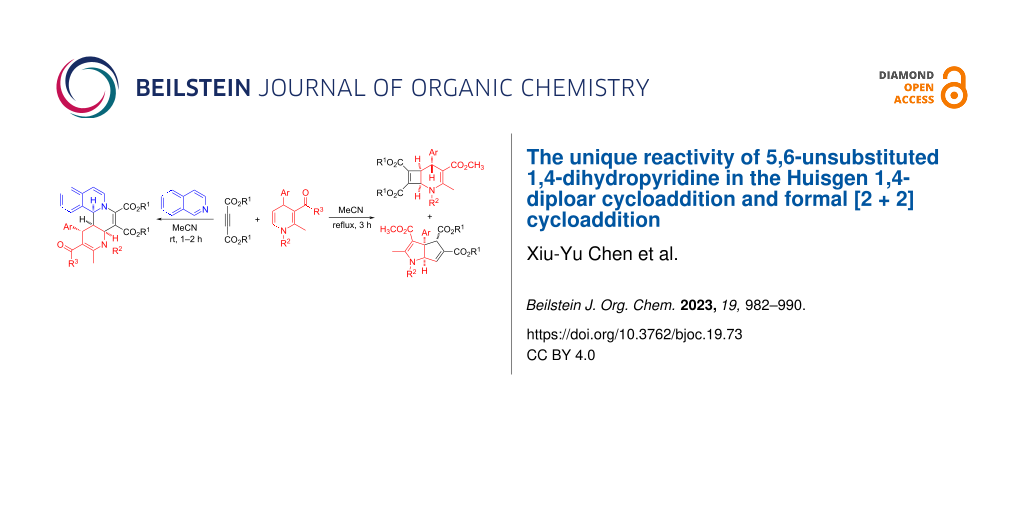
Abstract
The three-component reaction of isoquinolines, dialkyl acetylenedicarboxylates, and 5,6-unsubstituted 1,4-dihydropyridines in acetonitrile at room temperature afforded functionalized isoquinolino[1,2-f][1,6]naphthyridines in good yields and with high diastereoselectivity. More importantly, the formal [2 + 2] cycloaddition reaction of dialkyl acetylenedicarboxylates and 5,6-unsubstituted 1,4-dihydropyridines in refluxing acetonitrile gave unique 2-azabicyclo[4.2.0]octa-3,7-dienes as major products and 1,3a,4,6a-tetrahydrocyclopenta[b]pyrroles as minor products via further rearrangement.
Graphical Abstract
Introduction
Among various well-known cycloaddition reactions such as the 1,3-dipolar cycloaddition reaction, Diels–Alder reaction, and the Povarov reaction, the cycloaddition reaction of Huisgen 1,4-dipoles with activated alkenes received increasing attention [1-3]. The well-known Huisgen 1,4-dipoles have a special kind of zwitterionic intermediates and are usually prepared by a nucleophilic addition of pyridine, quinoline, isoquinoline and other aza-arenes to electron-deficient alkynes [4-8]. The reactive Huisgen 1,4-dipoles have been widely employed as one of the most valuable synthons to construct diverse carbocyclic and heterocyclic systems as well as many open-chain compounds [9-15]. In recent years, in situ generated Huisgen 1,4-dipoles were also widely employed to design highly efficient multicomponent and domino reactions [16-25]. Recently, much attention has been devoted to the development of new domino reactions containing reactive Huisgen 1,4-dipoles as key components for the assembly of many biologically important nitrogen-containing six-membered heterocyclic compounds [26-30].
The 5,6-unsubstituted 1,4-dihydropyridines can be easily prepared from the three-component reaction of an arylamine, cinnamaldehyde, and methyl acetoacetate [31-34]. The unsubstituted C=C double bond in 5,6-unsubstituted 1,4-dihydropyridines exhibits high reactivity and could act as activated alkene to take part in various cycloaddition reactions [35-40]. For example, Lavilla and co-workers developed a Sc(OTf)3-catalyzed three-component reaction of 5,6-unsubstituted 1,4-dihydropyridines, arylamines and ethyl glyoxylate for the preparation of various pyrido-fused tetrahydroquinolines (reaction 1 in Scheme 1) [41,42]. Menéndez and co-workers reported a Yb(OTf)3-mediated Povarov reaction of imines and N-alkyl-1,4-dihydropyridines for the synthesis of hexahydrobenzo[h][1,6]naphthyridines (reaction 2 in Scheme 1) [43]. Khan and co-workers employed a one-pot Povarov reaction of 3-aminocoumarins, aldehydes, and 5,6-unsubstituted 1,4-dihydropyridine derivatives for the construction of exo-hexahydrochromeno[3,4-h][1,6]naphthyridine-3-carboxylate derivatives (reaction 3 in Scheme 1) [44]. In these reactions, the 5,6-unsubstituted 1,4-dihydropyridines usually behaved as an activated electron-rich dienophile. Inspired by these elegant synthetic methodologies and in continuation of our aim to develop well-known Huisgen 1,4-dipoles for the construction of diverse nitrogen-containing heterocyclic compounds [45-64], herein, we wish to report the use of 5,6-unsubstituted 1,4-dihydropyridines as electron-deficient alkenes in the Huisgen 1,4-diploar cycloaddition and as electron-rich alkenes in formal [2 + 2] cycloadditions for the efficient synthesis of isoquinolino[1,2-f][1,6]naphthyridine and 2-azabicyclo[4.2.0]octa-3,7-diene derivatives.
Scheme 1: Various cycloaddition reactions of 5,6-unsymmetric 1,4-dihydropyridines.
Scheme 1: Various cycloaddition reactions of 5,6-unsymmetric 1,4-dihydropyridines.
Results and Discussion
Initially, the reaction conditions were briefly examined by using isoquinoline (1), dimethyl acetylenedicarboxylate (DMAD, 2) and 5,6-unsubstituted 1,4-dihydropyridine 3 as standard reaction (Table 1). The three-component reaction was carried out in common solvents such as ethanol, methanol, dichloromethane, and chloroform at room temperature for two hours. The expected isoquinolino[1,2-f][1,6]naphthyridine derivative 4a was successfully obtained in 35%, 40% 70% and 65% yields, respectively (Table 1, entries 1–4). The reaction in acetonitrile afforded the product 4a in 75% yield (Table 1, entry 5). When the reaction time was extended to 12 h at room temperature, the yield of product 4a did not increase (Table 1, entry 6). When the reaction was carried out in acetonitrile at elevated temperatures, the yield of product 4a decreased to 68% and 55% (Table 1, entries 7 and 8). Therefore, the optimal reaction conditions for this three-component reaction were simply carrying out the reaction in acetonitrile at room temperature for two hours.
