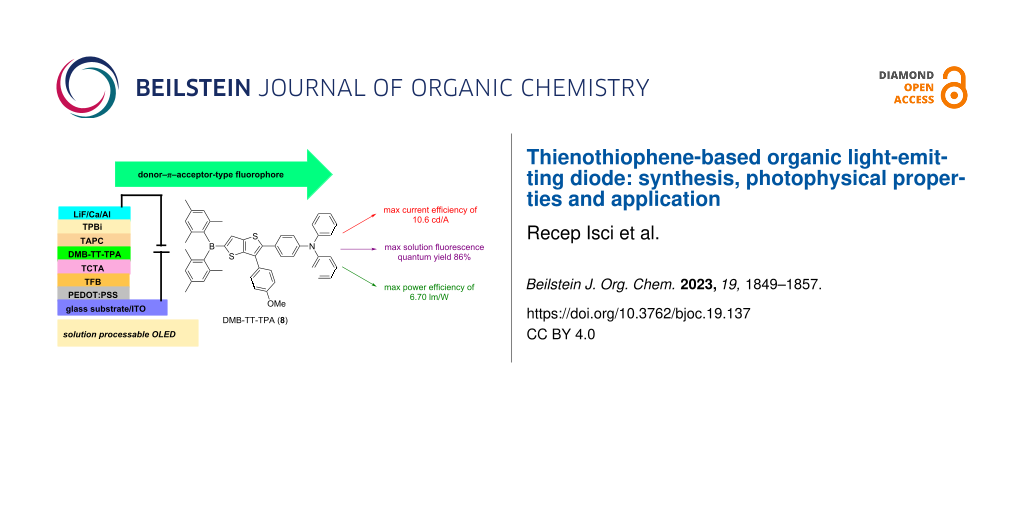
A donor–π–acceptor (D–π–A)-type pull–push compound, DMB-TT-TPA (8), comprising triphenylamine as donor and dimesitylboron as acceptor linked through a thieno[3,2-b]thiophene (TT) π-conjugated linker bearing a 4-MeOPh group, was designed, synthesized, and fabricated as an emitter via a solution process for an organic light-emitting diode (OLED) application. DMB-TT-TPA (8) exhibited absorption and emission maxima of 411 and 520 nm, respectively, with a mega Stokes shift of 109 nm and fluorescence quantum yields both in the solid state (41%) and in solution (86%). The optical properties were supported by computational chemistry using density functional theory for optimized geometry and absorption. A solution-processed OLED was fabricated using low turn-on voltage, which had performances with maximum power, current, and external quantum efficiencies of 6.70 lm/W, 10.6 cd/A, and 4.61%, respectively.
Graphical Abstract
Introduction
In recent years, organic electronics have become very attractive due to their various advantages such as high flexibility, easy designability, low fabrication cost, easy processing and large-scale fabrication [1-4]. Especially in display technology, organic-based materials have found use in many applications such as OLEDs, micro-LEDs, LCDs, lasers, and photodiodes by applying thin film methods and solution processes [5-8]. The performance of organic electronics is based on the active layer composition as well as the fabrication methods and processing parameters. The organic active layers are composed of various aromatic π-conjugated small molecules/polymers including thiophene, anthracene, carbazole, and triphenylamine [9-13].
Thienothiophenes are two annulated thiophene rings having four isomers, among which the most widely used isomer is thieno[3,2-b]thiophene (TT) [14-19]. These compounds are electron-rich, flat and electron-delocalized systems, properties that make them promising materials for the construction of conjugated energy-based semiconductors for OLEDs [20-23], perovskite solar cells [24,25], organic field-effect transistors (OFETs) [26-28], capacitors [29,30], hybrid films [31], and photosensitizers [32-34]. Another important π-conjugated unit is triphenylamine (TPA), having an ionization potential of 6.80 eV, which is lower compared to many other organic cores, thus providing a strong electron-donating ability for organic electronic applications [12,35]. Dimesitylboron (DMB), with its unoccupied p-orbital, is an electron-acceptor organoboron compound used in several donor–acceptor systems to provide the system with pull–push interaction [36,37].
In this work, we have designed and synthesized a D–π–A model pull–push fluorophore, DMB-TT-TPA (8), having TPA and DMB units as donor and acceptor units that were linked through a 4-MeOPh-substituted TT core as a π-spacer. The photophysical properties of the fluorophore were investigated by spectroscopic methods. Moreover, DMB-TT-TPA (8) was fabricated as an emitter for an organic light-emitting diode through a solution process. DMB-TT-TPA (8) displayed excellent performance in both device application and photophysical properties, i.e., a maximum solution fluorescence quantum yield of 86% in THF, maximum solid-state fluorescence quantum yield of 41%, maximum current efficiency of 10.6 cd/A, and maximum power efficiency of 6.70 lm/W.
Results and Discussion
Design and synthesis
The OLED fluorophore, DMB-TT-TPA (8, Scheme 1), having a donor–π–acceptor (D–π–A) system, was synthesized according to our previously reported methods [20-23,36,38]. The synthesis commenced with the treatment of 3-bromothiophene (1) with n-butyllithium at −78 °C, followed by the addition of elemental sulfur and subsequent reaction with 2-bromo-1-(4-methoxyphenyl)ethanone to produce compound 2 in 83% yield. The following ring-closure reaction was conducted in the presence of polyphosphoric acid (PPA) in refluxing chlorobenzene to give 3 (TT) in 86% yield. The brominated TT 4 was obtained through selective monobromination of compound 3 using NBS at −10 °C in DMF in 88% yield. The boronated triphenylamine 6 was constructed in a one-pot two-step reaction in 77% yield, by lithiation of 4-bromo-N,N-diphenylaniline (5) with n-butyllithium at −78 °C and addition of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane. The Suzuki-coupling reaction of TT 4 with borolane 6 produced the intermediate 7 in 81% yield. The target D–π–A-type fluorophore, DMB-TT-TPA (8), was produced by lithiation of 7 and following reaction with dimesitylboron fluoride in 85% yield (Scheme 1).
