Abstract
Catalysis is dominated by the use of rare and potentially toxic transition metals. The main group offers a potentially sustainable alternative for catalysis, due to the generally higher abundance and lower toxicity of these elements. Group 13 elements have a rich catalogue of stoichiometric addition reactions to unsaturated bonds but cannot undergo the redox chemistry which underpins transition-metal catalysis. Group 13 exchange reactions transfer one or more groups from one group 13 element to another, through σ-bond metathesis; where boron is both of the group 13 elements, this is termed transborylation. These redox-neutral processes are increasingly being used to render traditionally stoichiometric group 13-mediated processes catalytic and develop new catalytic processes, examples of which are the focus of this review.
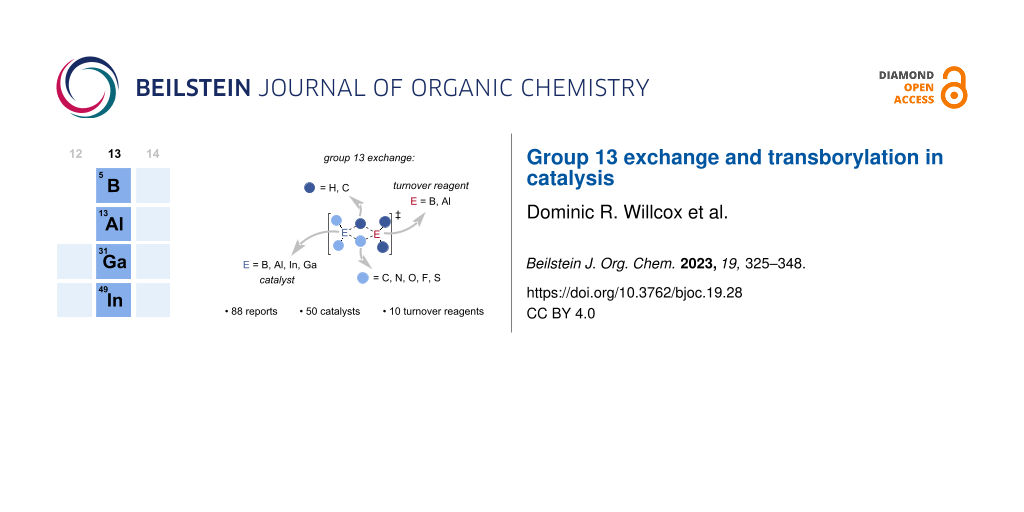
Graphical Abstract
Introduction
Group 13 compounds have found widespread use in stoichiometric organic transformations, typically in the functionalisation of unsaturated bonds [1-5], and, more recently, frustrated Lewis pair (FLP) chemistries including small molecule activations and C–H insertion reactions [6-10]. Group 13 exchange is the transfer of one or more substituents from one group 13 element to another group 13 element by σ-bond metathesis, a redox neutral process (Scheme 1). Stoichiometric group 13 exchange reactions are key to the synthesis of group 13 reagents including in organoboron chemistry [11-28], and more recently with aluminium [29-39], gallium [36,40-44], and indium [36,45] reagents being used for the preparation of group 13 reagents. Group 13 exchange has recently been used to enable catalytic turnover in traditionally stoichiometric reactions, expanding the use of group 13 compounds in catalysis beyond their typical use as Lewis acids [46]. This strategy has allowed the synthesis of bench-stable boronic ester products, rather than sensitive alkylboranes, and enabled the use of substoichiometric amounts of enantioenriched boron reagents, which can be challenging to prepare. This review will explore the use of group 13 exchange reactions as a general method for catalytic turnover, and serves to expand on the previously published review on transborylation-enabled boron catalysis to include a broader range of catalysts and turnover reagents [47].
Review
Boron catalysis
The borane-catalysed hydroboration of alkynes was first reported by Periasamy using N,N-diethylaniline·BH3 as the catalyst and HBcat as the turnover reagent (terminal reductant) [48,49]. This was followed by Hoshi who used dialkylboranes, 9-borabicyclo(3.3.1)nonane (H-B-9-BBN) and dicyclohexylborane (Cy2BH) to catalyse the hydroboration of alkynes with HBcat [50]. Hoshi later reported that Cy2BH [51] and in situ generated bis(pentafluorophenyl)borane, Piers’ borane [52], catalysed the hydroboration of alkynes with HBpin, to give alkenyl pinacol boronic esters. Tris(2,4,6-trifluorophenyl)borane [53], tris(3,4,5-trifluorophenyl)borane [54], BH3 [55-57], and H-B-9-BBN [58] have also been reported as catalysts for the hydroboration of alkynes with HBpin (Scheme 2). Lloyd-Jones et al. investigated the mechanism of this reaction and found transborylation, group 13 exchange between boron atoms, enabled catalytic turnover [58]. The alkyne 1 and dialkylborane reacted to give an alkenylborane 2. Transborylation with HBpin gave the alkenyl boronic ester 3 and regenerated the catalyst, HBR2. Isotopic labelling (H10Bpin) confirmed B–C(sp2)/B–H transborylation proceeded by σ-bond metathesis, and not ligand exchange. Using kinetic and computational analyses, the B‒C(sp2)/B‒H transborylation transition state was determined to have a free energy barrier of approximately 20 kcal mol−1 (ΔG‡calc = 19.7 kcal mol−1; ΔG‡exp = 20.3 kcal mol−1) (Scheme 2).
Scheme 2: Borane-catalysed hydroboration of alkynes and the proposed mechanism.
Scheme 2: Borane-catalysed hydroboration of alkynes and the proposed mechanism.
The borane-catalysed hydroboration of alkenes has been less explored, with tris[3,5-bis(trifluoromethyl)phenyl]borane [59], tris(3,4,5-trifluorophenyl)borane [54], and BH3 [55,56] found to be competent catalysts of this transformation (Scheme 3a). The mechanism was proposed to be analogous to that of borane-catalysed alkyne hydroboration; alkene 4 hydroboration, followed by transborylation with HBpin to give the alkylboronic ester 6 and regenerate the catalyst (Scheme 3a). Thomas also reported that alkynes undergo double hydroboration using H-B-9-BBN as the catalyst with HBpin to give gem-diborylalkanes 8 (Scheme 3b), and this was proposed to occur through transborylation, with an experimentally determined free energy barrier of 28 kcal mol−1 for the second transborylation reaction (Scheme 3b) [60].
Scheme 3: a) Borane-catalysed hydroboration of alkenes and the proposed mechanism. b) H-B-9-BBN-catalysed double hydroboration of alkynes and the proposed mechanism.
Scheme 3: a) Borane-catalysed hydroboration of alkenes and the proposed mechanism. b) H-B-9-BBN-catalysed dou...
The seminal work from Fontaine reported that [1-(N-2,2,6,6-tetramethylpiperidinyl)-2-BH2-C6H4]2 catalysed the C–H borylation of heterocycles with HBpin [61], the first example of a catalytic frustrated Lewis pair (FLP)-mediated C‒H functionalisation (Scheme 4a). Using computational analysis, the mechanism of the reaction was proposed to occur by borane dimer [9]2 dissociation, followed by a concerted deprotonation of the heterocycle 10 to give a zwitterionic intermediate 11. The zwitterion then loses dihydrogen to give a neutral borane 12, followed by B–C(sp2)/B–H transborylation with HBpin (ΔG‡ = 14.7 kcal mol−1) to give the borylated arene 13 and regenerate the catalyst (Scheme 4a). Fontaine showed that the steric bulk of the Lewis base had a significant effect on the rate of the reaction; changing the 2,2,6,6-tetramethylpiperidinyl group for a piperidinyl gave a large rate enhancement [62]. Fontaine also showed that bench-stable salts [1-(NR2)-2-BF2Y-C6H4]H (NR2 = 2,2,6,6-tetramethylpiperidinyl, pyridinyl, diethylamino, dimethylamino; Y = F, OMe, OH) could be used as precatalysts for the C‒H borylation, with an initial B‒Y/B‒H transborylation activating the precatalyst [62-65]. Zhang showed that benzoic acid decomposed HBpin to BH3 in situ to catalyse the C2‒H borylation of indoles (Scheme 4b) [66,67].
Scheme 4: a) Amine-borane-catalysed C‒H borylation of heterocycles and the proposed mechanism. b) Benzoic acid-promoted C‒H borylation of N-heterocycles and the proposed mechanism, where the active catalyst BH3 was formed in situ from HBpin decomposition.
Scheme 4: a) Amine-borane-catalysed C‒H borylation of heterocycles and the proposed mechanism. b) Benzoic aci...
Gellrich reported the bis(pentafluorophenyl)borane-catalysed dimerisation of allenes, using various boronates as the terminal reductant (Scheme 5) [68]. Experimental and computational studies suggested the reaction proceeded by hydroboration of the allene 14 by bis(pentafluorophenyl)borane to give an allylborane 15, which underwent allylation of a second equivalent of the allene 14, giving a boryl diene 16. A Cope rearrangement of the boryl diene 16 followed by transborylation gave the dienyl boronic ester 18 and regenerated the catalyst (Scheme 5).
Scheme 5: Bis(pentafluorophenyl)borane-catalysed dimerisation of allenes and the proposed mechanism.
Scheme 5: Bis(pentafluorophenyl)borane-catalysed dimerisation of allenes and the proposed mechanism.
Chang reported the alkoxide-promoted hydroboration of N-heteroarenes with HBpin, the first explicit example of a B‒N/B‒H transborylation in catalysis (Scheme 6) [69]. Reactive intermediates were characterised and BH3 was observed to be generated in situ by the decomposition of HBpin. The proposed catalytic cycle involved nucleophile-promoted decomposition of HBpin to various borohydride species 19, which reacted with the BH3-coordinated heterocycle 21. Hydride transfer from the BH3-amide 22 to HBpin regenerated the borohydride catalyst 19, and gave a neutral aminoborane 23, which then underwent B‒N/B‒H transborylation with HBpin to give the N‒Bpin dihydropyridine 24 and BH3 (Scheme 6).
Scheme 6: Alkoxide-promoted hydroboration of heterocycles and the proposed mechanism.
Scheme 6: Alkoxide-promoted hydroboration of heterocycles and the proposed mechanism.
The mechanism of stoichiometric indole reduction with Me2S·BH3 was investigated by Fontaine, and applied to a catalytic variant using HBpin as the turnover reagent (Scheme 7) [70]. Computational analysis showed two plausible, cooperative catalytic cycles: 1) hydroboration of indole 25 with BH3 to give a H2B-N-indoline 26, which then underwent B‒N/B‒H transborylation with HBpin to regenerate BH3 and give the N-Bpin-indoline product 27; 2) two molecules of H2B-N-indoline underwent rearrangement to regenerate BH3 and gave a bisindolinylborane 28. The bis-N-indolinylborane then underwent B‒N/B‒H transborylation with HBpin to regenerate H2B-N-indoline and gave the Bpin-N-indoline product 27; this was suggested as the major pathway (Scheme 7).
Scheme 7: Borane-catalysed reduction of indoles and the proposed mechanism.
Scheme 7: Borane-catalysed reduction of indoles and the proposed mechanism.
Thomas et al. reported the H-B-9-BBN-catalysed reductive cyanation of enones with HBpin and N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) (Scheme 8) [71]. The reaction was proposed to proceed by 1,4-hydroboration of the enone 29 with H-B-9-BBN to give an O-B-9-BBN enolate 30. Electrophilic cyanation of the enolate 30 with NCTS 31, and elimination gave the β-ketonitrile 33 and TsN(Ph)-9-B-BBN 34, which underwent B‒N/B‒H transborylation with HBpin to regenerate the catalyst and give TsN(Ph)-Bpin 35 (Scheme 8).
