Abstract
Various push–pull chromophores can be synthesized in a single and atom-economical step through [2 + 2] cycloaddition–retroelectrocyclization (CA–RE) reactions involving diverse electron-rich alkynes and electron-deficient alkenes. In this review, a comprehensive investigation of the recent and noteworthy advancements in the research on push–pull chromophores prepared via the [2 + 2] CA–RE reaction is conducted. In particular, an overview of the physicochemical properties of the family of these compounds that have been investigated is provided to clarify their potential for future applications.
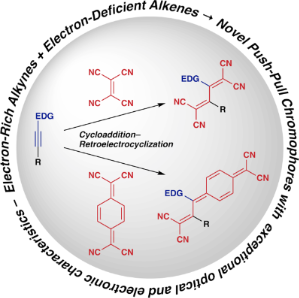
Graphical Abstract
Introduction
Push–pull chromophores, wherein both electron-donating and electron-accepting substituents are incorporated into a π-electron-conjugated system, exhibit exceptional optical and electronic properties. They hold significant promise for applications in diverse fields, particularly materials science (specifically in optoelectronics) [1,2]. The synthesis of push–pull chromophores is often achieved through click-type reactions between electron-rich alkynes and electron-deficient alkenes, which is a reliable and atom-economical method. Diverse chromophores can be obtained via this method, depending upon the choice of alkynes and alkenes. The pioneering work by Bruce et al. in 1981 revealed that the cleavage of tetracyanoethylene (TCNE) under mild conditions via its reaction with metal acetylides yields metal complexes featuring the tetracyanobuta-1,3-diene (TCBD) structural motif [3]. Subsequently, numerous researchers have explored the synthesis of various push–pull chromophores, primarily via reactions between alkynes and alkenes. Several comprehensive reviews of nonplanar push–pull chromophores have since enriched the scientific literature [4-6]. In 2018, Michinobu and Diederich provided an exemplary overview of click-type [2 + 2] cycloaddition (CA)–retroelectrocyclization (RE) reactions, offering means for preparing these coveted push–pull chromophores [7]. A review conducted in 2023 comprehensively explored the optoelectronic properties of TCBDs along with their photovoltaic applications [8]. The landscape of [2 + 2] CA–RE reactions for the preparation of push–pull chromophores is rapidly evolving and expanding. This evolution necessitates an update on the latest insights for materials science researchers. Furthermore, the diverse research advancements related to the distinctive physicochemical properties of push–pull chromophores remain fragmented. Thus, the primary objective of this review is to consolidate the developments in the research on push–pull chromophores prepared via [2 + 2] CA–RE reactions between electron-rich alkynes and electron-deficient alkenes across various domains of chemistry. This endeavor aims to delineate new directions for prospective applications of push–pull chromophores. Notably, in polymer chemistry, significant progress has been made in employing [2 + 2] CA–RE reactions for polymers as a valuable post-functionalization treatment [9,10]. However, this topic lies beyond the scope of this review.
Review
Click-type [2 + 2] CA–RE reactions
Click-type [2 + 2] CA–RE and associated reactions were comprehensively elucidated in a previous review [7]. Succinctly, the [2 + 2] CA reaction is postulated to be a stepwise process, whereas RE is postulated to be a concerted process. The [2 + 2] CA–RE sequence proceeds successively, as depicted in Scheme 1, where electron-donating groups are denoted as EDGs. During the [2 + 2] CA process, the nucleophilic attack by the terminal alkyne carbon of an electron-rich alkyne on an electron-deficient alkene, such as TCNE and 7,7,8,8-tetracyano-p-quinodimethane (TCNQ), results in the formation of a zwitterionic intermediate, wherein the negative charge evolves into a stabilized carbanion (dicyanomethide anion). Subsequently, the ring closure of the zwitterionic intermediate generates the corresponding cyclobutene intermediate. Finally, the ensuing RE process yields the corresponding TCBD derivatives. The resulting TCBDs and related products exhibit strong light absorption, resulting from the intramolecular charge transfer (ICT) in the visible region; they also exhibit a rich redox chemistry [11]. In the [2 + 2] CA–RE reaction of TCNQ with electron-rich alkynes, the alkyne terminal carbon executes a nucleophilic attack on the exocyclic carbon of the dicyanovinyl (DCV) group of TCNQ, affording dicyanoquinodimetanes (DCNQs) [12,13]. Intense ICT bands of TCBD and DCNQ are observed at around 450–470 nm and 680–710 nm, respectively. A study confirmed that with appropriate molecular design, the π-conjugation relationship between the donor and acceptor moieties in TCBDs and DCNQs can be retained despite their non-planarity [14]. Diederich et al. synthesized a plethora of push–pull chromophores by employing anilino groups as EDGs [4,5,7]. Notably, the N,N-dimethylanilino (DMA) moiety activates the reactivity of the neighboring alkyne moiety so strongly that the [2 + 2] CA–RE reactions with electron-deficient olefins proceed seamlessly [15,16] even when the terminus of the alkyne moiety is substituted by a cyano group [17]. In addition to anilino groups, a myriad of donor entities, including urea-substituted phenyl groups [18,19], carbazoles [20], phenothiazines [21,22], thiophenes [23-26], tetrathiafulvalenes (TTFs) [27], extended TTFs [28], ferrocenes [27,29-46], azulenes and their homologous compounds [32,47-58], boron dipyrromethenes (BODIPYs) [41,59-61], porphyrins [62-64], chlorophylls [65,66], triazenes [67,68], ynamides [69-71], arylynamines [72], indoles [73], and γ-pyranylidenes [74], have been identified as effective donor components for [2 + 2] CA–RE reactions. Concerning electron-deficient alkenes, 2,3,5,6-tetrafluoro-7,7,8,8-tetracyano-p-quinodimethane [75], DCV derivatives [76-80], tricyanovinyl derivatives [79,80], polyenic DCVs [81], N,N’-dicyanoquinone diimides [82], and 6,6-dicyanopentafulvenes (DCFs) [83,84] have been employed. Notably, the [2 + 2] CA–RE reaction of DMA-ethynyl-appended porphyrin with TCNQ is observed to occur on a metal surface (specifically Au(111)) under high-vacuum conditions, with successful visualization achieved through scanning tunneling microscopy [85]. For TCBDs bearing unsubstituted anilino (p-H2NC6H4–) groups, their conversion into the p-iodophenyl derivatives via the Sandmeyer reaction and subsequent post-functionalization via the Suzuki and Sonogashira coupling reactions are achieved [86]. In the reaction of bisanilino-end-capped buta-1,3-diynes with TCNE, simultaneous [2 + 2] CA–RE reactions proceed for each of the two alkyne moieties of the buta-1,3-diyne skeletons, forming octacyano[4]dendralene molecules corresponding to TCBD dimers [87,88]. A well-recognized potent electron acceptor, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, and its homologous compounds have been employed in chemical transformation reactions involving electron-rich alkynes. In particular, a [2 + 2] CA adduct was prepared through the [2 + 2] CA–RE reaction. Studies have shown that the thermal treatment of the [2 + 2] CA adduct leads to the formation of a spiro compound [89-94]. Ester-substituted, electron-deficient alkenes have also been employed in [2 + 2] CA–RE reactions involving electron-rich alkynes. Alkenes featuring either one or two ester substitutions exclusively catalyze [2 + 2] CA–RE reactions, yielding multicyanated ethenes [95]. Contrarily, alkenes bearing three or four ester substitutions partake in a [4 + 2]-type hetero-Diels–Alder (DA) reaction, yielding a third product, presumably through a [3 + 2] cycloaddition reaction, followed by rearrangement. The [2 + 2] CA–RE reactions involving cumulenes and TCNE also present noteworthy chemical transformation reactions [96-99]. However, the chemistry of cumulenes is not discussed in detail here; please refer to a previous review [7] for comprehensive insights.
Scheme 1: Pathway of the [2 + 2] CA–RE reaction of an electron-rich alkyne with TCNE or TCNQ. EDG = electron-donating group.
Scheme 1: Pathway of the [2 + 2] CA–RE reaction of an electron-rich alkyne with TCNE or TCNQ. EDG = electron-...
Upon replacing TCNE with tetracyanoethylene oxide (TCNEO), the [2 + 2] CA–RE reaction is extremely slow when applied to mono-substituted alkynes bearing an DMA group. Conversely, for bis-substituted alkynes, the reaction occurs readily at room temperature, yielding the corresponding TCBD products, as shown in Scheme 2. This reaction required the presence of two equivalents of TCNEO relative to the alkyne substrate for the generation of the TCBD products [100]. For the reaction, the formal [3 + 2] cycloaddition reaction is postulated to initiate through the initial nucleophilic attack of the alkyne carbon on the electrophilic TCNEO carbon, yielding a zwitterionic intermediate. Subsequently, following the production of an oxide ion through the ring-opening reaction of the 2,5-dihydrofuran ring, the oxide ion attacks another TCNEO molecule. This sequence culminates in the elimination of the tetracyanodioxetane moiety (either as dioxetane or carbonyl dicyanide molecules in the form of degraded fragments), affording the TCBD products.
Scheme 2: Reaction pathway for DMA-appended acetylene and TCNEO.
Scheme 2: Reaction pathway for DMA-appended acetylene and TCNEO.
A distinguishing feature of the [2 + 2] CA–RE reaction involving electron-rich alkynes and DCFs is the alternation of regioselectivity, which is contingent upon the specific substituents incorporated onto the cyclopentadienyl moiety of the DCF molecule [83,84], as shown in Scheme 3. In the reaction between 4-ethynyl-N,N-dimethylaniline (1) and triisopropylsilylethynyl-substituted DCF 2a, heating at 80 °C in acetonitrile selectively yields the corresponding adduct 3a with 64% yield. In 3a, the anilino group forms a covalent linkage, engaging in conjugation with the fulvene moiety. Conversely, the reaction of phenyl-substituted DCF 2b with 1 under the same reaction conditions selectively affords the corresponding adduct 3b with 64% yield. In 3b, the anilino group is conjugated with the DCV group rather than the fulvene moiety. This variation in the products arises from the difference in the initial reaction step, in particular, whether the nucleophilic attack of the alkyne carbon in 1 occurs at C(6) or C(1) of the DCF framework. This differentiation can be attributed to the efficient electron conjugation between the alkyne and cyclopentadiene moieties.
Scheme 3: Pathway of the [2 + 2] CA–RE reaction between 1 and DCFs.
Scheme 3: Pathway of the [2 + 2] CA–RE reaction between 1 and DCFs.
