Abstract
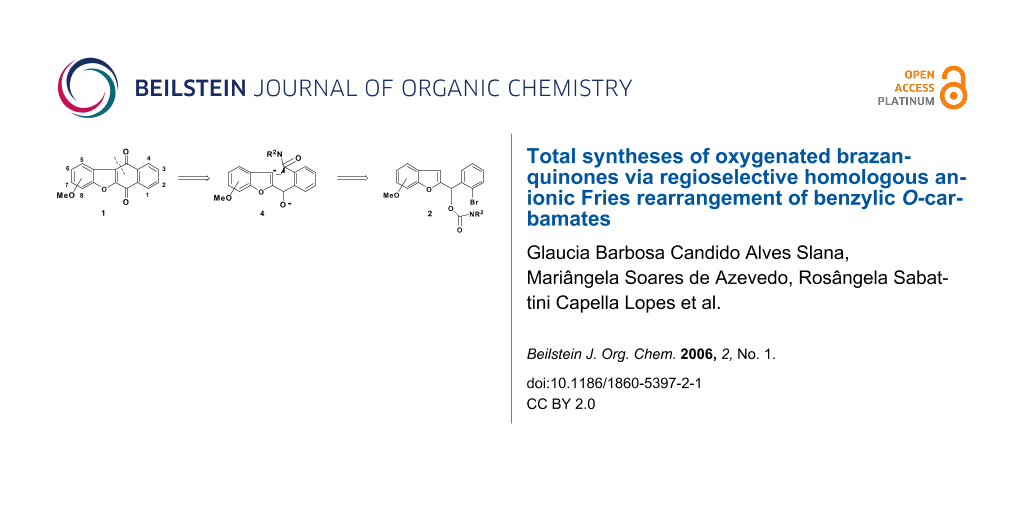
Using new variations of anionic aromatic chemistry, the total synthesis of oxygenated brazanquinones (1a-1c), derived from β-brasan, a natural product isolated from Caesalpina echinata, via carbamates 2a-2c is described.
Graphical Abstract
Introduction
The search for new synthetic routes for the total synthesis of biologically active natural products has been growing in recent years, often stimulated by the lack of synthetic drugs for the cure of diseases.
In various biological tests, several natural and synthetic brazanquinones have shown high biological activity. Cheng [1,2] in his work, evaluated (in vitro) a series of brazanquinones and their inhibitory activity against a series of cancer cell lines. Their activity has been attributed to their structure, in which the two ring systems are coplanar, and has attracted considerable attention as interesting synthetic targets. [3-11] However, the routes described in the literature do not offer the possibility of facile syntheses of other oxygenated analogues.
In this present work, the versatility of a new variation of anionic aromatic chemistry developed in our research group [12] will be applied in the context of rapid and efficient construction of these bioactive compounds for future biological evaluation.
The emerging carbanionic aromatic chemistry (anionic ortho-Fries, [13] homologous anionic Fries, [14] remote anionic Fries rearrangements [15] and carbamoyl Baker-Venkataraman reaction [16]) originating from the Directed ortho Metalation (DoM) strategy, [17] offer a mild and regioselective complement to classical Friedel-Crafts approaches for the rational construction of polysubstituted aromatics, biaryls, and several classes of heterocycles (Figure 1).
Figure 1: Examples of carbanionic aromatic chemistry rearrangements.
Figure 1: Examples of carbanionic aromatic chemistry rearrangements.
In 1993, Gawley showed that O-benzylcarbamates in the presence of directed metalation groups (DMGs) undergo competitive anionic [1,2] and [1,4] Wittig – carbamoyl rearrangements (paths a and b) [18,19] orientated by the groups R and DMGs (Figure 1).
Conceptual combination of path b and the well established tandem DoM route to anthraquinones and heteroanthraquinones [20] led to the conjecture that, barring the competitive [1,2]–Wittig rearrangement, and one-pot route, 3 → 4 → 1, may be established in a direct manner without resort to DoM-derived benzamide intermediate, thereby establishing new carbonyl dianion equivalency 4 (see Scheme 1).
Scheme 1: Retrosyntheses of Brazanquinones (1).
Scheme 1: Retrosyntheses of Brazanquinones (1).