Scheme 8: H-B-9-BBN-catalysed hydrocyanation of enones and the proposed mechanism.
Scheme 8: H-B-9-BBN-catalysed hydrocyanation of enones and the proposed mechanism.
Thomas and Gunanathan independently reported the borane-catalysed double hydroboration of nitriles using either Me2S·BH3 or H-B-9-BBN, respectively, as the catalyst and HBpin as the turnover reagent (Scheme 9) [72,73]. Both reports proposed similar mechanisms for the Me2S·BH3- and H-B-9-BBN-catalysed pathways (Scheme 9), where two pathways were operative. The hydroboration of the nitrile 36 gave a borylimine 37, which underwent a second hydroboration with the borane catalyst to give a diborylamine 38. Double B‒N/B‒H transborylation of the diborylamine 38 with HBpin regenerated the catalyst and gave the diborylamine 40 product. Alternatively, borylimine 37 underwent a formal B‒N/B‒H transborylation to give a borane-coordinated borylimine-Bpin complex 41, which underwent hydroboration to give a mixed diborylamine 39, followed by B‒N/B‒H transborylation to give the diborylamine 40.
Scheme 9: Borane-catalysed hydroboration of nitriles and the proposed mechanism.
Scheme 9: Borane-catalysed hydroboration of nitriles and the proposed mechanism.
The first explicit example of a B‒O/B‒H transborylation in catalysis was the catalytic Midland reduction of propargylic ketones developed by Thomas to give enantioenriched propargylic alcohols (Scheme 10) [74]. The reaction was proposed to occur by enantioselective reduction of the propargylic ketone 42 by myrtanyl borane 43 to give an enantioenriched borinic ester 44 and β-pinene 45. The borinic ester 44 underwent B‒O/B‒H transborylation (ΔG‡exp = 22.7 kcal mol−1) with HBpin giving H-B-9-BBN and the alkoxy boronic ester 46. Hydroboration of β-pinene 45 by H-B-9-BBN regenerated the myrtanylborane 43 catalyst. Reaction of H10Bpin with the borinic ester intermediate 44 showed no scrambling of the 10B label, suggesting a σ-bond metathesis mechanism for this transborylation reaction (Scheme 10).
Scheme 10: Myrtanylborane-catalysed asymmetric reduction of propargylic ketones and the proposed mechanism.
Scheme 10: Myrtanylborane-catalysed asymmetric reduction of propargylic ketones and the proposed mechanism.
Thomas et al. reported the H-B-9-BBN-catalysed esterification of alkyl fluorides, using carboxylic acids and HBpin (Scheme 11) [75]. Through a series of single-turnover experiments a reaction mechanism was proposed where H-B-9-BBN catalysed the dehydrocoupling of carboxylic acids 47 with HBpin through B‒O/B‒H transborylation, to give the acyloxy boronic ester 49. This underwent direct defluoronative carboxylation with the alkyl fluoride 50 to give the ester 51 and FBpin (Scheme 11).
Scheme 11: H-B-9-BBN-catalysed C–F esterification of alkyl fluorides and the proposed mechanism.
Scheme 11: H-B-9-BBN-catalysed C–F esterification of alkyl fluorides and the proposed mechanism.
The H-B-9-BBN-catalysed 1,4-hydroboration of enones with HBpin was shown by Thomas, including the subsequent functionalisation of the intermediate Bpin-enolate 52 (Scheme 12) [76]. The proposed mechanism began by 1,4-hydroboration of the enone 29 with H-B-9-BBN, followed by B‒O/B‒H transborylation with HBpin to give the Bpin-enolate 52 and regenerate H-B-9-BBN (Scheme 12). Isotopic labelling with DBpin and H10Bpin supported this proposal.
Scheme 12: H-B-9-BBN-catalysed 1,4-hydroboration of enones and the proposed mechanism.
Scheme 12: H-B-9-BBN-catalysed 1,4-hydroboration of enones and the proposed mechanism.
Fontaine reported that boric acid could be used as a precatalyst for the BH3-catalysed hydroboration of esters, lactones, and carbonates with HBpin under microwave irradiation (Scheme 13) [57]. When HBpin and boric acid were reacted together, BH3-coordinated HBpin and O(Bpin)2 were detected by 11B NMR spectroscopy. Supported by computational analysis and single-turnover experiments, the reaction was proposed to occur by hydroboration of the carbonyl compound 53 with BH3, followed by B‒O/B‒H transborylation with HBpin (ΔG‡ = 24.5 kcal mol−1), to give the alkoxy boronic ester 56 (Scheme 13).
Scheme 13: Boric acid-promoted reduction of esters, lactones, and carbonates and the proposed mechanism.
Scheme 13: Boric acid-promoted reduction of esters, lactones, and carbonates and the proposed mechanism.
Nicholson, Thomas and co-workers reported the H-B-9-BBN-catalysed diastereoselective reductive aldol-type reaction of enones and esters or lactones (Scheme 14) [77]. Through a series of single-turnover reactions, a mechanism was proposed (Scheme 14): H-B-9-BBN underwent 1,4-hydroboration with the enone 29, followed by B‒O/B‒H transborylation with HBpin to give an O-Bpin enolate 52 and regenerate H-B-9-BBN. Alongside this, H-B-9-BBN underwent reduction of the ester or lactone 57, to give a hemiacetal intermediate 58, which underwent B‒O/B‒H transborylation with HBpin to give an O-Bpin hemiacetal 59. Borane-mediated collapse of the O-Bpin hemiacetal gave an aldehyde 60 which reacted with the O-Bpin enolate 52 to give aldol-type products 61.
Scheme 14: H-B-9-BBN-catalysed reductive aldol-type reaction and the proposed mechanism.
Scheme 14: H-B-9-BBN-catalysed reductive aldol-type reaction and the proposed mechanism.
Thomas reported the borane-catalysed diastereo- and enantioselective allylation of ketones with allenes and HBpin to give diastereo- and enantioenriched allylic alcohols, after workup (Scheme 15) [78]. The mechanism was investigated by single-turnover experiments and isotopic labelling and proposed to proceed by hydroboration of the allene 62 by the borane catalyst (H-B-9-BBN or 10-phenyl-9-borabicyclo[3.3.2]decane [Ph-BBD]) followed by rapid isomerisation from the (Z)-63 to (E)-allylborane 64 which underwent allylation of the ketone 65 to give an allylic borinic ester 66. B‒O/B‒H transborylation with HBpin gave the O-Bpin-protected allylic alcohol 67 and regenerated the borane catalyst (Scheme 15).
Scheme 15: H-B-9-BBN-catalysed diastereoselective allylation of ketones and the Ph-BBD-catalysed enantioselective allylation of ketones and the proposed mechanism.
Scheme 15: H-B-9-BBN-catalysed diastereoselective allylation of ketones and the Ph-BBD-catalysed enantioselect...
The only example of a B‒F/B‒H transborylation in catalysis comes from Willcox, Thomas and co-workers in the H-B-9-BBN-catalysed arylation of alkyl fluorides with HBpin (Scheme 16) [75]. The reaction was proposed to occur through activation of the alkyl fluoride 68 with H-B-9-BBN, followed by electrophilic substitution of the arene 69 to give a Wheland intermediate and a fluoroborohydride 70 (Scheme 16). Loss of H2 gave the arylated product 71, dihydrogen, and F-B-9-BBN 72, which underwent B‒F/B‒H transborylation with HBpin, to give FBpin and regenerate H-B-9-BBN. The mechanism was confirmed through single-turnover experiments and computational analysis.
Scheme 16: H-B-9-BBN-catalysed C–F arylation of benzyl fluorides and the proposed mechanism.
Scheme 16: H-B-9-BBN-catalysed C–F arylation of benzyl fluorides and the proposed mechanism.
B‒S/B‒H Transborylation in catalysis is also limited to a single example in Fontaine’s FLP-catalysed S‒H borylation of thiols with HBpin (Scheme 17) [79]. Through computational analysis, a mechanism was proposed whereby the ambiphilic amine-borane 73 underwent concerted addition to the thiol 74 S–H bond, to give a zwitterion 75. After loss of H2, a neutral thioborane 76 was generated, which underwent B‒S/B‒H transborylation with HBpin, to give the thioBpin 77 product and regenerate the amine-borane catalyst 73 (Scheme 17).
Scheme 17: Borane-catalysed S‒H borylation of thiols and the proposed mechanism.
Scheme 17: Borane-catalysed S‒H borylation of thiols and the proposed mechanism.
Yamamoto reported the borane-catalysed hydroalumination of alkenes and allenes (Scheme 18) [80-83] in which the organoaluminium products were reacted in situ with various electrophiles to give formal hydrofunctionalisation products (Scheme 18) [80-83]. Although no mechanism was proposed, a rare B‒C/Al‒H exchange may be responsible for catalytic turnover.
Scheme 18: Borane-catalysed hydroalumination of alkenes and allenes.
Scheme 18: Borane-catalysed hydroalumination of alkenes and allenes.
Aluminium catalysis
The aluminium-catalysed hydroboration of alkynes with HBpin has been well studied with a variety of aluminium complexes having been shown to be catalytically active (Scheme 19a) [84-93]. Roesky reported the first example, using an N,N′-bis-2,6-diisopropylphenyl diketiminate (NacNac)-supported aluminium dihydride complex as the catalyst [84]. Through computational analysis, the mechanism was proposed to occur by dehydrocoupling between the aluminium dihydride and the alkyne 1 to give an alkynylaluminium species 78. Direct hydroboration of the alkynyl aluminium species by HBpin gave a gem-aluminyl-boryl-alkene 80 which underwent selective protodemetallation with another molecule of alkyne 1 to give the alkenylboronic ester 3 and regenerate an alkynylaluminium species 78 (Scheme 19b). Thomas et al. proposed a different mechanism for the diisobutylaluminium hydride (DIBALH)- or Et3Al·DABCO-catalysed hydroboration of alkynes [86], whereby an aluminium hydride 81 underwent hydroalumination of the alkyne 1, followed by Al‒C/B‒H exchange with HBpin, to give the alkenylboronic ester 3 and regenerate the aluminium hydride 81 (Scheme 19c). Single-turnover experiments and a lack of observable H2 production supported this hypothesis. It should also be noted that nucleophilic bases, including LiAlH4, promoted the decomposition of HBpin to BH3 which can mediate hidden catalysis [56].
Scheme 19: a) Aluminium-catalysed hydroboration of alkynes and example catalysts. b) Deprotonation mechanistic proposal. c) Hydroalumination mechanistic proposal.
Scheme 19: a) Aluminium-catalysed hydroboration of alkynes and example catalysts. b) Deprotonation mechanistic...
Thomas et al. reported the aluminium-catalysed hydroboration of alkenes, using HBpin and LiAlH4 as the catalyst (Scheme 20) [94]. Through single-turnover experiments they suggested a mechanism similar to aluminium-catalysed alkyne hydroboration; hydroalumination of the alkene 4 by the alane catalyst 80, Al‒C/B‒H exchange with HBpin, to give the alkylboronic ester 6 and regenerate the alane catalyst 80. A hydride-mediated decomposition of HBpin and hidden catalysis were not ruled out, as the use of LiH or NaH in place of LiAlH4 gave moderate yields of the hydroboration product, however, comparison of the rates of reaction showed the aluminium had an active catalytic role (Scheme 20) [56]. Shi et al. reported that triethylaluminium catalysed the hydroboration of alkenes, under similar conditions to those of Thomas et al. [91]. Other ligand frameworks and aluminate species have shown competence for aluminium-catalysed alkene hydroboration [92,95], with Panda reporting the only reaction which proceeded at room temperature reaction using [κ2-{Ph2P(Se)NCH2(C5H4N)}Al(CH3)2] as the catalyst [90].