Table 1: Optimizing the reaction conditions.a
|
|
||||
| Entry | Solvent | Temp. [°C] | Time [h] | Yield [%]b |
| 1 | EtOH | rt | 2 | 35 |
| 2 | MeOH | rt | 2 | 40 |
| 3 | CH2Cl2 | rt | 2 | 70 |
| 4 | CHCl3 | rt | 2 | 65 |
| 5 | MeCN | rt | 2 | 75 |
| 6 | MeCN | rt | 12 | 72 |
| 7 | MeCN | 50 | 2 | 68 |
| 8 | MeCN | 80 | 2 | 55 |
aReaction conditions: isoquinoline (0.5 mmol), DMAD (0.6 mmol), 5,6-unsubstituted 1,4-dihydropyridine (0.5 mmol), solvent (5.0 mL). bIsolated yields.
Under the optimal reaction conditions, various substrates were employed in the reaction for developing the scope of the reaction and the results are summarized in Table 2. It can be seen that all reactions gave the desired isoquinolino[1,2-f][1,6]naphthyridine derivatives 4a–o in good to excellent yields. Isoquinoline itself and its 4-, 5-, and 6-bromo-substituted derivatives were successfully used in the reaction. Dimethyl or diethyl acetylenedicarboxylates gave the products in comparable yields in the reaction. The 5,6-unsubstituted 1,4-dihydropyridines with an N-benzyl group usually gave the products in good yields (Table 2, entries 1–12). It should be pointed out that 6-unsubstituted 1,4-dihydropyridines with an N-(3,4-(CH3O)2C6H3CH2CH2) group also afforded the desired product 4h in 88% yield (Table 2, entry 8). Even 5,6-unsubstituted 1,4-dihydropyridines with an N-n-Bu group also gave the products 4m and 4n in satisfactory yields. At last, 5,6-unsubstituted 1,4-dihydropyridines derived from the condensation of acetylacetone also afforded the expected product 4o in 65% yield. The chemical structures of the obtained isoquinoline[2,1-h][1,7]naphthyridines 4a–o were fully characterized by various spectroscopy methods and further confirmed by determination of the single crystal structure of compound 4k (Figure 1, CCDC 2059918). Though there are four chiral centers in the product structure of the isoquinolino[1,2-f][1,6]naphthyridine, the 1H NMR spectra of the products all showed that only one diastereomer was produced in the reaction, which showed that this reaction has a high diastereoselectivity. From Figure 1, it can be seen that the three protons and the phenyl group have cis-configuration in the hexahydro-1,6-naphthyridyl ring.
Table 2: Synthesis of isoquinolino[1,2-f][1,6]naphthyridines 4a–o.a
|
|
|||||||
| Entry | Compd | R1 | R2 | Ar | R3 | R4 | Yield [%]b |
| 1 | 4a | H | CH3 | C6H5 | OCH3 | Bn | 75 |
| 2 | 4b | H | CH3 | C6H5 | OEt | Bn | 65 |
| 3 | 4c | H | CH3 | p-FC6H4 | OCH3 | Bn | 58 |
| 4 | 4d | H | CH3 | p-CH3OC6H4 | OCH3 | Bn | 88 |
| 5 | 4e | H | C2H5 | C6H5 | OCH3 | Bn | 80 |
| 6 | 4f | H | C2H5 | C6H5 | OC2H5 | Bn | 82 |
| 7 | 4g | H | CH3 | C6H5 | OCH3 | p-CH3OC6H4CH2 | 84 |
| 8 | 4h | H | CH3 | C6H5 | OCH3 | 3,4-(CH3O)2C6H3(CH2)2 | 88 |
| 9 | 4i | 4-Br | CH3 | C6H5 | OCH3 | Bn | 78 |
| 10 | 4j | 5-Br | CH3 | C6H5 | OCH3 | Bn | 67 |
| 11 | 4k | 6-Br | CH3 | C6H5 | OCH3 | Bn | 65 |
| 12 | 4l | 4-Br | CH3 | C6H5 | OC2H5 | Bn | 74 |
| 13 | 4m | H | CH3 | C6H5 | OCH3 | n-Bu | 77 |
| 14 | 4n | 5-Br | CH3 | C6H5 | OCH3 | n-Bu | 78 |
| 15 | 4o | H | CH3 | C6H5 | CH3 | Bn | 65 |
aReaction conditions: isoquinoline (0.5 mmol), dialkyl acetylenedicarboxylate (0.6 mmol), 5,6-unsubstituted 1,4-dihydropyridine (0.5 mmol), CH3CN (5.0 mL), rt, 2 h. bIsolated yields.
![[1860-5397-19-73-1]](/bjoc/content/figures/1860-5397-19-73-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Single crystal structure of the compound 4k.
Figure 1: Single crystal structure of the compound 4k.
During the investigation of the above three-component reaction, we found that the three-component reaction of isoquinoline, dimethyl acetylenedicarboxylate and 5,6-unsubstituted 1,4-dihydropyridines with N–Ar groups did not give the above isoquinolino[1,2-f][1,6]naphthyridines, but the unique 2-azabicyclo[4.2.0]octa-3,7-diene-7,8-dicarboxylates were isolated in moderate yields. These products were obviously produced from the formal [2 + 2] cycloaddition between dimethyl acetylenedicarboxylate and the 5,6-unsubstituted 1,4-dihydropyridine. Therefore, the reactions of dimethyl acetylenedicarboxylate and 5,6-unsubstituted 1,4-dihydropyridines were carefully explored. After adjusting the reaction conditions, we were pleased to find that 2-azabicyclo[4.2.0]octa-3,7-dienes 5a–o could be successfully obtained in moderate to good yields by carrying out the reaction of dimethyl acetylenedicarboxylate and 5,6-unsubstituted 1,4-dihydropyridines in refluxing acetonitrile for three hours (Table 3). As can be seen, unsymmetric 1,4-dihydropyridines with N–Bn and N-4-(CH3OC6H4CH2) groups can be successfully employed in the reaction (Table 3, entries 1–10). Additionally, 5,6-unsubstituted 1,4-dihydropyridines with various N–Ar groups also gave the expected products 5k–o in good yields. Sometimes, as unexpected byproducts, 1,3a,4,6a-tetrahydrocyclopenta[b]pyrrole derivatives 6e, 6f, 6i, 6k, 6l, and 6m were isolated in 23–39% yield from the reaction mixture. In other cases, the corresponding 1,3a,4,6a-tetrahydrocyclopenta[b]pyrrole derivatives could not be isolated due to too low yields. By analyzing the chemical structures of the 1,3a,4,6a-tetrahydrocyclopenta[b]pyrrole derivatives 6, it was found that the 1,4-dihydropyridinyl ring of the substrate was converted to a fused pyrrole ring, which might be a result from a rearrangement process of the formed 2-azabicyclo[4.2.0]octa-3,7-diene-7,8-dicarboxylates 5a–o at elevated temperature. The chemical structures of both bicyclic compounds 5a–o and 6a–o were fully characterized by various spectroscopy methods. The single crystal structures of compounds 5a (Figure 2) and 6f (Figure 3) were successfully determined by X-ray diffraction analysis. From Figure 2 (compound 5a), it can be seen that the cyclobutenyl ring and the 1,4-dihydropyridyl ring exist on the fused position. The two protons at the bridged position and the phenyl group are cis-configured. From Figure 3 (compound 6f), it can be seen that the fused pyrrole ring and the cyclopentyl ring are butterfly shaped. The unusual feature is that the C=C double bond is not located between the two carbon atoms substituted with the methoxycarbonyl groups, but between the methylene carbon atom and one carbon atom connected with an electron-withdrawing methoxycarbonyl group. The aryl group and the neighbouring methoxycarbonyl group are cis-configured.
