Abstract
The past decade witnessed remarkable success in synthetic molecular nanographenes. Encouraged by the widespread application of chiral nanomaterials, the design, and construction of chiral nanographenes is a hot topic recently. As a classic nanographene unit, hexa-peri-hexabenzocoronene generally serves as the building block for nanographene synthesis. This review summarizes the representative examples of hexa-peri-hexabenzocoronene-based chiral nanographenes.
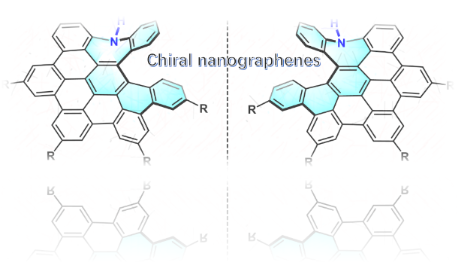
Graphical Abstract
Introduction
Graphene, an allotrope of carbon, has captured widespread attention since it was first experimentally demonstrated as a monolayer of carbon atoms [1]. Graphene and graphene-based materials are consisted of sp2 carbons and have shown a variety of outstanding applications, particularly in electronic devices as conductor materials with a zero band gap [2,3]. Compared to infinite graphene, nanoscale graphene fragments exhibit size-dependent non-zero bandgaps, which significantly enhanced their semiconducting character [4,5]. Hence, the preparation of graphene segments, so-called nanographenes (NGs), has received much attention. In materials science, graphene fragments have been obtained by “cutting” graphene materials through specific chemical methods, which leads to non-homogeneous NGs [6]. To have structurally well-defined NGs, the chemical synthetic approach offers an alternative and superior access. As a result, a variety of synthetic molecular NGs have been prepared through a bottom-up approach in recent years [7-9]. These newly designed NGs with tailor-made sizes and structures not only show planar conformation as fragments of graphene, but also evolved with a diversity of other shapes, including bowls [10], saddles [11-13], helixes [14,15], belts [16], rings [17], and their hybrid conformations such as helical bilayers [18], and nanoribbons [19]. Meanwhile, to modulate the properties of synthetic hydrocarbon NGs, a variety of heteroatom-doped NGs have been constructed [8].
Chirality is a fundamental phenomenon in nature and plays an important role in science, especially in living systems. The chirality in chemistry refers to the molecules which cannot be superimposed on their own mirror image. Nanomaterials with chiroptical properties have shown potential applications in different fields such as optical devices, polarization-based information encryption, circularly polarized luminescence (CPL) lasers, or biosensing [20-24]. Currently, there is a great interest in the introduction of chirality into large conjugated polyaromatics to obtain chiral NGs with chiroptical properties. Among these chiral NGs, helicenes represent the dominant chiral compounds by virtue of their inherent helical chirality [25-28]. Meanwhile, the introduction of strain into polyaromatic systems to generate Gaussian curvature or twistedness offers an alternative approach to forming chiral NGs [14].
As a classic NG unit, hexa-peri-hexabenzocoronene (p-HBC), a D6h-symmetry, planar π-scaffold (Scheme 1), and its derivatives have attracted increasing attention due to their specific optoelectronic properties [5,29]. HBC thus generally serves as a reference structure or building block to construct chiral NGs which are currently receiving a lot of interest in carbon-based materials science. This review will mainly focus on representative examples of HBC-based chiral molecular NGs. All the examples in the context can be treated either as HBC-like monomers, or HBC-based dimers, trimers, tetramers, or oligomers.
Scheme 1: Construction of HBC by Scholl reaction from hexaphenylbenzene.
Scheme 1: Construction of HBC by Scholl reaction from hexaphenylbenzene.
The synthesis of HBC is shown in Scheme 1. Pioneered by Scholl [30] and Clar [31] and further developed by Müllen [29], HBC was simply constructed through the Scholl-type oxidative intramolecular cyclodehydrogenation of hexaphenylbenzene. Accordingly, in the current cases in this review, the HBC-based chiral NGs were all established through oxidative cyclodehydrogenation by employing different oligophenylene precursors.
Review
seco-HBC-based chiral NGs
In the HBC synthesis, a small fraction of a partially cyclodehydrogenated intermediate was indeed isolated during the conversion of hexaphenylbenzene to HBC [32]. Conceptually, the partially ring-closed intermediate by cutting one C–C bond of HBC can be treated as seco-HBC, which gives a helical chiral structure. Based on this incomplete cyclizing strategy, types of seco-HBC-based chiral NGs were synthesized. Shown in Scheme 2 are the [5]helicenes that were synthesized from the corresponding hexaarylbenzenes as incompletely cyclized products during the Scholl reaction. To prevent planarization during the final oxidative cyclodehydrogenation reaction step, Jux and co-workers synthesized hexaarylbenzene precursor 3 with sterically demanding tert-butyl groups through a standard [4 + 2] Diels–Alder reaction of tetra-tert-butyltolane 1 and tetracyclone 2 in an 81% yield. The Scholl reaction of compound 3 in the presence of FeCl3 and nitromethane led to the [5]helicene containing, seco-HBC-based chiral NG 4 in an 80% yield [33]. Miao and co-workers reported a twisted chiral NG 7 by a partially cyclized Scholl reaction. By treating compound 6 in 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) and methanesulfonic acid in dichloromethane, the helical structure 7 was obtained in a 72% yield [34]. The possible reason for this incomplete cyclization is the electronic effect of the alkoxy groups. Meanwhile, the methoxy version was also synthesized from precursor 5. The helical conformation was confirmed by the single-crystal X-ray crystallography of its methoxy-substituted analogue.
Scheme 2: Synthesis of seco-HBC-based chiral nanographenes.
Scheme 2: Synthesis of seco-HBC-based chiral nanographenes.
Another method to obtain the incompletely cyclized seco-HBC is the introduction of a non-hexagonal ring into the precursors of HBC. Due to the large ring strain, the precursors are not prone to form planar structures through the oxidative cyclodehydrogenation reaction. Campaña, Morcillo, and co-workers synthesized a series of seco-HBC-based chiral NGs by introducing an octagon or a nonagon ring into the HBC skeleton (Scheme 2). The octagon-containing hexaphenylbenzene 9 was prepared from dibenzocyclooctyne 8 and tetracyclone 2 in a 91% yield. After a subsequent sequence of deprotection and oxidation, ketone 10 was obtained. Through the oxidative cyclodehydrogenation reaction of 10 in the presence of DDQ and trifluoromethanesulfonic acid (TfOH), a saddle-helix hybrid nanographene 11, bearing an octagon-containing carbo[5]helicene was isolated in a 61% yield, which can be further transformed into other chiral hydrocarbon analogues 12 and 13 [35]. Chiral resolution of these helicenes was accomplished by chiral high-performance liquid chromatography (HPLC) and the isomerization barriers were determined for the enantiopure helicenes. For compound 12, the racemization barrier was 24.8 kcal mol−1 at 298 K, which provides a racemization half-life (t1/2) of 24.4 h. For compounds 11 and 13, neither racemization nor decomposition was observed by chiral HPLC after heating hexadecane solutions of each compound at 200 °C for 5 h, which gives a lower limit to the barrier of racemization of ΔG > 38.3 kcal mol−1 at 473 K. The circularly polarized luminescence (CPL) of these structures were also evaluated with the luminescence dissymmetry factors (glum) values of 4 × 10−4 for compound 11 and 7 × 10−4 for compound 12. Meanwhile, Campaña, Morcillo, and co-workers converted the eight-membered ring 11 to nonagon-containing carbohelicene 14 through a single ring expansion reaction by treating 11 with TMSCHN2 (Scheme 2). By reacting with Tebbe’s reagent, compound 14 could be converted to all-carbon analogue 15. And the derivative 16 was also synthesized by allylic oxidation of compound 15 using selenium dioxide. As helical chiral NGs, helicene 14 and its derivatives 15 and 16 showed highly distorted helical conformation and also exhibited a relatively high isomerization barrier (over 28.9 kcal/mol determined at 90 °C) [36].