TCBD 4, obtained through the [2 + 2] CA–RE reaction, continues to function as an electron-accepting alkene, as shown in Scheme 4. Subsequent [2 + 2] CA–RE reactions involving electron-rich alkynes yield tetracyano-1,3,5-hexatrienes (TCHTs). These reactions occur seamlessly in a one-pot fashion. For instance, the reaction of one equivalent of TCNE with two equivalents of 1 in chloroform at 50 °C for 36 h yields the corresponding TCHT 5 with 80% yield [101]. In this reaction, the nucleophilic attack of the alkyne carbon of 1 occurs at the C(2) carbon of 4. Moreover, when TCBD 6, exhibiting enhanced electron-accepting capabilities, reacts with 1, it displays a more intricate reaction profile compared with that of 4 (Scheme 5). In addition to pentacyano-1,3-5-hexatriene (PCHT) compound 7, compounds 8 and 9 are generated [102]. This reaction is notably influenced by the different conditions, particularly the solvent employed. For instance, when toluene is used as the reaction solvent, a reaction at 100 °C for 15 h affords an E/Z mixture of 7 (E/Z ≈ 1:1) with 90% yield, 8 with 10% yield, and 9 with 0% yield. In contrast, a reaction at 25 °C for 15 h in N,N-dimethylformamide (DMF) affords 7 with 16% yield, 8 with 25% yield, and 9 with 46% yield. Furthermore, 7 and 8 are efficiently converted into 10 and 11, respectively, with high yields (80% and 95%, respectively) via column chromatography employing silica gel in acetonitrile, thereby instigating further molecular transformation reactions. In these reactions, the nucleophilic attack of the alkyne carbon of 1 occurs at the C(1) carbon of 6. When the formal [2 + 2] cycloaddition, as delineated in path A, occurs for the zwitterionic intermediate featuring the 1,1,3-tricyanoallyl anion obtained through the nucleophilic attack, subsequent RE affords 7. Conversely, if the formal [4 + 2] cycloaddition occurs along the course elucidated in path B, the generation of 8 is expected. Similarly, when the formal [4 + 2] cycloaddition follows the course depicted in path C, the generation of 9 is envisaged. Among these compounds, 10 features a DCF structure and is amenable to further molecular transformations through a reaction with 1 [103].
Scheme 4: Sequential double [2 + 2] CA–RE reactions between 1 and TCNE.
Scheme 4: Sequential double [2 + 2] CA–RE reactions between 1 and TCNE.
Scheme 5: Divergent chemical transformation pathways of TCBD 6.
Scheme 5: Divergent chemical transformation pathways of TCBD 6.
Considering the structure of 7, the possibility of a multistep [2 + 2] CA–RE reaction via a reaction with 1 becomes feasible. To execute such a multistep [2 + 2] CA–RE reaction, it becomes imperative to suppress the generation of 8 and 9, as well as the conversion reaction of 7 into 10. Therefore, it is important to use toluene as the solvent. Accordingly, pentacyanoocta-1,3,5,7-tetraene (PCOT) 12 was synthesized with 75% yield as a mixture of 3Z,5E/3E,5E isomers in the ratio of 71:29 via a one-pot reaction involving TCNE and one equivalent of 3-(4-(dimethylamino)phenyl)propionitrile in toluene at 90 °C for 24 h. Subsequently, one equivalent of 1 was added at 90 °C for 24 h (Scheme 6) [101]. While the separation of the isomers was not achieved, the structure of the 3Z,5E isomer was successfully characterized through single-crystal X-ray crystallography.
A mechanistic investigation of the [2 + 2] CA–RE reactions involving DCV compounds was undertaken by Diederich et al. in 2010 [77]. Their investigation unveiled that the reaction between 1 and arylated DCV derivatives followed second-order kinetics, indicating a bimolecular process. Furthermore, their findings elucidated a compelling linear free-energy relationship between the rate constants and electronic characteristics of the para-substituents of the DCV electrophiles, implying a dipolar, zwitterionic mechanism. The researchers also performed theoretical calculations, unveiling that the ring-opening step presented a higher energy barrier compared with the ring-formation step, particularly when the DCV compounds lacked aryl substituents. Subsequently, they successfully isolated the corresponding cyclobutene intermediate 13 through the reaction of 1 with 1,1-dicyanoethene (14). They demonstrated that this intermediate was converted into the corresponding TCBD 15 upon further heating, as depicted in Scheme 7. The kinetics of the RE step conformed well to that of a first-order reaction. However, notably, they underscored that generalizing the elucidated reaction mechanism to other [2 + 2] CA–RE reactions involving TCNE and TCNQ as electrophiles might be difficult. They emphasized the significance of considering a pre-equilibrium state of the charge-transfer complexes between the alkynes and alkenes and mentioned that the positioning of the [2 + 2] CA or RE step as the rate-determining step may depend upon the structural attributes of the electrophiles and nucleophiles.
Scheme 7: [2 + 2] CA–RE reaction of 1 with 14. TCE = 1,1,2,2-tetrachloroethane.
Scheme 7: [2 + 2] CA–RE reaction of 1 with 14. TCE = 1,1,2,2-tetrachloroethane.