Table 3: Synthesis of the bicyclic compounds 5a–o and 6a–o.a
|
|
|||||||
| Entry | R1 | Ar | R2 | Compd | Yield (%)b | Compd | Yield (%)b |
| 1 | CH3 | C6H5 | Bn | 5a | 89% | 6a | – |
| 2 | CH2CH3 | C6H5 | Bn | 5b | 70% | 6b | – |
| 3 | CH3 | o-CH3OC6H4 | Bn | 5c | 80% | 6c | – |
| 4 | CH2CH3 | o-CH3OC6H4 | Bn | 5d | 65% | 6d | – |
| 5 | CH2CH3 | p-NO2C6H4 | Bn | 5e | 36% | 6e | 35% |
| 6 | CH3 | C6H5 | p-CH3OC6H4CH2 | 5f | 40% | 6f | 23% |
| 7 | CH3 | o-CH3OC6H4 | p-CH3OC6H4CH2 | 5g | 57% | 6g | – |
| 8 | CH2CH3 | o-CH3OC6H4 | p-CH3OC6H4CH2 | 5h | 62% | 6h | – |
| 9 | CH2CH3 | p-NO2C6H4 | p-CH3OC6H4CH2 | 5i | 41% | 6i | 39% |
| 10 | CH3 | p-NO2C6H4 | p-CH3OC6H4CH2 | 5j | 64% | 6j | – |
| 11 | CH3 | C6H5 | p-CH3OC6H4 | 5k | 35% | 6k | 36% |
| 12 | CH2CH3 | C6H5 | p-CH3C6H4 | 5l | 31% | 6l | 33% |
| 13 | CH3 | C6H5 | p-BrC6H4 | 5m | 33% | 6m | 35% |
| 14 | CH3 | C6H5 | m-ClC6H4 | 5n | 55% | 6n | – |
| 15 | CH3 | o-CH3OC6H4 | o-CH3C6H4 | 5o | 64% | 6o | – |
aReaction conditions: dialkyl acetylenedicarboxylate (0.9 mmol), 5,6-unsubstituted 1,4-dihydropyridine (0.3 mmol), CH3CN (5.0 mL), reflux, 3 h. bIsolated yields.
![[1860-5397-19-73-2]](/bjoc/content/figures/1860-5397-19-73-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Single crystal structure of compound 5a.
Figure 2: Single crystal structure of compound 5a.
![[1860-5397-19-73-3]](/bjoc/content/figures/1860-5397-19-73-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Single crystal structure of compound 6f.
Figure 3: Single crystal structure of compound 6f.
For explaining the formation of the various cyclic compounds, a plausible reaction mechanism was proposed on the base of the previously reported works [41-44] and the present experiments (Scheme 2). Initially, the nucleophilic addition of isoquinoline to dimethyl acetylenedicarboxylate gives the well-known Huisgen 1,4-dipole A. Secondly, Michael addition of the 1,4-dipole A to 5,6-unsubstituted 1,4-dihydropyridine gives the adduct intermediate B. At last, the intramolecular coupling of the negative and the positive charges in intermediate B directly affords isoquinolino[1,2-f][1,6]naphthyridines 4. Because all reactions in this process are retro-equilibrium reactions, the most thermodynamically stable diastereomer is preferentially produced in the reaction. In the absence of isoquinoline, the 5,6-unsubstituted 1,4-dihydropyridine acts as an active enamine, which adds to dimethyl acetylenedicarboxylate to give the adduct C. Then, the direct coupling of the positive charge and the negative charge affords the 2-azabicyclo[4.2.0]octa-3,7-diene 5. On the other hand, a carbenium ion D can be formed by migration of a hydrogen atom in intermediate C, which in turn converts into a fused bicyclic intermediate E by a charge coupling process. At elevated temperature, the ring-opening of the unstable cyclobutenyl ring gives a 1,2-dihydroazocine intermediate F, which is transformed into a 1,4-dihydroazocine intermediate G by a 1,5-H shift process. At last, the tetrahydrocyclopenta[b]pyrrole 6 is formed by an intramolecular Michael addition process.
Scheme 2: Plausible reaction mechanism for the various products 4, 5, and 6.
Scheme 2: Plausible reaction mechanism for the various products 4, 5, and 6.
Conclusion
In summary, we have investigated the three-component reaction of isoquinoline, dialkyl acetylenedicarboxylate and 5,6-unsubstituted 1,4-dihydropyridines. This reaction successfully provided an efficient protocol for the synthesis of functionalized isoquinolino[1,2-f][1,6]naphthyridines in good yields and with high diastereoselectivity. We also found that the unique 2-azabicyclo[4.2.0]octa-3,7-diene and 1,3a,4,6a-tetrahydrocyclopenta[b]pyrrole derivatives can be conveniently produced by the cycloaddition reaction of dialkyl acetylenedicarboxylates and 5,6-unsubstituted 1,4-dihydropyridines. The advantages of the reaction include the use of readily available starting materials, simple reaction conditions, without using any catalyst, high molecular diversity and atomic economy. Therefore, this reaction not only successfully developed unprecedented synthetic reactivity of the electron-deficient alkynes, but also provides efficient synthetic methodologies for complex nitrogen-containing heterocycles. The potential application of this reaction in organic synthesis and medicinal chemistry might be significant.
Experimental
General procedure for the three-component reaction of isoquinoline, dialkyl acetylenedicarboxylate and 5,6-unsubstituted 1,4-dihydropyridine
A mixture of isoquinoline (0.5 mmol), dialkyl acetylenedicarboxylate (0.6 mmol), 5,6-unsubstituted 1,4-dihydropyridine (0.5 mmol) in acetonitrile (5.0 mL) was stirred at room temperature for two hours. After removing the solvent by rotatory evaporation at reduced pressure, the residue was subjected to column chromatography with petroleum ether and ethyl acetate (v/v = 5:1) as eluent to give the pure product for analysis.
