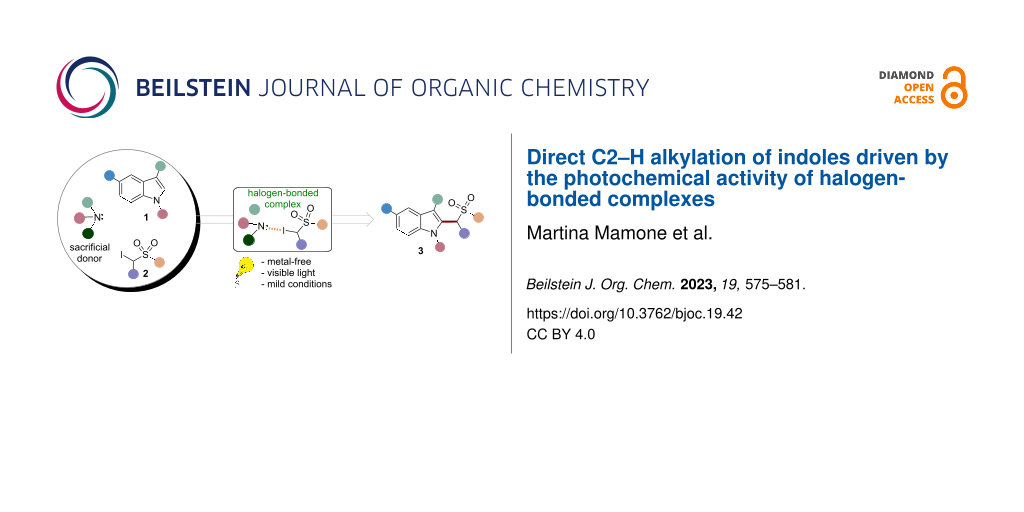
Abstract
A light-driven metal-free protocol for the synthesis of sulfone-containing indoles under mild conditions is reported. Specifically, the process is driven by the photochemical activity of halogen-bonded complexes formed upon complexation of a sacrificial donor, namely 1,4-diazabicyclo[2.2.2]octane (DABCO), with α-iodosulfones. The reaction provides a variety of densely functionalized products in good yields (up to 96% yield). Mechanistic investigations are reported. These studies provide convincing evidences for the photochemical formation of reactive open-shell species.
Graphical Abstract
Findings
Direct replacement of carbon–hydrogen (C–H) bonds with new carbon–carbon (C–C) and carbon–heteroatom (C–X) bonds has been and still is a central topic in organic synthesis [1,2]. Historically, organic chemists have extensively relied on the use of noble-metal-based catalysts (e.g., Pd, Rh, Ir, among others) to achieve such type of functionalization [3-5]. However, reliance on noble metal complexes has been constantly declined over recent years due to cost, availability, and toxicity, therefore discouraged by the modern guidelines towards implementation of sustainable chemical production schemes [6]. In the last decades, organic photochemistry has become a prominent tool to guide the development of greener and more convenient synthetic protocols [7-12]. In this context, photochemical approaches based on electron donor–acceptor (EDA) complexes have been successfully exploited to drive the direct C–H functionalization of a large number of organic substrates [13-18]. In this approach, an electron acceptor substrate (“A”) and a donor molecule (“D”) interact to form a new aggregate defined as EDA complex (Figure 1a). Although the two molecular entities might not directly absorb visible light, the newly formed complex usually presents a charge transfer state which results in a bathochromic shift of the absorption towards the visible range [19,20]. Upon light irradiation, the EDA complex may undergo an intramolecular single-electron-transfer (SET) process to produce a radical ion pair (D•+, A•−). To avoid the occurrence of a back-electron-transfer (BET), a suitable leaving group (LG) needs to be included in one of the precursors. In this manner, reactive intermediates (e.g., radical species) may be generated in solution through the irreversible fragmentation of the substrates [15,21,22]. These intermediates eventually react to yield the final products "A–D". This approach is not limited to reagents with appropriate donor–acceptor characteristics [13,19].