Scheme 20: Aluminium-catalysed hydroboration of alkenes and the proposed mechanism.
Scheme 20: Aluminium-catalysed hydroboration of alkenes and the proposed mechanism.
Using an ambiphilic aluminium precatalyst, (Me2N)C6H4AlMe2, Thomas et al. were able to shut down hydroalumination by the alane and catalyse the C–H borylation of terminal alkynes with HBpin (Scheme 21) [96]. Through kinetic analysis, it was found that the rate of the alkynyl-Bpin product formation was fastest during catalyst activation, rather than during catalysis, leading to an in-depth investigation of catalyst activation using variable time normalisation analysis (VTNA) and kinetic isotope effects. A catalytic cycle was proposed in which (Me2N)C6H4AlH2 83 underwent deprotonation of the alkyne 1 to give a zwitterion 84. Loss of dihydrogen gave an alkynylaluminium species 85 which underwent Al‒C/B‒H exchange with HBpin to give an alkynylboronic ester 86 and regenerate the catalyst 83 (Scheme 21).
Scheme 21: Aluminium-catalysed C–H borylation of terminal alkynes and the proposed mechanism.
Scheme 21: Aluminium-catalysed C–H borylation of terminal alkynes and the proposed mechanism.
Roesky reported the first example of Al‒N/B‒H exchange in catalysis: a NacNac-supported aluminium dihydride-catalysed the dehydrocoupling of HBpin or H-B-9-BBN with primary and secondary amines (Scheme 22) [84]. The reaction was proposed to proceed by double dehydrocoupling of the amine 87 and aluminium dihydride 88 to give a bisamido aluminium species 89 which underwent Al‒N/B‒H exchange with HBpin to give the borylated amide 90 and regenerate the aluminium hydride 88 (Scheme 22). This method was also applied to the dehydrocoupling of alcohols and thiols, with this being the only example of an Al‒S/B‒H exchange in catalysis.
Scheme 22: Aluminium-catalysed dehydrocoupling of amines, alcohols, and thiols with H-B-9-BBN or HBpin and the proposed mechanism.
Scheme 22: Aluminium-catalysed dehydrocoupling of amines, alcohols, and thiols with H-B-9-BBN or HBpin and the...
A number of aluminium hydride species has been used for the catalytic hydroboration of imines [87,92,97], nitriles [92,98-101], carbodiimides [92,100,102], pyridine [92], and isocyanides [92] with HBpin (Scheme 23). These generally follow a similar proposed catalytic cycle; aluminium-mediated reduction, followed by Al‒N/B‒H exchange with HBpin (Scheme 23).
Scheme 23: Aluminium-catalysed hydroboration of unsaturated compounds and the general reaction mechanism.
Scheme 23: Aluminium-catalysed hydroboration of unsaturated compounds and the general reaction mechanism.
The first example of Al‒O/B‒H exchange in catalysis was reported by Woodward, in the enantioselective catalytic hydroboration of ketones with HBcat as the terminal reductant (Scheme 23) [103]. A mixture of 1,1′-bi-2-naphthol (BINOL), 1,1'-binaphthalene-2,2'-dithiol (DTBH2), or 2-hydroxy-2'-mercapto-1,1'-binaphthyl (MTBH2) with LiAlH4 as the catalyst gave good yields of the alcohol (70–80%) after workup, but in low enantioselectivities (1–6% ee). A mechanism was proposed whereby reduction of the ketone 91 by the aluminium hydride 92 was followed by Al‒O/B‒H exchange with HBcat (Scheme 23).
Roesky reported the aluminium-catalysed reduction of aldehydes and ketones with HBpin, using a NacNac-supported aluminium hydride catalyst (Scheme 23) [104]. Using computational analysis, the reaction was proposed to proceed through reduction of the carbonyl 91 by the aluminium hydride 92, to give an alkoxy aluminium species 93, followed by Al‒O/B‒H exchange with HBpin to give the alkoxy boronic ester 94 and regenerate the aluminium hydride 92 (Scheme 23). Several aluminium hydride compounds have been reported as competent carbonyl hydroboration catalysts, with proposed mechanisms similar to Roesky’s initial report [85,88,89,97,101,105-109]. The aluminium-catalysed hydroboration of CO2 was reported by Fontaine using tris(triphenylphosphine)aluminium as the catalyst and HBcat as the terminal reductant [110]. So et al. reported the bis(phosphoranyl)methanido aluminium hydride-catalysed reduction of CO2 with Me2S·BH3 as the terminal reductant [101].
Gallium catalysis
Pioneering studies by Woodward reported the enantioselective reduction of ketones using HBcat and a mixture of MTBH2/LiGaH4 as the catalyst, achieving high yields (up to 96%) and enantioselectivities (up to 93% ee) (Scheme 24a) [111]. The reaction was proposed to proceed through the enantioselective reduction of the ketone 95 by gallium hydride 96, followed by Ga‒O/B‒H exchange with HBcat to give an enantioenriched alkoxy catechol borane 98, affording the alcohol after workup (Scheme 24a). The mechanism was later explored in more detail, and the scope expanded, suggesting the reaction proceeded in a similar manner to the Corey–Bakshi–Shibata (CBS) reduction [112], whereby the gallium complex acts as an ambiphilic species coordinated to a ketone, activating it towards reaction with pre-coordinated HBcat [103].
Scheme 24: a) Gallium-catalysed asymmetric hydroboration of ketones and the proposed mechanism. b) Gallium-catalysed hydroboration of CO2. c) Gallium-catalysed hydroboration of ketones and imines.
Scheme 24: a) Gallium-catalysed asymmetric hydroboration of ketones and the proposed mechanism. b) Gallium-cat...
Aldrich expanded the use of gallium in reductive catalysis by showing that a NacNac-supported gallium hydride catalysed the hydroboration of CO2 with HBpin to give MeOBpin and O(Bpin)2 (Scheme 24b) [113]. Through single-turnover experiments, the gallium hydride was observed to reduce CO2 giving a gallium formate complex, which underwent Ga‒O/B‒H exchange with HBpin to afford O-Bpin formate and regenerate the gallium hydride. The analogous NacNac-supported aluminium complex was not catalytically competent for the hydroboration of CO2, which was rationalised by the unfavourable thermodynamics of the analogous Al‒O/B‒H exchange [114]. Hevia reported a combination of a tris(alkyl)gallium species and bulky N-heterocyclic carbene acted as an FLP for B‒H insertion, and was used subsequently as a catalyst in the hydroboration of ketones, aldehydes, esters, and imines with HBpin [115]. Using an ONO-pincer-supported gallium hydride, Goicoechea showed the catalytic hydroboration of ketones and CO2 with HBpin. This was also proposed to proceed by carbonyl reduction and Ga‒O/B‒H exchange (Scheme 24c) [116].
Schneider has shown that a mixture of Ga0, AgOTf, and 18-crown-6 catalysed the allylation of acetals, ketals, or aminals with allylic or allenylboronic esters (Scheme 25) [117]. The reaction was proposed to proceed by an activation of elemental gallium to a GaI species [(18-crown-6)-GaI·(dioxane)nOTf] 99, which abstracted methoxide from the acetal 100, to give an oxocarbenium 101 and GaIOMe 102. The gallium(I) methoxide (102) underwent Ga‒O/B‒C exchange with allyl-Bpin 103 to give MeOBpin and an allylic gallium(I) species 104, which reacted with the oxocarbenium 103 to give the allylic ether 105 and regenerate the GaI catalyst 99 (Scheme 25). Using allenylBpin, the selective propargylation of acetals was also achieved. When AgOTf was replaced with silver (R)-BINOL phosphate, the asymmetric allylation proceeded in a moderate yield (60%) and enantioselectivity (40% ee). The structure of the ‘GaIOTf’ species was explored in more detail by Slattery, and a monovalent [GaI(18-crown-6)OTf] complex was isolated and characterised by X-ray crystallography, lending support to the mechanism proposed by Schneider [118].
Scheme 25: Gallium(I)-catalysed allylation/propargylation of acetals and aminals and the proposed mechanism.
Scheme 25: Gallium(I)-catalysed allylation/propargylation of acetals and aminals and the proposed mechanism.
Indium catalysis
Examples of group 13 exchange are limited with indium, even stoichiometrically [36,45], however Kobayashi demonstrated the InI-catalysed addition of allylic and allenylboranes to ketals, acetals, aminals, and alkyl ethers (Scheme 26) [119-121]. The proposed mechanism was analogous to the GaI catalysis by Schneider, with an In‒O/B‒C exchange proposed to drive catalytic turnover.
Scheme 26: Indium(I)-catalysed allylation/propargylation of acetals, aminals, and alkyl ethers.
Scheme 26: Indium(I)-catalysed allylation/propargylation of acetals, aminals, and alkyl ethers.
Nakazawa reported an iron–indium cooperative catalytic system for the hydroboration of nitriles with HBpin and HBcat (Scheme 27) [122]. The precatalyst ([Fe(CH3CN)6][cis-Fe(CO)4(InCl3)2]) was activated in situ with HBpin to give ClBpin and HInCl2 107 by In‒Cl/B‒H exchange. The indium hydride 107 underwent hydroelementation of an iron-coordinated nitrile 108, to give an indylimine iron complex 109, which after In‒N/B‒H exchange with HBpin gave a borylimine iron complex 110. A second hydroelementation and In‒N/B‒H exchange gave the bisborylamine 113 and regenerated the HInCl2 107 co-catalyst (Scheme 27).
Scheme 27: Iron–indium cocatalysed double hydroboration of nitriles and the proposed mechanism.
Scheme 27: Iron–indium cocatalysed double hydroboration of nitriles and the proposed mechanism.
Conclusion
Increasing concerns over the sustainability and toxicity of many transition-metal catalysts has led synthetic chemists to seek alternative elements for catalysis. Group 13 compounds have been at the forefront of chemical research for the past century as organic reagents and functional handles. Group 13 exchange reactions have enabled these reagents to move beyond stoichiometric reactivity to be rendered catalytic, and exhibit catalysis outwith Lewis acid-type activation. These exchange reactions have allowed redox-neutral catalysis complementary to and beyond the redox catalysis of the transition metals.
Boron, aluminium, gallium, and indium have all been demonstrated in catalytic transformations using group 13 exchange from alkene functionalisation to carbonyl reduction. The subtle differences in reactivity of the group 13 catalysts were used to enable unique catalytic reactivity and/or reaction chemo- or stereoselectivity, including cases where the stoichiometric reaction was rendered catalytic and, more significantly, where no stoichiometric precedent was known. Group 13 exchange reactions being the driver for new chemical reactivity and unique molecular disconnections. This is not to say that all stoichiometric group 13 reactions have been rendered catalytic, or all new reactivity discovered, leaving an exciting future for main group catalysis underpinned by group 13 exchange and transborylation reactions (Figure 1).
Figure 1: a) The number of reports for a given group 13 exchange in catalysis. b) Average free energy barrier to group 13 exchange in catalysis (blank where no free energy value was reported).
Figure 1: a) The number of reports for a given group 13 exchange in catalysis. b) Average free energy barrier...
References
-
Negishi, E.-I.; Idacavage, M. J. Org. React. 1985, 33, 1–246. doi:10.1002/0471264180.or033.01
Return to citation in text: [1] -
Suzuki, A. Acc. Chem. Res. 1982, 15, 178–184. doi:10.1021/ar00078a003
Return to citation in text: [1] -
Fernández, E.; Whiting, A. Synthesis and Application of Organoboron Compounds; Topics in Organometallic Chemistry, Vol. 49; Springer International Publishing: Cham, Switzerland, 2015. doi:10.1007/978-3-319-13054-5
Return to citation in text: [1] -
Mole, T.; Jeffery, E. A. Organoaluminium Compounds; Elsevier: New York, NY, USA, 1972.