Trimethyl 4-benzyl-3-methyl-1-phenyl-4,4a,13b,13c-tetrahydro-1H-isoquinolino[1,2-f][1,6]naphthyridine-2,5,6-tricarboxylate (4a): orange solid, 75%; mp 174–175 °C; 1H NMR (400 MHz, CDCl3) δ 7.48–7.42 (m, 4H, ArH), 7.39–7.35 (m, 1H, ArH), 6.80 (t, J = 7.6 Hz, 1H, ArH), 6.75–6.71 (m, 4H, ArH), 6.63 (d, J = 6.4 Hz, 2H, ArH), 6.39 (t, J = 7.6 Hz, 1H, ArH), 6.22 (d, J = 8.0 Hz, 1H, ArH), 5.91 (d, J = 8.0 Hz, 1H, CH), 5.43 (d, J = 8.0 Hz, 1H, CH), 5.25 (s, 1H, CH), 5.04 (d, J = 15.6 Hz, 1H, CH2), 4.60 (d, J = 6.0 Hz, 1H, CH), 4.45 (d, J = 15.6 Hz, 1H, CH2), 4.17 (d, J = 8.8 Hz, 1H, CH), 3.93 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 3.28 (s, 3H, OCH3), 2.60 (t, J = 7.6 Hz, 1H, CH), 2.44 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3) δ 168.7, 166.2, 164.9, 150.2, 146.1, 145.7, 138.7, 129.3, 128.8, 128.1, 128.0, 127.8, 127.5, 127.3, 127.0, 126.3, 126.1, 125.1, 124.7, 124.6, 106.3, 104.6, 102.8, 61.3, 57.6, 56.0, 53.0, 51.6, 50.0, 44.8, 39.5, 17.9 ppm. IR (KBr) ν: 3732, 3023, 2952, 2843, 1967, 1737, 1678, 1556, 1372, 1147, 1097, 1010, 957, 866, 759 cm−1; HRESIMS (m/z): [M + Na]+ calcd for C36H34NaN2O6, 613.2315; found, 613.2309.
General procedure for the reaction of dialkyl acetylenedicarboxylate and 5,6-unsubstituted 1,4-dihydropyridine
A mixture of dialkyl acetylenedicarboxylate (0.9 mmol), 5,6-unsubstituted 1,4-dihydropyridine (0.3 mmol) in acetonitrile (5.0 mL) was heated a reflux for three hours. After removing the solvent by rotatory evaporation at reduced pressure, the residue was subjected to column chromatography with petroleum ether and ethyl acetate (v/v = 6:1) as eluent to give the pure product for analysis.
7,8-Diethyl 4-methyl 2-benzyl-3-methyl-5-(4-nitrophenyl)-2-azabicyclo[4.2.0]octa-3,7-diene-4,7,8-tricarboxylate (5e): yellow solid, 36%; mp 161–163 °C; 1H NMR (400 MHz, CDCl3) δ 8.07–8.05 (m, 2H, ArH), 7.37–7.34 (m, 2H, ArH), 7.33–7.29 (m, 3H, ArH), 7.13–7.10 (m, 2H, ArH) 4.86 (d, J = 15.2 Hz, 1H, CH2), 4.59 (s, 1H, CH), 4.48 (d, J = 15.2 Hz, 1H, CH2), 4.37–4.34 (m, 1H, CH), 4.34–4.31 (m, 2H, CH2), 4.31–4.26 (m, 2H, CH2), 3.71 (d, J = 4.4 Hz, 1H, CH), 3.63 (s, 3H, OCH3), 2.51 (s, 3H, CH3), 1.40–1.37 (m, 3H, CH3), 1.37–1.33 (m, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3) δ 169.3, 160.9, 160.7, 156.2, 152.0, 146.4, 144.5, 137.2, 136.1, 128.7, 128.5, 128.1, 127.9, 123.4, 101.1, 61.5, 61.4, 56.6, 53.8, 51.8, 51.0, 38.9, 17.3, 14.1, 14.1 ppm; IR (KBr) ν: 3746, 2983, 2945, 1729, 1651, 1557, 1434, 1347, 1251, 1129, 1088, 841, 732, 709 cm−1; HRESIMS (m/z): [M + H]+) calcd for C29H31N2O8, 535.2075; found, 535.2076.
4,5-Diethyl 3-methyl 1-benzyl-2-methyl-3a-(4-nitrophenyl)-1,3a,4,6a-tetrahydrocyclopenta[b]pyrrole-3,4,5-tricarboxylate (6e): yellow solid, 35%; mp 153–155 °C; 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.8 Hz, ArH), 7.45–7.37 (m, 5H, ArH), 7.30–7.27 (m, 2H, ArH), 6.70 (s, 1H, CH2), 5.07–5.05 (m, 1H, CH), 5.00–4.98 (m, 1H, CH), 4.76 (d, J = 15.2 Hz, 1H, CH2), 4.37 (d, J = 15.6 Hz, 1H, CH2), 4.29–4.21 (m, 1H, CH2), 4.21–4.14 (m, 1H, CH2), 3.91–3.82 (m, 1H, CH2), 3.82–3.72 (m, 1H, CH2), 3.69 (s, 3H, OCH3), 2.34 (s, 3H, CH3), 1.29 (t, J = 7.2 Hz, CH3), 0.99 (t, J = 7.2 Hz, CH3) ppm; 13C NMR (100 MHz, CDCl3) δ 172.2, 166.1, 163.5, 158.0, 149.9, 146.3, 137.8, 137.4, 135. 9, 129.2, 128.5, 128.0, 127.2, 123.0, 101.9, 75.5, 62.9, 61.1, 61.0, 58.3, 50.3, 49.2, 14.1, 13.8, 13.4 ppm; IR (KBr) ν: 3069, 2981, 1736, 1660, 1552, 1514, 1344, 1222, 1121, 1040, 914, 854, 769, 704 cm−1; HRESIMS (m/z): [M + Na]+ calcd for C29H30NaN2O8, 557.1894; found, 557.1891.