In 2023, Nielsen et al. conducted an exhaustive kinetic analysis of the [2 + 2] CA–RE reaction involving 4-trimethylsilylethynylaniline and TCNE by leveraging 1H nuclear magnetic resonance (NMR) spectroscopy. Their investigation revealed that the product plays a pivotal role as an autocatalyst in the kinetics, as illustrated in Scheme 8 [104-106]. The traditional model posits that an alkyne (A) and TCNE (designated as B in Scheme 8) initially form a charge-transfer complex AB. Subsequently, this complex forms a zwitterion intermediate (C1), followed by the formation of a cyclobutene intermediate (C2), ultimately forming the product P. However, the researchers strongly advocated for an alternative pathway, which entails the formation of a complex between AB and P, from which C1 is generated. Notably, this additional route significantly accelerates the overall reaction. The researchers reported that the conversion of the ABP complex into C1 transpired at a markedly accelerated rate compared with the conversion of AB alone, with a kinetic constant ratio of k4/k2 = 62. Consequently, it is posited that P assumes the role of a template, directing the arrangement of reactants in a precise manner conducive to the generation of C1.
Scheme 8: Autocatalytic model proposed by Nielsen et al.
Scheme 8: Autocatalytic model proposed by Nielsen et al.
Leveraging the irreversibility inherent in the [2 + 2] CA–RE reaction, 1 can be adeptly employed as a proficient trapping agent for TCNE. Anthracene-based ynamide 16 offers two potential reactive sites for TCNE, one residing at the anthracene moiety and other at the alkyne moiety, as shown in Scheme 9 [71]. As exemplified by Trolez et al., the introduction of one equivalent of TCNE to 16 at room temperature initiates a [4 + 2] cycloaddition reaction with the anthracene moiety, yielding the DA cycloadduct 17. To achieve the conversion of the alkyne moiety of 16 into TCBD, the addition of five equivalents of TCNE to 16 or one equivalent of TCNE to 17 is required. This results in the formation of TCBD compound 18 bearing a derivatized anthracene moiety. Subsequently, to restore the anthracene structure of 18, the retro-DA reaction must occur efficiently. Therefore, through the addition of 1, followed by heating, the generated TCNE is effectively captured by 1. This maneuver concurrently suppresses the progression of the DA reaction, leading to the efficient generation of the anthracenyl TCBD 19.
Scheme 9: Synthesis of anthracene-embedded TCBD compound 19.
Scheme 9: Synthesis of anthracene-embedded TCBD compound 19.
Concerning [2 + 2] CA–RE reactions, studies have shown that alkynes exhibiting angular distortion exhibit high reactivity toward electron-deficient alkenes independent of the presence or absence of electron-donating substituents on the alkynes. The remarkable reactivity displayed by angle-strained alkynes makes them highly valuable tools in biorthogonal chemistry [107-109]. The reaction between dibenzo-fused cyclooctyne 20 and TCNE was observed to occur at room temperature, quantitatively yielding 22, wherein the TCBD moiety was effectively integrated, as shown in Scheme 10 [110]. During this reaction, an intermediate 21 was successfully captured experimentally along with the occurrence of only the [2 + 2] CA reaction. Similarly, the reaction involving cyclooctadiyne (23) and TCNE progressed in a stepwise manner, ultimately affording 27 with 90% yield. The formation of intermediates 24, 25, and 26 was elucidated through 1H NMR analysis, of which 24 and 26 were successfully isolated and characterized. A comprehensive analysis of the [2 + 2] CA–RE reaction activation parameters via 1H NMR spectroscopy revealed that the rate-determining step in the transformation of 20 into 22 was the first-order RE step, which was primarily governed by enthalpy changes. Furthermore, it was observed that the ring strain significantly accelerated the second-order [2 + 2] CA step. The rate enhancement in the [2 + 2] CA step was approximately 3300 times that for an acyclic model compound, at 298 K. Regarding the conversion reaction from 23 to 27, the rate-determining step was identified to be the final RE step: the conversion of 26 to 27. Comparative analysis using a linear model compound revealed rate enhancements of 5500 times that for the initial [2 + 2] CA step and 80 times that for the subsequent [2 + 2] CA step at 298 K, which was attributable to the ring strain.
Scheme 10: Sequence of the [2 + 2] CA–RE reaction between dibenzo-fused cyclooctyne or cyclooctadiyne and TCNE. All reactions were performed in TCE-d2.
Scheme 10: Sequence of the [2 + 2] CA–RE reaction between dibenzo-fused cyclooctyne or cyclooctadiyne and TCNE...
Jasti et al. synthesized an array of cycloparaphenylenes (CPPs) that incorporated alkyne moieties (28a–c) within their ring structures and investigated their reactions with TCNE (Scheme 11) [111]. In these reactions, CPP derivatives 30a–c, featuring TCBD moieties, were obtained with 59–97% yields. The magnitude of the ring strain exerted a profound effect on the reactivity of the compounds. Notably, the largest macrocycle 28c required heating to 100 °C to generate 30c. Conversely, the transformation of 28b proceeded at 45 °C, and the smallest macrocycle 28a reacted with TCNE at 0 °C. Kinetic investigations revealed that the rate-determining step in the reaction involving 28a–c with TCNE was the second-order [2 + 2] CA step, succeeded by the rapid RE step; the result is in contrast to those obtained for 20 and 23. This divergence in the reactivity may be attributed to the pronounced steric repulsion between the DCV group and the aryl hydrogen, particularly discernible for 21 and 26 during the RE step. Conversely, for 29a, it is reasonable to posit that the phenyl rings possess a degree of rotational freedom, thereby circumventing steric repulsions.
Scheme 11: [2 + 2] CA–RE reaction between the CPP derivatives and TCNE. THF = tetrahydrofuran.
Scheme 11: [2 + 2] CA–RE reaction between the CPP derivatives and TCNE. THF = tetrahydrofuran.
