Abstract
Asymmetric organocatalyzed multicomponent reactions represent an important toolbox in the field of organic synthesis to build complex scaffolds starting from simple starting materials. The Enders three-component cascade reaction was a cornerstone in the field and a plethora of organocatalyzed cascade reactions followed. However, acetaldehyde was not shown as a successful reaction partner, probably because of its high reactivity. Herein, we report the Enders-type cascade reaction using acetaldehyde dimethyl acetal, as a masked form of acetaldehyde. This strategy directly converts acetaldehyde, nitroalkenes and enals into stereochemically dense cyclohexenals in good yield and excellent enantioselectivity.
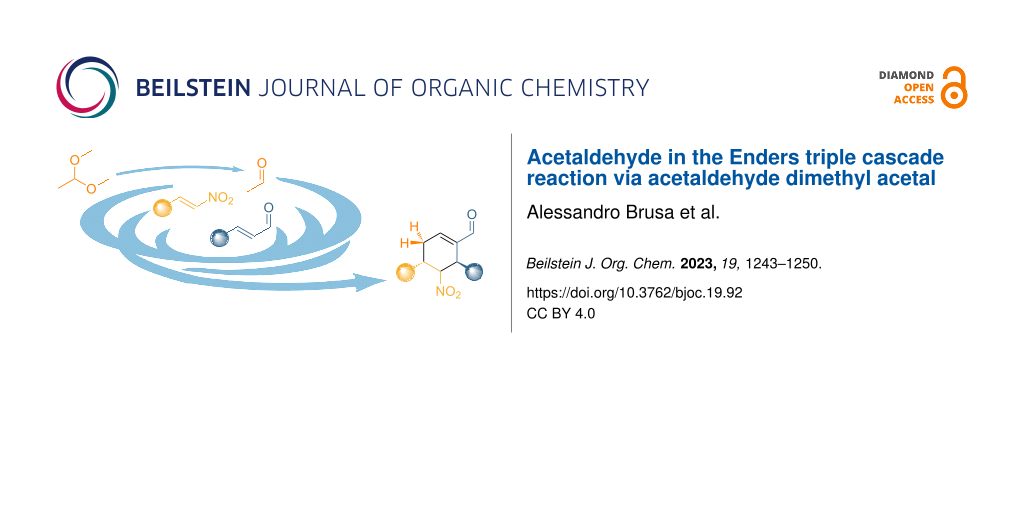
Graphical Abstract
Introduction
Multicomponent reactions (MCRs) are chemical processes that involve three or more compounds, in which the product contains all the atoms of the reagents, except for condensation coproducts, such as water, hydrogen chloride or other small molecules [1-3]. MCRs have a great advantage over the classical two-component reactions; they allow the construction of complex molecular motifs in only one synthetic operational step starting from simpler building blocks. For this reason, the use of MCRs is appealing in the construction of natural or synthetic products [2-5] or libraries of compounds [2], and is generally considered an advantage in organic synthesis for atom economy, waste reduction and time saving. Cascade reactions are defined as chemical processes in which two or more bond-forming steps happen under identical reaction conditions, and where a subsequent transformation takes place at the functionality obtained in the former bond-forming event. Cascade reactions are valuable tools for streamlining the synthesis of structurally complex molecules in a single operation and from readily available substrates. Their combination with asymmetric aminocatalysis [4,6-8] has recently led to innovative approaches for the one-step enantioselective preparation of stereochemically dense molecules. Nowadays, organocatalytic cascade processes provide a powerful tool for achieving molecular complexity. Their synthetic potential has been demonstrated by their application in the total synthesis of complex natural compounds [2,4,9-12].