It is now well-documented that the incorporation of heteroatoms into the graphene materials or NG molecules can effectively tune their electronic and optical properties. Examples in Scheme 3 show the nitrogen-doped, seco-HBC-based chiral NGs. By introducing a pyrrole ring, Jux and co-workers reported pyrrole-containing helical NGs 19 and 20. The precursors 17 and 18 were synthesized from pyrrole-containing alkynes and tetracyclone 2 through a typical Diels–Alder reaction. The pair of enantiomers of these aza-[5]helicenes was confirmed by the X-ray crystal structure of racemic structure 13 [37]. Draper’s group reported the pyrimidine-containing N-doped NGs (Scheme 3). Tri-pyrimidine precursors 21 and 23 were obtained by trimerization of 5-(phenylethynyl)pyrimidine in the presence of Co2(CO)8. Upon treatment of the tri-pyrimidine containing hexarylbenzene compounds 21 or 23 under Scholl reaction conditions (DDQ, H+; or FeCl3, CH3NO2), the aza-[5]helicenes 22 and 24 were obtained respectively with 60% and 23% yields [38]. It was noted that with the installation of two adjacent pyrimidines in this hexarylbenzene precursor, a fully cyclized planar NG was formed toward Scholl reaction [39]. Recently, An and co-workers reported a helical, azepine-containing NG 27 by the introduction of an NH in the seco-HBC framwork (Scheme 3). Single-crystal X-ray diffractometry revealed the mixed P/M enantiomers. Taking advantage of the grafting NH functional group, the optical resolution of this helical aza-NG was achieved by introducing a chiral auxiliary reagent at the nitrogen site [40], and the racemization barrier of one enantiomer was measured as 26.2 kcal/mol by monitoring the changes of CD spectra at 60–80 °C. The synthesis started with the Diels−Alder reaction of 5H-dibenzo[b,f]azepine and tetrabromothiophene-S,S-dioxide, followed by oxidative aromatization in the presence DDQ to afford compound 25 in an overall 75% yield. Suzuki−Miyaura cross-coupling reaction of compound 25 with (4-ethylphenyl)boronic acid in the presence of Pd(CH3CN)2Cl2, SPhos, and K3PO4 then furnished the target hexaphenylbenzene 26 in an 80% yield. Compound 26 was treated with excess DDQ (10.0 equiv) in the presence of triflic acid to furnish the fourfold cyclization product 27 in a 60% yield. Notably, no fivefold cyclized product was detected. Meanwhile, by introducing an aza-eight-membered ring in the hexarylbenzene derivative 30, An and co-workers also reported an azocine-embedded, [5]helicene containing NG 31 [41]. The precursor 30 was synthesized by Diels–Alder reaction of aza-alkyne 28 and tetracyclone 29. By treating compound 30 under Scholl reaction conditions, the helical structure 31 was obtained through oxidative cyclodehydrogenation and imine formation in a 72% yield. It was noted that no other regioisomer was detected for the asymmetrical precursor 30. The pair of enantiomers was confirmed by the racemic structure's single-crystal X-ray diffractometry and separated by chiral HPLC, and no racemization was observed by heating the enantiomer for 1 hour at 120 °C.
Scheme 3: Synthesis of nitrogen-doped, seco-HBC-based chiral nanographenes.
Scheme 3: Synthesis of nitrogen-doped, seco-HBC-based chiral nanographenes.
Except for [5]helicene, Narita and co-workers also reported [7]- or [9]helicene-containing chiral NGs [42]. As shown in Scheme 4, dibromo-functionalized 1,2,3,4-tetraphenylbenzene 32 was treated with DDQ and TfOH to produce dibromo 33 as the prefused building block in a 49% yield. Then naphthalene and phenanthrene residues were introduced into compound 33 through Suzuki coupling reaction, the corresponding precursors 34 and 36 were obtained respectively in high yields. The final cyclodehydrogenation using DDQ and TfOH proceeded regioselectively at 0 °C, affording the desired π-extended [7]helicene 35 and [9]helicene 37 in 76 and 84% yields, respectively. Single-crystal structures of racemic 35 and 37 revealed the P/M enantiomers repectively, and the P/M racemization barriers of 35 and 37 were determined as 42.4 and 41.6 kcal/mol, respectively by DFT calculation. The enantiopure isomers of the π-extended helicenes were evaluated as excellent circularly polarized luminescence (CPL) emitters with a glum of 7.44 × 10−3 for 37, which is around 10-fold higher than 35.
Scheme 4: Synthesis of π-extended [7]- and [9]helicene containing chiral nanographenes.
Scheme 4: Synthesis of π-extended [7]- and [9]helicene containing chiral nanographenes.
Chiral “HBC-dimers” and “HBC trimers”
The combination of two or more HBCs or HBC-like units into one ring system is a straightforward way to generate HBC-based chiral NGs. In general, different HBC units were fused together to form the final chiral structures by connecting with a linker or sharing the same phenyl ring. As shown in Scheme 5, Jux and co-workers synthesized the NG precursor 39 by heating dialkyne 38 and tetracyclone 2, which led to two hexarylbenzene units connected by an oxygen linker. Upon treatment of 39 under Scholl reaction conditions (DDQ, TfOH), an oxa-[7]helicene containing chiral NG 40 was obtained in high yield [43]. Meanwhile, they enlarged this [7]helicene family by introducing different linkers such as nitrogen, sulfur, sulfone, ketone, methylene, and derivatives of ketone [44]. Due to the varied nature of the different linkers, the photophysical and semiconductor properties can be effectively tuned. Feng and co-workers reported a helical NG 44 containing [6]helicene structure and an azulene unit (Scheme 5). Through a two-fold Diels–Alder cycloaddition from 1,4-bis(2-ethynylphenyl)buta-1,3-diyne (41) and tetracyclone 11, alkyne 42 was obtained in an 83% yield. Then unique diiodide precursor 43 was obtained by ICl-mediated benzannulation of diacetylene 42 in an 85% yield. The final NG 44 formed by treating compound 43 with DDQ/TfOH at 0 °C and the overall structure exhibits interesting photophysical and antiaromatic properties [45]. During the final Scholl reaction, an monoiodide structure 101 was also isolated (Scheme 11).
Scheme 5: Synthesis of “HBC-dimer”-based chiral nanographenes.
Scheme 5: Synthesis of “HBC-dimer”-based chiral nanographenes.
In the pursuit of HBC-tetramer-based supertwistacene (compound 115 in Scheme 12), Wang and co-workers synthesized a chiral HBC-dimer 46 [46], in which two HBC units structurally shared one benzene ring (Scheme 6). 1,4-Bis((4-(tert-butyl)phenyl)ethynyl)benzene reacted with tetracyclone 2 through a two-fold Diels–Alder cycloaddition to afford compound 45 in a 79% yield. Due to the steric hindrance from two bulky tert-butyl groups on the benzene rings in the adjacent hexaphenylbenzene monomers in precursor 45, two [5]helicenes were formed in the oxidative cyclodehydrognation reaction, which gave compound 46 as the P,P, and M,M enantiomers. The isomerization barrier was determined as 44.7 kcal/mol at 260 °C by HPLC analysis. Meanwhile, Campaña and co-workers reported a similar chiral, tropone-containing HBC-dimer 53 [47]. Ketone 47 was transformed to compound 48 by reacting with 1-iodo-4-(phenylethynyl)benzene through Co-catalyzed cyclotrimerization in a 45% yield. Then monoiodide NG 49 was obtained through oxidative cyclodehydronation in a high yield. From the heptagon-containing NG 49, Sonogashira coupling with p-tert-butylphenylacetylene (50) afforded 51 in a quantitative yield. Subsequent Diels–Alder reaction with cyclopentadienone 2 gave polyphenylene 52 in a 34% yield. Unlike the double helical structure of NG 48, the lack of the bulky tert-butyl group on one side in precursor 52, only one [5]helicene was generated in the Scholl reaction during the formation of the final structure 53. The Gibbs activation energy of enantiomer 53 for the racemization process was determined as 33.0 kcal mol−1 at 298 K. The CPL spectra of M-53 and P-53 showed an emission maximum at 560 nm with glum value of 2.3 × 10−4. Instead of helicene formation in the final Scholl-type ring formation step, Martín and co-workers synthesized a helical bilayer NG by using helicene in the initial step as the linker to fuse two HBC units [48]. As shown in Scheme 6, starting from the helical alkyne 54, Sonogashira coupling with 4-tert-butyliodobenzene (55) afforded structure 56 in a 77% yield. Subsequent Diels–Alder reaction with cyclopentadienone 2 generated compound 57. The final Scholl reaction of 57 afforded helical NG 58 in high yield. This folded NG 58 is composed of two HBC layers fused to a [10]helicene with an interlayer distance of 3.6 Å as determined by X-ray crystallography. Since the helicene was initially introduced as a linker, an optically pure chiral NG synthesis was accomplished using the same synthetic route by starting with an enantiopure helicene.