Photophysical properties
The UV–vis absorption and fluorescence spectra of DMB-TT-TPA (8) were recorded in THF (Figure 1 and Table 1) [38]. It showed maximum absorption and emission wavelengths of 411 and 520 nm (excitation at λmax), respectively, leading to a mega Stokes shift (>100 nm) of 109 nm, which could be explained to be due to a fast relaxation from the excited state to the ground state as a result of a powerful intramolecular energy transfer between the TPA and boron groups through the thieno[3,2-b]thiophene (TT) core. The optical band gap (Eoptic) of DMB-TT-TPA (8) was calculated to be 2.52 eV from the onset wavelength of the absorption spectrum at 491 nm. The compound demonstrates high quantum efficiencies in the solid-state and in solution (THF) of 41 and 86%, respectively. The considerable quantum efficiencies pointed out that DMB-TT-TPA (8) is among the best D–π–A modal fluorophores suitable for an OLED application. Moreover, the photophysical properties of DMB-TT-TPA (8) were investigated through time-resolved fluorescence studies (390 nm laser source in THF). The fluorescence lifetime (τ) of DMB-TT-TPA (8) exhibited a mono-exponential profile having a 3.20 ns fluorescence decay pattern (Figure S1 in Supporting Information File 1), demonstrating a strong pull–push interaction in steady-state time resolved fluorescence performance.
![[1860-5397-19-137-1]](/bjoc/content/figures/1860-5397-19-137-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Absorption and emission of DMB-TT-TPA (8) in THF. Figure 1 was adapted with permission of Institution of Chemical Engineers (IChemE) and The Royal Society of Chemistry from [38] (“Cationic and radical polymerization using a boron–thienothiophene–triphenylamine based D-π-A type photosensitizer under white LED irradiation”) by A. Suerkan et al., Mol. Syst. Des. Eng., vol. 8, issue 10, © 2023); permission conveyed through Copyright Clearance Center, Inc. This content is not subject to CC BY 4.0.
Figure 1: Absorption and emission of DMB-TT-TPA (8) in THF. Figure 1 was adapted with permission of Institution of Ch...
Table 1: Photophysical data of DMB-TT-TPA (8) [38].
| Compound | UVmaxa (nm) | UVonset (nm) | Flmaxa (nm) | Δνb (cm−1) | Eopticc (eV) | Φsolidd (%) | Φsole (%) |
| DMB-TT-TPA | 411 | 491 | 520 | 5100 | 2.52 | 41 | 86 |
aAbsorption and fluorescence maxima in THF. bStokes shift (cm−1) Δν = 1/λmax − 1/λem. cEoptic from the onset of the absorption spectrum. dSolid-state quantum yield. eSolution-state quantum yield in THF.
OLED application
An OLED was fabricated using a standard conventional device architecture of ITO/PEDOT:PSS/TFB/TAPC:TCTA:emitter (DMB-TT-TPA (8))/TPBi/LiF/Ca/Ag, where TFB, TCTA/TAPC, and TPBi acted as hole transport, hole transporting host, and electron transport materials, respectively (Figure S2 in Supporting Information File 1). The current efficiency–luminance–voltage (J–L–V) graph and power efficiency (PE), external quantum efficiency (EQE), and electroluminescence curves are depicted in Figure 2 and Figure 3, respectively. Although DMB-TT-TPA (8) was synthesized and OLED performance was examined in our previous study [23], a different device architecture and method, i.e., solution processing, was used in this study. In the previous study, the OLED of DMB-TT-TPA (8) was explained to demonstrate performance with the turn-on voltage, external quantum efficiency (EQE), and highest luminescence efficiency of 4.6 V, 0.15% and 0.40 cd/A, respectively, using a thermal evaporation method. On the other hand, in this study, the OLED of DMB-TT-TPA (8), prepared using a solution processing method, showed a low turn-on voltage (Von) of 2.90 V, a max current efficiency (CEmax) of 10.6 cd/A, a max luminance of 752 cd/m2, a max power efficiency (PEmax) of 6.70 lm/W, and an external quantum efficiency (EQE) of 4.61%, along with a green emitting luminescence at 512 nm (Table 2). According to the CIE color space chromaticity diagram, the device was located at the coordinates of 0.16 and 0.51. The obtained EL results are in good agreement with the fluorescence characteristic of DMB-TT-TPA (8). Additionally, OLED performances were significantly increased compared to the previous study [23]. In terms of the TT chemistry, the device results reached remarkable values for donor–π–acceptor-type solution processable emitters within the donor–acceptor family [39-42]. This approach also supports that the solution-processable OLED application is a perfectly suitable device preparation for DMB-TT-TPA (8).
![[1860-5397-19-137-2]](/bjoc/content/figures/1860-5397-19-137-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: (a) Current efficiency–luminance, (b) current efficiency–voltage, (c) luminance–voltage, and (d) current density–voltage characteristics of DMB-TT-TPA (8).
Figure 2: (a) Current efficiency–luminance, (b) current efficiency–voltage, (c) luminance–voltage, and (d) cu...
![[1860-5397-19-137-3]](/bjoc/content/figures/1860-5397-19-137-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) Power efficiency–luminance, (b) external quantum efficiency–luminescence, (c) electroluminescence–wavelength characteristics of DMB-TT-TPA (8).