A remarkable example of an amino-catalyzed cascade process was reported by Enders [11], a three-component cascade reaction for the synthesis of polyfunctionalized cyclohexenes bearing multiple stereocenters. The reaction is promoted by a chiral secondary amine, which is capable of catalyzing each step of the process activating the substrates through enamine and iminium ion catalysis towards a Michael/Michael/aldol process. The ingenious crafting of the reaction lies in the selection of the reactivity of the different nucleophiles and electrophiles present in the mixture, both as reagents and as intermediates. First, the chiral aminocatalyst 1 activates the saturated aldehyde 2 via enamine intermediate A, which intercepts the nitroalkene 3 in a Michael-type addition forming intermediate B. Hydrolysis regenerates catalyst 1 that can then selectively condense with the α,β-unsaturated aldehyde 4 to form chiral iminium ion intermediate C. Iminium ion C reacts with intermediate B in a further Michael-type reaction. The last step involves the enamine intermediate which drives an intramolecular aldol condensation to form the final product 5. In this elegant cascade process, catalyst 1 promotes three consecutive carbon–carbon bond forming steps generating four stereogenic centers with high diastereoselectivity and complete enantiocontrol (Scheme 1).
Scheme 1: Original triple organocatalytic cascade reaction developed by Enders.
Scheme 1: Original triple organocatalytic cascade reaction developed by Enders.
This elegantly designed example established a new direction in asymmetric aminocatalysis, leading to an impressive growth of methods based on organocascade processes [8,10,13-16]. The experimental simplicity of the strategy offers the potential of rapidly increasing structural and stereochemical complexity starting from readily available substrates. The scope of the process was shown to be successful with aliphatic aldehydes 2, aromatic nitroalkenes 3, and both aromatic and aliphatic unsaturated aldehydes 4.
Given our recent interest in the use of a surrogate of acetaldehyde [17-19] to address the challenges of working with free acetaldehyde, we wondered why the scope of this reaction did not include acetaldehyde and questioned whether a three-component triple cascade would indeed work employing this highly reactive substrate. The use of acetaldehyde as a reagent has always been challenging. The low boiling point and high volatility pose a problem with its handling and safety. The small steric hindrance gives rise to a high reactivity both as an electrophile and as a pro-nucleophile, hampering chemoselectivity (further to side reactions such as self-aldol condensations, polymerization and Tishchenko-type processes) and stereoselectivity [20]; the activation of acetaldehyde via aminocatalysis, furthermore, suffers from a lack of proper steric hindrance for the enantio-discrimination process. However, some methodologies enabling the use of acetaldehyde have been reported [20-24].
The safety and handling problems associated with acetaldehyde can be solved by synthetic equivalents that can be generated in situ through different paths. Some examples are represented by vinyl acetate [25], silyl vinyl ethers [26], ethanol, pyruvic acid, (E)-3-chloroacrylic acid, 2,4,6-trimethyl-1,3,5-trioxane (paraldehyde) [24,27], and acetaldehyde dimethyl acetal (6) [17-19]. On the basis of a long-term project based on masked reagents, our group has previously demonstrated the feasibility of the addition of a masked acetaldehyde 6 to nitroalkene derivatives with low reagent excess and high enantioselectivity; this reaction represents the first step in the Enders triple cascade catalytic cycle.
The use of acetaldehyde in a two-component cascade reaction was previously reported by Enders [27]; however, the scope of this reaction is limited to cyclohexene carbaldehydes bearing a methyl group on the C-6 atom (Figure 1a). On the other hand, these structural motifs can also be synthesized via the condensation of two equivalents of an enal and nitromethane (Figure 1b), although in this case C-4 and C-6 present the same substituent [28]. Interestingly, to the best of our knowledge, the use of acetaldehyde in the original Enders triple cascade reaction has not been explored despite its synthetic value. Indeed, this protocol would enable a wide structural variability in the synthesis of 3-substituted cyclohexene carbaldehydes (Figure 1c).
Figure 1: Approaches based on the original Enders cascade reaction to access trisubstituted cyclohexene carbaldehyde derivatives.
Figure 1: Approaches based on the original Enders cascade reaction to access trisubstituted cyclohexene carba...