Scheme 6: Synthesis of “HBC-dimer”-based chiral nanographenes.
Scheme 6: Synthesis of “HBC-dimer”-based chiral nanographenes.
Except for the helicene-based chirality, chirality stemming from a chiral axis is relatively rare in designing chiral NGs. Notably, Martín and co-workers reported a new family of chiral NGs, which constituted by two orthogonal dibenzo[fg,ij]phenanthro-[9,10,1,2,3-pqrst]pentaphene (DBPP) units covalently connected through tetrafluorophenylene (61) [49] or octafluoro-9,10-anthracenylene (65) [50] linkers. The synthesis started with a double Pd-catalyzed Sonogashira cross-coupling reaction of phenylacetylene 50 and 1,4-dibromotetrafluorobenzene. The resulting bis[aryl(ethynyl)]tetrafluorobenzene 59 was able to undergo a 2-fold [4 + 2] cycloaddition reaction with cyclopentadienone 2, affording polyaromatic 60 in a 70% yield. The final step was the Scholl cyclodehydrogenation of polyphenylene 60 by reaction with DDQ in the presence of TfOH at 0 °C, to afford NG 61 in a good yield. The helical arrangement of these three covalently linked molecular fragments leads to the existence of a chiral axis which gives rise to a racemic mixture. The axis-based helical chiral structures were confirmed by single-crystal X-ray diffractometry and chiral HPLC. The rotational isomerization barrier of C6F4 ring for the analogue of 62, with two tert-butyl groups substituted by protons was determined as 24.6 kcal/mol at 40 °C with a half-life of t1/2 = 107 min. Employing the similar synthetic procedure, NG 65 was also synthesized as a chiral axis-based chiral NG by starting with octafluoroanthracen. Recently, An and co-workers reported an atropisomeric chiral nanographene with chirality purely stemming from an axially chiral binaphthyl. As shown in Scheme 7, the Diels–Alder reaction of benzo[b]naphtho[2,3-f]oxepine 66 with tetrabromothiophene-S,S-dioxide in toluene followed by oxidative aromatization in the presence of DDQ afforded tetrabrominated aromatics 67 in an 81% yield. Subsequently, fourfold Suzuki–Miyaura cross-coupling of polybrominated compound 67 was performed, affording hexaphenylbenzene 68 in an 85% yield. Then the Scholl cyclodehydrogenation of compound 68 was carried out in DDQ/TfOH to afford NG 69 in a 64% yield. By the selective dimerization of azopine-containing, HBC-monomer 69, the novel BINOL-like, atropisomeric chiral oxa-nanographene 70 was obtained in high yield. Meanwhile, this NG 70 can also be synthesized from compound 68 through oxidative cyclodehydrognation and dimerization in one pot. The isomerization barrier was determined as over 35 kcal/mol at 170 °C, and 37 kcal/mol by DFT calculation, indicating a high degree of optical stability of pure enantiomers [51].
Scheme 7: Synthesis of axis-based chiral nanographenes.
Scheme 7: Synthesis of axis-based chiral nanographenes.
When three HBCs were fused together linearly, the graphene nanoribbons would be constructed. Wang [46] and Campaña [52] groups separately reported the synthesis of graphene nanoribbon 73 (Scheme 8), which contains four [5]carbohelicenes due to bulky tert-butyl groups as lateral chains. In brief, diiodide NG 71 reacted with phenylacetylene 50 through Sonogashira cross-coupling, followed by Diels–Alder reaction with tetracyclone 2 to afford precursor 72 in an overall 22% yield. Then the final helical NG 73, which contains different conformations was obtained through Scholl reaction in the presence of DDQ and TfOH in a 29% yield. In careful checking of conformations of each fraction, except for achiral meso isomers (P,P,M,M), a pair of helical enantiomers were confirmed (M,M,M,M and P,P,P,P). Whereas, the nanoribbons 76 and 77, which contain a seven-membered ring in the molecular skeleton, following the similar synthetic procedure from the dibromo 74 only gave achiral meso isomers (P,P,M,M) [52].
Scheme 8: Synthesis of “HBC-trimers”-based nanoribbons.
Scheme 8: Synthesis of “HBC-trimers”-based nanoribbons.
Wang and co-workers synthesized the triangular NG 82 by oxidative cyclodehydrogenation of the hexaphenylbenzene-trimer 81 [53]. As shown in Scheme 9, two-fold Sonogashira cross-coupling reaction of trimethylsilylacetylene and monobromo 78 afforded HBD-dimer 79. Then the Diels–Alder reaction of compound 79 and tetracyclone 80 gave compound 81 in a medium yield. Due to the crowdedness of adjacent HBC units, a chiral [7]helicene 82 was formed during the Scholl reaction of precursor 81. Campaña and co-workers synthesized a [7]carbohelicene-centered HBC-trimer 86 with two tropones [54] in the HBC units. As shown in Scheme 10, Sonogashira cross-coupling reaction of alkyne 83 and iodide 49 afforded compound 84 in a high yield. Then the precursor 85 was obtained by Diels–Alder reaction of 84 and tetracyclone 2. The final Scholl cyclodehydrogenation of 85 afforded the HBC-trimer 86. The non-linear optics of NG 86 and the chiroptical properties of enantiomers were well studied. The CPL spectra centered at 610 nm were observed for both enantiomeric forms and the glum was determined as 2 × 10−3. Due to large racemization barrier for [7]helicene, neither racemization nor decomposition was observed for optical pure 86 at 180 or 200 °C for 24 or 4 hours. Recently, they reported another HBC-trimer 91 consisting of only one tropone [55]. Dibromo 87 was converted into its corresponding distorted hept-HBC 88 by oxidative cyclodehydrogenation in the presence of DDQ/TfOH in an 83% yield. Through two-fold Sonogashira coupling reaction with 4-tert-butylphenylacetylene (50), compound 88 was converted to alkyne 89. Then compound 89 was subjected to a double Diels–Alder reaction with cyclopentadienone 2, affording precursor 90 in a 64% yield. By the steric effect of the existing tert-butyl groups in precursor 90, another two extended carbo[5]helicenes were formed in the final Scholl reaction of precursor 90 except for the centred carbo[7]helicene in the final structure 91. Initial analysis of the final products by chiral stationary phase HPLC suggested four main peaks were detected, belonging to two pairs of enantiomers. Notably, one pair of enantiomers can transform to the thermodynamically more stable (M,M,P)-91 and (P,P,M)-91 enantiomers after 16 hours heating. Compared to structures 82 and 86, the photoluminescence quantum yields of 91 was significantly improved. The enantiomers of 91 were proved as NIR-CPL emitters with 600 to 800 nm range of emission and glum values of 3 × 10−3 both in solution and in the solid state.
Scheme 9: Synthesis of “HBC-trimers”-based, triangle-shaped chiral nanographenes.
Scheme 9: Synthesis of “HBC-trimers”-based, triangle-shaped chiral nanographenes.
Scheme 10: Synthesis of “HBC-trimers”-based, triangle-shaped chiral nanographenes.
Scheme 10: Synthesis of “HBC-trimers”-based, triangle-shaped chiral nanographenes.