Figure 1: (a) Exploitation of an EDA complex in organic synthesis. (b) This work: use of halogen-bonded complexes to photochemically initiate the C–H alkylation of indoles 1 with iodosulfones 2.
Figure 1: (a) Exploitation of an EDA complex in organic synthesis. (b) This work: use of halogen-bonded compl...
Indeed, sacrificial electron donors and electron-deficient radical precursors can be used to form photoactive EDA complexes. Specifically, these aggregates can be employed to photochemically generate electrophilic radicals that can drive the functionalization of suitable electron-rich substrates [23].
Exploiting this strategy, here we report a novel metal-free methodology for the direct homolytic aromatic substitution (HAS) reaction of indoles 1 with α-iodosulfones 2 to yield the alkylated derivatives 3 (Figure 1b). Indoles play a crucial role in many natural and industrial processes. Therefore, the direct chemical manipulation of the indole system is a matter of paramount importance [24-27]. Moreover, the sulfonyl group is an extremely versatile chemical moiety which may be easily transformed into different functionalities employing conventional synthetic methods. As an example, the sulfonyl group removal under simple reductive treatment may give access to important methylated compounds [12,21]. This operationally simple approach occurs at ambient temperature and under visible-light irradiation. Interestingly, this method employs 1,4-diazabicyclo[2.2.2]octane (DABCO) as sacrificial donor in the EDA complex formation with 2. To test the feasibility of our design plan, we focused on the reaction between 3-methylindole (1a, 2 equiv) and α-iodosulfone 2a (Table 1).
Table 1: Optimization of the reaction conditions and control experiments.
|
|
|||||
| entry | donor | [M] | 1a:2a:donor (equiv) | light source (nm) | yield (%)a |
| 1b | DBU | 0.5 | 1:2:1 | 456 | 56 |
| 2 | DBU | 0.5 | 1:2:1 | light off | 0 |
| 3 | – | 0.5 | 1:2:1 | 456 | 0 |
| 4c | DBU | 0.5 | 1:2:1 | 456 | 0 |
| 5d | DBU | 0.5 | 1:2:1 | 456 | 0 |
| 6 | 2,6-lutidine | 0.5 | 1:2:1 | 456 | 0 |
| 7 | TMG | 0.5 | 1:2:1 | 456 | 64 |
| 8 | NEt3 | 0.5 | 1:2:1 | 456 | 64 |
| 9 | DABCO | 0.5 | 1:2:1 | 456 | 77 |
| 10 | DABCO | 0.25 | 1:2:1 | 456 | 64 |
| 11 | DABCO | 1.0 | 1:2:1 | 456 | 69 |
| 12 | DABCO | 0.5 | 1:1:1 | 456 | 65 |
| 13 | DABCO | 0.5 | 2:1:1 | 456 | 73 |
| 14 | DABCO | 0.5 | 2:1:1.5 | 456 | 95 |
| 15e | DABCO | 0.5 | 2:1:1.5 | 456 | 18 |
| 16f | DABCO | 0.5 | 2:1:1.5 | 456 | 60 |
aYield determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as the internal standard. bConditions: indole 1a (0.1 mmol), α-iodosulfone 2a (0.2 mmol), acetonitrile (MeCN, 200 μL), donor (0.1 mmol), ambient temperature. cReaction in air. dReaction performed in the presence of 2 equiv of TEMPO. eReaction performed in hexane as solvent. fReaction performed in methanol as solvent.