Results and Discussion
In order to explore the feasibility of the triple cascade reaction with acetaldehyde (2a) as a substrate, we tested the original reaction conditions reported by Enders using trans-β-nitrostyrene (3) and trans-cinnamaldehyde (4) as the other substrates (Table 1, entry 1). The reaction proved to yield the desired product, indicating that the catalytic system may indeed be applicable. Lowering the amount of organocatalyst 1 to 10 mol % (Table 1, entry 2) resulted in a decrease of both yield and selectivity.
Table 1: Enders reaction with acetaldehyde.a
|
|
|||||
| Entry | 1 (mol %) | eeb (%) | Conversionc (%) | Yieldc (%) | Selectivityd (%) |
| 1 | 20 | 99 | 91 | 43 | 48 |
| 2 | 10 | 99 | 95 | 33 | 35 |
aReaction conditions: 3 (0.5 mmol, 1 equiv), 2a (0.6 mmol, 1.2 equiv), 4 (0.525 mmol, 1.05 equiv) were added to a solution of 1 (0.05 mmol, 0.1 equiv) in toluene (0.625 M wrt limiting reagent) and allowed to stir for 24 hours at room temperature. bDetermined by chiral HPLC analysis. cCalculated by 1H NMR using triphenylmethane as an internal standard. dRatio between yield and conversion.
Based on the results obtained (Table 1, entry 2) and the reaction conditions developed in our previous work [17], we tried to introduce 6 as an acetaldehyde equivalent, adding water in the reaction system and Amberlyst-15 as a catalyst to accelerate the hydrolysis process (Scheme 2).
Scheme 2: Acetaldehyde dimethyl acetal (6) as an acetaldehyde surrogate to effect a triple organocatalytic cascade reaction.
Scheme 2: Acetaldehyde dimethyl acetal (6) as an acetaldehyde surrogate to effect a triple organocatalytic ca...
To our delight, 6 as an acetaldehyde surrogate allows a slightly better yield with doubled selectivity, measured as the ratio between yield and conversion (Table 2, entry 1). This means that the productivity of the reaction using 6 is superior, since far more substrate was converted into the desired product. A solvent screening (see Supporting Information File 1) did not reveal any better alternative to toluene. The use of chloroform (Table 2, entry 2), as in our previous report [17], showed an improvement on selectivity, however, toluene was chosen as a more benign solvent. After further optimization of the acidic resin, stoichiometry, concentration, temperature, and reaction time (see Supporting Information File 1), we identified the best reaction conditions that yield the desired product in 44% yield, over 48 h (Table 2, entry 6). Unfortunately, side products and unwanted reactions do not allow to have a higher yield. All reactions provide the product in >99% ee, which is expected for a process which involves two consecutives stereoselective reactions. The control of the diastereomeric ratio is, however, difficult to attain as demonstrated by an extensive screening and is always near 50:50 without significant deviations changing the reaction conditions. On the contrary, the use of aldehydes other than acetaldehyde generates higher control [11]. It was previously shown that the first stereogenic center formed in the presented cascade process is formed with high control [17]. Therefore, the second carbon–carbon bond forming step, i.e., the organocatalyzed Michael addition of the nitronate to the α,β-unsaturated iminium ion, should be tackled to improve the diastereocontrol. All efforts to discriminate the two faces of the nitroenolate during the addition proved unproductive during the optimization, therefore, a potential epimerization was envisaged during the course of the reaction, but this hypothesis was discarded after further experimentation; submitting the two isolated diastereomers to the reaction conditions did not show any change. A slight improvement in the dr was found by exposing the mixture of the diastereomers to a strong basic environment (t-BuOK/MeOH) to force the formation of secondary nitronates. However, it could not be improved to synthetically interesting values. The observed diastereomeric ratio is, therefore, the direct result of the second Michael addition reaction, as previously reported [29], and no post-process epimerization event could be found.