Multilayer (e.g., bilayer and trilayer) graphenes that are composed of stacked graphenic layers exhibit outstanding electronic and optical features which are not available with single-layer graphenes. The helical bilayer NG 58 developed by Martín and co-workers has been described. Recently, some more complex multilayer NGs were reported. As shown in Scheme 11, Tan and co-workers reported trilayer chiral nanographenes 96 and 100 using helicene or oxa-helicene as the linkers, respectively [56]. The first π-extension started from the borylated penta-tert-butyl HBC 92. The chemical selective Suzuki−Miyaura cross-coupling reaction between 92 and 93 followed by Scholl oxidation produced compound 94 in an overall 50% yield. Alkyne 50 was coupled to 94 by Sonogashira coupling (Scheme 11), followed by Diels–Alder reaction with tetracyclone 2, giving the phenylethynyl-functionalized double-helical nanographene 95. The conversion of 95 into nanographene sheets by Scholl oxidation finally afforded the trilayer nanographene 96. And the trilayer nanographene 100 containing two oxa[6]helicenes was obtained through a synthetic route similar to that of 96. The structure of hydrocarbon NG 96 exhibited a typical overlapped structure with an interlayer distance of 4.1 Å and consisted of two [8]helicenes as the linker between two HBC layers suggested by DFT optimized structure. The furan containing NG 100 revealed a looser conformation than compound 96 with less overlap between two layers and a longer interlayer distance of 4.9 Å suggested by single crystal X-ray diffraction. The enantiomers of (P,P)-96 and (M,M)-96 or (P,P)-100 and (M,M)-100 were well separated by chiral HPLC, and the CD and CPL spectra were investigated for each enantiopure compound. The glum values were measured as 1 × 10−3 and 3 × 10−3 for 96 and 100, respectively. Following their reported structure 44, Feng, Ma, and co-workers reported a helical bilayer, non-benzenoid nanographene 58 which contains a [10]helicene with two embedded heptagons as a novel chiral moiety (Scheme 11). By using the same intermediate 43, the iodine-NG 101 together with NG 44 was obtained in a 22% yield. Then compound 101 was coupled with 4-tert-butylphenylacetylene through a Sonogashira reaction followed by Diels–Alder reaction with tetracyclone 2, to give the precursor 102 in an overall 40% yield. The Scholl reaction of 102 using DDQ/TfOH at 0 °C provided the NG 103. Single-crystal X-ray diffraction analysis of 103 clearly elucidates a closer interlayer distance of 3.2 Å compared with 96 and 100 [57]. The resolution of racemic 58 into its two enantiomers was achieved by chiral stationary phase HPLC. The CPL spectra showed a maximum centered at 688 nm with glum as 1.3 × 10−3.
Scheme 11: Synthesis of HBC-based multilayer nanographenes.
Scheme 11: Synthesis of HBC-based multilayer nanographenes.
Chiral NGs containing more than three HBC units
Recently, by fusing four HBC units together, Gong and co-workers synthesized a novel chiral NG 110 (Scheme 12) with a pentadecabenzo[9]helicene core fragment [58]. The synthesis of 110 started from iodohexabenzocoronene 104. A Sonogashira coupling between 104 and compound 105 gave compound 106. Then selective Diels–Alder reaction between compound 106 and tetracyclone 80, followed by removing the triisopropylsilane protective group using tetrabutylammonium fluoride (TBAF) provided compound 107. Then, a second Sonogashira coupling between 107 and 104 produced compound 108, followed by a second Diels–Alder reaction between 108 and 80, which formed intermediate 109 in a high yield. Finally, DDQ and TfOH induced the Scholl oxidation of 109 to generate 110 in a yield of 24%. Single-crystal X-ray diffraction unambiguously confirmed the helical conformation and a pair of enantiomers. Chiral resolution of enantiomers was achieved by chiral HPLC. Due to the large conjugated structure, NG 110 exhibited a significantly red-shifted emission in the near-infrared (NIR) region. Furthermore, the structure extension causes significant improvements both in the fluorescence quantum yield and CPL properties. The dissymmetry factors glum of (P)- and (M)-110 at 684 nm were up to 4.50 × 10−2 and 4.22 × 10−2, respectively. Wang and co-workers reported a chiral graphene nanoribbon 115 (Scheme 12) by linearly fusing four HBS units in a helical manner [46]. The synthesis started by construction of a HBC-dimer 111. para-Iodization of 111 gave compound 112 in a 91% yield. Scholl oxidation of 112, and then Sonogashira coupling of 113 yielded the bisalkyne, which was ready for a second Diels−Alder reaction to give precursor 114. Through dehydrocyclization induced by DDQ and TfOH, precursor 114 was transformed to nanoribbon 115 in a 5% yield. This nanoribbon is 4.3 nm in length, with an end-to-end twist of 117° as indicated by single-crystal X-ray diffraction. Except for the helical conformation, two other isomers, in the waggling or mixed configuration were also generated in the last oxidative cyclodehydrogenation step, and they could be separated via preparative thin-layer chromatography. The P- and M-isomers of the helical conformation of 115 were separated by chiral HPLC, and the thermal racemization barrier was determined as 44.7 kcal/mol at 260 °C.
Scheme 12: Synthesis of a chiral nanographene constructed by “HBC-tetramers”.
Scheme 12: Synthesis of a chiral nanographene constructed by “HBC-tetramers”.
Campaña and co-workers also reported tropone-containing, four HBC-fused NG 117 [59]. The synthesis started with the preparation of the distorted HBC analogue 49, bearing an aryl iodide for the subsequent Sonogashira cross-coupling reaction with alkyne 50 to give 51. The precursor 116 containing three pre-existing HBCs was synthesized through Co-catalyzed cyclotrimerization of compound 51. Finally, compound 116 was subjected to a final oxidative cyclodehydrogenation to create the central HBC unit, leading to NG 117 (Scheme 13) Three carbo[5]helicene moieties were formed during the final Scholl reaction due to the incorporation of bulky t-Bu substituents. For the final NG 117, two diastereomers, C3-symmetric (P,P,P/M,M,M)-117 and C1-asymmetric (P,P,M/M,M,P)-117 were separated and each racemic diastereomer was resolved into the enantiomers by chiral HPLC. The CPL spectra of both enantiomers show a maximum centered at 643 nm, a glum value estimated as 3 × 10−4 for (P,P,P/M,M,M)-117. The glum of (P,P,M/M,M,P)-117 was measured as 2 × 10−4.
Scheme 13: Synthesis of a triskelion-shaped nanographene constructed by four HBCs.
Scheme 13: Synthesis of a triskelion-shaped nanographene constructed by four HBCs.
Cyclooctatetraphenylene (COT-Ph) is a π-conjugated scaffold, whose three-dimensional (3D) geometry is based on its saddle shape. Martín and co-workers reported the 3D NG 121 by the introduction of four HBC units into COT-Ph [60]. As shown in Scheme 14, functionalized COT-Ph reacted with compound 50 through Sonogashira cross-coupling reaction affording compound 119 in a 73% yield. Then Diels–Alder reaction between 119 and 2 gave structure 120 as NG precursor. Compound 120 underwent intramolecular cyclodehydrogenation mediated by FeCl3, affording the final NG 121 in a high yield. Due to the two conformations of cyclooctatetraene saddles, NG 121 is a chiral structure and optically pure enantiomers were separated using chiral high-performance liquid chromatography (HPLC), which featured a perfect mirror-image circular dichroism (CD) for both enantiomers.
Scheme 14: Synthesis of a three-dimensional nanographene bearing four HBCs.
Scheme 14: Synthesis of a three-dimensional nanographene bearing four HBCs.
Wang and co-workers constructed double π-extended undecabenzo-[7]helicene 125, which composed of five HBC units and 186 sp2 carbon atoms [53]. As shown in Scheme 15, a hexaphenylbenzene with four alkyne substitutions was constructed initially. A Sonogashira coupling from 122 to 123, followed by a Diels–Alder reaction, yielded the polyphenylene precursor 124 in an overall 12% yield. At last, a Scholl oxidation, mediated by DDQ and TfOH, gave the target NG 125 in a 6% yield. Due to the extremely large conjugated structure, compound 125 shows an extraordinary panchromatic light absorption capability (ε = 844 000 M−1 cm−1 at 573 nm). A pair of enantiomers, (M,M)- and (P,P)-configuration was revealed by single crystal X-ray diffraction and optically pure samples of 125 were isolated by chiral HPLC. Meanwhile, a record high electronic circular dichroism (ECD) signal in the visible spectral range (Δε = 1375 M−1 cm−1 at 573 nm at 430 nm) for enantiopure 125 was obtained.
Scheme 15: Synthesis of a chiral nanographene constructed by five HBC units.
Scheme 15: Synthesis of a chiral nanographene constructed by five HBC units.