Figure 3: (a) Power efficiency–luminance, (b) external quantum efficiency–luminescence, (c) electroluminescen...
Table 2: Photophysical data of DMB-TT-TPA (8).
| compound | Vona (V) | CEb (cd/A) | Lc (cd/m2) | λELd (nm) | EQEe (%) | PEmaxf (lm/W) | CIEg (x, y) |
| DMB-TT-TPA | 2.9 | 10.6 | 752 | 512 | 4.61 | 6.70 | (0.16, 0.51) |
aTurn-on voltage, recorded at the luminance of 1 cd·m2. bMaximum current efficiency. cMaximum luminance. eMaximum electroluminescence wavelength. dMaximum current efficiency. eMaximum external quantum efficiency. fMaximum power efficiency. gChromaticity coordinates according to the CIE 1931 diagram.
Thermal properties
The thermal properties of DMB-TT-TPA (8) were investigated through thermal gravimetric analysis (TGA) at 750 °C at a heating rate of 10 °C min−1 under N2 atmosphere (Figure 4). The initial mass loss (5%) around 120 °C could be due to residual water and/or solvent. The highest decomposition was observed at around 405 oC and 14% of DMB-TT-TPA (8) remained without ash up to 750 °C, indicating that the compound has an excellent thermal stability. The high thermal stability is profitable for the preparation of stable and durable OLED devices.
![[1860-5397-19-137-4]](/bjoc/content/figures/1860-5397-19-137-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Thermal gravimetric analyses (TGA) of DMB-TT-TPA (8).
Figure 4: Thermal gravimetric analyses (TGA) of DMB-TT-TPA (8).
Computational chemistry
Ground-state geometry optimization of DMB-TT-TPA (8) was performed using density functional theory (DFT) calculations with the Gaussian 16 software at the B3LYP/6-31G (d,p) level (Figure S3 in Supporting Information File 1) [23,43]. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels were calculated to be −4.93 and −1.83 eV, respectively (Figure 5). While the HOMO electrons were distributed mainly on the triphenylamine and TT units, the LUMO was found to be delocalized through the dimesitylboron and TT ring, the results being in line with the experimental values of our previous study [23].
![[1860-5397-19-137-5]](/bjoc/content/figures/1860-5397-19-137-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: HOMO and LUMO diagrams calculated at the B3LYP/6-31G (d,p) level of theory.
Figure 5: HOMO and LUMO diagrams calculated at the B3LYP/6-31G (d,p) level of theory.
On the basis of the optimized ground-state geometry, time-dependent DFT (TD-DFT) calculations were conducted in THF to investigate the absorption properties and theoretical band gap (Table 3). The optical band gap value (Eoptic) was calculated to be 2.06 eV, considering the λonset (605 nm) of the UV–vis curve. The calculated absorption maximum was centered at 470 nm (Figure S4 in Supporting Information File 1), which was found to be in a good agreement with the experimentally determined UV–vis spectrum.
Conclusion
A small fluorophore molecule, DMB-TT-TPA (8), containing dimesitylboron as an acceptor and triphenylamine as a donor linked through a thieno[3,2-b]thiophene core having a 4-MeOPh group, was designed as a D–π–A model and synthesized in 85% yield. Its photophysical properties were investigated by UV–vis and fluorescence spectroscopy. The obtained experimental results were found to be in good agreement with computational investigations. An OLED fabrication, where DMB-TT-TPA (8) was employed as an emitter, showed a maximum luminescence efficiency of 752 cd/m2, a maximum external quantum efficiency of 4.61%, a maximum power efficiency of 6.70 lm/W, and a maximum current efficiency of 10.6 cd/A on 2.9 V turn on voltage with CIE coordinates of 0.16 and 0.51 at λEL = 512 nm. The OLED, optical and thermal properties indicated that the composition of thienothiophene, triphenylamine, and boron is a highly suitable combination for fluorescent organic electronics in display technology.
Experimental
General methods
1H and 13C NMR spectra were recorded on a Varian model NMR spectrometer (500 and 126 MHz) and chemical shift values are reported in ppm downfield from tetramethylsilane (TMS). UV–vis absorption spectra were obtained using a HITACHI U-0080D spectrophotometer. Fluorescence spectra were recorded on a HITACHI F-4500 fluorescence spectrophotometer. Time-resolved fluorescence studies were performed on a Horiba, FL3-2IHR fluorescence spectrophotometer. Solid-state and solution-state quantum yields were measured using a Hamamatsu Quantaurus-QY Absolute PL Quantum Yield Spectrometer. Thermal gravimetric analysis (TGA) was performed on a PerkinElmer Diamond TA/TGA with a heating rate of 10 °C min−1 under nitrogen flow.
Materials
3-Bromothiophene (97%, Across), 2-bromo-4′-methoxyacetophenone (97% Merck), N-bromosuccinimide (Sigma-Aldrich), polyphosphoric acid (PPA, 115% H3PO4 basis, Sigma-Aldrich), n-butyllithium (2.5 M in hexanes, Sigma-Aldrich), sodium sulfate (Merck), 4-bromotriphenylamine (Sigma-Aldrich), dimesitylboronfluoride (90%, Sigma-Aldrich), 4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Sigma-Aldrich), tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4, 99%, Sigma-Aldrich), were used as received. Diethyl ether and THF were dried over metallic sodium. Dimethylformamide (HPLC grade) was stored over activated molecular sieves (4 Å). Dichloromethane (Aldrich), methanol (Merck), and sodium carbonate (Merck) were used as received. Compounds 2–6 were synthesized following our previous reports [20-23,44-47]. The characterization data of 7 and 8 are compatible with the published data in ref. [23].