Table 2: Selected optimization reaction conditions.a
|
|
|||||
| Entry | Deviation from above | eeb (%) | Conversionc (%) | Yieldc (%) | Selectivityd (%) |
| 1 | none | 99 | 61 | 40 | 66 |
| 2 | CHCl3 | 99 | 54 | 43 | 79 |
| 3 | 0.25 M | 99 | 72 | 35 | 48 |
| 4 | 0.5 M | 99 | 93 | 39 | 42 |
| 5 | 1 M | 99 | 88 | 35 | 40 |
| 6 | 0.25 M, 48 h | 99 | 79 | 44 | 56 |
aReaction conditions: 3 (0.1 mmol, 1 equiv), 6 (0.2 mmol, 2 equiv), 4 (0.1 mmol, 1.05 equiv), H2O (0.6 mmol, 6 equiv), and Amberlyst-15 (10 mol %) were added to a solution of 1 (10 mol %) in 1 mL of solvent (0.1 M) and allowed to stir for 24 hours at room temperature. bDetermined by chiral HPLC analysis. cCalculated by 1H NMR using triphenylmethane as an internal standard. dRatio between yield and conversion.
With the best conditions in our hand, we evaluated the scope of our triple cascade reaction enabled by masked acetaldehyde. As highlighted in Figure 2, the reaction proceeded smoothly regardless of structural and electronic variations of both substrates, giving access to a variety of complex cyclohexenals. Although the scope is limited to aromatic nitroalkenes and enals, the yields are good considering the complexity of the domino process that involves highly reactive partners, and the ees are consistently very high. Finally, albeit there is low diastereocontrol, the diastereomers can be easily separated by simple flash chromatography.
Figure 2: Scope of the cascade reaction using 6 as an acetaldehyde equivalent. Reaction conditions: 3 (0.5 mmol, 1 equiv), 6 (1 mmol, 2 equiv), 4 (0.525 mmol, 1.05 equiv), H2O (3.0 mmol, 6 equiv), and Amberlyst-15 (10 mol %) were added to a solution of 1 (10 mol %) in 2 mL of solvent (0.25 M) and allowed to stir for 48 hours at room temperature. Yields of isolated compounds are given. Diastereomeric ratio (dr) determined by 1H NMR analysis. Enatiomeric excess (ee) determined by chiral HPLC analysis.
Figure 2: Scope of the cascade reaction using 6 as an acetaldehyde equivalent. Reaction conditions: 3 (0.5 mm...
Conclusion
An unprecedented methodology for the synthesis of 4,6-disubstituted 5-nitrocyclohexene carbaldehydes with three contiguous stereogenic centers using acetaldehyde as one of the reaction components of an Enders cascade reaction has been developed. The masked form of acetaldehyde, which is hydrolyzed in situ using Amberlyst-15 as an acid catalyst, instead of directly using acetaldehyde allows for higher yields and fewer byproducts. Using mild reaction conditions, it was possible to obtain a variety of functionalized cyclohexene carbaldehydes in good yields and very high enantiomeric excesses. Unfortunately, the developed methodology is currently limited to aromatic substrates and the formation of one stereocenter is difficult to control, leading to a mixture of two diastereomers. Current efforts in our laboratories are addressing these challenges.
Supporting Information
| Supporting Information File 1: Experimental part, NMR and HPLC spectra. | ||
| Format: PDF | Size: 5.5 MB | Download |
Funding
Fabio Pesciaioli thanks the Ministero dell'Università e della Ricerca (PON-AIM1842894, CUPE18D19000560001) for funding this research. Maria Edith Casacchia thanks the support of the Italian national inter-university Ph.D. course in Sustainable Development and Climate Change. Alessio Carioscia acknowledges Dipharma Francis for supporting his Ph.D. fellowship. Alessandro Brusa and Armando Carlone acknowledge funding by the European Union - NextGenerationEU under the Italian Ministry of University and Research (MUR) National Innovation Ecosystem grant ECS00000041 - VITALITY - CUP E13C22001060006. Giuliana Giorgianni thanks the Ministero dell'Università e della Ricerca (PON-DOT13OV2OC) for an industrial Ph.D. fellowship.