In 2019, Wang and co-workers reported the largest atomically precise three-dimensional conjugated chiral nanographene propeller 128, representing the largest three-dimensional conjugated polyaromatics everprepared using scalable solution chemistry [61]. As shown in Scheme 16, similar to 125, a hexaphenylbenzene 126 with six alkyne substitutions was prepared first. Through a six-fold Diels–Alder reaction with 2, compound 126 was converted to 127 in a 50% yield. Finally, NG 128 was synthesized from polybenzenes 127 through a stepwise Scholl reaction from 0 °C to room temperature by removing 84 hydrogens total. NG 128 is composed of seven HBCs and exhibit an extraordinary size, which reaches 3.50 nm in width and 1.25 nm in height. Single crystals X-ray diffraction indicates only one form (M or P) of enantiomer which can be separated by chiral HPLC. Optically pure enantiomers show a series of strong mirror-image Cotton effects across the whole UV−NIR spectral window.
Scheme 16: Synthesis of a chiral nanographene constructed by seven HBC units.
Scheme 16: Synthesis of a chiral nanographene constructed by seven HBC units.
Conclusion
As demonstrated by the aforementioned success in synthesizing numerous chiral nanographenes, the HBC is a well-established reference structure to exploit three-dimensional aesthetic structures. The continuously developed novel structures contented our interests and curiosity. To further tune the optoelectronic or photophysical properties of nanographenes for real applications, heteroatom doping was found to be an effective strategy. The introduction of different main group elements beyond nitrogen summarized above would provide more diverse structures and properties. Meanwhile, the methodologies for further functionalization of the existing nanographene molecules are still needed for application purposes.
References
-
Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grigorieva, I. V.; Firsov, A. A. Science 2004, 306, 666–669. doi:10.1126/science.1102896
Return to citation in text: [1] -
Zhang, Y.; Tan, Y.-W.; Stormer, H. L.; Kim, P. Nature 2005, 438, 201–204. doi:10.1038/nature04235
Return to citation in text: [1] -
Geim, A. K.; Novoselov, K. S. Nat. Mater. 2007, 6, 183–191. doi:10.1038/nmat1849
Return to citation in text: [1] -
Rieger, R.; Müllen, K. J. Phys. Org. Chem. 2010, 23, 315–325. doi:10.1002/poc.1644
Return to citation in text: [1] -
Diez-Perez, I.; Li, Z.; Hihath, J.; Li, J.; Zhang, C.; Yang, X.; Zang, L.; Dai, Y.; Feng, X.; Muellen, K.; Tao, N. Nat. Commun. 2010, 1, 31. doi:10.1038/ncomms1029
Return to citation in text: [1] [2] -
Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Science 2008, 319, 1229–1232. doi:10.1126/science.1150878
Return to citation in text: [1] -
Narita, A.; Wang, X.-Y.; Feng, X.; Müllen, K. Chem. Soc. Rev. 2015, 44, 6616–6643. doi:10.1039/c5cs00183h
Return to citation in text: [1] -
Wang, X.-Y.; Yao, X.; Narita, A.; Müllen, K. Acc. Chem. Res. 2019, 52, 2491–2505. doi:10.1021/acs.accounts.9b00322
Return to citation in text: [1] [2] -
Zhang, Y.; Pun, S. H.; Miao, Q. Chem. Rev. 2022, 122, 14554–14593. doi:10.1021/acs.chemrev.2c00186
Return to citation in text: [1] -
Stuparu, M. C. Acc. Chem. Res. 2021, 54, 2858–2870. doi:10.1021/acs.accounts.1c00207
Return to citation in text: [1] -
Miao, Q.; Yang, D. Prog. Chem. 2020, 32, 1835–1845.
Return to citation in text: [1] -
Kirschbaum, T.; Rominger, F.; Mastalerz, M. Angew. Chem., Int. Ed. 2020, 59, 270–274. doi:10.1002/anie.201912213
Return to citation in text: [1] -
Márquez, I. R.; Fuentes, N.; Cruz, C. M.; Puente-Muñoz, V.; Sotorrios, L.; Marcos, M. L.; Choquesillo-Lazarte, D.; Biel, B.; Crovetto, L.; Gómez-Bengoa, E.; González, M. T.; Martin, R.; Cuerva, J. M.; Campaña, A. G. Chem. Sci. 2017, 8, 1068–1074. doi:10.1039/c6sc02895k
Return to citation in text: [1] -
Rickhaus, M.; Mayor, M.; Juríček, M. Chem. Soc. Rev. 2016, 45, 1542–1556. doi:10.1039/c5cs00620a
Return to citation in text: [1] [2] -
Li, R.; Ma, B.; He, R.-Y.; Zhang, B.; Zhang, Y.-K.; Feng, S.-Y.; An, P. Chem. – Asian J. 2022, 17, e202101365. doi:10.1002/asia.202101365
Return to citation in text: [1] -
Wang, S.; Yuan, J.; Xie, J.; Lu, Z.; Jiang, L.; Mu, Y.; Huo, Y.; Tsuchido, Y.; Zhu, K. Angew. Chem., Int. Ed. 2021, 60, 18443–18447. doi:10.1002/anie.202104054
Return to citation in text: [1] -
Wang, J.; Zhang, X.; Jia, H.; Wang, S.; Du, P. Acc. Chem. Res. 2021, 54, 4178–4190. doi:10.1021/acs.accounts.1c00505
Return to citation in text: [1] -
Milton, M.; Schuster, N. J.; Paley, D. W.; Hernández Sánchez, R.; Ng, F.; Steigerwald, M. L.; Nuckolls, C. Chem. Sci. 2019, 10, 1029–1034. doi:10.1039/c8sc04216k
Return to citation in text: [1] -
Jolly, A.; Miao, D.; Daigle, M.; Morin, J.-F. Angew. Chem., Int. Ed. 2020, 59, 4624–4633. doi:10.1002/anie.201906379
Return to citation in text: [1] -
Sang, Y.; Han, J.; Zhao, T.; Duan, P.; Liu, M. Adv. Mater. (Weinheim, Ger.) 2020, 32, 1900110. doi:10.1002/adma.201900110
Return to citation in text: [1] -
MacKenzie, L. E.; Pal, R. Nat. Rev. Chem. 2021, 5, 109–124. doi:10.1038/s41570-020-00235-4
Return to citation in text: [1] -
Xu, L.; Feng, Y.; Yu, D.; Zheng, Z.; Chen, X.; Hong, W. Adv. Mater. Technol. (Weinheim, Ger.) 2020, 5, 2000373. doi:10.1002/admt.202000373
Return to citation in text: [1] -
Zhang, Y.-P.; Zheng, Y.-X. Dalton Trans. 2022, 51, 9966–9970. doi:10.1039/d2dt01582j
Return to citation in text: [1] -
Hao, C.; Xu, L.; Kuang, H.; Xu, C. Adv. Mater. (Weinheim, Ger.) 2020, 32, 1802075. doi:10.1002/adma.201802075