Synthesis of 7
Synthesized as described in [23]. To a mixture of thienothiophene 4 (250 mg, 0.770 mmol) and borolane 6 (320 mg, 0.845 mmol) dissolved in THF (25 mL) and degassed for 45 min with N2 was added K2CO3 (2.5 mL, 2.5 M) and Pd(PPh3)4 (0.077 mmol). The mixture was then saturated with N2, the reaction flask sealed and the mixture stirred at 75 °C for 48 h. Afterwards, the reaction mixture was filtered through celite eluting with CH2Cl2, extracted with CH2Cl2/water, and the organic phase was washed with sodium carbonate solution (10%) and water, dried over sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. The crude product was purified by column chromatography eluting with n-hexane/CH2Cl2 4:1 to obtain the title compound 7 (300 mg, 81%) as a white powder. Mp 141–142 °C; 1H NMR (500 MHz, CDCl3) δ 7.42 (d, J = 8.8 Hz, 2H), 7.35 (d, J = 5.2 Hz, 1H), 7.28 (t, J = 8.7 Hz, 5H), 7.20 (d, J = 8.7 Hz, 2H), 7.13 (d, J = 7.6 Hz, 4H), 7.05 (t, J = 7.3 Hz, 2H), 6.95 (d, J = 8.7 Hz, 2H), 6.92 (d, J = 8.8 Hz, 2H), 3.86 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 158.89, 147.36, 147.17, 142.04, 139.51, 135.73, 130.12, 129.87, 129.29, 128.34, 127.96, 125.86, 124.80, 123.24, 122.53, 119.81, 114.14, 55.22.
Synthesis of DMB-TT-TPA (8)
Synthesized as described in [23]. To a solution of compound 7 (200 mg, 0.410 mmol) in dry THF (50 mL) was added tert-butyllithium (0.3 mL, 1.7 M, 0.490 mmol) dropwise at −78 °C under a nitrogen atmosphere over a period of 45 min. Then, dimesitylborofluoride (130 mg, 0.490 mmol) was added rapidly. The mixture was further stirred at −78 °C for 1 h, then, allowed to warm to room temperature and stirring was continued overnight. The solution was extracted with dichloromethane, and the organic layer was washed with Na2CO3 solution (10%) and water. The organic layer was dried over Na2SO4, filtered and the solvent was evaporated under reduced pressure. The crude product was purified by flash column chromatography eluting with a mixture of n-hexane/CH2Cl2 3:1 and then crystallized from ethanol to give the title compound DMB-TT-TPA (8) as a yellow powder in 85% yield (256 mg). Mp 165–166 °C; 1H NMR (500 MHz, CDCl3) δ 7.59 (s, 1H), 7.39 (d, J = 8.8 Hz, 2H), 7.28 (d, J = 7.8 Hz, 4H), 7.19 (d, J = 8.7 Hz, 2H), 7.12 (d, J = 7.6 Hz, 4H), 7.05 (t, J = 7.3 Hz, 2H), 6.93 (d, J = 8.7 Hz, 2H), 6.87 (d, J = 8.8 Hz, 2H), 6.84 (s, 4H), 3.82 (s, 3H), 2.32 (s, 6H), 2.17 (s, 12H); 13C NMR (126 MHz, CDCl3) δ 158.92, 153.46, 151.26, 147.60, 147.20, 143.95, 141.05, 140.90, 138.50, 137.96, 132.59, 130.25, 129.86, 129.49, 129.33, 128.14, 127.85, 127.57, 125.01, 123.45, 122.08, 114.12, 55.23, 23.54, 21.22.
Supporting Information
| Supporting Information File 1: General experimental device methods, life time spectra, theoretical computation data, 1H and 13C NMR spectra. | ||
| Format: PDF | Size: 904.9 KB | Download |
Acknowledgements
The authors thank Dilara Gunturkun for DFT calculations, Istanbul Technical University (ITU).
Funding
The authors thank THD-2023-44904, PTA-2023-44900, TGA-2023-44077, TDA-2022-43696 numbered ITU BAP Projects and 122Z568 numbered TUBITAK 1001 Project and Unsped Global Lojistik, Turkey, for financial support. We also thank Higher Education Council of Turkey (YOK) and TUBITAK grants to Recep Isci (PhD, 100/2000 YOK, 2214A and 2211A BIDEP/TUBITAK) for financial support.