We now report the versatility of this strategy, in the context of rapid and efficient construction of new oxygenated brazanquinones (1a-1c) derived from β-brasan, a natural product isolated from Caesalpina echinata (Scheme 2).
Scheme 2: Total syntheses of brazanquinones (1 a-c).
Scheme 2: Total syntheses of brazanquinones (1 a-c).
Results and discussion
The synthesis of brazanquinones (1a-1c) is summarized in Scheme 2. According to our recent report, [12] the benzoylbenzofurans 5a-5d, were prepared in one-pot reaction from α-bromoacetophenone 6 and ortho-hydroxybenzaldehydes 7a-7d in excellent yields (90–96%). Reduction of benzoylbenzofurans 5a-5d in the presence of NaBH4 yielded the secondary alcohols 8a-8d, which upon treatment with N,N-diethylcarbamoyl chloride, and sodium hydride afforded the carbamates 2a-2d (Scheme 2).
The carbamates 2a-2c were cyclized in the presence of excess sec-BuLi in THF at -78°C to afford the desired brazanquinones 1a-1c in reasonable yields (54%–57%) (Scheme 2).
The mechanism suggested by us and shown in Scheme 1, involves first the preparation of aryllithium intermediates 3 from the carbamates 2a-2b by the reversible metathesis reaction known [21-23] as the lithium-halogen exchange which has been widely employed for replacement of a bromine or iodine atom in a substrate by lithium. The intermediate 3 then undergoes an intramolecular anionic Fries rearrangement to intermediate 4, that was isolated in our previous results. [12]
Snieckus reported the remote metalation and cyclization of diethyl N-methyl-O-tolylanthranilamide to N-methyl dibenzazepinone [24-26] developing a new regiospecific construction of condensed aromatics. We found this route very attractive and envisaged that by in situ treatment of intermediate 4 with the third equivalent of sec-BuLi, the cyclization to the desired quinone 1a-d would be obtained.
One unexpected result was the formation of the phthalide 9 when the dimethoxy carbamate 2d was treated with excess sec-BuLi under the same reaction conditions as 2a-2c. Presumably, the presence of 4-OMe inhibits the cyclization by shielding the remote position (Scheme 3).
Scheme 3: Total syntheses of phthalide (9).
Scheme 3: Total syntheses of phthalide (9).
Conclusion
An efficient synthesis of brazanquinones (1a-c) using new variations of anionic aromatic chemistry was described. This methodology could be expanded in the future for the construction of new molecules with similar structures.
Supporting Information
| Supporting Information File 1: contains the experimental section | ||
| Format: DOC | Size: 62.5 KB | Download |
References
-
Cheng, C. C.; Dong, Q.; Liu, D.-F.; Luo, Y.-L.; Liu, L. F.; Chen, A.-Y.; Yu, C.; Savaraj, N.; Chou, T.-C. J. Med. Chem. 1993, 36, 4108. doi:10.1021/jm00077a016
Return to citation in text: [1] -
Crawford, P. W.; Carlos, E.; Ellegood, J. C.; Cheng, C. C.; Dong, Q.; LruJ, D. F.; Luof, Y. L. Electrochim. Acta 1996, 41, 2399. doi:10.1016/0013-4686(96)00020-5
Return to citation in text: [1] -
Stadlbauer, W.; Kappe, T. Z. Naturforsch. 1975, 30b, 139.
Return to citation in text: [1] -
El-Wareth, A.; Sarhan, A. O.; El-Dean, A. M.; Abdel-Monem, M. I. Monatsh. Chem. 1998, 129, 205.
Return to citation in text: [1] -
Martínez, E.; Martínez, L.; Estévez, J. C.; Estévez, R. J.; Castedo, L. J. Med. Chem. Soc. 1978, 21, 291.
Return to citation in text: [1] -
Bentley, K. W.; Robinson, R. J. Chem. Soc. 1950, 1353. doi:10.1039/jr9500001353
Return to citation in text: [1] -
Yoshida, K.; Adachi, T.; Oga, N.; Kubo, Y. Chem. Lett. 1990, 2049.