Return to citation in text: [1] -
Li, C.; Yang, Y.; Miao, Q. Chem. – Asian J. 2018, 13, 884–894. doi:10.1002/asia.201800073
Return to citation in text: [1] -
Fernández-García, J. M.; Evans, P. J.; Filippone, S.; Herranz, M. Á.; Martín, N. Acc. Chem. Res. 2019, 52, 1565–1574. doi:10.1021/acs.accounts.9b00144
Return to citation in text: [1] -
Mori, T. Chem. Rev. 2021, 121, 2373–2412. doi:10.1021/acs.chemrev.0c01017
Return to citation in text: [1] -
Wu, Y.-F.; Zhang, L.; Zhang, Q.; Xie, S.-Y.; Zheng, L.-S. Org. Chem. Front. 2022, 9, 4726–4743. doi:10.1039/d2qo00988a
Return to citation in text: [1] -
Wu, J.; Pisula, W.; Müllen, K. Chem. Rev. 2007, 107, 718–747. doi:10.1021/cr068010r
Return to citation in text: [1] [2] -
Scholl, R.; Seer, C.; Weitzenböck, R. Ber. Dtsch. Chem. Ges. 1910, 43, 2202–2209. doi:10.1002/cber.191004302175
Return to citation in text: [1] -
Clar, E.; Stewart, D. G. J. Am. Chem. Soc. 1953, 75, 2667–2672. doi:10.1021/ja01107a035
Return to citation in text: [1] -
Kübel, C.; Eckhardt, K.; Enkelmann, V.; Wegner, G.; Müllen, K. J. Mater. Chem. 2000, 10, 879–886. doi:10.1039/a908941a
Return to citation in text: [1] -
Martin, M. M.; Hampel, F.; Jux, N. Chem. – Eur. J. 2020, 26, 10210–10212. doi:10.1002/chem.202001471
Return to citation in text: [1] -
Luo, J.; Xu, X.; Mao, R.; Miao, Q. J. Am. Chem. Soc. 2012, 134, 13796–13803. doi:10.1021/ja3054354
Return to citation in text: [1] -
Medel, M. A.; Tapia, R.; Blanco, V.; Miguel, D.; Morcillo, S. P.; Campaña, A. G. Angew. Chem., Int. Ed. 2021, 60, 6094–6100. doi:10.1002/anie.202015368
Return to citation in text: [1] -
Medel, M. A.; Cruz, C. M.; Miguel, D.; Blanco, V.; Morcillo, S. P.; Campaña, A. G. Angew. Chem., Int. Ed. 2021, 60, 22051–22056. doi:10.1002/anie.202109310
Return to citation in text: [1] -
Ammon, F.; Sauer, S. T.; Lippert, R.; Lungerich, D.; Reger, D.; Hampel, F.; Jux, N. Org. Chem. Front. 2017, 4, 861–870. doi:10.1039/c7qo00112f
Return to citation in text: [1] -
Wijesinghe, L. P.; Perera, S. D.; Larkin, E.; Ó Máille, G. M.; Conway-Kenny, R.; Lankage, B. S.; Wang, L.; Draper, S. M. RSC Adv. 2017, 7, 24163–24167. doi:10.1039/c7ra02648j
Return to citation in text: [1] -
Draper, S. M.; Gregg, D. J.; Madathil, R. J. Am. Chem. Soc. 2002, 124, 3486–3487. doi:10.1021/ja017394u
Return to citation in text: [1] -
Zhang, B.; Ruan, L.; Zhang, Y.-K.; Zhang, H.; Li, R.; An, P. Org. Lett. 2023, 25, 732–737. doi:10.1021/acs.orglett.2c04097
Return to citation in text: [1] -
An, P.; Li, R.; Ma, B.; He, R.-Y.; Zhang, Y.-K.; Xiao, M.-J.; Zhang, B. Angew. Chem., Int. Ed. 2021, 60, 24478–24483. doi:10.1002/anie.202110538
Return to citation in text: [1] -
Qiu, Z.; Ju, C.-W.; Frédéric, L.; Hu, Y.; Schollmeyer, D.; Pieters, G.; Müllen, K.; Narita, A. J. Am. Chem. Soc. 2021, 143, 4661–4667. doi:10.1021/jacs.0c13197
Return to citation in text: [1] -
Reger, D.; Haines, P.; Heinemann, F. W.; Guldi, D. M.; Jux, N. Angew. Chem., Int. Ed. 2018, 57, 5938–5942. doi:10.1002/anie.201800585
Return to citation in text: [1] -
Reger, D.; Haines, P.; Amsharov, K. Y.; Schmidt, J. A.; Ullrich, T.; Bönisch, S.; Hampel, F.; Görling, A.; Nelson, J.; Jelfs, K. E.; Guldi, D. M.; Jux, N. Angew. Chem., Int. Ed. 2021, 60, 18073–18081. doi:10.1002/anie.202103253
Return to citation in text: [1] -
Ma, J.; Fu, Y.; Dmitrieva, E.; Liu, F.; Komber, H.; Hennersdorf, F.; Popov, A. A.; Weigand, J. J.; Liu, J.; Feng, X. Angew. Chem., Int. Ed. 2020, 59, 5637–5642. doi:10.1002/anie.201914716
Return to citation in text: [1] -
Ma, S.; Gu, J.; Lin, C.; Luo, Z.; Zhu, Y.; Wang, J. J. Am. Chem. Soc. 2020, 142, 16887–16893. doi:10.1021/jacs.0c08555
Return to citation in text: [1] [2] [3] -
Cruz, C. M.; Márquez, I. R.; Mariz, I. F. A.; Blanco, V.; Sánchez-Sánchez, C.; Sobrado, J. M.; Martín-Gago, J. A.; Cuerva, J. M.; Maçôas, E.; Campaña, A. G. Chem. Sci. 2018, 9, 3917–3924. doi:10.1039/c8sc00427g
Return to citation in text: [1] -
Evans, P. J.; Ouyang, J.; Favereau, L.; Crassous, J.; Fernández, I.; Perles, J.; Martín, N. Angew. Chem., Int. Ed. 2018, 57, 6774–6779. doi:10.1002/anie.201800798
Return to citation in text: [1] -
Izquierdo-García, P.; Fernández-García, J. M.; Fernández, I.; Perles, J.; Martín, N. J. Am. Chem. Soc. 2021, 143, 11864–11870. doi:10.1021/jacs.1c05977
Return to citation in text: [1] -
Izquierdo-García, P.; Fernández-García, J. M.; Perles, J.; Fernández, I.; Martín, N. Angew. Chem., Int. Ed. 2023, 62, e202215655. doi:10.1002/anie.202215655
Return to citation in text: [1] -
Li, S.; Li, R.; Zhang, Y.-K.; Wang, S.; Ma, B.; Zhang, B.; An, P. Chem. Sci. 2023, 14, 3286–3292. doi:10.1039/d2sc06244e
Return to citation in text: [1] -
Castro‐Fernández, S.; Cruz, C. M.; Mariz, I. F. A.; Márquez, I. R.; Jiménez, V. G.; Palomino‐Ruiz, L.; Cuerva, J. M.; Maçôas, E.; Campaña, A. G. Angew. Chem., Int. Ed. 2020, 59, 7139–7145. doi:10.1002/anie.202000105
Return to citation in text: [1] [2] -
Chen, Y.; Lin, C.; Luo, Z.; Yin, Z.; Shi, H.; Zhu, Y.; Wang, J. Angew. Chem., Int. Ed. 2021, 60, 7796–7801. doi:10.1002/anie.202014621
Return to citation in text: [1] [2] -
Cruz, C. M.; Castro-Fernández, S.; Maçôas, E.; Cuerva, J. M.; Campaña, A. G. Angew. Chem., Int. Ed. 2018, 57, 14782–14786. doi:10.1002/anie.201808178
Return to citation in text: [1] -
Míguez-Lago, S.; Mariz, I. F. A.; Medel, M. A.; Cuerva, J. M.; Maçôas, E.; Cruz, C. M.; Campaña, A. G. Chem. Sci. 2022, 13, 10267–10272. doi:10.1039/d2sc03452b
Return to citation in text: [1] -