The steric congestion surrounding TCBD moieties can also induce further skeletal rearrangement. For instance, the spatial constraints proximal to the surface of fullerene derivatives lead to novel reactions arising from steric congestion. In a reaction involving ethynylfullerene derivatives featuring a DMA group (31) and TCNE, the conventional [2 + 2] CA–RE reaction was observed to transpire at 20 °C, yielding the corresponding TCBD derivatives 32, as shown in Scheme 12. Conversely, upon refluxing in chlorobenzene, a rearrangement ensued, yielding tetrakis-substituted fullerene derivatives 33 [112]. During this rearrangement, it was postulated that a zwitterionic intermediate was formed from the nucleophilic attack of the carbon of the dicyanomethylidene adjacent to the DMA group on the fullerene sphere. This was followed by a nucleophilic attack of the fullerene carbanion on one of the cyano groups, ultimately yielding 33. Computational calculations also suggested that the rearrangement was facilitated by the destabilization of the reactant due to steric interactions.
Scheme 12: [2 + 2] CA–RE reaction between ethynylfullerenes 31 and TCNE and subsequent thermal rearrangement.
Scheme 12: [2 + 2] CA–RE reaction between ethynylfullerenes 31 and TCNE and subsequent thermal rearrangement.
Additional skeletal transformation reactions in TCBD structures, such as those shown in Scheme 12, have also been reported [113-116], which enable the construction of a wide array of push–pull chromophore structures. For instance, Shoji et al. reported that the reaction between 3(4-(N,N-dimethylanilino))prop-2-yn-1-ol (34) and TCNE in dichloromethane at room temperature yielded 35, which was characterized by a furan skeleton (Scheme 13) [116]. This transformation occurred through the intermediate formation of the corresponding TCBD with a high yield of 85%. Furthermore, when 35 reacted with pyrrolidine at 0 °C, the pentafulvene derivative 36 was obtained with 63% yield via an additional ring-opening process. Similar skeletal transformation reactions were observed for azulene derivatives bearing similar substructures.
Scheme 13: Pathway of the [2 + 2] CA–RE reaction between TCNE and 34, followed by additional skeletal transformation.
Scheme 13: Pathway of the [2 + 2] CA–RE reaction between TCNE and 34, followed by additional skeletal transfor...
The reaction of 1 with TCNE in an aqueous medium containing a surfactant results in the formation of a 6,6-dicyano-heteropentafulvene derivative 37 as an additional byproduct along with the typical TCBD adduct 4 (Scheme 14) [117]. The yields and formation ratios of 4 and 37 depend on the type and concentration of the surfactant employed. For instance, when the nonionic surfactant, Brij 30, was used as the reaction solvent at a concentration of 20 mM, exceeding the critical micelle concentration, 4 and 37 were obtained in a ratio of 53:47, with 64% yield. Notably, 37 exhibits instability in silica gel and polar solvents. In particular, when 37 is kept in ethyl acetate or acetonitrile for 8 h, 38 is obtained with 98% yield. Microscopic observations suggest that the vesicle structure, with water as the core, plays a pivotal role in the transformation of 4 into 37. A similar reaction of TCBD with water was also reported by Bruce et al. [118].
Scheme 14: Synthesis scheme for heterocycle 38 from the reaction between TCNE and 1 in water and a surfactant.
Scheme 14: Synthesis scheme for heterocycle 38 from the reaction between TCNE and 1 in water and a surfactant.
The reaction of the anthracene derivative 39, wherein a DMA–ethynyl group is introduced at the 9-position, with TCNE at 40 °C in THF yielded the corresponding TCBD 40 with 91% yield. Subsequent heating of 40 in toluene at 70 °C led to an intramolecular cyano-DA (CDA) reaction, which quantitatively afforded the CDA product 41 (Scheme 15) [119]. The quantitative CDA reaction progression was attributed to be due to the presence of multiple cyano groups on the skeleton, which increased the dienophile character of the cyano groups and stabilized the structure of the product. The thermal conversion of 40 to 41 was also accelerated by the addition of B(C6H5)3, which may be due to the enhancement of the dienophilic nature of the cyano group due to coordination with borane.
Scheme 15: Synthesis scheme of the CDA product 41.
Scheme 15: Synthesis scheme of the CDA product 41.
Rotaxane synthesis
Push–pull chromophores with nonplanar configurations have been reported as effective stoppering motifs for rotaxane synthesis. A rational approach toward rotaxane synthesis involves affixing stoppers at both termini substituents and threading a molecular thread through a macrocycle. In this threading–stoppering strategy, a mild yet high-yield reaction is required for the stoppering process. The [2 + 2] CA–RE reaction, which yields push–pull chromophores, is the preferred method for stoppering. Accordingly, Li et al. synthesized a rotaxane 44 terminated using push–pull chromophores by incorporating concise peptide and DCNQ moieties and employing the threading–stoppering method (Scheme 16). In 44, the rod moiety and macrocycle feature short peptide substructures. Consequently, the macrocycle was tethered to the peptide segment through amide–amide hydrogen bonding, and 44 was obtained with 30% yield. The generation of 44 was accompanied by the formation of the corresponding free thread with 62% yield, resulting from the reaction between the thread precursor 42 and macrocycle 43, followed by an additional reaction of the alkyne moiety with TCNQ [120]. Rotaxane 46 terminated using push–pull chromophores, which exhibited a solvent-driven molecular shuttling phenomenon, was synthesized from the thread precursor 45 using a clipping approach; however, the yield for the ring-formation process was modest at 5% (see Scheme 16) [121,122]. The macrocycle in 46 was observed to engage with the peptide segment in low-polarity solvents. In contrast, 1H NMR analysis suggested that the macrocycle disengaged from the peptide segment and relocated along the alkyl chain in dimethyl sulfoxide. Furthermore, the solvent-driven shuttling motion of the macrocycle was observed to exert a discernible effect on the aggregation of the amphiphilic molecular system. In particular, changing the solvent led to the generation of interlaced nanofibers, perforated capsules, and wormlike nanoparticles.