References
-
Tavasli, A.; Gurunlu, B.; Gunturkun, D.; Isci, R.; Faraji, S. Electronics (Basel, Switz.) 2022, 11, 316. doi:10.3390/electronics11030316
Return to citation in text: [1] -
Chen, Z.; Huang, Y.; Gao, J.; Zhang, L.; Ma, Z.; Liu, M.; Emrick, T.; Liu, Y. ACS Energy Lett. 2022, 7, 4052–4060. doi:10.1021/acsenergylett.2c02153
Return to citation in text: [1] -
M’Baye, G.; Klymchenko, A. S.; Yushchenko, D. A.; Shvadchak, V. V.; Ozturk, T.; Mély, Y.; Duportail, G. Photochem. Photobiol. Sci. 2007, 6, 71–76. doi:10.1039/b611699j
Return to citation in text: [1] -
Takimiya, K.; Osaka, I.; Nakano, M. Chem. Mater. 2014, 26, 587–593. doi:10.1021/cm4021063
Return to citation in text: [1] -
Monkman, A. ACS Appl. Mater. Interfaces 2022, 14, 20463–20467. doi:10.1021/acsami.1c09189
Return to citation in text: [1] -
Yu, L.; Portale, G.; Stingelin, N. J. Mater. Chem. C 2021, 9, 10547–10556. doi:10.1039/d1tc01418h
Return to citation in text: [1] -
Liu, X.; Lin, R.; Chen, H.; Zhang, S.; Qian, Z.; Zhou, G.; Chen, X.; Zhou, X.; Zheng, L.; Liu, R.; Tian, P. ACS Photonics 2019, 6, 3186–3195. doi:10.1021/acsphotonics.9b00799
Return to citation in text: [1] -
Oyama, Y.; Mamada, M.; Shukla, A.; Moore, E. G.; Lo, S.-C.; Namdas, E. B.; Adachi, C. ACS Mater. Lett. 2020, 2, 161–167. doi:10.1021/acsmaterialslett.9b00536
Return to citation in text: [1] -
Isci, R.; Rahimi Varzeghani, A.; Kaya, K.; Sütay, B.; Tekin, E.; Ozturk, T. ACS Sustainable Chem. Eng. 2022, 10, 1605–1615. doi:10.1021/acssuschemeng.1c07240
Return to citation in text: [1] -
Jiang, H.; Tao, P.; Wong, W.-Y. ACS Mater. Lett. 2023, 5, 822–845. doi:10.1021/acsmaterialslett.2c01070
Return to citation in text: [1] -
Tsai, M.-H.; Ke, T.-H.; Lin, H.-W.; Wu, C.-C.; Chiu, S.-F.; Fang, F.-C.; Liao, Y.-L.; Wong, K.-T.; Chen, Y.-H.; Wu, C.-I. ACS Appl. Mater. Interfaces 2009, 1, 567–574. doi:10.1021/am800124q
Return to citation in text: [1] -
Wang, J.; Liu, K.; Ma, L.; Zhan, X. Chem. Rev. 2016, 116, 14675–14725. doi:10.1021/acs.chemrev.6b00432
Return to citation in text: [1] [2] -
Schipper, D. J.; Fagnou, K. Chem. Mater. 2011, 23, 1594–1600. doi:10.1021/cm103483q
Return to citation in text: [1] -
Cinar, M. E.; Ozturk, T. Chem. Rev. 2015, 115, 3036–3140. doi:10.1021/cr500271a
Return to citation in text: [1] -
Isci, R.; Unal, M.; Kucukcakir, G.; Gurbuz, N. A.; Gorkem, S. F.; Ozturk, T. J. Phys. Chem. B 2021, 125, 13309–13319. doi:10.1021/acs.jpcb.1c09448
Return to citation in text: [1] -
Isci, R.; Gunturkun, D.; Yalin, A. S.; Ozturk, T. J. Polym. Sci. (Hoboken, NJ, U. S.) 2021, 59, 117–123. doi:10.1002/pol.20200635
Return to citation in text: [1] -
Gunturkun, D.; Isci, R.; Sütay, B.; Majewski, L. A.; Faraji, S.; Ozturk, T. Eur. Polym. J. 2022, 170, 111167. doi:10.1016/j.eurpolymj.2022.111167
Return to citation in text: [1] -
Ozturk, T.; Rice, C. R.; Wallis, J. D. J. Mater. Chem. 1995, 5, 1553–1556. doi:10.1039/jm9950501553
Return to citation in text: [1] -
Schneider, J. A.; Dadvand, A.; Wen, W.; Perepichka, D. F. Macromolecules 2013, 46, 9231–9239. doi:10.1021/ma402018n
Return to citation in text: [1] -
Isci, R.; Tekin, E.; Kaya, K.; Piravadili Mucur, S.; Gorkem, S. F.; Ozturk, T. J. Mater. Chem. C 2020, 8, 7908–7915. doi:10.1039/d0tc01715a
Return to citation in text: [1] [2] [3] -
Isci, R.; Tekin, E.; Mucur, S. P.; Ozturk, T. ChemistrySelect 2020, 5, 13091–13098. doi:10.1002/slct.202003273
Return to citation in text: [1] [2] [3] -
Isci, R.; Wan, L.; Topal, S.; Gunturkun, D.; Campbell, A. J.; Ozturk, T. J. Mater. Chem. C 2022, 10, 10719–10727. doi:10.1039/d2tc02371g
Return to citation in text: [1] [2] [3] -
Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] -
Isci, R.; Unal, M.; Yesil, T.; Ekici, A.; Sütay, B.; Zafer, C.; Ozturk, T. Front. Mater. 2023, 10, 1125462. doi:10.3389/fmats.2023.1125462
Return to citation in text: [1] -