Ju, Y.-Y.; Chai, L.; Li, K.; Xing, J.-F.; Ma, X.-H.; Qiu, Z.-L.; Zhao, X.-J.; Zhu, J.; Tan, Y.-Z. J. Am. Chem. Soc. 2023, 145, 2815–2821. doi:10.1021/jacs.2c08746
Return to citation in text: [1] -
Yang, L.; Ju, Y.-Y.; Medel, M. A.; Fu, Y.; Komber, H.; Dmitrieva, E.; Zhang, J.-J.; Obermann, S.; Campaña, A. G.; Ma, J.; Feng, X. Angew. Chem., Int. Ed. 2023, 62, e202216193. doi:10.1002/anie.202216193
Return to citation in text: [1] -
Shen, Y.-J.; Yao, N.-T.; Diao, L.-N.; Yang, Y.; Chen, X.-L.; Gong, H.-Y. Angew. Chem., Int. Ed. 2023, 62, e202300840. doi:10.1002/anie.202300840
Return to citation in text: [1] -
Cruz, C. M.; Márquez, I. R.; Castro‐Fernández, S.; Cuerva, J. M.; Maçôas, E.; Campaña, A. G. Angew. Chem., Int. Ed. 2019, 58, 8068–8072. doi:10.1002/anie.201902529
Return to citation in text: [1] -
Urieta-Mora, J.; Krug, M.; Alex, W.; Perles, J.; Fernández, I.; Molina-Ontoria, A.; Guldi, D. M.; Martín, N. J. Am. Chem. Soc. 2020, 142, 4162–4172. doi:10.1021/jacs.9b10203
Return to citation in text: [1] -
Zhu, Y.; Guo, X.; Li, Y.; Wang, J. J. Am. Chem. Soc. 2019, 141, 5511–5517. doi:10.1021/jacs.9b01266
Return to citation in text: [1]
| 39. | Draper, S. M.; Gregg, D. J.; Madathil, R. J. Am. Chem. Soc. 2002, 124, 3486–3487. doi:10.1021/ja017394u |
| 40. | Zhang, B.; Ruan, L.; Zhang, Y.-K.; Zhang, H.; Li, R.; An, P. Org. Lett. 2023, 25, 732–737. doi:10.1021/acs.orglett.2c04097 |
| 41. | An, P.; Li, R.; Ma, B.; He, R.-Y.; Zhang, Y.-K.; Xiao, M.-J.; Zhang, B. Angew. Chem., Int. Ed. 2021, 60, 24478–24483. doi:10.1002/anie.202110538 |
| 48. | Evans, P. J.; Ouyang, J.; Favereau, L.; Crassous, J.; Fernández, I.; Perles, J.; Martín, N. Angew. Chem., Int. Ed. 2018, 57, 6774–6779. doi:10.1002/anie.201800798 |
| 49. | Izquierdo-García, P.; Fernández-García, J. M.; Fernández, I.; Perles, J.; Martín, N. J. Am. Chem. Soc. 2021, 143, 11864–11870. doi:10.1021/jacs.1c05977 |
| 46. | Ma, S.; Gu, J.; Lin, C.; Luo, Z.; Zhu, Y.; Wang, J. J. Am. Chem. Soc. 2020, 142, 16887–16893. doi:10.1021/jacs.0c08555 |
| 47. | Cruz, C. M.; Márquez, I. R.; Mariz, I. F. A.; Blanco, V.; Sánchez-Sánchez, C.; Sobrado, J. M.; Martín-Gago, J. A.; Cuerva, J. M.; Maçôas, E.; Campaña, A. G. Chem. Sci. 2018, 9, 3917–3924. doi:10.1039/c8sc00427g |
| 44. | Reger, D.; Haines, P.; Amsharov, K. Y.; Schmidt, J. A.; Ullrich, T.; Bönisch, S.; Hampel, F.; Görling, A.; Nelson, J.; Jelfs, K. E.; Guldi, D. M.; Jux, N. Angew. Chem., Int. Ed. 2021, 60, 18073–18081. doi:10.1002/anie.202103253 |
| 45. | Ma, J.; Fu, Y.; Dmitrieva, E.; Liu, F.; Komber, H.; Hennersdorf, F.; Popov, A. A.; Weigand, J. J.; Liu, J.; Feng, X. Angew. Chem., Int. Ed. 2020, 59, 5637–5642. doi:10.1002/anie.201914716 |
| 42. | Qiu, Z.; Ju, C.-W.; Frédéric, L.; Hu, Y.; Schollmeyer, D.; Pieters, G.; Müllen, K.; Narita, A. J. Am. Chem. Soc. 2021, 143, 4661–4667. doi:10.1021/jacs.0c13197 |
| 43. | Reger, D.; Haines, P.; Heinemann, F. W.; Guldi, D. M.; Jux, N. Angew. Chem., Int. Ed. 2018, 57, 5938–5942. doi:10.1002/anie.201800585 |
| 50. | Izquierdo-García, P.; Fernández-García, J. M.; Perles, J.; Fernández, I.; Martín, N. Angew. Chem., Int. Ed. 2023, 62, e202215655. doi:10.1002/anie.202215655 |
| 51. | Li, S.; Li, R.; Zhang, Y.-K.; Wang, S.; Ma, B.; Zhang, B.; An, P. Chem. Sci. 2023, 14, 3286–3292. doi:10.1039/d2sc06244e |
| 46. | Ma, S.; Gu, J.; Lin, C.; Luo, Z.; Zhu, Y.; Wang, J. J. Am. Chem. Soc. 2020, 142, 16887–16893. doi:10.1021/jacs.0c08555 |
| 57. | Yang, L.; Ju, Y.-Y.; Medel, M. A.; Fu, Y.; Komber, H.; Dmitrieva, E.; Zhang, J.-J.; Obermann, S.; Campaña, A. G.; Ma, J.; Feng, X. Angew. Chem., Int. Ed. 2023, 62, e202216193. doi:10.1002/anie.202216193 |
| 58. | Shen, Y.-J.; Yao, N.-T.; Diao, L.-N.; Yang, Y.; Chen, X.-L.; Gong, H.-Y. Angew. Chem., Int. Ed. 2023, 62, e202300840. doi:10.1002/anie.202300840 |
| 55. | Míguez-Lago, S.; Mariz, I. F. A.; Medel, M. A.; Cuerva, J. M.; Maçôas, E.; Cruz, C. M.; Campaña, A. G. Chem. Sci. 2022, 13, 10267–10272. doi:10.1039/d2sc03452b |
| 56. | Ju, Y.-Y.; Chai, L.; Li, K.; Xing, J.-F.; Ma, X.-H.; Qiu, Z.-L.; Zhao, X.-J.; Zhu, J.; Tan, Y.-Z. J. Am. Chem. Soc. 2023, 145, 2815–2821. doi:10.1021/jacs.2c08746 |
| 53. | Chen, Y.; Lin, C.; Luo, Z.; Yin, Z.; Shi, H.; Zhu, Y.; Wang, J. Angew. Chem., Int. Ed. 2021, 60, 7796–7801. doi:10.1002/anie.202014621 |
| 54. | Cruz, C. M.; Castro-Fernández, S.; Maçôas, E.; Cuerva, J. M.; Campaña, A. G. Angew. Chem., Int. Ed. 2018, 57, 14782–14786. doi:10.1002/anie.201808178 |
| 52. | Castro‐Fernández, S.; Cruz, C. M.; Mariz, I. F. A.; Márquez, I. R.; Jiménez, V. G.; Palomino‐Ruiz, L.; Cuerva, J. M.; Maçôas, E.; Campaña, A. G. Angew. Chem., Int. Ed. 2020, 59, 7139–7145. doi:10.1002/anie.202000105 |
| 52. | Castro‐Fernández, S.; Cruz, C. M.; Mariz, I. F. A.; Márquez, I. R.; Jiménez, V. G.; Palomino‐Ruiz, L.; Cuerva, J. M.; Maçôas, E.; Campaña, A. G. Angew. Chem., Int. Ed. 2020, 59, 7139–7145. doi:10.1002/anie.202000105 |
| 59. | Cruz, C. M.; Márquez, I. R.; Castro‐Fernández, S.; Cuerva, J. M.; Maçôas, E.; Campaña, A. G. Angew. Chem., Int. Ed. 2019, 58, 8068–8072. doi:10.1002/anie.201902529 |
| 60. | Urieta-Mora, J.; Krug, M.; Alex, W.; Perles, J.; Fernández, I.; Molina-Ontoria, A.; Guldi, D. M.; Martín, N. J. Am. Chem. Soc. 2020, 142, 4162–4172. doi:10.1021/jacs.9b10203 |
| 46. | Ma, S.; Gu, J.; Lin, C.; Luo, Z.; Zhu, Y.; Wang, J. J. Am. Chem. Soc. 2020, 142, 16887–16893. doi:10.1021/jacs.0c08555 |
| 1. | Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grigorieva, I. V.; Firsov, A. A. Science 2004, 306, 666–669. doi:10.1126/science.1102896 |