Scheme 16: Synthesis of rotaxanes 44 and 46 via the [2 + 2] CA–RE reaction.
Scheme 16: Synthesis of rotaxanes 44 and 46 via the [2 + 2] CA–RE reaction.
In rotaxanes, the utilization of metal–ligand bonding involving CuI is a common strategy for immobilizing a thread moiety within a macrocycle. However, the efficacy of such a bonding is compromised when catalysts are used in stoppering reactions, e.g., the copper-catalyzed azide–alkyne cycloaddition reaction. Consequently, a [2 + 2] CA–RE reaction that can yield push–pull chromophores without the use of a catalyst is exceedingly valuable as a stoppering method, even for systems featuring metal–ligand bonding. Accordingly, Diederich et al. demonstrated the synthesis of a CuI bis-phenanthroline-based rotaxane 50 by employing the [2 + 2] CA–RE reaction, as shown in Scheme 17 [123]. The initial threading reaction involving the rod molecule 47 and macrocycle 48, constructed using [Cu(MeCN)4]PF6, resulted in the formation of the pseudorotaxane 49. The ensuing [2 + 2] CA–RE reaction of 49 with TCNQ in dichloromethane at room temperature afforded 50 with an impressive yield of 84%.
Scheme 17: Synthesis of a CuI bisphenanthroline-based rotaxane 50.
Scheme 17: Synthesis of a CuI bisphenanthroline-based rotaxane 50.
Chiroptical properties
Push–pull chromophores synthesized through the [2 + 2] CA–RE reaction can, in certain cases, exhibit distinct chiroptical properties, attributable to their nonplanar geometry and steric congestion. In previous studies, chiral induction in TCBD structures was accomplished by introducing chiral allene (51) [124,125] or binaphthyl (52 and 53) [126,127] moieties, as shown in Figure 1. These molecules exhibited Cotton effects related to ICT absorptions, and chiral induction in TCBD moieties resulted from optically active constituents. Compound 53, due to its elongated rigid structure, holds potential for use as a chiral dopant in nematic liquid crystals (LCs); however, the helical twisting powers of 53 within nematic LCs are limited. Recently, Alonso-Gómez et al. reported the synthesis and optical resolutions of spirobifluorenes featuring two TCBD units located at the 2,2’-positions, designated as 54 [128]. Furthermore, Autschbach et al. prepared chiral carbo[6]helicene–TCBD derivatives 55 and 56 from enantiopure precursors [129]. For such intricate molecular systems, the presence of multiple chromophore units induces complexity in the interpretation of the resulting circular dichroism (CD) spectra because of the overlap of several exciton couplets. Thus, exciton coupling CD signals were not discernible for 51–55. Further, regarding 56, the exciton coupling CD signal in the ICT region may not be apparent at a glance. Nevertheless, comprehensive computational analyses employing the matrix method suggest that the intense long-wavelength CD signal observed for 56 is due to the coupling of individual helicene-to-TCBD electric-transition dipole moments.
Figure 1: Structures of the chiral push–pull chromophores 51–56.
Figure 1: Structures of the chiral push–pull chromophores 51–56.
The emergence of axial chirality in TCBDs and DCNQs and their optical resolutions were first realized in 2010 through their conjugation with methylated fullerenes, as shown in Figure 2 [130]. The optical resolution was realized using a chiral high-performance liquid chromatography (HPLC) system equipped with an (S,S)-WHELK-O1 column. The absolute configurations of the axially chiral TCBD and DCNQ derivatives were ascertained by a comparative analysis of the experimental CD spectra against the spectra derived from time-dependent density functional theory (TD-DFT) calculations. The axial chirality was stabilized by the steric congestion on the surface of fullerene, with its endurance contingent upon the bulkiness of the substituent incorporated onto the fullerene core. This observation was supported by the absence of axial chirality in molecules where an additional alkyne moiety is intercalated between the C60 core and TCBD or DCNQ unit or where a methyl group on the C60 surface is replaced by a hydrogen atom. The C60–TCBD conjugate 57 and C60–DCNQ conjugate 58 exhibited substantial optical rotations at 589 nm, presenting +770 for (P)-57 and +5,800 for (P)-58. In concordance, robust Cotton effects related to ICT absorption in the visible region were evident in their CD spectra. Using the Arrhenius and Eyring formalisms, the kinetic parameters for the thermal racemization of 57 and 58 were obtained. The activation free enthalpy (ΔG‡) for the racemization of 57 (ΔG‡298 K = 24.8 kcal mol−1) was ≈1.5 kcal mol−1 larger than that for 58 (ΔG‡298 K = 23.3 kcal mol−1), indicating that the cylohexa-2,5-diene-1,4-diylidene moiety is more flexible and its distortion by out-of-plane bending is energetically less favored compared to the DCV moiety.
Figure 2: Structures of the axially chiral TCBD 57 and DCNQ 58 bearing a C60 core.