Return to citation in text: [1] -
Downs, A. J. Chemistry of Aluminium, Gallium, Indium and Thallium; Springer: Dordrecht, Netherlands, 1993.
Return to citation in text: [1] -
Fyfe, J. W. B.; Watson, A. J. B. Chem 2017, 3, 31–55. doi:10.1016/j.chempr.2017.05.008
Return to citation in text: [1] -
Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46–76. doi:10.1002/anie.200903708
Return to citation in text: [1] -
Stephan, D. W. J. Am. Chem. Soc. 2015, 137, 10018–10032. doi:10.1021/jacs.5b06794
Return to citation in text: [1] -
Jupp, A. R.; Stephan, D. W. Trends Chem. 2019, 1, 35–48. doi:10.1016/j.trechm.2019.01.006
Return to citation in text: [1] -
Li, N.; Zhang, W.-X. Chin. J. Chem. 2020, 38, 1360–1370. doi:10.1002/cjoc.202000027
Return to citation in text: [1] -
Schlesinger, H. I.; Burg, A. B. J. Am. Chem. Soc. 1931, 53, 4321–4332. doi:10.1021/ja01363a009
Return to citation in text: [1] -
Schlesinger, H. I.; Walker, A. O. J. Am. Chem. Soc. 1935, 57, 621–625. doi:10.1021/ja01307a009
Return to citation in text: [1] -
Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1970, 92, 6983–6984. doi:10.1021/ja00726a052
Return to citation in text: [1] -
Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1971, 93, 1816–1818. doi:10.1021/ja00736a061
Return to citation in text: [1] -
Wrackmeyer, B. J. Organomet. Chem. 1976, 117, 313–320. doi:10.1016/s0022-328x(00)87208-3
Return to citation in text: [1] -
Contreras, R.; Wrackmeyer, B. Spectrochim. Acta, Part A 1982, 38, 941–951. doi:10.1016/0584-8539(82)80119-0
Return to citation in text: [1] -
Männig, D.; Nöth, H. J. Chem. Soc., Dalton Trans. 1985, 1689–1692. doi:10.1039/dt9850001689
Return to citation in text: [1] -
Garrett, C. E.; Fu, G. C. J. Org. Chem. 1996, 61, 3224–3225. doi:10.1021/jo960386p
Return to citation in text: [1] -
Hoshi, M.; Shirakawa, K.; Arase, A. Chem. Commun. 1998, 1225–1226. doi:10.1039/a801939h
Return to citation in text: [1] -
Kanth, J. V. B.; Periasamy, M.; Brown, H. C. Org. Process Res. Dev. 2000, 4, 550–553. doi:10.1021/op000291w
Return to citation in text: [1] -
Schlesinger, H. I.; Horvitz, L.; Burg, A. B. J. Am. Chem. Soc. 1936, 58, 407–409. doi:10.1021/ja01294a007
Return to citation in text: [1] -
McCusker, P. A.; Ashby, E. C.; Makowski, H. S. J. Am. Chem. Soc. 1957, 79, 5179–5181. doi:10.1021/ja01576a026
Return to citation in text: [1] -
Hennion, G. F.; McCusker, P. A.; Ashby, E. C.; Rutkowski, A. J. J. Am. Chem. Soc. 1957, 79, 5190–5191. doi:10.1021/ja01576a030
Return to citation in text: [1] -
McCusker, P. A.; Hennion, G. F.; Ashby, E. C. J. Am. Chem. Soc. 1957, 79, 5192–5194. doi:10.1021/ja01576a031
Return to citation in text: [1] -
Hennion, G. F.; McCusker, P. A.; Ashby, E. C.; Rutkowski, A. J. J. Am. Chem. Soc. 1957, 79, 5194–5196. doi:10.1021/ja01576a032
Return to citation in text: [1] -
Brown, H. C.; Klender, G. J. Inorg. Chem. 1962, 1, 204–214. doi:10.1021/ic50002a003
Return to citation in text: [1] -
McCusker, P. A.; Bright, J. H. J. Org. Chem. 1964, 29, 2093–2094. doi:10.1021/jo01030a555
Return to citation in text: [1] -
Pasto, D. J.; Balasubramaniyan, V.; Wojtkowski, P. W. Inorg. Chem. 1969, 8, 594–598. doi:10.1021/ic50073a038
Return to citation in text: [1] -
Gamble, E. L.; Gilmont, P.; Stiff, J. F. J. Am. Chem. Soc. 1940, 62, 1257–1258. doi:10.1021/ja01862a078
Return to citation in text: [1] -
Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1971, 93, 1818–1819. doi:10.1021/ja00736a062
Return to citation in text: [1] -
Chen, J.; Chen, E. Y.-X. Dalton Trans. 2016, 45, 6105–6110. doi:10.1039/c5dt03895b
Return to citation in text: [1] -
Synoradzki, L.; Bolesławski, M.; Lewínski, J. J. Organomet. Chem. 1985, 284, 1–4. doi:10.1016/0022-328x(85)80006-1
Return to citation in text: [1] -
Uhl, W.; Layh, M. J. Organomet. Chem. 1991, 415, 181–190. doi:10.1016/0022-328x(91)80118-4
Return to citation in text: [1] -
Feulner, H.; Metzler, N.; Nöth, H. J. Organomet. Chem. 1995, 489, 51–62. doi:10.1016/0022-328x(94)05147-4
Return to citation in text: [1] -
Bochmann, M.; Sarsfield, M. J. Organometallics 1998, 17, 5908–5912. doi:10.1021/om980400j
Return to citation in text: [1] -
Anulewicz-Ostrowska, R.; Luliński, S.; Serwatowski, J. Inorg. Chem. 1999, 38, 3796–3800. doi:10.1021/ic981454o
Return to citation in text: [1] [2] [3] [4] -
Kim, J. S.; Wojcinski, L. M.; Liu, S.; Sworen, J. C.; Sen, A. J. Am. Chem. Soc. 2000, 122, 5668–5669. doi:10.1021/ja0010960
Return to citation in text: [1] -
Klosin, J.; Roof, G. R.; Chen, E. Y.-X.; Abboud, K. A. Organometallics 2000, 19, 4684–4686. doi:10.1021/om000573k
Return to citation in text: [1] -
McGuinness, D. S.; Rucklidge, A. J.; Tooze, R. P.; Slawin, A. M. Z. Organometallics 2007, 26, 2561–2569. doi:10.1021/om070029c
Return to citation in text: [1] -
Beachley, O. T., Jr.; Royster, T. L., Jr.; Arhar, J. R. J. Organomet. Chem. 1992, 434, 11–17. doi:10.1016/0022-328x(92)83348-l
Return to citation in text: [1] -
Elms, F. M.; Koutsantonis, G. A.; Raston, C. L. J. Chem. Soc., Chem. Commun. 1995, 1669–1670. doi:10.1039/c39950001669
Return to citation in text: [1] -
Beachley, O. T., Jr.; Rosenblum, D. B.; Churchill, M. R.; Lake, C. H.; Krajkowski, L. M. Organometallics 1995, 14, 4402–4408. doi:10.1021/om00009a050
Return to citation in text: [1] -
Beachley, O. T.; Mosscrop, M. T. Organometallics 2000, 19, 4550–4556. doi:10.1021/om0005304
Return to citation in text: [1] -
Althoff, A.; Jutzi, P.; Lenze, N.; Neumann, B.; Stammler, A.; Stammler, H.-G. Organometallics 2003, 22, 2766–2774. doi:10.1021/om030115m
Return to citation in text: [1] -
Beachley, O. T.; MacRae, D. J.; Zhang, Y.; Li, X. Organometallics 2002, 21, 4632–4640. doi:10.1021/om0202322
Return to citation in text: [1] [2] -
Friedel, C.; Crafts, J. M. C. R. Hebd. Seances Acad. Sci. 1877, 84, 1392–1395.
Return to citation in text: [1] -
Bage, A. D.; Nicholson, K.; Hunt, T. A.; Langer, T.; Thomas, S. P. Synthesis 2023, 55, 62–74. doi:10.1055/s-0040-1720046
Return to citation in text: [1] -
Suseela, Y.; Prasad, A. S. B.; Periasamy, M. J. Chem. Soc., Chem. Commun. 1990, 446–447. doi:10.1039/c39900000446
Return to citation in text: [1] -
Suseela, Y.; Periasamy, M. J. Organomet. Chem. 1993, 450, 47–52. doi:10.1016/0022-328x(93)80135-x
Return to citation in text: [1] -
Arase, A.; Hoshi, M.; Mijin, A.; Nishi, K. Synth. Commun. 1995, 25, 1957–1962. doi:10.1080/00397919508015872
Return to citation in text: [1] -
Shirakawa, K.; Arase, A.; Hoshi, M. Synthesis 2004, 1814–1820. doi:10.1055/s-2004-829165
Return to citation in text: [1] -
Hoshi, M.; Shirakawa, K.; Okimoto, M. Tetrahedron Lett. 2007, 48, 8475–8478. doi:10.1016/j.tetlet.2007.09.176
Return to citation in text: [1] -
Lawson, J. R.; Wilkins, L. C.; Melen, R. L. Chem. – Eur. J. 2017, 23, 10997–11000. doi:10.1002/chem.201703109
Return to citation in text: [1] -
Carden, J. L.; Gierlichs, L. J.; Wass, D. F.; Browne, D. L.; Melen, R. L. Chem. Commun. 2019, 55, 318–321. doi:10.1039/c8cc09459d
Return to citation in text: [1] [2] -
Ang, N. W. J.; Buettner, C. S.; Docherty, S.; Bismuto, A.; Carney, J. R.; Docherty, J. H.; Cowley, M. J.; Thomas, S. P. Synthesis 2018, 50, 803–808. doi:10.1055/s-0036-1591719
Return to citation in text: [1] [2] -
Bage, A. D.; Hunt, T. A.; Thomas, S. P. Org. Lett. 2020, 22, 4107–4112. doi:10.1021/acs.orglett.0c01168
Return to citation in text: [1] [2] [3] [4] -
Légaré Lavergne, J.; To, H.-M.; Fontaine, F.-G. RSC Adv. 2021, 11, 31941–31949. doi:10.1039/d1ra05945a
Return to citation in text: [1] [2] -
Nieto-Sepulveda, E.; Bage, A. D.; Evans, L. A.; Hunt, T. A.; Leach, A. G.; Thomas, S. P.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2019, 141, 18600–18611. doi:10.1021/jacs.9b10114
Return to citation in text: [1] [2] -
Yin, Q.; Kemper, S.; Klare, H. F. T.; Oestreich, M. Chem. – Eur. J. 2016, 22, 13840–13844. doi:10.1002/chem.201603466