Supporting Information
The crystallographic data of compounds 4k (CCDC 2260340), 5a (CCDC 2260341), and 5f (CCDC 2260342) have been deposited at the Cambridge Crystallographic Data Center (https://www.ccdc.cam.ac.uk).
| Supporting Information File 1: Characterization data and 1H NMR, 13C NMR, HRMS spectra of the synthesized compounds. | ||
| Format: PDF | Size: 6.0 MB | Download |
References
-
Huisgen, R.; Morikawa, M.; Herbig, K.; Brunn, E. Chem. Ber. 1967, 100, 1094–1106. doi:10.1002/cber.19671000406
Return to citation in text: [1] -
Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p
Return to citation in text: [1] -
Nair, V.; Menon, R. S.; Sreekanth, A. R.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520–530. doi:10.1021/ar0502026
Return to citation in text: [1] -
Kielland, N.; Lavilla, R. Top. Heterocycl. Chem. 2010, 25, 127–168. doi:10.1007/7081_2010_42
Return to citation in text: [1] -
Shaabani, A.; Maleki, A.; Rezayan, A. H.; Sarvary, A. Mol. Diversity 2011, 15, 41–68. doi:10.1007/s11030-010-9258-1
Return to citation in text: [1] -
Nair, V.; Deepthi, A.; Ashok, D.; Raveendran, A. E.; Paul, R. R. Tetrahedron 2014, 70, 3085–3105. doi:10.1016/j.tet.2014.03.014
Return to citation in text: [1] -
Sharma, U. K.; Ranjan, P.; Van der Eycken, E. V.; You, S.-L. Chem. Soc. Rev. 2020, 49, 8721–8748. doi:10.1039/d0cs00128g
Return to citation in text: [1] -
Piltan, M.; Yavari, I.; Moradi, L. Chin. Chem. Lett. 2013, 24, 979–983. doi:10.1016/j.cclet.2013.06.023
Return to citation in text: [1] -
Sun, J.; Hu, H.; Wang, F.; Wu, H.; Liu, Y. RSC Adv. 2014, 4, 36498–36501. doi:10.1039/c4ra06048b
Return to citation in text: [1] -
Xing, Y.; Zhang, M.; Ciccarelli, S.; Lee, J.; Catano, B. Eur. J. Org. Chem. 2017, 781–785. doi:10.1002/ejoc.201601416
Return to citation in text: [1] -
Ghafouri, S. S.; Afshari, M. A.; Saeedi, N.; Djahaniani, H.; Mohtat, B. Asian J. Chem. 2013, 25, 4111–4112. doi:10.14233/ajchem.2013.13772
Return to citation in text: [1] -
Sun, J.; Wang, F.; Hu, H.; Wang, X.; Wu, H.; Liu, Y. J. Org. Chem. 2014, 79, 3992–3998. doi:10.1021/jo500456d
Return to citation in text: [1] -
Yang, H.-B.; Guan, X.-Y.; Wei, Y.; Shi, M. Eur. J. Org. Chem. 2012, 2792–2800. doi:10.1002/ejoc.201200185
Return to citation in text: [1] -
Wang, G.-W.; Li, J.-X. Org. Biomol. Chem. 2006, 4, 4063–4064. doi:10.1039/b612641c
Return to citation in text: [1] -
Douglas, T.; Pordea, A.; Dowden, J. Org. Lett. 2017, 19, 6396–6399. doi:10.1021/acs.orglett.7b03252
Return to citation in text: [1] -
Dong, S.; Huang, J.; Sha, H.; Qiu, L.; Hu, W.; Xu, X. Org. Biomol. Chem. 2020, 18, 1926–1932. doi:10.1039/d0ob00222d
Return to citation in text: [1] -
Cheng, B.; Li, Y.; Wang, T.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Zhai, H. J. Org. Chem. 2020, 85, 6794–6802. doi:10.1021/acs.joc.0c00374
Return to citation in text: [1] -
Heo, N.; Jung, I.; Kim, D. K.; Han, S. H.; Lee, K.; Lee, P. H. Org. Lett. 2020, 22, 6562–6567. doi:10.1021/acs.orglett.0c02333
Return to citation in text: [1] -
Cheng, B.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Sun, H.; Wang, T.; Zhai, H. Adv. Synth. Catal. 2020, 362, 4668–4672. doi:10.1002/adsc.202000655
Return to citation in text: [1] -
Duan, S.; Chen, C.; Chen, Y.; Jie, Y.; Luo, H.; Xu, Z.-F.; Cheng, B.; Li, C.-Y. Org. Chem. Front. 2021, 8, 6962–6967. doi:10.1039/d1qo01237a
Return to citation in text: [1] -
Yavari, I.; Hojati, M.; Azad, L.; Halvagar, M. Synlett 2018, 29, 1024–1027. doi:10.1055/s-0037-1609302
Return to citation in text: [1] -
Cao, H.; Cheng, Q.; Studer, A. Science 2022, 378, 779–785. doi:10.1126/science.ade6029
Return to citation in text: [1] -
Sun, S.; Wei, Y.; Xu, J. Org. Lett. 2022, 24, 6024–6030. doi:10.1021/acs.orglett.2c02321
Return to citation in text: [1] -
Galeev, A. R.; Moroz, A. A.; Dmitriev, M. V.; Maslivets, A. N. RSC Adv. 2022, 12, 578–587. doi:10.1039/d1ra08384h
Return to citation in text: [1] -
Li, T.-T.; You, Y.; Sun, T.-J.; Zhang, Y.-P.; Zhao, J.-Q.; Wang, Z.-H.; Yuan, W.-C. Org. Lett. 2022, 24, 5120–5125. doi:10.1021/acs.orglett.2c01959
Return to citation in text: [1] -
He, B.; Huang, J.; Zhang, J.; Liu, X.; Wang, D.; Sung, H. H. Y.; Liu, Y.; Qin, A.; Lam, J. W. Y.; Tang, B. Z. Sci. China: Chem. 2022, 65, 789–795. doi:10.1007/s11426-021-1225-4
Return to citation in text: [1] -
Wei, Y.; Sun, S.; Xu, J. Tetrahedron Lett. 2023, 120, 154444. doi:10.1016/j.tetlet.2023.154444
Return to citation in text: [1] -
He, B.; Huang, J.; Zhang, J.; Sung, H. H. Y.; Lam, J. W. Y.; Zhang, Z.; Yan, S.; Wang, D.; Zhang, J.; Tang, B. Z. Angew. Chem., Int. Ed. 2022, 61, e202117709. doi:10.1002/anie.202117709