| 7. | Narita, A.; Wang, X.-Y.; Feng, X.; Müllen, K. Chem. Soc. Rev. 2015, 44, 6616–6643. doi:10.1039/c5cs00183h |
| 8. | Wang, X.-Y.; Yao, X.; Narita, A.; Müllen, K. Acc. Chem. Res. 2019, 52, 2491–2505. doi:10.1021/acs.accounts.9b00322 |
| 9. | Zhang, Y.; Pun, S. H.; Miao, Q. Chem. Rev. 2022, 122, 14554–14593. doi:10.1021/acs.chemrev.2c00186 |
| 25. | Li, C.; Yang, Y.; Miao, Q. Chem. – Asian J. 2018, 13, 884–894. doi:10.1002/asia.201800073 |
| 26. | Fernández-García, J. M.; Evans, P. J.; Filippone, S.; Herranz, M. Á.; Martín, N. Acc. Chem. Res. 2019, 52, 1565–1574. doi:10.1021/acs.accounts.9b00144 |
| 27. | Mori, T. Chem. Rev. 2021, 121, 2373–2412. doi:10.1021/acs.chemrev.0c01017 |
| 28. | Wu, Y.-F.; Zhang, L.; Zhang, Q.; Xie, S.-Y.; Zheng, L.-S. Org. Chem. Front. 2022, 9, 4726–4743. doi:10.1039/d2qo00988a |
| 6. | Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Science 2008, 319, 1229–1232. doi:10.1126/science.1150878 |
| 14. | Rickhaus, M.; Mayor, M.; Juríček, M. Chem. Soc. Rev. 2016, 45, 1542–1556. doi:10.1039/c5cs00620a |
| 4. | Rieger, R.; Müllen, K. J. Phys. Org. Chem. 2010, 23, 315–325. doi:10.1002/poc.1644 |
| 5. | Diez-Perez, I.; Li, Z.; Hihath, J.; Li, J.; Zhang, C.; Yang, X.; Zang, L.; Dai, Y.; Feng, X.; Muellen, K.; Tao, N. Nat. Commun. 2010, 1, 31. doi:10.1038/ncomms1029 |
| 8. | Wang, X.-Y.; Yao, X.; Narita, A.; Müllen, K. Acc. Chem. Res. 2019, 52, 2491–2505. doi:10.1021/acs.accounts.9b00322 |
| 2. | Zhang, Y.; Tan, Y.-W.; Stormer, H. L.; Kim, P. Nature 2005, 438, 201–204. doi:10.1038/nature04235 |
| 3. | Geim, A. K.; Novoselov, K. S. Nat. Mater. 2007, 6, 183–191. doi:10.1038/nmat1849 |
| 20. | Sang, Y.; Han, J.; Zhao, T.; Duan, P.; Liu, M. Adv. Mater. (Weinheim, Ger.) 2020, 32, 1900110. doi:10.1002/adma.201900110 |
| 21. | MacKenzie, L. E.; Pal, R. Nat. Rev. Chem. 2021, 5, 109–124. doi:10.1038/s41570-020-00235-4 |
| 22. | Xu, L.; Feng, Y.; Yu, D.; Zheng, Z.; Chen, X.; Hong, W. Adv. Mater. Technol. (Weinheim, Ger.) 2020, 5, 2000373. doi:10.1002/admt.202000373 |
| 23. | Zhang, Y.-P.; Zheng, Y.-X. Dalton Trans. 2022, 51, 9966–9970. doi:10.1039/d2dt01582j |
| 24. | Hao, C.; Xu, L.; Kuang, H.; Xu, C. Adv. Mater. (Weinheim, Ger.) 2020, 32, 1802075. doi:10.1002/adma.201802075 |
| 16. | Wang, S.; Yuan, J.; Xie, J.; Lu, Z.; Jiang, L.; Mu, Y.; Huo, Y.; Tsuchido, Y.; Zhu, K. Angew. Chem., Int. Ed. 2021, 60, 18443–18447. doi:10.1002/anie.202104054 |
| 18. | Milton, M.; Schuster, N. J.; Paley, D. W.; Hernández Sánchez, R.; Ng, F.; Steigerwald, M. L.; Nuckolls, C. Chem. Sci. 2019, 10, 1029–1034. doi:10.1039/c8sc04216k |
| 14. | Rickhaus, M.; Mayor, M.; Juríček, M. Chem. Soc. Rev. 2016, 45, 1542–1556. doi:10.1039/c5cs00620a |
| 15. | Li, R.; Ma, B.; He, R.-Y.; Zhang, B.; Zhang, Y.-K.; Feng, S.-Y.; An, P. Chem. – Asian J. 2022, 17, e202101365. doi:10.1002/asia.202101365 |
| 19. | Jolly, A.; Miao, D.; Daigle, M.; Morin, J.-F. Angew. Chem., Int. Ed. 2020, 59, 4624–4633. doi:10.1002/anie.201906379 |
| 11. | Miao, Q.; Yang, D. Prog. Chem. 2020, 32, 1835–1845. |
| 12. | Kirschbaum, T.; Rominger, F.; Mastalerz, M. Angew. Chem., Int. Ed. 2020, 59, 270–274. doi:10.1002/anie.201912213 |
| 13. | Márquez, I. R.; Fuentes, N.; Cruz, C. M.; Puente-Muñoz, V.; Sotorrios, L.; Marcos, M. L.; Choquesillo-Lazarte, D.; Biel, B.; Crovetto, L.; Gómez-Bengoa, E.; González, M. T.; Martin, R.; Cuerva, J. M.; Campaña, A. G. Chem. Sci. 2017, 8, 1068–1074. doi:10.1039/c6sc02895k |
| 53. | Chen, Y.; Lin, C.; Luo, Z.; Yin, Z.; Shi, H.; Zhu, Y.; Wang, J. Angew. Chem., Int. Ed. 2021, 60, 7796–7801. doi:10.1002/anie.202014621 |
| 10. | Stuparu, M. C. Acc. Chem. Res. 2021, 54, 2858–2870. doi:10.1021/acs.accounts.1c00207 |
| 17. | Wang, J.; Zhang, X.; Jia, H.; Wang, S.; Du, P. Acc. Chem. Res. 2021, 54, 4178–4190. doi:10.1021/acs.accounts.1c00505 |
| 61. | Zhu, Y.; Guo, X.; Li, Y.; Wang, J. J. Am. Chem. Soc. 2019, 141, 5511–5517. doi:10.1021/jacs.9b01266 |
| 31. | Clar, E.; Stewart, D. G. J. Am. Chem. Soc. 1953, 75, 2667–2672. doi:10.1021/ja01107a035 |
| 5. | Diez-Perez, I.; Li, Z.; Hihath, J.; Li, J.; Zhang, C.; Yang, X.; Zang, L.; Dai, Y.; Feng, X.; Muellen, K.; Tao, N. Nat. Commun. 2010, 1, 31. doi:10.1038/ncomms1029 |
| 29. | Wu, J.; Pisula, W.; Müllen, K. Chem. Rev. 2007, 107, 718–747. doi:10.1021/cr068010r |
| 30. | Scholl, R.; Seer, C.; Weitzenböck, R. Ber. Dtsch. Chem. Ges. 1910, 43, 2202–2209. doi:10.1002/cber.191004302175 |
| 37. | Ammon, F.; Sauer, S. T.; Lippert, R.; Lungerich, D.; Reger, D.; Hampel, F.; Jux, N. Org. Chem. Front. 2017, 4, 861–870. doi:10.1039/c7qo00112f |
| 38. | Wijesinghe, L. P.; Perera, S. D.; Larkin, E.; Ó Máille, G. M.; Conway-Kenny, R.; Lankage, B. S.; Wang, L.; Draper, S. M. RSC Adv. 2017, 7, 24163–24167. doi:10.1039/c7ra02648j |
| 35. | Medel, M. A.; Tapia, R.; Blanco, V.; Miguel, D.; Morcillo, S. P.; Campaña, A. G. Angew. Chem., Int. Ed. 2021, 60, 6094–6100. doi:10.1002/anie.202015368 |
| 36. | Medel, M. A.; Cruz, C. M.; Miguel, D.; Blanco, V.; Morcillo, S. P.; Campaña, A. G. Angew. Chem., Int. Ed. 2021, 60, 22051–22056. doi:10.1002/anie.202109310 |
| 33. | Martin, M. M.; Hampel, F.; Jux, N. Chem. – Eur. J. 2020, 26, 10210–10212. doi:10.1002/chem.202001471 |
| 34. | Luo, J.; Xu, X.; Mao, R.; Miao, Q. J. Am. Chem. Soc. 2012, 134, 13796–13803. doi:10.1021/ja3054354 |
| 29. | Wu, J.; Pisula, W.; Müllen, K. Chem. Rev. 2007, 107, 718–747. doi:10.1021/cr068010r |
| 32. | Kübel, C.; Eckhardt, K.; Enkelmann, V.; Wegner, G.; Müllen, K. J. Mater. Chem. 2000, 10, 879–886. doi:10.1039/a908941a |
© 2023 Li et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.