Return to citation in text: [1] -
Docherty, J. H.; Nicholson, K.; Dominey, A. P.; Thomas, S. P. ACS Catal. 2020, 10, 4686–4691. doi:10.1021/acscatal.0c00869
Return to citation in text: [1] -
Légaré, M.-A.; Courtemanche, M.-A.; Rochette, É.; Fontaine, F.-G. Science 2015, 349, 513–516. doi:10.1126/science.aab3591
Return to citation in text: [1] -
Légaré Lavergne, J.; Jayaraman, A.; Misal Castro, L. C.; Rochette, É.; Fontaine, F.-G. J. Am. Chem. Soc. 2017, 139, 14714–14723. doi:10.1021/jacs.7b08143
Return to citation in text: [1] [2] -
Légaré, M.-A.; Rochette, É.; Légaré Lavergne, J.; Bouchard, N.; Fontaine, F.-G. Chem. Commun. 2016, 52, 5387–5390. doi:10.1039/c6cc01267a
Return to citation in text: [1] -
Jayaraman, A.; Misal Castro, L. C.; Fontaine, F.-G. Org. Process Res. Dev. 2018, 22, 1489–1499. doi:10.1021/acs.oprd.8b00248
Return to citation in text: [1] -
Bouchard, N.; Fontaine, F.-G. Dalton Trans. 2019, 48, 4846–4856. doi:10.1039/c9dt00484j
Return to citation in text: [1] -
Zou, Y.; Zhang, B.; Wang, L.; Zhang, H. Org. Lett. 2021, 23, 2821–2825. doi:10.1021/acs.orglett.1c00809
Return to citation in text: [1] -
Hoyt, C. B.; Sarazen, M. L.; Jones, C. W. J. Catal. 2019, 369, 493–500. doi:10.1016/j.jcat.2018.11.021
Return to citation in text: [1] -
Phatake, R. S.; Averdunk, A.; Würtele, C.; Gellrich, U. ACS Catal. 2022, 12, 13961–13968. doi:10.1021/acscatal.2c04605
Return to citation in text: [1] -
Jeong, E.; Heo, J.; Park, S.; Chang, S. Chem. – Eur. J. 2019, 25, 6320–6325. doi:10.1002/chem.201901214
Return to citation in text: [1] -
Jayaraman, A.; Powell-Davies, H.; Fontaine, F.-G. Tetrahedron 2019, 75, 2118–2127. doi:10.1016/j.tet.2019.02.048
Return to citation in text: [1] -
Benn, K.; Nicholson, K.; Langer, T.; Thomas, S. P. Chem. Commun. 2021, 57, 9406–9409. doi:10.1039/d1cc03649a
Return to citation in text: [1] -
Meger, F.; Kwok, A. C. W.; Gilch, F.; Willcox, D. R.; Hendy, A. J.; Nicholson, K.; Bage, A. D.; Langer, T.; Hunt, T. A.; Thomas, S. P. Beilstein J. Org. Chem. 2022, 18, 1332–1337. doi:10.3762/bjoc.18.138
Return to citation in text: [1] -
Pradhan, S.; Sankar, R. V.; Gunanathan, C. J. Org. Chem. 2022, 87, 12386–12396. doi:10.1021/acs.joc.2c01655
Return to citation in text: [1] -
Nicholson, K.; Dunne, J.; DaBell, P.; Garcia, A. B.; Bage, A. D.; Docherty, J. H.; Hunt, T. A.; Langer, T.; Thomas, S. P. ACS Catal. 2021, 11, 2034–2040. doi:10.1021/acscatal.0c05168
Return to citation in text: [1] -
Willcox, D. R.; Nichol, G. S.; Thomas, S. P. ACS Catal. 2021, 11, 3190–3197. doi:10.1021/acscatal.1c00282
Return to citation in text: [1] [2] -
Nicholson, K.; Langer, T.; Thomas, S. P. Org. Lett. 2021, 23, 2498–2504. doi:10.1021/acs.orglett.1c00446
Return to citation in text: [1] -
Moreno González, A.; Nicholson, K.; Llopis, N.; Nichol, G. S.; Langer, T.; Baeza, A.; Thomas, S. P. Angew. Chem., Int. Ed. 2022, 61, e202209584. doi:10.1002/anie.202209584
Return to citation in text: [1] -
Nicholson, K.; Peng, Y.; Llopis, N.; Willcox, D. R.; Nichol, G. S.; Langer, T.; Baeza, A.; Thomas, S. P. ACS Catal. 2022, 12, 10887–10893. doi:10.1021/acscatal.2c03158
Return to citation in text: [1] -
Rochette, É.; Boutin, H.; Fontaine, F.-G. Organometallics 2017, 36, 2870–2876. doi:10.1021/acs.organomet.7b00346
Return to citation in text: [1] -
Maruoka, K.; Sano, H.; Shinoda, K.; Nakai, S.; Yamamoto, H. J. Am. Chem. Soc. 1986, 108, 6036–6038. doi:10.1021/ja00279a061
Return to citation in text: [1] [2] -
Maruoka, K.; Sano, H.; Shinoda, K.; Yamamoto, H. Chem. Lett. 1987, 16, 73–76. doi:10.1246/cl.1987.73
Return to citation in text: [1] [2] -
Maruoka, K.; Shinoda, K.; Yamamoto, H. Synth. Commun. 1988, 18, 1029–1033. doi:10.1080/00397918808060887
Return to citation in text: [1] [2] -
Nagahara, S.; Maruoka, K.; Doi, Y.; Yamamoto, H. Chem. Lett. 1990, 19, 1595–1598. doi:10.1246/cl.1990.1595
Return to citation in text: [1] [2] -
Yang, Z.; Zhong, M.; Ma, X.; Nijesh, K.; De, S.; Parameswaran, P.; Roesky, H. W. J. Am. Chem. Soc. 2016, 138, 2548–2551. doi:10.1021/jacs.6b00032
Return to citation in text: [1] [2] [3] -
Franz, D.; Sirtl, L.; Pöthig, A.; Inoue, S. Z. Anorg. Allg. Chem. 2016, 642, 1245–1250. doi:10.1002/zaac.201600313
Return to citation in text: [1] [2] -
Bismuto, A.; Thomas, S. P.; Cowley, M. J. Angew. Chem., Int. Ed. 2016, 55, 15356–15359. doi:10.1002/anie.201609690
Return to citation in text: [1] [2] -
Jaladi, A. K.; Kim, H.; Lee, J. H.; Shin, W. K.; Hwang, H.; An, D. K. New J. Chem. 2019, 43, 16524–16529. doi:10.1039/c9nj03931g
Return to citation in text: [1] [2] -
Lemmerz, L. E.; McLellan, R.; Judge, N. R.; Kennedy, A. R.; Orr, S. A.; Uzelac, M.; Hevia, E.; Robertson, S. D.; Okuda, J.; Mulvey, R. E. Chem. – Eur. J. 2018, 24, 9940–9948. doi:10.1002/chem.201801541
Return to citation in text: [1] [2] -
Zhang, G.; Wu, J.; Zeng, H.; Neary, M. C.; Devany, M.; Zheng, S.; Dub, P. A. ACS Catal. 2019, 9, 874–884. doi:10.1021/acscatal.8b04096
Return to citation in text: [1] [2] -
Harinath, A.; Banerjee, I.; Bhattacharjee, J.; Panda, T. K. New J. Chem. 2019, 43, 10531–10536. doi:10.1039/c9nj01859j
Return to citation in text: [1] [2] -
Li, F.; Bai, X.; Cai, Y.; Li, H.; Zhang, S.-Q.; Liu, F.-H.; Hong, X.; Xu, Y.; Shi, S.-L. Org. Process Res. Dev. 2019, 23, 1703–1708. doi:10.1021/acs.oprd.9b00205
Return to citation in text: [1] [2] -
Sarkar, N.; Bera, S.; Nembenna, S. J. Org. Chem. 2020, 85, 4999–5009. doi:10.1021/acs.joc.0c00234
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Hobson, K.; Carmalt, C. J.; Bakewell, C. Inorg. Chem. 2021, 60, 10958–10969. doi:10.1021/acs.inorgchem.1c00619
Return to citation in text: [1] -
Bismuto, A.; Cowley, M. J.; Thomas, S. P. ACS Catal. 2018, 8, 2001–2005. doi:10.1021/acscatal.7b04279
Return to citation in text: [1] -
Jaladi, A. K.; Shin, W. K.; An, D. K. RSC Adv. 2019, 9, 26483–26486. doi:10.1039/c9ra04699b
Return to citation in text: [1] -
Willcox, D. R.; De Rosa, D. M.; Howley, J.; Levy, A.; Steven, A.; Nichol, G. S.; Morrison, C. A.; Cowley, M. J.; Thomas, S. P. Angew. Chem., Int. Ed. 2021, 60, 20672–20677. doi:10.1002/anie.202106216
Return to citation in text: [1] -
Pollard, V. A.; Fuentes, M. Á.; Kennedy, A. R.; McLellan, R.; Mulvey, R. E. Angew. Chem., Int. Ed. 2018, 57, 10651–10655. doi:10.1002/anie.201806168
Return to citation in text: [1] [2] -
Harinath, A.; Bhattacharjee, J.; Panda, T. K. Adv. Synth. Catal. 2019, 361, 850–857. doi:10.1002/adsc.201801252
Return to citation in text: [1] -
Liu, W.; Ding, Y.; Jin, D.; Shen, Q.; Yan, B.; Ma, X.; Yang, Z. Green Chem. 2019, 21, 3812–3815. doi:10.1039/c9gc01659g
Return to citation in text: [1] -
Ding, Y.; Ma, X.; Liu, Y.; Liu, W.; Yang, Z.; Roesky, H. W. Organometallics 2019, 38, 3092–3097. doi:10.1021/acs.organomet.9b00421
Return to citation in text: [1] [2] -
Chia, C.-C.; Teo, Y.-C.; Cham, N.; Ho, S. Y.-F.; Ng, Z.-H.; Toh, H.-M.; Mézailles, N.; So, C.-W. Inorg. Chem. 2021, 60, 4569–4577. doi:10.1021/acs.inorgchem.0c03507
Return to citation in text: [1] [2] [3] -
Shen, Q.; Ma, X.; Li, W.; Liu, W.; Ding, Y.; Yang, Z.; Roesky, H. W. Chem. – Eur. J. 2019, 25, 11918–11923. doi:10.1002/chem.201902000
Return to citation in text: [1] -
Blake, A. J.; Cunningham, A.; Ford, A.; Teat, S. J.; Woodward, S. Chem. – Eur. J. 2000, 6, 3586–3594. doi:10.1002/1521-3765(20001002)6:19<3586::aid-chem3586>3.0.co;2-s
Return to citation in text: [1] [2] -
Yang, Z.; Zhong, M.; Ma, X.; De, S.; Anusha, C.; Parameswaran, P.; Roesky, H. W. Angew. Chem., Int. Ed. 2015, 54, 10225–10229. doi:10.1002/anie.201503304
Return to citation in text: [1] -
Jakhar, V. K.; Barman, M. K.; Nembenna, S. Org. Lett. 2016, 18, 4710–4713. doi:10.1021/acs.orglett.6b02310
Return to citation in text: [1] -
Pollard, V. A.; Orr, S. A.; McLellan, R.; Kennedy, A. R.; Hevia, E.; Mulvey, R. E. Chem. Commun. 2018, 54, 1233–1236. doi:10.1039/c7cc08214b