Figure 2: Structures of the axially chiral TCBD 57 and DCNQ 58 bearing a C60 core.
Guldi et al. reported that the atropisomerism in the TCBD structure was observed when the TCBD moiety was incorporated at the axial position of the subphthalocyanine (SubPc) core (Figure 3) [131]. Axially chiral SubPc–TCBD–aniline conjugates 59 and 60 were characterized via optical-resolution analysis through chiral HPLC using a Chiralpak IC column. The researchers unequivocally determined the absolute configurations of these atropisomers through the X-ray crystallographic analyses of (Sa)-59 and (Ra)-60. Surprisingly, heating the enantiomers of 69 and 60 at 80 °C for 3 h resulted in negligible thermally induced racemization. Meanwhile, in O2-free toluene solution under illumination, the racemization was observed at 80 °C. Torres et al. performed detailed theoretical calculations and showed that the racemization observed in 59 and 60 is caused by triplet-state photogeneration, which leads to the rotation around the sterically hindered buta-1,3-diene chiral axis [132]. In fact, the estimated energy barrier of ≈37 kcal mol−1 is reduced to ≈15 kcal mol−1 upon the electronic excitation of 59 and 60 to the T1 state. It is reasonable to consider that the phototriggered racemization is inhibited in the presence of O2 because O2 acts as a triplet-state scavenger. Osuka et al. synthesized two types of subporphyrin derivatives 61 and 62, wherein the subporphyrin skeleton was functionalized at its meso or axial position with the TCBD moiety (Figure 3). Although the optical resolution of 61 failed, they succeeded in achieving the optical resolution of 62 via chiral HPLC using a Chiralpak IC column. In 62, unlike 59 and 60, thermally induced racemization was observed and kinetic parameters were obtained (ΔG‡298 K = 25.4 kcal mol−1), which were similar to those reported for 57 and 58 [133].
Figure 3: Structures of the axially chiral SubPc–TCBD–aniline conjugates 59 and 60 and the subporphyrin–TCBD–aniline conjugates 61 and 62.
Figure 3: Structures of the axially chiral SubPc–TCBD–aniline conjugates 59 and 60 and the subporphyrin–TCBD–...
Photoluminescence properties
Most anilino-substituted TCBD molecules exhibit negligible fluorescence in chloroform, while only a few exhibit weak fluorescence in hexane [15]. These nonluminescence characteristics are attributable to the low-energy state of the twisted ICT (TICT) state, which is capable of deactivating the ground state through accessible conical intersections (CIs) [15,134-136]. In particular, Diederich et al. conducted a theoretical investigation on the photophysical properties of 1,1,2,4,4-pentacyanobuta-1,3-diene (PCBD) (63) [134]. Compound 63 and the TCBD 64 demonstrated no discernible luminescence at room temperature and 77 K (Figure 4). Transient absorption spectral measurements of 63 in toluene revealed that the lowest singlet excited state (S1) decays mono-exponentially to the ground state (S0) within approximately 1 ps, suggesting the existence of an accessible CI between S1 and S0. In the S1 minimum conformation of 63, the PCBD moiety adopted a planar orientation perpendicular to that of the aniline moiety. The calculations indicated the presence of accessible S1/S0 CIs. In the lowest-energy S1/S0 CI geometry, the aniline moiety exhibited a pronounced quinoidal character and the carbon atom directly linked to the butadiene moiety exhibited a conspicuous radical nature. Concurrently, the excited electron was extensively delocalized over the entire pentacyanobutadiene moiety. The researchers postulated the following description for the photophysical properties of 64: a nonradiative deactivation process occurred via a CI, which was similar to that observed in the case of 63.
Figure 4: Structures of 63 and the TCBD 64.
Figure 4: Structures of 63 and the TCBD 64.
Notably, a rigid molecular environment, which induces constraints on molecular motions, serves to mitigate nonradiative losses in push–pull chromophores. Li et al. synthesized an oligo(p-phenylenevinylene)-based TCBD derivative denoted as 65, which is noteworthy for its lack of photoluminescence in solution as well as contradictory propensity to form luminescent nanostructure suspensions in hexane (Figure 5) [137]. It was posited that 65 features hollow vesicles, with vesicle fusion releasing curvature energy, leading to a thermodynamically more stable tubular structure. Such aggregation-induced-emission (AIE)-active aggregates exhibit emissive behavior in hexane, with an emission peak at 679 nm and a shoulder at 717 nm along with a fluorescence quantum yield of 8.5%. This luminescence phenomenon can be aptly considered to be AIE, considering the pronounced constraints imposed on molecular rotation in the aggregate state, including rotation around the central single bond of the TCBD moiety.
Figure 5: Structures of the fluorophore-containing TCBDs 65–67.
Figure 5: Structures of the fluorophore-containing TCBDs 65–67.
Regarding the anthracene-embedded TCBD 19 in dichloromethane, no emission signal was observed. However, in the powdered form or sparsely distributed rigid matrices, a broad emission spectrum was observed. In particular, the powders of 19 exhibited photoluminescence in the near-infrared (NIR) region centered at 865 nm, with a long tail extending up to 1,550 nm. This enhancement in photoluminescence can be attributed to the restriction of molecular motion, resulting in the inhibition of nonradiative processes. Analogously, the TCBDs 66 and 67, consisting of pyrene and perylene moieties, respectively, have been reported to exhibit luminescence in the NIR region in their powdered forms, with the maxima at 810 nm and 890 nm for 66 and 67, respectively, despite their nonemissive behavior in dichloromethane [138].