Return to citation in text: [1] -
Yao, Y.; Lin, B.; Wu, M.; Zhang, Y.; Huang, Y.; Han, X.; Weng, Z. Org. Biomol. Chem. 2022, 20, 8761–8765. doi:10.1039/d2ob01749k
Return to citation in text: [1] -
Sun, S.; Zhang, M.; Xu, J. Org. Chem. Front. 2022, 9, 3335–3341. doi:10.1039/d2qo00556e
Return to citation in text: [1] -
Kumar, A.; Maurya, R. A. Tetrahedron 2008, 64, 3477–3482. doi:10.1016/j.tet.2008.02.022
Return to citation in text: [1] -
Yang, S.-H.; Zhao, F.-Y.; Lü, H.-Y.; Deng, J.; Zhang, Z.-H. J. Heterocycl. Chem. 2012, 49, 1126–1129. doi:10.1002/jhet.953
Return to citation in text: [1] -
Ramesh, D.; Rajaram, S.; Narasimhulu, M.; Reddy, T. S.; Mahesh, K. C.; Manasa, G.; Venkateswarlu, Y. Chin. J. Chem. 2011, 29, 2471–2475. doi:10.1002/cjoc.201100032
Return to citation in text: [1] -
Kumar, A.; Maurya, R. A.; Sharma, S.; Kumar, M.; Bhatia, G. Eur. J. Med. Chem. 2010, 45, 501–509. doi:10.1016/j.ejmech.2009.10.036
Return to citation in text: [1] -
Lavilla, R.; Carranco, I.; Díaz, J. L.; Bernabeu, M. C.; de la Rosa, G. Mol. Diversity 2003, 6, 171–175. doi:10.1023/b:modi.0000006756.83821.80
Return to citation in text: [1] -
Di Pietro, O.; Viayna, E.; Vicente-García, E.; Bartolini, M.; Ramón, R.; Juárez-Jiménez, J.; Clos, M. V.; Pérez, B.; Andrisano, V.; Luque, F. J.; Lavilla, R.; Muñoz-Torrero, D. Eur. J. Med. Chem. 2014, 73, 141–152. doi:10.1016/j.ejmech.2013.12.008
Return to citation in text: [1] -
Vicente-García, E.; Catti, F.; Ramón, R.; Lavilla, R. Org. Lett. 2010, 12, 860–863. doi:10.1021/ol902913j
Return to citation in text: [1] -
Jiang, J.; Yu, J.; Sun, X.-X.; Rao, Q.-Q.; Gong, L.-Z. Angew. Chem., Int. Ed. 2008, 47, 2458–2462. doi:10.1002/anie.200705300
Return to citation in text: [1] -
Vohra, R. K.; Bruneau, C.; Renaud, J.-L. Adv. Synth. Catal. 2006, 348, 2571–2574. doi:10.1002/adsc.200600343
Return to citation in text: [1] -
Basu, S.; Chatterjee, S.; Ray, S.; Maity, S.; Ghosh, P.; Bhaumik, A.; Mukhopadhyay, C. Beilstein J. Org. Chem. 2022, 18, 133–142. doi:10.3762/bjoc.18.14
Return to citation in text: [1] -
Lavilla, R.; Bernabeu, M. C.; Carranco, I.; Díaz, J. L. Org. Lett. 2003, 5, 717–720. doi:10.1021/ol027545d
Return to citation in text: [1] [2] -
Carranco, I.; Díaz, J. L.; Jiménez, O.; Vendrell, M.; Albericio, F.; Royo, M.; Lavilla, R. J. Comb. Chem. 2005, 7, 33–41. doi:10.1021/cc049877a
Return to citation in text: [1] [2] -
Maiti, S.; Sridharan, V.; Menéndez, J. C. J. Comb. Chem. 2010, 12, 713–722. doi:10.1021/cc100084b
Return to citation in text: [1] [2] -
Islam, K.; Das, D. K.; Akram, E.; Khan, A. T. Synthesis 2015, 47, 2745–2755. doi:10.1055/s-0034-1380431
Return to citation in text: [1] [2] -
Sun, J.; Sun, Y.; Gong, H.; Xie, Y.-J.; Yan, C.-G. Org. Lett. 2012, 14, 5172–5175. doi:10.1021/ol302530m
Return to citation in text: [1] -
Sun, J.; Gong, H.; Sun, Y.; Yan, C.-G. Mol. Diversity 2013, 17, 627–639. doi:10.1007/s11030-013-9459-5
Return to citation in text: [1] -
Sun, J.; Gong, H.; Yan, C.-G. Tetrahedron 2013, 69, 10235–10244. doi:10.1016/j.tet.2013.10.044
Return to citation in text: [1] -
Sun, J.; Zhu, D.; Gong, H.; Yan, C.-G. Tetrahedron 2013, 69, 10565–10572. doi:10.1016/j.tet.2013.10.040
Return to citation in text: [1] -
Gong, H.; Sun, J.; Yan, C.-G. Tetrahedron 2014, 70, 6641–6650. doi:10.1016/j.tet.2014.06.097
Return to citation in text: [1] -
Yang, F.; Zhang, L.-J.; Yan, C.-G. RSC Adv. 2014, 4, 64466–64475. doi:10.1039/c4ra12278j
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Gong, H.; Yan, C.-G. J. Heterocycl. Chem. 2015, 52, 1278–1285. doi:10.1002/jhet.2140
Return to citation in text: [1] -
Zhu, D.; Sun, J.; Yan, C.-G. Synthesis 2015, 47, 193–198. doi:10.1055/s-0034-1379234
Return to citation in text: [1] -
Sun, J.; Gong, H.; Yan, C. Chin. J. Chem. 2015, 33, 1049–1056. doi:10.1002/cjoc.201500332
Return to citation in text: [1] -
Yang, F.; Sun, J.; Yan, C. Chin. J. Chem. 2015, 33, 1371–1379. doi:10.1002/cjoc.201500664
Return to citation in text: [1] -
Zhang, Y.-Y.; Han, Y.; Sun, J.; Yan, C.-G. ChemistrySelect 2017, 2, 7382–7386. doi:10.1002/slct.201701348
Return to citation in text: [1] -
Fang, H.-L.; Sun, J.; Yan, C.-G. ChemistrySelect 2021, 6, 10537–10541. doi:10.1002/slct.202102255
Return to citation in text: [1] -
Xu, F.-S.; Yan, C.; Sun, J.; Yan, C.-G. New J. Chem. 2021, 45, 19666–19670. doi:10.1039/d1nj03772b
Return to citation in text: [1] -
Zheng, H.; Han, Y.; Sun, J.; Yan, C.-G. Chin. Chem. Lett. 2021, 32, 1683–1686. doi:10.1016/j.cclet.2020.12.024
Return to citation in text: [1] -
Liu, D.; Sun, J.; Xie, J.; Shi, H.; Yan, C.-G. J. Org. Chem. 2021, 86, 1827–1842. doi:10.1021/acs.joc.0c02645
Return to citation in text: [1] -