The experiments were conducted at ambient temperature in acetonitrile (0.5 M) and under irradiation by a Kessil lamp at 456 nm. When adding 1,8-diazabiciclo[5.4.0]undec-7-ene (DBU) as sacrificial donor (1 equiv), the desired product 3a was formed in good chemical yield (entry 1, Table 1). Control experiments were conducted to obtain more mechanistic clues (entries 2–5, Table 1). An experiment revealed how the exclusion of light completely suppressed the process, therefore establishing the photochemical nature of the transformation (entry 2, Table 1). In addition, we confirmed that DBU was essential for the reactivity, since no reaction occurred in its absence (entry 3, Table 1). Reactivity was also inhibited under an aerobic atmosphere and in the presence of 2,2,6,6‐tetramethylpiperidinyloxyl (TEMPO). These experiments are consonant with the occurrence of a radical mechanism (entries 4 and 5, Table 1) [28]. Afterwards, the effect of the chemical nature of the sacrificial donor on the reaction was investigated (entries 6–9, Table 1). In particular, we employed 2,6-lutidine, 1,1,3,3-tetramethylguanidine (TMG), triethylamine (NEt3), and DABCO. Interestingly, the use of DABCO provided the best result in terms of reactivity, yielding compound 3a in 77% yield. We also observed that either increasing or decreasing the concentration of the reaction mixture did not bring any improvement (entries 10 and 11, Table 1). Then, the ratio between the reagents was optimized. In particular, the use of 2a:1a in a 1:1 ratio resulted in the formation of 3a in 65% yield (entry 12, Table 1). Moreover, we found that employing an excess of 1a (2 equiv) led to the formation of 3a in a 73% yield (entry 13, Table 1). Due to an easier purification of product 3a from the reaction crude by flash column chromatography, we decided to keep optimizing the transformation using the stoichiometric ratio indicated in entry 13 of Table 1. Importantly, product 3a was obtained in excellent yield (95%) using 1.5 equivalents of DABCO (entry 14, Table 1). In addition, the use of hexane as solvent provided the desired product 3a in low chemical yield (entry 15, Table 1). On the other hand, 3a was obtained in moderate yield (60%) using methanol as solvent (entry 16, Table 1). To shed light on the reaction mechanism, the formation of an EDA complex between the α-iodosulfone 2a and DABCO was investigated using both UV–vis and nuclear magnetic resonance (NMR) spectroscopy [29].
In particular, the optical absorption spectra of substrate 2a (green dotted line), DABCO (red dotted line), and the solution containing both 2a and DABCO (blue line) were recorded in acetonitrile (Figure 2). Specifically, it was observed that the addition of DABCO to the solution of 2a induced a bathochromic shift of the absorption spectrum towards the visible region, thus indicating the formation of an EDA complex between these chemical species. Importantly, we also confirmed that indole 1a and 2a do not form a photoactive EDA complex when mixed in solution (see Figure S1 in Supporting Information File 1). To further corroborate the hypothesis of an EDA complex being at the roots of the observed reactivity, NMR studies were also performed on samples containing the α-iodosufone 2a and different concentrations of DABCO in deuterated acetonitrile (Figure 3).
Figure 2: Optical absorption spectra recorded in acetonitrile in 1 cm path quartz cuvettes. [DABCO]: 0.5 M; [2a]: 0.5 M.
Figure 2: Optical absorption spectra recorded in acetonitrile in 1 cm path quartz cuvettes. [DABCO]: 0.5 M; [...
Figure 3: 1H NMR titration of DABCO in a solution of 2a in ACN-d3 to detect their halogen-bonding association through the shift of the signal of Hα.
Figure 3: 1H NMR titration of DABCO in a solution of 2a in ACN-d3 to detect their halogen-bonding association...
Interestingly, a change in chemical shift of the diagnostic α-protons of 2a was displayed upon addition of increasing amounts of DABCO, suggesting the presence of the halogen-bonding interaction [30]. More precisely, the 1H NMR signal of the α-hydrogens (Hα) within 2a was found to shift to lower ppm values because the Hα nuclei have been affected by higher electron density caused by the formation of the halogen-bonded complex between 2a and DABCO. To confirm that the shift of Hα was indeed produced by a halogen-bonding interaction, 19F NMR analysis of compound 2d, which presents a difluoromethylene group (–CF2–) in the alpha position to the iodine, was performed (see Figure S3 in Supporting Information File 1). Even in this case, an important shift of the fluorine signal was observed. Thus, from a mechanistic point of view, the reaction is driven by the formation of a halogen-bonded EDA complex (Ia) between the sulfone 2a and DABCO (Figure 4).