Chi, W.-J.; Zheng, D.-Y.; Chen, X.-F.; Li, Z.-S. J. Mater. Chem. C 2017, 5, 10055–10060. doi:10.1039/c7tc03232c
Return to citation in text: [1] -
Ozturk, S. E.; Isci, R.; Faraji, S.; Sütay, B.; Majewski, L. A.; Ozturk, T. Eur. Polym. J. 2023, 191, 112028. doi:10.1016/j.eurpolymj.2023.112028
Return to citation in text: [1] -
Amna, B.; Isci, R.; Siddiqi, H. M.; Majewski, L. A.; Faraji, S.; Ozturk, T. J. Mater. Chem. C 2022, 10, 8254–8265. doi:10.1039/d2tc01222g
Return to citation in text: [1] -
Zhong, H.; Li, C.-Z.; Carpenter, J.; Ade, H.; Jen, A. K.-Y. J. Am. Chem. Soc. 2015, 137, 7616–7619. doi:10.1021/jacs.5b04209
Return to citation in text: [1] -
Isci, R.; Balkan, T.; Tafazoli, S.; Sütay, B.; Eroglu, M. S.; Ozturk, T. ACS Appl. Energy Mater. 2022, 5, 13284–13292. doi:10.1021/acsaem.2c01830
Return to citation in text: [1] -
Topal, S.; Isci, R.; Sezer, E.; Ozturk, T.; Ustamehmetoglu, B. Electrochim. Acta 2021, 389, 138688. doi:10.1016/j.electacta.2021.138688
Return to citation in text: [1] -
Isci, R.; Baysak, E.; Kesan, G.; Minofar, B.; Eroglu, M. S.; Duygulu, O.; Gorkem, S. F.; Ozturk, T. Nanoscale 2022, 14, 16602–16610. doi:10.1039/d2nr04582f
Return to citation in text: [1] -
Celiker, T.; Isci, R.; Kaya, K.; Ozturk, T.; Yagci, Y. J. Polym. Sci. (Hoboken, NJ, U. S.) 2020, 58, 2327–2334. doi:10.1002/pol.20200398
Return to citation in text: [1] -
Kutahya, C.; Allushi, A.; Isci, R.; Kreutzer, J.; Ozturk, T.; Yilmaz, G.; Yagci, Y. Macromolecules 2017, 50, 6903–6910. doi:10.1021/acs.macromol.7b01335
Return to citation in text: [1] -
Beyazit, S.; Aydogan, B.; Osken, I.; Ozturk, T.; Yagci, Y. Polym. Chem. 2011, 2, 1185–1189. doi:10.1039/c1py00019e
Return to citation in text: [1] -
Farokhi, A.; Shahroosvand, H.; Monache, G. D.; Pilkington, M.; Nazeeruddin, M. K. Chem. Soc. Rev. 2022, 51, 5974–6064. doi:10.1039/d1cs01157j
Return to citation in text: [1] -
Turkoglu, G.; Cinar, M. E.; Ozturk, T. Eur. J. Org. Chem. 2017, 4552–4561. doi:10.1002/ejoc.201700679
Return to citation in text: [1] [2] -
Zhou, G.; Baumgarten, M.; Müllen, K. J. Am. Chem. Soc. 2008, 130, 12477–12484. doi:10.1021/ja803627x
Return to citation in text: [1] -
Suerkan, A.; Isci, R.; Ozturk, T.; Yagci, Y. Mol. Syst. Des. Eng. 2023, 8, 1319–1326. doi:10.1039/d3me00083d
Return to citation in text: [1] [2] [3] [4] -
Sonar, P.; Soh, M. S.; Cheng, Y. H.; Henssler, J. T.; Sellinger, A. Org. Lett. 2010, 12, 3292–3295. doi:10.1021/ol1007179
Return to citation in text: [1] -
Lee, H.; Karthik, D.; Lampande, R.; Ryu, J. H.; Kwon, J. H. Front. Chem. (Lausanne, Switz.) 2020, 8, 373. doi:10.3389/fchem.2020.00373
Return to citation in text: [1] -
Xu, T.; Liang, X.; Xie, G. Front. Chem. (Lausanne, Switz.) 2021, 9, 691172. doi:10.3389/fchem.2021.691172
Return to citation in text: [1] -
Fu, Q.; Chen, J.; Shi, C.; Ma, D. ACS Appl. Mater. Interfaces 2012, 4, 6579–6586. doi:10.1021/am301703a
Return to citation in text: [1] -
Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, 2016.
Return to citation in text: [1] -
Gunturkun, D.; Isci, R.; Faraji, S.; Sütay, B.; Majewski, L. A.; Ozturk, T. J. Mater. Chem. C 2023, 11, 13129–13141. doi:10.1039/d3tc02109b
Return to citation in text: [1] -
Capan, A.; Ozturk, T. Synth. Met. 2014, 188, 100–103. doi:10.1016/j.synthmet.2013.11.018
Return to citation in text: [1] -
Turksoy, F.; Wallis, J. D.; Tunca, U.; Ozturk, T. Tetrahedron 2003, 59, 8107–8116. doi:10.1016/j.tet.2003.08.042
Return to citation in text: [1] -
Işçi, R.; Öztürk, T. Turk. J. Chem. 2023, 47, 1239–1248. doi:10.55730/1300-0527.3608
Return to citation in text: [1]
| 1. | Tavasli, A.; Gurunlu, B.; Gunturkun, D.; Isci, R.; Faraji, S. Electronics (Basel, Switz.) 2022, 11, 316. doi:10.3390/electronics11030316 |
| 2. | Chen, Z.; Huang, Y.; Gao, J.; Zhang, L.; Ma, Z.; Liu, M.; Emrick, T.; Liu, Y. ACS Energy Lett. 2022, 7, 4052–4060. doi:10.1021/acsenergylett.2c02153 |