Return to citation in text: [1] -
Simonitsch, E.; Eisenhuth, W.; Stamm, O. A. E.; Schmid, H. Helv. Chim. Acta 1960, 43, 58. doi:10.1002/hlca.19600430107
Return to citation in text: [1] -
Chatterjea, J. N. Experientia 1953, 9, 256. doi:10.1007/BF02172434
Return to citation in text: [1] -
Jha, O. P. J. Indian Chem. Soc. 1973, 44, 740.
Return to citation in text: [1] -
Chatterjea, J. N.; Jha, O. P. J. Indian Chem. Soc. 1970, 47, 6.
Return to citation in text: [1] -
Azevedo, M. S.; Lopes, C. C.; Lopes, R. S. C.; Alves, G. B. C. Synthesis 2004, 8, 1262–1268.
Return to citation in text: [1] [2] [3] -
Sibi, M. P.; Snieckus, V. J. Org. Chem. 1983, 48, 1935. doi:10.1021/jo00159a040
Return to citation in text: [1] -
Kalinin, A. V.; Miah, M. A. J.; Chattopadhyay, S.; Tsukazaki, M.; Wicki, M. Synlett 1997, 7, 839.
Return to citation in text: [1] -
Wang, W.; Snieckus, V. J. Org. Chem. 1992, 57, 424. doi:10.1021/jo00028a004
Return to citation in text: [1] -
Kalinin, A. V.; da Silva, A. J. M.; Lopes, C. C.; Lopes, R. S. C.; Snieckus, V. Tetrahedron Lett. 1998, 39, 4995. doi:10.1016/S0040-4039(98)00977-0
Return to citation in text: [1] -
Snieckus, V. Chem. Rev. 1990, 90, 897. doi:10.1021/cr00104a001
Return to citation in text: [1] -
Zhang, P.; Gawley, R. E. J. Org. Chem. 1993, 58, 3223. doi:10.1021/jo00064a001
Return to citation in text: [1] -
Superchi, S.; Sotomayor, N.; Maio, G.; Joseph, B.; Campbell, M. G.; Snieckus, V. Tetrahedron Lett. 1996, 37, 60.
Return to citation in text: [1] -
Watanabe, M.; Snieckus, V. J. Am. Chem. Soc. 1980, 102, 1457. doi:10.1021/ja00524a059
for a tandem DoM route to anthraquinones and heteroanthraquinones based on initial benzamide-aromatic aldehyde condensation.
Return to citation in text: [1] -
Wittig, G.; Pockels, D.; Dröge, H. Ber. Dtsch. Chem. Ges. B 1938, 71, 1903.
Return to citation in text: [1] -
Gilman, H.; Jacoby, A. L. J. Org. Chem. 1938, 3, 108. doi:10.1021/jo01219a003
Return to citation in text: [1] -
Gilman, H.; Langham, W.; Jacoby, A. L. J. Am. Chem. Soc. 1939, 61, 106. doi:10.1021/ja01870a036
Return to citation in text: [1] -
Fu, J. M.; Zhao, B. P.; Sharp, M. J.; Snieckus, V. J. Org. Chem. 1991, 56, 1683. doi:10.1021/jo00005a004
Return to citation in text: [1] -
Wang, W.; Snieckus, V. J. Org. Chem. 1992, 57, 424. doi:10.1021/jo00028a004
Return to citation in text: [1] -
Wang, X.; Snieckus, V. Tetrahedron Lett. 1991, 32, 4879. doi:10.1016/S0040-4039(00)93485-3
Return to citation in text: [1] -
Still, W. C.; Michael, K.; Abhiji, M. J. Org. Chem. 1978, 4, 2923. doi:10.1021/jo00408a041
-
Watson, S. C.; Eastman, J. F. J. Organomet. Chem. 1967, 9, 165. doi:10.1016/S0022-328X(00)92418-5
| 1. | Cheng, C. C.; Dong, Q.; Liu, D.-F.; Luo, Y.-L.; Liu, L. F.; Chen, A.-Y.; Yu, C.; Savaraj, N.; Chou, T.-C. J. Med. Chem. 1993, 36, 4108. doi:10.1021/jm00077a016 |