Figure 4: Proposed reaction mechanism for the photochemical alkylation of 1a with the α-iodosulfone 2a in the presence of DABCO.
Figure 4: Proposed reaction mechanism for the photochemical alkylation of 1a with the α-iodosulfone 2a in the...
When irradiated, this photoactive aggregate led to the formation of reactive alkyl radicals (IIa), which may react with indole 1a eventually yielding the product 3a through a classical HAS pathway [31-33]. Then, we demonstrated the synthetic potential of our photochemical method (Scheme 1).
Scheme 1: Study of scope of the HAS reaction between indoles 1 and α-iodosulfones 2. Yields in parentheses were determined by 1H NMR analyses, using 1,3,5-trimethoxybenzene as an internal standard.
Scheme 1: Study of scope of the HAS reaction between indoles 1 and α-iodosulfones 2. Yields in parentheses we...
The reaction could satisfactorily tolerate a diverse set of α-iodosulfones 2 to deliver the corresponding products 3a–d from moderate to excellent yields (up to 73% yield). We found that different indoles actively participated in the photochemical alkylation, leading to the products 3e–i (up to 96% yield). It is worth noting that derivatives 3e–g were isolated in moderate yields as single regioisomer since the alkylation step took place exclusively in position 2 of the starting indoles. As limitation, we observed that indole-3-carboxaldehyde (1g) was not a suitable substrate for this transformation.
Conclusion
In conclusion, we reported a novel photochemical method for the direct C–H alkylation of indoles with α-iodosulfones. This approach exploits the photochemical activity of halogen-bonded EDA complexes, formed between α-iodosulfones and DABCO, that are able to produce reactive C-centered radicals under mild reaction conditions.
Supporting Information
| Supporting Information File 1: General procedures and products characterization. | ||
| Format: PDF | Size: 2.6 MB | Download |
Funding
The authors gratefully acknowledge the University of Trieste, INSTM and the Maria de Maeztu Units of Excellence Program from the Spanish State Research Agency (Grant No. MDM-2017-0720). G.F. and J.D. kindly acknowledge FRA2022 funded by the University of Trieste. G.F kindly acknowledges Microgrants 2021 funded by Region FVG (LR 2/2011, ART. 4). J.D. kindly acknowledges the RTDa–PON “ricerca e innovazione” 2014–2020.
References
-
Godula, K.; Sames, D. Science 2006, 312, 67–72. doi:10.1126/science.1114731
Return to citation in text: [1] -
Davies, H. M. L.; Morton, D. J. Org. Chem. 2016, 81, 343–350. doi:10.1021/acs.joc.5b02818
Return to citation in text: [1] -
Wencel-Delord, J.; Glorius, F. Nat. Chem. 2013, 5, 369–375. doi:10.1038/nchem.1607
Return to citation in text: [1] -
He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754–8786. doi:10.1021/acs.chemrev.6b00622
Return to citation in text: [1] -
Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236–10254. doi:10.1002/anie.201203269
Return to citation in text: [1] -
Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Chem. Rev. 2022, 122, 3637–3710. doi:10.1021/acs.chemrev.1c00631