References
-
Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a
Return to citation in text: [1] -
Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515
Return to citation in text: [1] [2] [3] [4] -
Younus, H. A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K. M. Expert Opin. Ther. Pat. 2021, 31, 267–289. doi:10.1080/13543776.2021.1858797
Return to citation in text: [1] [2] -
Wang, Y.; Lu, H.; Xu, P.-F. Acc. Chem. Res. 2015, 48, 1832–1844. doi:10.1021/acs.accounts.5b00217
Return to citation in text: [1] [2] [3] -
Ramón, D. J.; Yus, M. Angew. Chem., Int. Ed. 2005, 44, 1602–1634. doi:10.1002/anie.200460548
Return to citation in text: [1] -
Volla, C. M. R.; Atodiresei, I.; Rueping, M. Chem. Rev. 2014, 114, 2390–2431. doi:10.1021/cr400215u
Return to citation in text: [1] -
Guillena, G.; Ramón, D. J.; Yus, M. Tetrahedron: Asymmetry 2007, 18, 693–700. doi:10.1016/j.tetasy.2007.03.002
Return to citation in text: [1] -
Enders, D.; Grondal, C.; Hüttl, M. R. M. Angew. Chem., Int. Ed. 2007, 46, 1570–1581. doi:10.1002/anie.200603129
Return to citation in text: [1] [2] -
Grondal, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 2, 167–178. doi:10.1038/nchem.539
Return to citation in text: [1] -
Zhu, Q.-N.; Zhang, Y.-C.; Xu, M.-M.; Sun, X.-X.; Yang, X.; Shi, F. J. Org. Chem. 2016, 81, 7898–7907. doi:10.1021/acs.joc.6b01598
Return to citation in text: [1] [2] -
Enders, D.; Hüttl, M. R. M.; Grondal, C.; Raabe, G. Nature 2006, 441, 861–863. doi:10.1038/nature04820
Return to citation in text: [1] [2] [3] -
Ishikawa, H.; Suzuki, T.; Orita, H.; Uchimaru, T.; Hayashi, Y. Chem. – Eur. J. 2010, 16, 12616–12626. doi:10.1002/chem.201001108
Return to citation in text: [1] -
Carlone, A.; Cabrera, S.; Marigo, M.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2007, 46, 1101–1104. doi:10.1002/anie.200604479
Return to citation in text: [1] -
Tseliou, V.; Faraone, A.; Kqiku, L.; Vilím, J.; Simionato, G.; Melchiorre, P. Angew. Chem., Int. Ed. 2022, 61, e202212176. doi:10.1002/anie.202212176
Return to citation in text: [1] -
Kumar, M.; Chauhan, P.; Bailey, S. J.; Jafari, E.; von Essen, C.; Rissanen, K.; Enders, D. Org. Lett. 2018, 20, 1232–1235. doi:10.1021/acs.orglett.8b00175
Return to citation in text: [1] -
Barløse, C. L.; Faghtmann, J.; Bitsch, R. S.; Gbubele, J. D.; Jørgensen, K. A. Org. Lett. 2023, 25, 1209–1213. doi:10.1021/acs.orglett.3c00220
Return to citation in text: [1] -
Giorgianni, G.; Nori, V.; Baschieri, A.; Palombi, L.; Carlone, A. Catalysts 2020, 10, 1296. doi:10.3390/catal10111296
Return to citation in text: [1] [2] [3] [4] [5] -