Return to citation in text: [1] -
Prashanth, B.; Bhandari, M.; Ravi, S.; Shamasundar, K. R.; Singh, S. Chem. – Eur. J. 2018, 24, 4794–4799. doi:10.1002/chem.201800299
Return to citation in text: [1] -
Jin, D.; Ma, X.; Liu, Y.; Peng, J.; Yang, Z. Appl. Organomet. Chem. 2019, 33, e4637. doi:10.1002/aoc.4637
Return to citation in text: [1] -
Peddarao, T.; Sarkar, N.; Nembenna, S. Inorg. Chem. 2020, 59, 4693–4702. doi:10.1021/acs.inorgchem.9b03778
Return to citation in text: [1] -
Courtemanche, M.-A.; Larouche, J.; Légaré, M.-A.; Bi, W.; Maron, L.; Fontaine, F.-G. Organometallics 2013, 32, 6804–6811. doi:10.1021/om400645s
Return to citation in text: [1] -
Ford, A.; Woodward, S. Angew. Chem., Int. Ed. 1999, 38, 335–336. doi:10.1002/(sici)1521-3773(19990201)38:3<335::aid-anie335>3.0.co;2-t
Return to citation in text: [1] -
Corey, E. J.; Helal, C. J. Angew. Chem., Int. Ed. 1998, 37, 1986–2012. doi:10.1002/(sici)1521-3773(19980817)37:15<1986::aid-anie1986>3.0.co;2-z
Return to citation in text: [1] -
Abdalla, J. A. B.; Riddlestone, I. M.; Tirfoin, R.; Aldridge, S. Angew. Chem., Int. Ed. 2015, 54, 5098–5102. doi:10.1002/anie.201500570
Return to citation in text: [1] -
Caise, A.; Jones, D.; Kolychev, E. L.; Hicks, J.; Goicoechea, J. M.; Aldridge, S. Chem. – Eur. J. 2018, 24, 13624–13635. doi:10.1002/chem.201802603
Return to citation in text: [1] -
Bole, L. J.; Uzelac, M.; Hernán-Gómez, A.; Kennedy, A. R.; O’Hara, C. T.; Hevia, E. Inorg. Chem. 2021, 60, 13784–13796. doi:10.1021/acs.inorgchem.1c01276
Return to citation in text: [1] -
Liu, L.; Lo, S.-K.; Smith, C.; Goicoechea, J. M. Chem. – Eur. J. 2021, 27, 17379–17385. doi:10.1002/chem.202103009
Return to citation in text: [1] -
Qin, B.; Schneider, U. J. Am. Chem. Soc. 2016, 138, 13119–13122. doi:10.1021/jacs.6b06767
Return to citation in text: [1] -
Boronski, J. T.; Stevens, M. P.; van IJzendoorn, B.; Whitwood, A. C.; Slattery, J. M. Angew. Chem., Int. Ed. 2021, 60, 1567–1572. doi:10.1002/anie.202010837
Return to citation in text: [1] -
Schneider, U.; Dao, H. T.; Kobayashi, S. Org. Lett. 2010, 12, 2488–2491. doi:10.1021/ol100450s
Return to citation in text: [1] -
Dao, H. T.; Schneider, U.; Kobayashi, S. Chem. Commun. 2011, 47, 692–694. doi:10.1039/c0cc03673k
Return to citation in text: [1] -
Huang, Y.-Y.; Chakrabarti, A.; Morita, N.; Schneider, U.; Kobayashi, S. Angew. Chem., Int. Ed. 2011, 50, 11121–11124. doi:10.1002/anie.201105182
Return to citation in text: [1] -
Ito, M.; Itazaki, M.; Nakazawa, H. Inorg. Chem. 2017, 56, 13709–13714. doi:10.1021/acs.inorgchem.7b01369
Return to citation in text: [1]
| 70. | Jayaraman, A.; Powell-Davies, H.; Fontaine, F.-G. Tetrahedron 2019, 75, 2118–2127. doi:10.1016/j.tet.2019.02.048 |
| 71. | Benn, K.; Nicholson, K.; Langer, T.; Thomas, S. P. Chem. Commun. 2021, 57, 9406–9409. doi:10.1039/d1cc03649a |
| 122. | Ito, M.; Itazaki, M.; Nakazawa, H. Inorg. Chem. 2017, 56, 13709–13714. doi:10.1021/acs.inorgchem.7b01369 |
| 72. | Meger, F.; Kwok, A. C. W.; Gilch, F.; Willcox, D. R.; Hendy, A. J.; Nicholson, K.; Bage, A. D.; Langer, T.; Hunt, T. A.; Thomas, S. P. Beilstein J. Org. Chem. 2022, 18, 1332–1337. doi:10.3762/bjoc.18.138 |
| 73. | Pradhan, S.; Sankar, R. V.; Gunanathan, C. J. Org. Chem. 2022, 87, 12386–12396. doi:10.1021/acs.joc.2c01655 |
| 36. | Anulewicz-Ostrowska, R.; Luliński, S.; Serwatowski, J. Inorg. Chem. 1999, 38, 3796–3800. doi:10.1021/ic981454o |
| 45. | Beachley, O. T.; MacRae, D. J.; Zhang, Y.; Li, X. Organometallics 2002, 21, 4632–4640. doi:10.1021/om0202322 |
| 119. | Schneider, U.; Dao, H. T.; Kobayashi, S. Org. Lett. 2010, 12, 2488–2491. doi:10.1021/ol100450s |
| 120. | Dao, H. T.; Schneider, U.; Kobayashi, S. Chem. Commun. 2011, 47, 692–694. doi:10.1039/c0cc03673k |
| 121. | Huang, Y.-Y.; Chakrabarti, A.; Morita, N.; Schneider, U.; Kobayashi, S. Angew. Chem., Int. Ed. 2011, 50, 11121–11124. doi:10.1002/anie.201105182 |
| 118. | Boronski, J. T.; Stevens, M. P.; van IJzendoorn, B.; Whitwood, A. C.; Slattery, J. M. Angew. Chem., Int. Ed. 2021, 60, 1567–1572. doi:10.1002/anie.202010837 |
| 75. | Willcox, D. R.; Nichol, G. S.; Thomas, S. P. ACS Catal. 2021, 11, 3190–3197. doi:10.1021/acscatal.1c00282 |
| 79. | Rochette, É.; Boutin, H.; Fontaine, F.-G. Organometallics 2017, 36, 2870–2876. doi:10.1021/acs.organomet.7b00346 |
| 77. | Moreno González, A.; Nicholson, K.; Llopis, N.; Nichol, G. S.; Langer, T.; Baeza, A.; Thomas, S. P. Angew. Chem., Int. Ed. 2022, 61, e202209584. doi:10.1002/anie.202209584 |
| 78. | Nicholson, K.; Peng, Y.; Llopis, N.; Willcox, D. R.; Nichol, G. S.; Langer, T.; Baeza, A.; Thomas, S. P. ACS Catal. 2022, 12, 10887–10893. doi:10.1021/acscatal.2c03158 |
| 76. | Nicholson, K.; Langer, T.; Thomas, S. P. Org. Lett. 2021, 23, 2498–2504. doi:10.1021/acs.orglett.1c00446 |
| 57. | Légaré Lavergne, J.; To, H.-M.; Fontaine, F.-G. RSC Adv. 2021, 11, 31941–31949. doi:10.1039/d1ra05945a |
| 74. | Nicholson, K.; Dunne, J.; DaBell, P.; Garcia, A. B.; Bage, A. D.; Docherty, J. H.; Hunt, T. A.; Langer, T.; Thomas, S. P. ACS Catal. 2021, 11, 2034–2040. doi:10.1021/acscatal.0c05168 |
| 75. | Willcox, D. R.; Nichol, G. S.; Thomas, S. P. ACS Catal. 2021, 11, 3190–3197. doi:10.1021/acscatal.1c00282 |
| 80. | Maruoka, K.; Sano, H.; Shinoda, K.; Nakai, S.; Yamamoto, H. J. Am. Chem. Soc. 1986, 108, 6036–6038. doi:10.1021/ja00279a061 |
| 81. | Maruoka, K.; Sano, H.; Shinoda, K.; Yamamoto, H. Chem. Lett. 1987, 16, 73–76. doi:10.1246/cl.1987.73 |
| 82. | Maruoka, K.; Shinoda, K.; Yamamoto, H. Synth. Commun. 1988, 18, 1029–1033. doi:10.1080/00397918808060887 |
| 83. | Nagahara, S.; Maruoka, K.; Doi, Y.; Yamamoto, H. Chem. Lett. 1990, 19, 1595–1598. doi:10.1246/cl.1990.1595 |
| 80. | Maruoka, K.; Sano, H.; Shinoda, K.; Nakai, S.; Yamamoto, H. J. Am. Chem. Soc. 1986, 108, 6036–6038. doi:10.1021/ja00279a061 |
| 81. | Maruoka, K.; Sano, H.; Shinoda, K.; Yamamoto, H. Chem. Lett. 1987, 16, 73–76. doi:10.1246/cl.1987.73 |
| 82. | Maruoka, K.; Shinoda, K.; Yamamoto, H. Synth. Commun. 1988, 18, 1029–1033. doi:10.1080/00397918808060887 |
| 83. | Nagahara, S.; Maruoka, K.; Doi, Y.; Yamamoto, H. Chem. Lett. 1990, 19, 1595–1598. doi:10.1246/cl.1990.1595 |
| 84. | Yang, Z.; Zhong, M.; Ma, X.; Nijesh, K.; De, S.; Parameswaran, P.; Roesky, H. W. J. Am. Chem. Soc. 2016, 138, 2548–2551. doi:10.1021/jacs.6b00032 |
| 85. | Franz, D.; Sirtl, L.; Pöthig, A.; Inoue, S. Z. Anorg. Allg. Chem. 2016, 642, 1245–1250. doi:10.1002/zaac.201600313 |
| 86. | Bismuto, A.; Thomas, S. P.; Cowley, M. J. Angew. Chem., Int. Ed. 2016, 55, 15356–15359. doi:10.1002/anie.201609690 |
| 87. | Jaladi, A. K.; Kim, H.; Lee, J. H.; Shin, W. K.; Hwang, H.; An, D. K. New J. Chem. 2019, 43, 16524–16529. doi:10.1039/c9nj03931g |
| 88. | Lemmerz, L. E.; McLellan, R.; Judge, N. R.; Kennedy, A. R.; Orr, S. A.; Uzelac, M.; Hevia, E.; Robertson, S. D.; Okuda, J.; Mulvey, R. E. Chem. – Eur. J. 2018, 24, 9940–9948. doi:10.1002/chem.201801541 |
| 89. | Zhang, G.; Wu, J.; Zeng, H.; Neary, M. C.; Devany, M.; Zheng, S.; Dub, P. A. ACS Catal. 2019, 9, 874–884. doi:10.1021/acscatal.8b04096 |
| 90. | Harinath, A.; Banerjee, I.; Bhattacharjee, J.; Panda, T. K. New J. Chem. 2019, 43, 10531–10536. doi:10.1039/c9nj01859j |
| 91. | Li, F.; Bai, X.; Cai, Y.; Li, H.; Zhang, S.-Q.; Liu, F.-H.; Hong, X.; Xu, Y.; Shi, S.-L. Org. Process Res. Dev. 2019, 23, 1703–1708. doi:10.1021/acs.oprd.9b00205 |
| 92. | Sarkar, N.; Bera, S.; Nembenna, S. J. Org. Chem. 2020, 85, 4999–5009. doi:10.1021/acs.joc.0c00234 |
| 93. | Hobson, K.; Carmalt, C. J.; Bakewell, C. Inorg. Chem. 2021, 60, 10958–10969. doi:10.1021/acs.inorgchem.1c00619 |
| 92. | Sarkar, N.; Bera, S.; Nembenna, S. J. Org. Chem. 2020, 85, 4999–5009. doi:10.1021/acs.joc.0c00234 |
| 95. | Jaladi, A. K.; Shin, W. K.; An, D. K. RSC Adv. 2019, 9, 26483–26486. doi:10.1039/c9ra04699b |