Return to citation in text: [1] -
Bortolato, T.; Cuadros, S.; Simionato, G.; Dell’Amico, L. Chem. Commun. 2022, 58, 1263–1283. doi:10.1039/d1cc05850a
Return to citation in text: [1] -
Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075–10166. doi:10.1021/acs.chemrev.6b00057
Return to citation in text: [1] -
Rosso, C.; Cuadros, S.; Barison, G.; Costa, P.; Kurbasic, M.; Bonchio, M.; Prato, M.; Dell’Amico, L.; Filippini, G. ACS Catal. 2022, 12, 4290–4295. doi:10.1021/acscatal.2c00565
Return to citation in text: [1] -
Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898–6926. doi:10.1021/acs.joc.6b01449
Return to citation in text: [1] -
Rosso, C.; Filippini, G.; Prato, M. Eur. J. Org. Chem. 2021, 1193–1200. doi:10.1002/ejoc.202001616
Return to citation in text: [1] -
Filippini, G.; Silvi, M.; Melchiorre, P. Angew. Chem., Int. Ed. 2017, 56, 4447–4451. doi:10.1002/anie.201612045
Return to citation in text: [1] [2] -
Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. J. Am. Chem. Soc. 2020, 142, 5461–5476. doi:10.1021/jacs.0c01416
Return to citation in text: [1] [2] -
Yang, Z.; Liu, Y.; Cao, K.; Zhang, X.; Jiang, H.; Li, J. Beilstein J. Org. Chem. 2021, 17, 771–799. doi:10.3762/bjoc.17.67
Return to citation in text: [1] -
Kandukuri, S. R.; Bahamonde, A.; Chatterjee, I.; Jurberg, I. D.; Escudero-Adán, E. C.; Melchiorre, P. Angew. Chem., Int. Ed. 2015, 54, 1485–1489. doi:10.1002/anie.201409529
Return to citation in text: [1] [2] -
Arceo, E.; Jurberg, I. D.; Álvarez-Fernández, A.; Melchiorre, P. Nat. Chem. 2013, 5, 750–756. doi:10.1038/nchem.1727
Return to citation in text: [1] -
Yuan, Y.-q.; Majumder, S.; Yang, M.-h.; Guo, S.-r. Tetrahedron Lett. 2020, 61, 151506. doi:10.1016/j.tetlet.2019.151506
Return to citation in text: [1] -
Tian, Y.-M.; Hofmann, E.; Silva, W.; Pu, X.; Touraud, D.; Gschwind, R. M.; Kunz, W.; König, B. Angew. Chem., Int. Ed. 2023, 62, e202218775. doi:10.1002/anie.202218775
Return to citation in text: [1] -
Lima, C. G. S.; de M. Lima, T.; Duarte, M.; Jurberg, I. D.; Paixão, M. W. ACS Catal. 2016, 6, 1389–1407. doi:10.1021/acscatal.5b02386
Return to citation in text: [1] [2] -
Slama-Schwok, A.; Blanchard-Desce, M.; Lehn, J.-M. J. Phys. Chem. 1990, 94, 3894–3902. doi:10.1021/j100373a007
Return to citation in text: [1] -
Cuadros, S.; Rosso, C.; Barison, G.; Costa, P.; Kurbasic, M.; Bonchio, M.; Prato, M.; Filippini, G.; Dell’Amico, L. Org. Lett. 2022, 24, 2961–2966. doi:10.1021/acs.orglett.2c00604
Return to citation in text: [1] [2] -
Nappi, M.; Bergonzini, G.; Melchiorre, P. Angew. Chem., Int. Ed. 2014, 53, 4921–4925. doi:10.1002/anie.201402008
Return to citation in text: [1] -
Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Org. Lett. 2016, 18, 4638–4641. doi:10.1021/acs.orglett.6b02271
Return to citation in text: [1] -
Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608–9644. doi:10.1002/anie.200901843
Return to citation in text: [1] -
Su, Y.-M.; Hou, Y.; Yin, F.; Xu, Y.-M.; Li, Y.; Zheng, X.; Wang, X.-S. Org. Lett. 2014, 16, 2958–2961. doi:10.1021/ol501094z
Return to citation in text: [1] -