Nori, V.; Sinibaldi, A.; Giorgianni, G.; Pesciaioli, F.; Di Donato, F.; Cocco, E.; Biancolillo, A.; Landa, A.; Carlone, A. Chem. – Eur. J. 2022, 28, e202104524. doi:10.1002/chem.202104524
Return to citation in text: [1] [2] -
Casacchia, M. E.; Giorgianni, G.; Allegritti, E.; Giansanti, L.; Carlone, A.; Pesciaioli, F. SynOpen 2023, 07, 29–32. doi:10.1055/a-1996-8940
Return to citation in text: [1] [2] -
Kumar, M.; Kumar, A.; Rizvi, M. A.; Shah, B. A. RSC Adv. 2015, 5, 55926–55937. doi:10.1039/c5ra05695k
Return to citation in text: [1] [2] -
García-García, P.; Ladépêche, A.; Halder, R.; List, B. Angew. Chem., Int. Ed. 2008, 47, 4719–4721. doi:10.1002/anie.200800847
Return to citation in text: [1] -
Hayashi, Y.; Itoh, T.; Ohkubo, M.; Ishikawa, H. Angew. Chem., Int. Ed. 2008, 47, 4722–4724. doi:10.1002/anie.200801130
Return to citation in text: [1] -
Hayashi, Y.; Itoh, T.; Aratake, S.; Ishikawa, H. Angew. Chem., Int. Ed. 2008, 47, 2082–2084. doi:10.1002/anie.200704870
Return to citation in text: [1] -
Fan, X.; Rodríguez-Escrich, C.; Sayalero, S.; Pericàs, M. A. Chem. – Eur. J. 2013, 19, 10814–10817. doi:10.1002/chem.201302087
Return to citation in text: [1] [2] -
Zhang, W.; Wang, N.; Yang, Z.-J.; Li, Y.-R.; Yu, Y.; Pu, X.-M.; Yu, X.-Q. Adv. Synth. Catal. 2017, 359, 3397–3406. doi:10.1002/adsc.201700599
Return to citation in text: [1] -
Boxer, M. B.; Yamamoto, H. J. Am. Chem. Soc. 2006, 128, 48–49. doi:10.1021/ja054725k
Return to citation in text: [1] -
Enders, D.; Krüll, R.; Bettray, W. Synthesis 2010, 567–572. doi:10.1055/s-0029-1217146
Return to citation in text: [1] [2] -
Enders, D.; Jeanty, M.; Bats, J. Synlett 2009, 3175–3178. doi:10.1055/s-0029-1218282
Return to citation in text: [1] -
Świderek, K.; Nödling, A. R.; Tsai, Y.-H.; Luk, L. Y. P.; Moliner, V. J. Phys. Chem. A 2018, 122, 451–459. doi:10.1021/acs.jpca.7b11803
Return to citation in text: [1]
| 11. | Enders, D.; Hüttl, M. R. M.; Grondal, C.; Raabe, G. Nature 2006, 441, 861–863. doi:10.1038/nature04820 |
| 17. | Giorgianni, G.; Nori, V.; Baschieri, A.; Palombi, L.; Carlone, A. Catalysts 2020, 10, 1296. doi:10.3390/catal10111296 |
| 17. | Giorgianni, G.; Nori, V.; Baschieri, A.; Palombi, L.; Carlone, A. Catalysts 2020, 10, 1296. doi:10.3390/catal10111296 |
| 1. | Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a |
| 2. | Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515 |
| 3. | Younus, H. A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K. M. Expert Opin. Ther. Pat. 2021, 31, 267–289. doi:10.1080/13543776.2021.1858797 |
| 2. | Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515 |