| 2. | Crawford, P. W.; Carlos, E.; Ellegood, J. C.; Cheng, C. C.; Dong, Q.; LruJ, D. F.; Luof, Y. L. Electrochim. Acta 1996, 41, 2399. doi:10.1016/0013-4686(96)00020-5 |
| 14. | Kalinin, A. V.; Miah, M. A. J.; Chattopadhyay, S.; Tsukazaki, M.; Wicki, M. Synlett 1997, 7, 839. |
| 13. | Sibi, M. P.; Snieckus, V. J. Org. Chem. 1983, 48, 1935. doi:10.1021/jo00159a040 |
| 12. | Azevedo, M. S.; Lopes, C. C.; Lopes, R. S. C.; Alves, G. B. C. Synthesis 2004, 8, 1262–1268. |
| 12. | Azevedo, M. S.; Lopes, C. C.; Lopes, R. S. C.; Alves, G. B. C. Synthesis 2004, 8, 1262–1268. |
| 3. | Stadlbauer, W.; Kappe, T. Z. Naturforsch. 1975, 30b, 139. |
| 4. | El-Wareth, A.; Sarhan, A. O.; El-Dean, A. M.; Abdel-Monem, M. I. Monatsh. Chem. 1998, 129, 205. |
| 5. | Martínez, E.; Martínez, L.; Estévez, J. C.; Estévez, R. J.; Castedo, L. J. Med. Chem. Soc. 1978, 21, 291. |
| 6. | Bentley, K. W.; Robinson, R. J. Chem. Soc. 1950, 1353. doi:10.1039/jr9500001353 |
| 7. | Yoshida, K.; Adachi, T.; Oga, N.; Kubo, Y. Chem. Lett. 1990, 2049. |
| 8. | Simonitsch, E.; Eisenhuth, W.; Stamm, O. A. E.; Schmid, H. Helv. Chim. Acta 1960, 43, 58. doi:10.1002/hlca.19600430107 |
| 9. | Chatterjea, J. N. Experientia 1953, 9, 256. doi:10.1007/BF02172434 |
| 10. | Jha, O. P. J. Indian Chem. Soc. 1973, 44, 740. |
| 11. | Chatterjea, J. N.; Jha, O. P. J. Indian Chem. Soc. 1970, 47, 6. |
| 24. | Fu, J. M.; Zhao, B. P.; Sharp, M. J.; Snieckus, V. J. Org. Chem. 1991, 56, 1683. doi:10.1021/jo00005a004 |
| 25. | Wang, W.; Snieckus, V. J. Org. Chem. 1992, 57, 424. doi:10.1021/jo00028a004 |
| 26. | Wang, X.; Snieckus, V. Tetrahedron Lett. 1991, 32, 4879. doi:10.1016/S0040-4039(00)93485-3 |
| 18. | Zhang, P.; Gawley, R. E. J. Org. Chem. 1993, 58, 3223. doi:10.1021/jo00064a001 |
| 19. | Superchi, S.; Sotomayor, N.; Maio, G.; Joseph, B.; Campbell, M. G.; Snieckus, V. Tetrahedron Lett. 1996, 37, 60. |
| 12. | Azevedo, M. S.; Lopes, C. C.; Lopes, R. S. C.; Alves, G. B. C. Synthesis 2004, 8, 1262–1268. |
| 21. | Wittig, G.; Pockels, D.; Dröge, H. Ber. Dtsch. Chem. Ges. B 1938, 71, 1903. |
| 22. | Gilman, H.; Jacoby, A. L. J. Org. Chem. 1938, 3, 108. doi:10.1021/jo01219a003 |
| 23. | Gilman, H.; Langham, W.; Jacoby, A. L. J. Am. Chem. Soc. 1939, 61, 106. doi:10.1021/ja01870a036 |
| 16. | Kalinin, A. V.; da Silva, A. J. M.; Lopes, C. C.; Lopes, R. S. C.; Snieckus, V. Tetrahedron Lett. 1998, 39, 4995. doi:10.1016/S0040-4039(98)00977-0 |
| 20. |
Watanabe, M.; Snieckus, V. J. Am. Chem. Soc. 1980, 102, 1457. doi:10.1021/ja00524a059
for a tandem DoM route to anthraquinones and heteroanthraquinones based on initial benzamide-aromatic aldehyde condensation. |
© 2006 Slana et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)