Liu, F.; Li, P. J. Org. Chem. 2016, 81, 6972–6979. doi:10.1021/acs.joc.6b00689
Return to citation in text: [1] -
Mi, X.; Kong, Y.; Zhang, J.; Pi, C.; Cui, X. Chin. Chem. Lett. 2019, 30, 2295–2298. doi:10.1016/j.cclet.2019.09.040
Return to citation in text: [1] -
Buzzetti, L.; Crisenza, G. E. M.; Melchiorre, P. Angew. Chem., Int. Ed. 2019, 58, 3730–3747. doi:10.1002/anie.201809984
Return to citation in text: [1] -
Rosokha, S. V.; Kochi, J. K. Acc. Chem. Res. 2008, 41, 641–653. doi:10.1021/ar700256a
Return to citation in text: [1] -
Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. Chem. Rev. 2016, 116, 2478–2601. doi:10.1021/acs.chemrev.5b00484
Return to citation in text: [1] -
Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Acc. Chem. Res. 2005, 38, 386–395. doi:10.1021/ar0400995
Return to citation in text: [1] -
Bowman, W. R.; Storey, J. M. D. Chem. Soc. Rev. 2007, 36, 1803–1822. doi:10.1039/b605183a
Return to citation in text: [1] -
Studer, A.; Bossart, M. Homolytic Aromatic Substitutions. In Radicals in Organic Synthesis; Renaud, P.; Sibi, M. P., Eds.; Wiley-VCH: Weinheim, Germany, 2001; Vol. 2, pp 62–80. doi:10.1002/9783527618293.ch29
Return to citation in text: [1]
| 1. | Godula, K.; Sames, D. Science 2006, 312, 67–72. doi:10.1126/science.1114731 |
| 2. | Davies, H. M. L.; Morton, D. J. Org. Chem. 2016, 81, 343–350. doi:10.1021/acs.joc.5b02818 |
| 13. | Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. J. Am. Chem. Soc. 2020, 142, 5461–5476. doi:10.1021/jacs.0c01416 |
| 14. | Yang, Z.; Liu, Y.; Cao, K.; Zhang, X.; Jiang, H.; Li, J. Beilstein J. Org. Chem. 2021, 17, 771–799. doi:10.3762/bjoc.17.67 |
| 15. | Kandukuri, S. R.; Bahamonde, A.; Chatterjee, I.; Jurberg, I. D.; Escudero-Adán, E. C.; Melchiorre, P. Angew. Chem., Int. Ed. 2015, 54, 1485–1489. doi:10.1002/anie.201409529 |
| 16. | Arceo, E.; Jurberg, I. D.; Álvarez-Fernández, A.; Melchiorre, P. Nat. Chem. 2013, 5, 750–756. doi:10.1038/nchem.1727 |
| 17. | Yuan, Y.-q.; Majumder, S.; Yang, M.-h.; Guo, S.-r. Tetrahedron Lett. 2020, 61, 151506. doi:10.1016/j.tetlet.2019.151506 |
| 18. | Tian, Y.-M.; Hofmann, E.; Silva, W.; Pu, X.; Touraud, D.; Gschwind, R. M.; Kunz, W.; König, B. Angew. Chem., Int. Ed. 2023, 62, e202218775. doi:10.1002/anie.202218775 |
| 31. | Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Acc. Chem. Res. 2005, 38, 386–395. doi:10.1021/ar0400995 |
| 32. | Bowman, W. R.; Storey, J. M. D. Chem. Soc. Rev. 2007, 36, 1803–1822. doi:10.1039/b605183a |
| 33. | Studer, A.; Bossart, M. Homolytic Aromatic Substitutions. In Radicals in Organic Synthesis; Renaud, P.; Sibi, M. P., Eds.; Wiley-VCH: Weinheim, Germany, 2001; Vol. 2, pp 62–80. doi:10.1002/9783527618293.ch29 |
| 7. | Bortolato, T.; Cuadros, S.; Simionato, G.; Dell’Amico, L. Chem. Commun. 2022, 58, 1263–1283. doi:10.1039/d1cc05850a |
| 8. | Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075–10166. doi:10.1021/acs.chemrev.6b00057 |
| 9. | Rosso, C.; Cuadros, S.; Barison, G.; Costa, P.; Kurbasic, M.; Bonchio, M.; Prato, M.; Dell’Amico, L.; Filippini, G. ACS Catal. 2022, 12, 4290–4295. doi:10.1021/acscatal.2c00565 |