| 3. | M’Baye, G.; Klymchenko, A. S.; Yushchenko, D. A.; Shvadchak, V. V.; Ozturk, T.; Mély, Y.; Duportail, G. Photochem. Photobiol. Sci. 2007, 6, 71–76. doi:10.1039/b611699j |
| 4. | Takimiya, K.; Osaka, I.; Nakano, M. Chem. Mater. 2014, 26, 587–593. doi:10.1021/cm4021063 |
| 20. | Isci, R.; Tekin, E.; Kaya, K.; Piravadili Mucur, S.; Gorkem, S. F.; Ozturk, T. J. Mater. Chem. C 2020, 8, 7908–7915. doi:10.1039/d0tc01715a |
| 21. | Isci, R.; Tekin, E.; Mucur, S. P.; Ozturk, T. ChemistrySelect 2020, 5, 13091–13098. doi:10.1002/slct.202003273 |
| 22. | Isci, R.; Wan, L.; Topal, S.; Gunturkun, D.; Campbell, A. J.; Ozturk, T. J. Mater. Chem. C 2022, 10, 10719–10727. doi:10.1039/d2tc02371g |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 38. | Suerkan, A.; Isci, R.; Ozturk, T.; Yagci, Y. Mol. Syst. Des. Eng. 2023, 8, 1319–1326. doi:10.1039/d3me00083d |
| 14. | Cinar, M. E.; Ozturk, T. Chem. Rev. 2015, 115, 3036–3140. doi:10.1021/cr500271a |
| 15. | Isci, R.; Unal, M.; Kucukcakir, G.; Gurbuz, N. A.; Gorkem, S. F.; Ozturk, T. J. Phys. Chem. B 2021, 125, 13309–13319. doi:10.1021/acs.jpcb.1c09448 |
| 16. | Isci, R.; Gunturkun, D.; Yalin, A. S.; Ozturk, T. J. Polym. Sci. (Hoboken, NJ, U. S.) 2021, 59, 117–123. doi:10.1002/pol.20200635 |
| 17. | Gunturkun, D.; Isci, R.; Sütay, B.; Majewski, L. A.; Faraji, S.; Ozturk, T. Eur. Polym. J. 2022, 170, 111167. doi:10.1016/j.eurpolymj.2022.111167 |
| 18. | Ozturk, T.; Rice, C. R.; Wallis, J. D. J. Mater. Chem. 1995, 5, 1553–1556. doi:10.1039/jm9950501553 |
| 19. | Schneider, J. A.; Dadvand, A.; Wen, W.; Perepichka, D. F. Macromolecules 2013, 46, 9231–9239. doi:10.1021/ma402018n |
| 38. | Suerkan, A.; Isci, R.; Ozturk, T.; Yagci, Y. Mol. Syst. Des. Eng. 2023, 8, 1319–1326. doi:10.1039/d3me00083d |
| 9. | Isci, R.; Rahimi Varzeghani, A.; Kaya, K.; Sütay, B.; Tekin, E.; Ozturk, T. ACS Sustainable Chem. Eng. 2022, 10, 1605–1615. doi:10.1021/acssuschemeng.1c07240 |
| 10. | Jiang, H.; Tao, P.; Wong, W.-Y. ACS Mater. Lett. 2023, 5, 822–845. doi:10.1021/acsmaterialslett.2c01070 |
| 11. | Tsai, M.-H.; Ke, T.-H.; Lin, H.-W.; Wu, C.-C.; Chiu, S.-F.; Fang, F.-C.; Liao, Y.-L.; Wong, K.-T.; Chen, Y.-H.; Wu, C.-I. ACS Appl. Mater. Interfaces 2009, 1, 567–574. doi:10.1021/am800124q |
| 12. | Wang, J.; Liu, K.; Ma, L.; Zhan, X. Chem. Rev. 2016, 116, 14675–14725. doi:10.1021/acs.chemrev.6b00432 |
| 13. | Schipper, D. J.; Fagnou, K. Chem. Mater. 2011, 23, 1594–1600. doi:10.1021/cm103483q |
| 20. | Isci, R.; Tekin, E.; Kaya, K.; Piravadili Mucur, S.; Gorkem, S. F.; Ozturk, T. J. Mater. Chem. C 2020, 8, 7908–7915. doi:10.1039/d0tc01715a |
| 21. | Isci, R.; Tekin, E.; Mucur, S. P.; Ozturk, T. ChemistrySelect 2020, 5, 13091–13098. doi:10.1002/slct.202003273 |
| 22. | Isci, R.; Wan, L.; Topal, S.; Gunturkun, D.; Campbell, A. J.; Ozturk, T. J. Mater. Chem. C 2022, 10, 10719–10727. doi:10.1039/d2tc02371g |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 36. | Turkoglu, G.; Cinar, M. E.; Ozturk, T. Eur. J. Org. Chem. 2017, 4552–4561. doi:10.1002/ejoc.201700679 |
| 38. | Suerkan, A.; Isci, R.; Ozturk, T.; Yagci, Y. Mol. Syst. Des. Eng. 2023, 8, 1319–1326. doi:10.1039/d3me00083d |
| 5. | Monkman, A. ACS Appl. Mater. Interfaces 2022, 14, 20463–20467. doi:10.1021/acsami.1c09189 |
| 6. | Yu, L.; Portale, G.; Stingelin, N. J. Mater. Chem. C 2021, 9, 10547–10556. doi:10.1039/d1tc01418h |
| 7. | Liu, X.; Lin, R.; Chen, H.; Zhang, S.; Qian, Z.; Zhou, G.; Chen, X.; Zhou, X.; Zheng, L.; Liu, R.; Tian, P. ACS Photonics 2019, 6, 3186–3195. doi:10.1021/acsphotonics.9b00799 |
| 8. | Oyama, Y.; Mamada, M.; Shukla, A.; Moore, E. G.; Lo, S.-C.; Namdas, E. B.; Adachi, C. ACS Mater. Lett. 2020, 2, 161–167. doi:10.1021/acsmaterialslett.9b00536 |
| 38. | Suerkan, A.; Isci, R.; Ozturk, T.; Yagci, Y. Mol. Syst. Des. Eng. 2023, 8, 1319–1326. doi:10.1039/d3me00083d |