Han, Y.; Zheng, H.; Zhang, Y.-Y.; Yan, C.-G. Chin. Chem. Lett. 2020, 31, 1337–1341. doi:10.1016/j.cclet.2019.10.042
Return to citation in text: [1] -
Zheng, H.; Han, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2022, 18, 669–679. doi:10.3762/bjoc.18.68
Return to citation in text: [1] -
Zheng, H.; Han, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2022, 18, 991–998. doi:10.3762/bjoc.18.99
Return to citation in text: [1] -
Liu, X.; Sun, J.; Yan, C.-G. New J. Chem. 2022, 46, 11877–11882. doi:10.1039/d2nj01135b
Return to citation in text: [1] -
Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2022, 18, 796–808. doi:10.3762/bjoc.18.80
Return to citation in text: [1]
| 1. | Huisgen, R.; Morikawa, M.; Herbig, K.; Brunn, E. Chem. Ber. 1967, 100, 1094–1106. doi:10.1002/cber.19671000406 |
| 2. | Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p |
| 3. | Nair, V.; Menon, R. S.; Sreekanth, A. R.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520–530. doi:10.1021/ar0502026 |
| 26. | He, B.; Huang, J.; Zhang, J.; Liu, X.; Wang, D.; Sung, H. H. Y.; Liu, Y.; Qin, A.; Lam, J. W. Y.; Tang, B. Z. Sci. China: Chem. 2022, 65, 789–795. doi:10.1007/s11426-021-1225-4 |
| 27. | Wei, Y.; Sun, S.; Xu, J. Tetrahedron Lett. 2023, 120, 154444. doi:10.1016/j.tetlet.2023.154444 |
| 28. | He, B.; Huang, J.; Zhang, J.; Sung, H. H. Y.; Lam, J. W. Y.; Zhang, Z.; Yan, S.; Wang, D.; Zhang, J.; Tang, B. Z. Angew. Chem., Int. Ed. 2022, 61, e202117709. doi:10.1002/anie.202117709 |
| 29. | Yao, Y.; Lin, B.; Wu, M.; Zhang, Y.; Huang, Y.; Han, X.; Weng, Z. Org. Biomol. Chem. 2022, 20, 8761–8765. doi:10.1039/d2ob01749k |
| 30. | Sun, S.; Zhang, M.; Xu, J. Org. Chem. Front. 2022, 9, 3335–3341. doi:10.1039/d2qo00556e |
| 16. | Dong, S.; Huang, J.; Sha, H.; Qiu, L.; Hu, W.; Xu, X. Org. Biomol. Chem. 2020, 18, 1926–1932. doi:10.1039/d0ob00222d |
| 17. | Cheng, B.; Li, Y.; Wang, T.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Zhai, H. J. Org. Chem. 2020, 85, 6794–6802. doi:10.1021/acs.joc.0c00374 |
| 18. | Heo, N.; Jung, I.; Kim, D. K.; Han, S. H.; Lee, K.; Lee, P. H. Org. Lett. 2020, 22, 6562–6567. doi:10.1021/acs.orglett.0c02333 |
| 19. | Cheng, B.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Sun, H.; Wang, T.; Zhai, H. Adv. Synth. Catal. 2020, 362, 4668–4672. doi:10.1002/adsc.202000655 |
| 20. | Duan, S.; Chen, C.; Chen, Y.; Jie, Y.; Luo, H.; Xu, Z.-F.; Cheng, B.; Li, C.-Y. Org. Chem. Front. 2021, 8, 6962–6967. doi:10.1039/d1qo01237a |
| 21. | Yavari, I.; Hojati, M.; Azad, L.; Halvagar, M. Synlett 2018, 29, 1024–1027. doi:10.1055/s-0037-1609302 |
| 22. | Cao, H.; Cheng, Q.; Studer, A. Science 2022, 378, 779–785. doi:10.1126/science.ade6029 |
| 23. | Sun, S.; Wei, Y.; Xu, J. Org. Lett. 2022, 24, 6024–6030. doi:10.1021/acs.orglett.2c02321 |
| 24. | Galeev, A. R.; Moroz, A. A.; Dmitriev, M. V.; Maslivets, A. N. RSC Adv. 2022, 12, 578–587. doi:10.1039/d1ra08384h |
| 25. | Li, T.-T.; You, Y.; Sun, T.-J.; Zhang, Y.-P.; Zhao, J.-Q.; Wang, Z.-H.; Yuan, W.-C. Org. Lett. 2022, 24, 5120–5125. doi:10.1021/acs.orglett.2c01959 |
| 9. | Sun, J.; Hu, H.; Wang, F.; Wu, H.; Liu, Y. RSC Adv. 2014, 4, 36498–36501. doi:10.1039/c4ra06048b |
| 10. | Xing, Y.; Zhang, M.; Ciccarelli, S.; Lee, J.; Catano, B. Eur. J. Org. Chem. 2017, 781–785. doi:10.1002/ejoc.201601416 |
| 11. | Ghafouri, S. S.; Afshari, M. A.; Saeedi, N.; Djahaniani, H.; Mohtat, B. Asian J. Chem. 2013, 25, 4111–4112. doi:10.14233/ajchem.2013.13772 |
| 12. | Sun, J.; Wang, F.; Hu, H.; Wang, X.; Wu, H.; Liu, Y. J. Org. Chem. 2014, 79, 3992–3998. doi:10.1021/jo500456d |
| 13. | Yang, H.-B.; Guan, X.-Y.; Wei, Y.; Shi, M. Eur. J. Org. Chem. 2012, 2792–2800. doi:10.1002/ejoc.201200185 |
| 14. | Wang, G.-W.; Li, J.-X. Org. Biomol. Chem. 2006, 4, 4063–4064. doi:10.1039/b612641c |
| 15. | Douglas, T.; Pordea, A.; Dowden, J. Org. Lett. 2017, 19, 6396–6399. doi:10.1021/acs.orglett.7b03252 |
| 4. | Kielland, N.; Lavilla, R. Top. Heterocycl. Chem. 2010, 25, 127–168. doi:10.1007/7081_2010_42 |
| 5. | Shaabani, A.; Maleki, A.; Rezayan, A. H.; Sarvary, A. Mol. Diversity 2011, 15, 41–68. doi:10.1007/s11030-010-9258-1 |
| 6. | Nair, V.; Deepthi, A.; Ashok, D.; Raveendran, A. E.; Paul, R. R. Tetrahedron 2014, 70, 3085–3105. doi:10.1016/j.tet.2014.03.014 |
| 7. | Sharma, U. K.; Ranjan, P.; Van der Eycken, E. V.; You, S.-L. Chem. Soc. Rev. 2020, 49, 8721–8748. doi:10.1039/d0cs00128g |
| 8. | Piltan, M.; Yavari, I.; Moradi, L. Chin. Chem. Lett. 2013, 24, 979–983. doi:10.1016/j.cclet.2013.06.023 |
| 43. | Maiti, S.; Sridharan, V.; Menéndez, J. C. J. Comb. Chem. 2010, 12, 713–722. doi:10.1021/cc100084b |