| 10. | Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898–6926. doi:10.1021/acs.joc.6b01449 |
| 11. | Rosso, C.; Filippini, G.; Prato, M. Eur. J. Org. Chem. 2021, 1193–1200. doi:10.1002/ejoc.202001616 |
| 12. | Filippini, G.; Silvi, M.; Melchiorre, P. Angew. Chem., Int. Ed. 2017, 56, 4447–4451. doi:10.1002/anie.201612045 |
| 6. | Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Chem. Rev. 2022, 122, 3637–3710. doi:10.1021/acs.chemrev.1c00631 |
| 29. | Rosokha, S. V.; Kochi, J. K. Acc. Chem. Res. 2008, 41, 641–653. doi:10.1021/ar700256a |
| 3. | Wencel-Delord, J.; Glorius, F. Nat. Chem. 2013, 5, 369–375. doi:10.1038/nchem.1607 |
| 4. | He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754–8786. doi:10.1021/acs.chemrev.6b00622 |
| 5. | Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236–10254. doi:10.1002/anie.201203269 |
| 30. | Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. Chem. Rev. 2016, 116, 2478–2601. doi:10.1021/acs.chemrev.5b00484 |
| 23. | Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Org. Lett. 2016, 18, 4638–4641. doi:10.1021/acs.orglett.6b02271 |
| 12. | Filippini, G.; Silvi, M.; Melchiorre, P. Angew. Chem., Int. Ed. 2017, 56, 4447–4451. doi:10.1002/anie.201612045 |
| 21. | Cuadros, S.; Rosso, C.; Barison, G.; Costa, P.; Kurbasic, M.; Bonchio, M.; Prato, M.; Filippini, G.; Dell’Amico, L. Org. Lett. 2022, 24, 2961–2966. doi:10.1021/acs.orglett.2c00604 |
| 13. | Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. J. Am. Chem. Soc. 2020, 142, 5461–5476. doi:10.1021/jacs.0c01416 |
| 19. | Lima, C. G. S.; de M. Lima, T.; Duarte, M.; Jurberg, I. D.; Paixão, M. W. ACS Catal. 2016, 6, 1389–1407. doi:10.1021/acscatal.5b02386 |
| 28. | Buzzetti, L.; Crisenza, G. E. M.; Melchiorre, P. Angew. Chem., Int. Ed. 2019, 58, 3730–3747. doi:10.1002/anie.201809984 |
| 15. | Kandukuri, S. R.; Bahamonde, A.; Chatterjee, I.; Jurberg, I. D.; Escudero-Adán, E. C.; Melchiorre, P. Angew. Chem., Int. Ed. 2015, 54, 1485–1489. doi:10.1002/anie.201409529 |
| 21. | Cuadros, S.; Rosso, C.; Barison, G.; Costa, P.; Kurbasic, M.; Bonchio, M.; Prato, M.; Filippini, G.; Dell’Amico, L. Org. Lett. 2022, 24, 2961–2966. doi:10.1021/acs.orglett.2c00604 |
| 22. | Nappi, M.; Bergonzini, G.; Melchiorre, P. Angew. Chem., Int. Ed. 2014, 53, 4921–4925. doi:10.1002/anie.201402008 |
| 19. | Lima, C. G. S.; de M. Lima, T.; Duarte, M.; Jurberg, I. D.; Paixão, M. W. ACS Catal. 2016, 6, 1389–1407. doi:10.1021/acscatal.5b02386 |
| 20. | Slama-Schwok, A.; Blanchard-Desce, M.; Lehn, J.-M. J. Phys. Chem. 1990, 94, 3894–3902. doi:10.1021/j100373a007 |
| 24. | Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608–9644. doi:10.1002/anie.200901843 |
| 25. | Su, Y.-M.; Hou, Y.; Yin, F.; Xu, Y.-M.; Li, Y.; Zheng, X.; Wang, X.-S. Org. Lett. 2014, 16, 2958–2961. doi:10.1021/ol501094z |
| 26. | Liu, F.; Li, P. J. Org. Chem. 2016, 81, 6972–6979. doi:10.1021/acs.joc.6b00689 |
| 27. | Mi, X.; Kong, Y.; Zhang, J.; Pi, C.; Cui, X. Chin. Chem. Lett. 2019, 30, 2295–2298. doi:10.1016/j.cclet.2019.09.040 |
© 2023 Mamone et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.