| 31. | Isci, R.; Baysak, E.; Kesan, G.; Minofar, B.; Eroglu, M. S.; Duygulu, O.; Gorkem, S. F.; Ozturk, T. Nanoscale 2022, 14, 16602–16610. doi:10.1039/d2nr04582f |
| 12. | Wang, J.; Liu, K.; Ma, L.; Zhan, X. Chem. Rev. 2016, 116, 14675–14725. doi:10.1021/acs.chemrev.6b00432 |
| 35. | Farokhi, A.; Shahroosvand, H.; Monache, G. D.; Pilkington, M.; Nazeeruddin, M. K. Chem. Soc. Rev. 2022, 51, 5974–6064. doi:10.1039/d1cs01157j |
| 29. | Isci, R.; Balkan, T.; Tafazoli, S.; Sütay, B.; Eroglu, M. S.; Ozturk, T. ACS Appl. Energy Mater. 2022, 5, 13284–13292. doi:10.1021/acsaem.2c01830 |
| 30. | Topal, S.; Isci, R.; Sezer, E.; Ozturk, T.; Ustamehmetoglu, B. Electrochim. Acta 2021, 389, 138688. doi:10.1016/j.electacta.2021.138688 |
| 36. | Turkoglu, G.; Cinar, M. E.; Ozturk, T. Eur. J. Org. Chem. 2017, 4552–4561. doi:10.1002/ejoc.201700679 |
| 37. | Zhou, G.; Baumgarten, M.; Müllen, K. J. Am. Chem. Soc. 2008, 130, 12477–12484. doi:10.1021/ja803627x |
| 26. | Ozturk, S. E.; Isci, R.; Faraji, S.; Sütay, B.; Majewski, L. A.; Ozturk, T. Eur. Polym. J. 2023, 191, 112028. doi:10.1016/j.eurpolymj.2023.112028 |
| 27. | Amna, B.; Isci, R.; Siddiqi, H. M.; Majewski, L. A.; Faraji, S.; Ozturk, T. J. Mater. Chem. C 2022, 10, 8254–8265. doi:10.1039/d2tc01222g |
| 28. | Zhong, H.; Li, C.-Z.; Carpenter, J.; Ade, H.; Jen, A. K.-Y. J. Am. Chem. Soc. 2015, 137, 7616–7619. doi:10.1021/jacs.5b04209 |
| 24. | Isci, R.; Unal, M.; Yesil, T.; Ekici, A.; Sütay, B.; Zafer, C.; Ozturk, T. Front. Mater. 2023, 10, 1125462. doi:10.3389/fmats.2023.1125462 |
| 25. | Chi, W.-J.; Zheng, D.-Y.; Chen, X.-F.; Li, Z.-S. J. Mater. Chem. C 2017, 5, 10055–10060. doi:10.1039/c7tc03232c |
| 32. | Celiker, T.; Isci, R.; Kaya, K.; Ozturk, T.; Yagci, Y. J. Polym. Sci. (Hoboken, NJ, U. S.) 2020, 58, 2327–2334. doi:10.1002/pol.20200398 |
| 33. | Kutahya, C.; Allushi, A.; Isci, R.; Kreutzer, J.; Ozturk, T.; Yilmaz, G.; Yagci, Y. Macromolecules 2017, 50, 6903–6910. doi:10.1021/acs.macromol.7b01335 |
| 34. | Beyazit, S.; Aydogan, B.; Osken, I.; Ozturk, T.; Yagci, Y. Polym. Chem. 2011, 2, 1185–1189. doi:10.1039/c1py00019e |
| 39. | Sonar, P.; Soh, M. S.; Cheng, Y. H.; Henssler, J. T.; Sellinger, A. Org. Lett. 2010, 12, 3292–3295. doi:10.1021/ol1007179 |
| 40. | Lee, H.; Karthik, D.; Lampande, R.; Ryu, J. H.; Kwon, J. H. Front. Chem. (Lausanne, Switz.) 2020, 8, 373. doi:10.3389/fchem.2020.00373 |
| 41. | Xu, T.; Liang, X.; Xie, G. Front. Chem. (Lausanne, Switz.) 2021, 9, 691172. doi:10.3389/fchem.2021.691172 |
| 42. | Fu, Q.; Chen, J.; Shi, C.; Ma, D. ACS Appl. Mater. Interfaces 2012, 4, 6579–6586. doi:10.1021/am301703a |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 20. | Isci, R.; Tekin, E.; Kaya, K.; Piravadili Mucur, S.; Gorkem, S. F.; Ozturk, T. J. Mater. Chem. C 2020, 8, 7908–7915. doi:10.1039/d0tc01715a |
| 21. | Isci, R.; Tekin, E.; Mucur, S. P.; Ozturk, T. ChemistrySelect 2020, 5, 13091–13098. doi:10.1002/slct.202003273 |
| 22. | Isci, R.; Wan, L.; Topal, S.; Gunturkun, D.; Campbell, A. J.; Ozturk, T. J. Mater. Chem. C 2022, 10, 10719–10727. doi:10.1039/d2tc02371g |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 44. | Gunturkun, D.; Isci, R.; Faraji, S.; Sütay, B.; Majewski, L. A.; Ozturk, T. J. Mater. Chem. C 2023, 11, 13129–13141. doi:10.1039/d3tc02109b |
| 45. | Capan, A.; Ozturk, T. Synth. Met. 2014, 188, 100–103. doi:10.1016/j.synthmet.2013.11.018 |
| 46. | Turksoy, F.; Wallis, J. D.; Tunca, U.; Ozturk, T. Tetrahedron 2003, 59, 8107–8116. doi:10.1016/j.tet.2003.08.042 |
| 47. | Işçi, R.; Öztürk, T. Turk. J. Chem. 2023, 47, 1239–1248. doi:10.55730/1300-0527.3608 |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
| 43. | Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, 2016. |
| 23. | Turkoglu, G.; Cinar, M. E.; Buyruk, A.; Tekin, E.; Mucur, S. P.; Kaya, K.; Ozturk, T. J. Mater. Chem. C 2016, 4, 6045–6053. doi:10.1039/c6tc01285j |
© 2023 Isci and Ozturk; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.