| 4. | Wang, Y.; Lu, H.; Xu, P.-F. Acc. Chem. Res. 2015, 48, 1832–1844. doi:10.1021/acs.accounts.5b00217 |
| 9. | Grondal, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 2, 167–178. doi:10.1038/nchem.539 |
| 10. | Zhu, Q.-N.; Zhang, Y.-C.; Xu, M.-M.; Sun, X.-X.; Yang, X.; Shi, F. J. Org. Chem. 2016, 81, 7898–7907. doi:10.1021/acs.joc.6b01598 |
| 11. | Enders, D.; Hüttl, M. R. M.; Grondal, C.; Raabe, G. Nature 2006, 441, 861–863. doi:10.1038/nature04820 |
| 12. | Ishikawa, H.; Suzuki, T.; Orita, H.; Uchimaru, T.; Hayashi, Y. Chem. – Eur. J. 2010, 16, 12616–12626. doi:10.1002/chem.201001108 |
| 27. | Enders, D.; Krüll, R.; Bettray, W. Synthesis 2010, 567–572. doi:10.1055/s-0029-1217146 |
| 4. | Wang, Y.; Lu, H.; Xu, P.-F. Acc. Chem. Res. 2015, 48, 1832–1844. doi:10.1021/acs.accounts.5b00217 |
| 6. | Volla, C. M. R.; Atodiresei, I.; Rueping, M. Chem. Rev. 2014, 114, 2390–2431. doi:10.1021/cr400215u |
| 7. | Guillena, G.; Ramón, D. J.; Yus, M. Tetrahedron: Asymmetry 2007, 18, 693–700. doi:10.1016/j.tetasy.2007.03.002 |
| 8. | Enders, D.; Grondal, C.; Hüttl, M. R. M. Angew. Chem., Int. Ed. 2007, 46, 1570–1581. doi:10.1002/anie.200603129 |
| 28. | Enders, D.; Jeanty, M.; Bats, J. Synlett 2009, 3175–3178. doi:10.1055/s-0029-1218282 |
| 2. | Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515 |
| 24. | Fan, X.; Rodríguez-Escrich, C.; Sayalero, S.; Pericàs, M. A. Chem. – Eur. J. 2013, 19, 10814–10817. doi:10.1002/chem.201302087 |
| 27. | Enders, D.; Krüll, R.; Bettray, W. Synthesis 2010, 567–572. doi:10.1055/s-0029-1217146 |
| 2. | Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515 |
| 3. | Younus, H. A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K. M. Expert Opin. Ther. Pat. 2021, 31, 267–289. doi:10.1080/13543776.2021.1858797 |
| 4. | Wang, Y.; Lu, H.; Xu, P.-F. Acc. Chem. Res. 2015, 48, 1832–1844. doi:10.1021/acs.accounts.5b00217 |
| 5. | Ramón, D. J.; Yus, M. Angew. Chem., Int. Ed. 2005, 44, 1602–1634. doi:10.1002/anie.200460548 |
| 17. | Giorgianni, G.; Nori, V.; Baschieri, A.; Palombi, L.; Carlone, A. Catalysts 2020, 10, 1296. doi:10.3390/catal10111296 |
| 18. | Nori, V.; Sinibaldi, A.; Giorgianni, G.; Pesciaioli, F.; Di Donato, F.; Cocco, E.; Biancolillo, A.; Landa, A.; Carlone, A. Chem. – Eur. J. 2022, 28, e202104524. doi:10.1002/chem.202104524 |
| 19. | Casacchia, M. E.; Giorgianni, G.; Allegritti, E.; Giansanti, L.; Carlone, A.; Pesciaioli, F. SynOpen 2023, 07, 29–32. doi:10.1055/a-1996-8940 |
| 20. | Kumar, M.; Kumar, A.; Rizvi, M. A.; Shah, B. A. RSC Adv. 2015, 5, 55926–55937. doi:10.1039/c5ra05695k |
| 25. | Zhang, W.; Wang, N.; Yang, Z.-J.; Li, Y.-R.; Yu, Y.; Pu, X.-M.; Yu, X.-Q. Adv. Synth. Catal. 2017, 359, 3397–3406. doi:10.1002/adsc.201700599 |