| 90. | Harinath, A.; Banerjee, I.; Bhattacharjee, J.; Panda, T. K. New J. Chem. 2019, 43, 10531–10536. doi:10.1039/c9nj01859j |
| 56. | Bage, A. D.; Hunt, T. A.; Thomas, S. P. Org. Lett. 2020, 22, 4107–4112. doi:10.1021/acs.orglett.0c01168 |
| 91. | Li, F.; Bai, X.; Cai, Y.; Li, H.; Zhang, S.-Q.; Liu, F.-H.; Hong, X.; Xu, Y.; Shi, S.-L. Org. Process Res. Dev. 2019, 23, 1703–1708. doi:10.1021/acs.oprd.9b00205 |
| 56. | Bage, A. D.; Hunt, T. A.; Thomas, S. P. Org. Lett. 2020, 22, 4107–4112. doi:10.1021/acs.orglett.0c01168 |
| 94. | Bismuto, A.; Cowley, M. J.; Thomas, S. P. ACS Catal. 2018, 8, 2001–2005. doi:10.1021/acscatal.7b04279 |
| 84. | Yang, Z.; Zhong, M.; Ma, X.; Nijesh, K.; De, S.; Parameswaran, P.; Roesky, H. W. J. Am. Chem. Soc. 2016, 138, 2548–2551. doi:10.1021/jacs.6b00032 |
| 86. | Bismuto, A.; Thomas, S. P.; Cowley, M. J. Angew. Chem., Int. Ed. 2016, 55, 15356–15359. doi:10.1002/anie.201609690 |
| 84. | Yang, Z.; Zhong, M.; Ma, X.; Nijesh, K.; De, S.; Parameswaran, P.; Roesky, H. W. J. Am. Chem. Soc. 2016, 138, 2548–2551. doi:10.1021/jacs.6b00032 |
| 87. | Jaladi, A. K.; Kim, H.; Lee, J. H.; Shin, W. K.; Hwang, H.; An, D. K. New J. Chem. 2019, 43, 16524–16529. doi:10.1039/c9nj03931g |
| 92. | Sarkar, N.; Bera, S.; Nembenna, S. J. Org. Chem. 2020, 85, 4999–5009. doi:10.1021/acs.joc.0c00234 |
| 97. | Pollard, V. A.; Fuentes, M. Á.; Kennedy, A. R.; McLellan, R.; Mulvey, R. E. Angew. Chem., Int. Ed. 2018, 57, 10651–10655. doi:10.1002/anie.201806168 |
| 96. | Willcox, D. R.; De Rosa, D. M.; Howley, J.; Levy, A.; Steven, A.; Nichol, G. S.; Morrison, C. A.; Cowley, M. J.; Thomas, S. P. Angew. Chem., Int. Ed. 2021, 60, 20672–20677. doi:10.1002/anie.202106216 |
| 1. | Negishi, E.-I.; Idacavage, M. J. Org. React. 1985, 33, 1–246. doi:10.1002/0471264180.or033.01 |
| 2. | Suzuki, A. Acc. Chem. Res. 1982, 15, 178–184. doi:10.1021/ar00078a003 |
| 3. | Fernández, E.; Whiting, A. Synthesis and Application of Organoboron Compounds; Topics in Organometallic Chemistry, Vol. 49; Springer International Publishing: Cham, Switzerland, 2015. doi:10.1007/978-3-319-13054-5 |
| 4. | Mole, T.; Jeffery, E. A. Organoaluminium Compounds; Elsevier: New York, NY, USA, 1972. |
| 5. | Downs, A. J. Chemistry of Aluminium, Gallium, Indium and Thallium; Springer: Dordrecht, Netherlands, 1993. |
| 36. | Anulewicz-Ostrowska, R.; Luliński, S.; Serwatowski, J. Inorg. Chem. 1999, 38, 3796–3800. doi:10.1021/ic981454o |
| 40. | Beachley, O. T., Jr.; Royster, T. L., Jr.; Arhar, J. R. J. Organomet. Chem. 1992, 434, 11–17. doi:10.1016/0022-328x(92)83348-l |
| 41. | Elms, F. M.; Koutsantonis, G. A.; Raston, C. L. J. Chem. Soc., Chem. Commun. 1995, 1669–1670. doi:10.1039/c39950001669 |
| 42. | Beachley, O. T., Jr.; Rosenblum, D. B.; Churchill, M. R.; Lake, C. H.; Krajkowski, L. M. Organometallics 1995, 14, 4402–4408. doi:10.1021/om00009a050 |
| 43. | Beachley, O. T.; Mosscrop, M. T. Organometallics 2000, 19, 4550–4556. doi:10.1021/om0005304 |
| 44. | Althoff, A.; Jutzi, P.; Lenze, N.; Neumann, B.; Stammler, A.; Stammler, H.-G. Organometallics 2003, 22, 2766–2774. doi:10.1021/om030115m |
| 55. | Ang, N. W. J.; Buettner, C. S.; Docherty, S.; Bismuto, A.; Carney, J. R.; Docherty, J. H.; Cowley, M. J.; Thomas, S. P. Synthesis 2018, 50, 803–808. doi:10.1055/s-0036-1591719 |
| 56. | Bage, A. D.; Hunt, T. A.; Thomas, S. P. Org. Lett. 2020, 22, 4107–4112. doi:10.1021/acs.orglett.0c01168 |
| 57. | Légaré Lavergne, J.; To, H.-M.; Fontaine, F.-G. RSC Adv. 2021, 11, 31941–31949. doi:10.1039/d1ra05945a |
| 85. | Franz, D.; Sirtl, L.; Pöthig, A.; Inoue, S. Z. Anorg. Allg. Chem. 2016, 642, 1245–1250. doi:10.1002/zaac.201600313 |
| 88. | Lemmerz, L. E.; McLellan, R.; Judge, N. R.; Kennedy, A. R.; Orr, S. A.; Uzelac, M.; Hevia, E.; Robertson, S. D.; Okuda, J.; Mulvey, R. E. Chem. – Eur. J. 2018, 24, 9940–9948. doi:10.1002/chem.201801541 |
| 89. | Zhang, G.; Wu, J.; Zeng, H.; Neary, M. C.; Devany, M.; Zheng, S.; Dub, P. A. ACS Catal. 2019, 9, 874–884. doi:10.1021/acscatal.8b04096 |
| 97. | Pollard, V. A.; Fuentes, M. Á.; Kennedy, A. R.; McLellan, R.; Mulvey, R. E. Angew. Chem., Int. Ed. 2018, 57, 10651–10655. doi:10.1002/anie.201806168 |
| 101. | Chia, C.-C.; Teo, Y.-C.; Cham, N.; Ho, S. Y.-F.; Ng, Z.-H.; Toh, H.-M.; Mézailles, N.; So, C.-W. Inorg. Chem. 2021, 60, 4569–4577. doi:10.1021/acs.inorgchem.0c03507 |
| 105. | Jakhar, V. K.; Barman, M. K.; Nembenna, S. Org. Lett. 2016, 18, 4710–4713. doi:10.1021/acs.orglett.6b02310 |
| 106. | Pollard, V. A.; Orr, S. A.; McLellan, R.; Kennedy, A. R.; Hevia, E.; Mulvey, R. E. Chem. Commun. 2018, 54, 1233–1236. doi:10.1039/c7cc08214b |
| 107. | Prashanth, B.; Bhandari, M.; Ravi, S.; Shamasundar, K. R.; Singh, S. Chem. – Eur. J. 2018, 24, 4794–4799. doi:10.1002/chem.201800299 |
| 108. | Jin, D.; Ma, X.; Liu, Y.; Peng, J.; Yang, Z. Appl. Organomet. Chem. 2019, 33, e4637. doi:10.1002/aoc.4637 |
| 109. | Peddarao, T.; Sarkar, N.; Nembenna, S. Inorg. Chem. 2020, 59, 4693–4702. doi:10.1021/acs.inorgchem.9b03778 |
| 29. | Gamble, E. L.; Gilmont, P.; Stiff, J. F. J. Am. Chem. Soc. 1940, 62, 1257–1258. doi:10.1021/ja01862a078 |
| 30. | Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1971, 93, 1818–1819. doi:10.1021/ja00736a062 |
| 31. | Chen, J.; Chen, E. Y.-X. Dalton Trans. 2016, 45, 6105–6110. doi:10.1039/c5dt03895b |
| 32. | Synoradzki, L.; Bolesławski, M.; Lewínski, J. J. Organomet. Chem. 1985, 284, 1–4. doi:10.1016/0022-328x(85)80006-1 |
| 33. | Uhl, W.; Layh, M. J. Organomet. Chem. 1991, 415, 181–190. doi:10.1016/0022-328x(91)80118-4 |
| 34. | Feulner, H.; Metzler, N.; Nöth, H. J. Organomet. Chem. 1995, 489, 51–62. doi:10.1016/0022-328x(94)05147-4 |
| 35. | Bochmann, M.; Sarsfield, M. J. Organometallics 1998, 17, 5908–5912. doi:10.1021/om980400j |
| 36. | Anulewicz-Ostrowska, R.; Luliński, S.; Serwatowski, J. Inorg. Chem. 1999, 38, 3796–3800. doi:10.1021/ic981454o |
| 37. | Kim, J. S.; Wojcinski, L. M.; Liu, S.; Sworen, J. C.; Sen, A. J. Am. Chem. Soc. 2000, 122, 5668–5669. doi:10.1021/ja0010960 |
| 38. | Klosin, J.; Roof, G. R.; Chen, E. Y.-X.; Abboud, K. A. Organometallics 2000, 19, 4684–4686. doi:10.1021/om000573k |
| 39. | McGuinness, D. S.; Rucklidge, A. J.; Tooze, R. P.; Slawin, A. M. Z. Organometallics 2007, 26, 2561–2569. doi:10.1021/om070029c |
| 58. | Nieto-Sepulveda, E.; Bage, A. D.; Evans, L. A.; Hunt, T. A.; Leach, A. G.; Thomas, S. P.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2019, 141, 18600–18611. doi:10.1021/jacs.9b10114 |
| 11. | Schlesinger, H. I.; Burg, A. B. J. Am. Chem. Soc. 1931, 53, 4321–4332. doi:10.1021/ja01363a009 |
| 12. | Schlesinger, H. I.; Walker, A. O. J. Am. Chem. Soc. 1935, 57, 621–625. doi:10.1021/ja01307a009 |
| 13. | Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1970, 92, 6983–6984. doi:10.1021/ja00726a052 |
| 14. | Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1971, 93, 1816–1818. doi:10.1021/ja00736a061 |
| 15. | Wrackmeyer, B. J. Organomet. Chem. 1976, 117, 313–320. doi:10.1016/s0022-328x(00)87208-3 |
| 16. | Contreras, R.; Wrackmeyer, B. Spectrochim. Acta, Part A 1982, 38, 941–951. doi:10.1016/0584-8539(82)80119-0 |
| 17. | Männig, D.; Nöth, H. J. Chem. Soc., Dalton Trans. 1985, 1689–1692. doi:10.1039/dt9850001689 |
| 18. | Garrett, C. E.; Fu, G. C. J. Org. Chem. 1996, 61, 3224–3225. doi:10.1021/jo960386p |
| 19. | Hoshi, M.; Shirakawa, K.; Arase, A. Chem. Commun. 1998, 1225–1226. doi:10.1039/a801939h |
| 20. | Kanth, J. V. B.; Periasamy, M.; Brown, H. C. Org. Process Res. Dev. 2000, 4, 550–553. doi:10.1021/op000291w |
| 21. | Schlesinger, H. I.; Horvitz, L.; Burg, A. B. J. Am. Chem. Soc. 1936, 58, 407–409. doi:10.1021/ja01294a007 |
| 22. | McCusker, P. A.; Ashby, E. C.; Makowski, H. S. J. Am. Chem. Soc. 1957, 79, 5179–5181. doi:10.1021/ja01576a026 |
| 23. | Hennion, G. F.; McCusker, P. A.; Ashby, E. C.; Rutkowski, A. J. J. Am. Chem. Soc. 1957, 79, 5190–5191. doi:10.1021/ja01576a030 |