| 45. | Sun, J.; Sun, Y.; Gong, H.; Xie, Y.-J.; Yan, C.-G. Org. Lett. 2012, 14, 5172–5175. doi:10.1021/ol302530m |
| 46. | Sun, J.; Gong, H.; Sun, Y.; Yan, C.-G. Mol. Diversity 2013, 17, 627–639. doi:10.1007/s11030-013-9459-5 |
| 47. | Sun, J.; Gong, H.; Yan, C.-G. Tetrahedron 2013, 69, 10235–10244. doi:10.1016/j.tet.2013.10.044 |
| 48. | Sun, J.; Zhu, D.; Gong, H.; Yan, C.-G. Tetrahedron 2013, 69, 10565–10572. doi:10.1016/j.tet.2013.10.040 |
| 49. | Gong, H.; Sun, J.; Yan, C.-G. Tetrahedron 2014, 70, 6641–6650. doi:10.1016/j.tet.2014.06.097 |
| 50. | Yang, F.; Zhang, L.-J.; Yan, C.-G. RSC Adv. 2014, 4, 64466–64475. doi:10.1039/c4ra12278j |
| 51. | Sun, J.; Sun, Y.; Gong, H.; Yan, C.-G. J. Heterocycl. Chem. 2015, 52, 1278–1285. doi:10.1002/jhet.2140 |
| 52. | Zhu, D.; Sun, J.; Yan, C.-G. Synthesis 2015, 47, 193–198. doi:10.1055/s-0034-1379234 |
| 53. | Sun, J.; Gong, H.; Yan, C. Chin. J. Chem. 2015, 33, 1049–1056. doi:10.1002/cjoc.201500332 |
| 54. | Yang, F.; Sun, J.; Yan, C. Chin. J. Chem. 2015, 33, 1371–1379. doi:10.1002/cjoc.201500664 |
| 55. | Zhang, Y.-Y.; Han, Y.; Sun, J.; Yan, C.-G. ChemistrySelect 2017, 2, 7382–7386. doi:10.1002/slct.201701348 |
| 56. | Fang, H.-L.; Sun, J.; Yan, C.-G. ChemistrySelect 2021, 6, 10537–10541. doi:10.1002/slct.202102255 |
| 57. | Xu, F.-S.; Yan, C.; Sun, J.; Yan, C.-G. New J. Chem. 2021, 45, 19666–19670. doi:10.1039/d1nj03772b |
| 58. | Zheng, H.; Han, Y.; Sun, J.; Yan, C.-G. Chin. Chem. Lett. 2021, 32, 1683–1686. doi:10.1016/j.cclet.2020.12.024 |
| 59. | Liu, D.; Sun, J.; Xie, J.; Shi, H.; Yan, C.-G. J. Org. Chem. 2021, 86, 1827–1842. doi:10.1021/acs.joc.0c02645 |
| 60. | Han, Y.; Zheng, H.; Zhang, Y.-Y.; Yan, C.-G. Chin. Chem. Lett. 2020, 31, 1337–1341. doi:10.1016/j.cclet.2019.10.042 |
| 61. | Zheng, H.; Han, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2022, 18, 669–679. doi:10.3762/bjoc.18.68 |
| 62. | Zheng, H.; Han, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2022, 18, 991–998. doi:10.3762/bjoc.18.99 |
| 63. | Liu, X.; Sun, J.; Yan, C.-G. New J. Chem. 2022, 46, 11877–11882. doi:10.1039/d2nj01135b |
| 64. | Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2022, 18, 796–808. doi:10.3762/bjoc.18.80 |
| 41. | Lavilla, R.; Bernabeu, M. C.; Carranco, I.; Díaz, J. L. Org. Lett. 2003, 5, 717–720. doi:10.1021/ol027545d |
| 42. | Carranco, I.; Díaz, J. L.; Jiménez, O.; Vendrell, M.; Albericio, F.; Royo, M.; Lavilla, R. J. Comb. Chem. 2005, 7, 33–41. doi:10.1021/cc049877a |
| 41. | Lavilla, R.; Bernabeu, M. C.; Carranco, I.; Díaz, J. L. Org. Lett. 2003, 5, 717–720. doi:10.1021/ol027545d |
| 42. | Carranco, I.; Díaz, J. L.; Jiménez, O.; Vendrell, M.; Albericio, F.; Royo, M.; Lavilla, R. J. Comb. Chem. 2005, 7, 33–41. doi:10.1021/cc049877a |
| 43. | Maiti, S.; Sridharan, V.; Menéndez, J. C. J. Comb. Chem. 2010, 12, 713–722. doi:10.1021/cc100084b |
| 44. | Islam, K.; Das, D. K.; Akram, E.; Khan, A. T. Synthesis 2015, 47, 2745–2755. doi:10.1055/s-0034-1380431 |
| 35. | Lavilla, R.; Carranco, I.; Díaz, J. L.; Bernabeu, M. C.; de la Rosa, G. Mol. Diversity 2003, 6, 171–175. doi:10.1023/b:modi.0000006756.83821.80 |
| 36. | Di Pietro, O.; Viayna, E.; Vicente-García, E.; Bartolini, M.; Ramón, R.; Juárez-Jiménez, J.; Clos, M. V.; Pérez, B.; Andrisano, V.; Luque, F. J.; Lavilla, R.; Muñoz-Torrero, D. Eur. J. Med. Chem. 2014, 73, 141–152. doi:10.1016/j.ejmech.2013.12.008 |
| 37. | Vicente-García, E.; Catti, F.; Ramón, R.; Lavilla, R. Org. Lett. 2010, 12, 860–863. doi:10.1021/ol902913j |
| 38. | Jiang, J.; Yu, J.; Sun, X.-X.; Rao, Q.-Q.; Gong, L.-Z. Angew. Chem., Int. Ed. 2008, 47, 2458–2462. doi:10.1002/anie.200705300 |
| 39. | Vohra, R. K.; Bruneau, C.; Renaud, J.-L. Adv. Synth. Catal. 2006, 348, 2571–2574. doi:10.1002/adsc.200600343 |
| 40. | Basu, S.; Chatterjee, S.; Ray, S.; Maity, S.; Ghosh, P.; Bhaumik, A.; Mukhopadhyay, C. Beilstein J. Org. Chem. 2022, 18, 133–142. doi:10.3762/bjoc.18.14 |
| 31. | Kumar, A.; Maurya, R. A. Tetrahedron 2008, 64, 3477–3482. doi:10.1016/j.tet.2008.02.022 |
| 32. | Yang, S.-H.; Zhao, F.-Y.; Lü, H.-Y.; Deng, J.; Zhang, Z.-H. J. Heterocycl. Chem. 2012, 49, 1126–1129. doi:10.1002/jhet.953 |
| 33. | Ramesh, D.; Rajaram, S.; Narasimhulu, M.; Reddy, T. S.; Mahesh, K. C.; Manasa, G.; Venkateswarlu, Y. Chin. J. Chem. 2011, 29, 2471–2475. doi:10.1002/cjoc.201100032 |
| 34. | Kumar, A.; Maurya, R. A.; Sharma, S.; Kumar, M.; Bhatia, G. Eur. J. Med. Chem. 2010, 45, 501–509. doi:10.1016/j.ejmech.2009.10.036 |
| 44. | Islam, K.; Das, D. K.; Akram, E.; Khan, A. T. Synthesis 2015, 47, 2745–2755. doi:10.1055/s-0034-1380431 |
© 2023 Chen et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.