| 17. | Giorgianni, G.; Nori, V.; Baschieri, A.; Palombi, L.; Carlone, A. Catalysts 2020, 10, 1296. doi:10.3390/catal10111296 |
| 18. | Nori, V.; Sinibaldi, A.; Giorgianni, G.; Pesciaioli, F.; Di Donato, F.; Cocco, E.; Biancolillo, A.; Landa, A.; Carlone, A. Chem. – Eur. J. 2022, 28, e202104524. doi:10.1002/chem.202104524 |
| 19. | Casacchia, M. E.; Giorgianni, G.; Allegritti, E.; Giansanti, L.; Carlone, A.; Pesciaioli, F. SynOpen 2023, 07, 29–32. doi:10.1055/a-1996-8940 |
| 26. | Boxer, M. B.; Yamamoto, H. J. Am. Chem. Soc. 2006, 128, 48–49. doi:10.1021/ja054725k |
| 8. | Enders, D.; Grondal, C.; Hüttl, M. R. M. Angew. Chem., Int. Ed. 2007, 46, 1570–1581. doi:10.1002/anie.200603129 |
| 10. | Zhu, Q.-N.; Zhang, Y.-C.; Xu, M.-M.; Sun, X.-X.; Yang, X.; Shi, F. J. Org. Chem. 2016, 81, 7898–7907. doi:10.1021/acs.joc.6b01598 |
| 13. | Carlone, A.; Cabrera, S.; Marigo, M.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2007, 46, 1101–1104. doi:10.1002/anie.200604479 |
| 14. | Tseliou, V.; Faraone, A.; Kqiku, L.; Vilím, J.; Simionato, G.; Melchiorre, P. Angew. Chem., Int. Ed. 2022, 61, e202212176. doi:10.1002/anie.202212176 |
| 15. | Kumar, M.; Chauhan, P.; Bailey, S. J.; Jafari, E.; von Essen, C.; Rissanen, K.; Enders, D. Org. Lett. 2018, 20, 1232–1235. doi:10.1021/acs.orglett.8b00175 |
| 16. | Barløse, C. L.; Faghtmann, J.; Bitsch, R. S.; Gbubele, J. D.; Jørgensen, K. A. Org. Lett. 2023, 25, 1209–1213. doi:10.1021/acs.orglett.3c00220 |
| 17. | Giorgianni, G.; Nori, V.; Baschieri, A.; Palombi, L.; Carlone, A. Catalysts 2020, 10, 1296. doi:10.3390/catal10111296 |
| 11. | Enders, D.; Hüttl, M. R. M.; Grondal, C.; Raabe, G. Nature 2006, 441, 861–863. doi:10.1038/nature04820 |
| 20. | Kumar, M.; Kumar, A.; Rizvi, M. A.; Shah, B. A. RSC Adv. 2015, 5, 55926–55937. doi:10.1039/c5ra05695k |
| 21. | García-García, P.; Ladépêche, A.; Halder, R.; List, B. Angew. Chem., Int. Ed. 2008, 47, 4719–4721. doi:10.1002/anie.200800847 |
| 22. | Hayashi, Y.; Itoh, T.; Ohkubo, M.; Ishikawa, H. Angew. Chem., Int. Ed. 2008, 47, 4722–4724. doi:10.1002/anie.200801130 |
| 23. | Hayashi, Y.; Itoh, T.; Aratake, S.; Ishikawa, H. Angew. Chem., Int. Ed. 2008, 47, 2082–2084. doi:10.1002/anie.200704870 |
| 24. | Fan, X.; Rodríguez-Escrich, C.; Sayalero, S.; Pericàs, M. A. Chem. – Eur. J. 2013, 19, 10814–10817. doi:10.1002/chem.201302087 |
| 29. | Świderek, K.; Nödling, A. R.; Tsai, Y.-H.; Luk, L. Y. P.; Moliner, V. J. Phys. Chem. A 2018, 122, 451–459. doi:10.1021/acs.jpca.7b11803 |
© 2023 Brusa et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.