| 24. | McCusker, P. A.; Hennion, G. F.; Ashby, E. C. J. Am. Chem. Soc. 1957, 79, 5192–5194. doi:10.1021/ja01576a031 |
| 25. | Hennion, G. F.; McCusker, P. A.; Ashby, E. C.; Rutkowski, A. J. J. Am. Chem. Soc. 1957, 79, 5194–5196. doi:10.1021/ja01576a032 |
| 26. | Brown, H. C.; Klender, G. J. Inorg. Chem. 1962, 1, 204–214. doi:10.1021/ic50002a003 |
| 27. | McCusker, P. A.; Bright, J. H. J. Org. Chem. 1964, 29, 2093–2094. doi:10.1021/jo01030a555 |
| 28. | Pasto, D. J.; Balasubramaniyan, V.; Wojtkowski, P. W. Inorg. Chem. 1969, 8, 594–598. doi:10.1021/ic50073a038 |
| 53. | Lawson, J. R.; Wilkins, L. C.; Melen, R. L. Chem. – Eur. J. 2017, 23, 10997–11000. doi:10.1002/chem.201703109 |
| 103. | Blake, A. J.; Cunningham, A.; Ford, A.; Teat, S. J.; Woodward, S. Chem. – Eur. J. 2000, 6, 3586–3594. doi:10.1002/1521-3765(20001002)6:19<3586::aid-chem3586>3.0.co;2-s |
| 6. | Fyfe, J. W. B.; Watson, A. J. B. Chem 2017, 3, 31–55. doi:10.1016/j.chempr.2017.05.008 |
| 7. | Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46–76. doi:10.1002/anie.200903708 |
| 8. | Stephan, D. W. J. Am. Chem. Soc. 2015, 137, 10018–10032. doi:10.1021/jacs.5b06794 |
| 9. | Jupp, A. R.; Stephan, D. W. Trends Chem. 2019, 1, 35–48. doi:10.1016/j.trechm.2019.01.006 |
| 10. | Li, N.; Zhang, W.-X. Chin. J. Chem. 2020, 38, 1360–1370. doi:10.1002/cjoc.202000027 |
| 54. | Carden, J. L.; Gierlichs, L. J.; Wass, D. F.; Browne, D. L.; Melen, R. L. Chem. Commun. 2019, 55, 318–321. doi:10.1039/c8cc09459d |
| 104. | Yang, Z.; Zhong, M.; Ma, X.; De, S.; Anusha, C.; Parameswaran, P.; Roesky, H. W. Angew. Chem., Int. Ed. 2015, 54, 10225–10229. doi:10.1002/anie.201503304 |
| 48. | Suseela, Y.; Prasad, A. S. B.; Periasamy, M. J. Chem. Soc., Chem. Commun. 1990, 446–447. doi:10.1039/c39900000446 |
| 49. | Suseela, Y.; Periasamy, M. J. Organomet. Chem. 1993, 450, 47–52. doi:10.1016/0022-328x(93)80135-x |
| 51. | Shirakawa, K.; Arase, A.; Hoshi, M. Synthesis 2004, 1814–1820. doi:10.1055/s-2004-829165 |
| 92. | Sarkar, N.; Bera, S.; Nembenna, S. J. Org. Chem. 2020, 85, 4999–5009. doi:10.1021/acs.joc.0c00234 |
| 47. | Bage, A. D.; Nicholson, K.; Hunt, T. A.; Langer, T.; Thomas, S. P. Synthesis 2023, 55, 62–74. doi:10.1055/s-0040-1720046 |
| 52. | Hoshi, M.; Shirakawa, K.; Okimoto, M. Tetrahedron Lett. 2007, 48, 8475–8478. doi:10.1016/j.tetlet.2007.09.176 |
| 92. | Sarkar, N.; Bera, S.; Nembenna, S. J. Org. Chem. 2020, 85, 4999–5009. doi:10.1021/acs.joc.0c00234 |
| 46. | Friedel, C.; Crafts, J. M. C. R. Hebd. Seances Acad. Sci. 1877, 84, 1392–1395. |
| 92. | Sarkar, N.; Bera, S.; Nembenna, S. J. Org. Chem. 2020, 85, 4999–5009. doi:10.1021/acs.joc.0c00234 |
| 98. | Harinath, A.; Bhattacharjee, J.; Panda, T. K. Adv. Synth. Catal. 2019, 361, 850–857. doi:10.1002/adsc.201801252 |
| 99. | Liu, W.; Ding, Y.; Jin, D.; Shen, Q.; Yan, B.; Ma, X.; Yang, Z. Green Chem. 2019, 21, 3812–3815. doi:10.1039/c9gc01659g |
| 100. | Ding, Y.; Ma, X.; Liu, Y.; Liu, W.; Yang, Z.; Roesky, H. W. Organometallics 2019, 38, 3092–3097. doi:10.1021/acs.organomet.9b00421 |
| 101. | Chia, C.-C.; Teo, Y.-C.; Cham, N.; Ho, S. Y.-F.; Ng, Z.-H.; Toh, H.-M.; Mézailles, N.; So, C.-W. Inorg. Chem. 2021, 60, 4569–4577. doi:10.1021/acs.inorgchem.0c03507 |
| 36. | Anulewicz-Ostrowska, R.; Luliński, S.; Serwatowski, J. Inorg. Chem. 1999, 38, 3796–3800. doi:10.1021/ic981454o |
| 45. | Beachley, O. T.; MacRae, D. J.; Zhang, Y.; Li, X. Organometallics 2002, 21, 4632–4640. doi:10.1021/om0202322 |
| 50. | Arase, A.; Hoshi, M.; Mijin, A.; Nishi, K. Synth. Commun. 1995, 25, 1957–1962. doi:10.1080/00397919508015872 |
| 92. | Sarkar, N.; Bera, S.; Nembenna, S. J. Org. Chem. 2020, 85, 4999–5009. doi:10.1021/acs.joc.0c00234 |
| 100. | Ding, Y.; Ma, X.; Liu, Y.; Liu, W.; Yang, Z.; Roesky, H. W. Organometallics 2019, 38, 3092–3097. doi:10.1021/acs.organomet.9b00421 |
| 102. | Shen, Q.; Ma, X.; Li, W.; Liu, W.; Ding, Y.; Yang, Z.; Roesky, H. W. Chem. – Eur. J. 2019, 25, 11918–11923. doi:10.1002/chem.201902000 |
| 54. | Carden, J. L.; Gierlichs, L. J.; Wass, D. F.; Browne, D. L.; Melen, R. L. Chem. Commun. 2019, 55, 318–321. doi:10.1039/c8cc09459d |
| 58. | Nieto-Sepulveda, E.; Bage, A. D.; Evans, L. A.; Hunt, T. A.; Leach, A. G.; Thomas, S. P.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2019, 141, 18600–18611. doi:10.1021/jacs.9b10114 |
| 59. | Yin, Q.; Kemper, S.; Klare, H. F. T.; Oestreich, M. Chem. – Eur. J. 2016, 22, 13840–13844. doi:10.1002/chem.201603466 |
| 111. | Ford, A.; Woodward, S. Angew. Chem., Int. Ed. 1999, 38, 335–336. doi:10.1002/(sici)1521-3773(19990201)38:3<335::aid-anie335>3.0.co;2-t |
| 112. | Corey, E. J.; Helal, C. J. Angew. Chem., Int. Ed. 1998, 37, 1986–2012. doi:10.1002/(sici)1521-3773(19980817)37:15<1986::aid-anie1986>3.0.co;2-z |
| 110. | Courtemanche, M.-A.; Larouche, J.; Légaré, M.-A.; Bi, W.; Maron, L.; Fontaine, F.-G. Organometallics 2013, 32, 6804–6811. doi:10.1021/om400645s |
| 101. | Chia, C.-C.; Teo, Y.-C.; Cham, N.; Ho, S. Y.-F.; Ng, Z.-H.; Toh, H.-M.; Mézailles, N.; So, C.-W. Inorg. Chem. 2021, 60, 4569–4577. doi:10.1021/acs.inorgchem.0c03507 |
| 68. | Phatake, R. S.; Averdunk, A.; Würtele, C.; Gellrich, U. ACS Catal. 2022, 12, 13961–13968. doi:10.1021/acscatal.2c04605 |
| 69. | Jeong, E.; Heo, J.; Park, S.; Chang, S. Chem. – Eur. J. 2019, 25, 6320–6325. doi:10.1002/chem.201901214 |
| 62. | Légaré Lavergne, J.; Jayaraman, A.; Misal Castro, L. C.; Rochette, É.; Fontaine, F.-G. J. Am. Chem. Soc. 2017, 139, 14714–14723. doi:10.1021/jacs.7b08143 |
| 63. | Légaré, M.-A.; Rochette, É.; Légaré Lavergne, J.; Bouchard, N.; Fontaine, F.-G. Chem. Commun. 2016, 52, 5387–5390. doi:10.1039/c6cc01267a |
| 64. | Jayaraman, A.; Misal Castro, L. C.; Fontaine, F.-G. Org. Process Res. Dev. 2018, 22, 1489–1499. doi:10.1021/acs.oprd.8b00248 |
| 65. | Bouchard, N.; Fontaine, F.-G. Dalton Trans. 2019, 48, 4846–4856. doi:10.1039/c9dt00484j |
| 116. | Liu, L.; Lo, S.-K.; Smith, C.; Goicoechea, J. M. Chem. – Eur. J. 2021, 27, 17379–17385. doi:10.1002/chem.202103009 |
| 66. | Zou, Y.; Zhang, B.; Wang, L.; Zhang, H. Org. Lett. 2021, 23, 2821–2825. doi:10.1021/acs.orglett.1c00809 |
| 67. | Hoyt, C. B.; Sarazen, M. L.; Jones, C. W. J. Catal. 2019, 369, 493–500. doi:10.1016/j.jcat.2018.11.021 |
| 117. | Qin, B.; Schneider, U. J. Am. Chem. Soc. 2016, 138, 13119–13122. doi:10.1021/jacs.6b06767 |
| 61. | Légaré, M.-A.; Courtemanche, M.-A.; Rochette, É.; Fontaine, F.-G. Science 2015, 349, 513–516. doi:10.1126/science.aab3591 |
| 114. | Caise, A.; Jones, D.; Kolychev, E. L.; Hicks, J.; Goicoechea, J. M.; Aldridge, S. Chem. – Eur. J. 2018, 24, 13624–13635. doi:10.1002/chem.201802603 |
| 62. | Légaré Lavergne, J.; Jayaraman, A.; Misal Castro, L. C.; Rochette, É.; Fontaine, F.-G. J. Am. Chem. Soc. 2017, 139, 14714–14723. doi:10.1021/jacs.7b08143 |
| 115. | Bole, L. J.; Uzelac, M.; Hernán-Gómez, A.; Kennedy, A. R.; O’Hara, C. T.; Hevia, E. Inorg. Chem. 2021, 60, 13784–13796. doi:10.1021/acs.inorgchem.1c01276 |
| 55. | Ang, N. W. J.; Buettner, C. S.; Docherty, S.; Bismuto, A.; Carney, J. R.; Docherty, J. H.; Cowley, M. J.; Thomas, S. P. Synthesis 2018, 50, 803–808. doi:10.1055/s-0036-1591719 |
| 56. | Bage, A. D.; Hunt, T. A.; Thomas, S. P. Org. Lett. 2020, 22, 4107–4112. doi:10.1021/acs.orglett.0c01168 |
| 103. | Blake, A. J.; Cunningham, A.; Ford, A.; Teat, S. J.; Woodward, S. Chem. – Eur. J. 2000, 6, 3586–3594. doi:10.1002/1521-3765(20001002)6:19<3586::aid-chem3586>3.0.co;2-s |
| 60. | Docherty, J. H.; Nicholson, K.; Dominey, A. P.; Thomas, S. P. ACS Catal. 2020, 10, 4686–4691. doi:10.1021/acscatal.0c00869 |
| 113. | Abdalla, J. A. B.; Riddlestone, I. M.; Tirfoin, R.; Aldridge, S. Angew. Chem., Int. Ed. 2015, 54, 5098–5102. doi:10.1002/anie.201500570 |
© 2023 Willcox and Thomas; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.