Compounds 55 and 56 remain devoid of emissive characteristics even when in a solution or a solid state [129]. When compound 50 was characterized using a standard steady-state fluorimeter, no emission signals were observed across the visible and NIR regions extending up to 1,600 nm, both in a room-temperature solution (dichloromethane) and as a frozen matrix at 77 K [123]. This is in contrast with the typical homoleptic phenanthroline-based CuI complexes renowned for their emissions from a triplet metal-to-ligand charge transfer excited state. The absence of luminescence may be attributed to the presence of competitive processes, such as energy or electron transfer.
Trolez et al. investigated the photoluminescence properties of various fluorophore-containing TCBDs synthesized via reactions between ynamides and TCNE [139]. The study revealed that numerous fluorenyl derivatives and their phenanthrenyl and terphenyl counterparts exhibited noteworthy emission behavior in solid form and in solutions (Figure 6). For instance, the fluorenyl derivatives 68 and 69, phenanthrenyl derivative 70, and terphenyl derivative 71 exhibited fluorescence characterized by emission peaks at 596, 595, 639, and 594 nm, with fluorescence quantum yields of 6.1%, 7.5%, 1.6%, and 7.8%, respectively, when dissolved in cyclohexane. Notably, the photoluminescence characteristics of these compounds depend on the solvent polarities, as evidenced by the observed decrease in the fluorescence quantum yields in toluene. This phenomenon aligns with the notion that the TICT states experience stabilization with increasing solvent polarity. Conversely, in contrast to 68–70, and 71, a TCBD derivative 72 incorporating a tetraphenylethylene (TPE) moiety exhibited no emission in toluene, possibly due to the presence of intramolecular motion within the propeller core of TPE, which promoted nonradiative decay [140].
Figure 6: Structures of the fluorophore-containing TCBDs 68–72.
Figure 6: Structures of the fluorophore-containing TCBDs 68–72.
A subgroup of compounds that exhibit exceptional luminescence in solution includes 73, 74, and 75, each containing urea-substituted phenyl groups (Figure 7) [18,19]. When excited at 380 nm, these compounds show broad emission spectra encompassing the entire visible region, featuring two peaks centered at 430–472 and 633 nm. The fluorescence quantum yields estimated for 73, 74, and 75 in acetonitrile are 3.4%, 3.3%, and 4.3%, respectively. Notably, in the case of 74 and 75, the intensity of the long-wavelength emission increases when they are excited at 420 nm, resulting in white-light emission. The underlying mechanism governing these luminescence properties remains unknown. It has been established that 75 does not exhibit luminescence in its solid-state form, which is attributed to the quenching effects arising from hydrogen bonding and π–π stacking interactions. In contrast, when 75 is incorporated into a nanocomposite with polystyrene serving as the matrix, luminescent properties are observed [141].
Figure 7: Structures of the urea-containing TCBDs 73–75.
Figure 7: Structures of the urea-containing TCBDs 73–75.
Photoinduced intramolecular energy and electron transfer
Exploiting the electron-accepting property of TCBD, donor–acceptor conjugated systems have been systematically developed by coupling the TCBD motif with an electron donor, resulting in the experimental observation of photoinduced electron- and energy-transfer events. In 2014, comprehensive investigations on the photophysical properties of conjugates featuring diethylamino groups and TCBD or DCNQ structures covalently linked to C60 (76 and 77) and conjugates incorporating octamethylferrocenyl (OEF) groups and TCBD or DCNQ structures bonded to C60 (78 and 79) in addition to their respective reference compounds (80–83) were reported (Figure 8) [142]. In these conjugates, the push–pull chromophores and the C60 unit were effectively spatially isolated from each other – a feat achieved through the strategic incorporation of a pyrrolidine ring as the connecting bridge. Thorough examinations via steady-state fluorescence spectroscopy in toluene unequivocally demonstrated that compounds 77–83 exhibited negligible emissions upon excitation in the charge-transfer (CT) band. In contrast, compound 76 emitted radiations corresponding to fluorescence exhibited by fullerenes, with a peak wavelength of 708 nm, upon CT-band excitation. This observation confirms the plausible energy transfer from the local charge-separated (CS) state of the push–pull chromophore (namely N,N-diethylanilino (DEA)•+–TCBD•−) to the singlet excited state of C60 (1C60*). In transient absorption spectral measurements conducted via the femtosecond laser-flash photolysis of compound 80 in toluene, the occurrence of CT-band bleaching along with increased absorption in the 500–700 nm spectral range and a net decrease in absorption within the 850–1,200 nm region has been observed upon excitation at 420 nm, which is proximate to the CT band. For compound 76, in addition to the CT-band bleaching and increased absorption within the 500–700 nm range, an increase in the absorption intensity at 1,021 nm, corresponding to the characteristics of the C60 radical anion, was observed initially (20 ps), indicating the formation of the DEA•+–C60•− CS state. Furthermore, an increase in the absorption intensity at 700 nm, corresponding to the triplet excited state of C60 (3C60*), was observed along with the decay of the CS state with a lifetime of 1,380 ps. Conversely, for compound 77, excitation at 387 nm was required for the formation of the CS state and an increase in the absorption intensity at 890 nm, indicating the emergence of 1C60* state with a lifetime of 115 ps, was accompanied by a subsequent increase in the absorption intensity at 1,021 nm, corresponding to the formation of the C60 radical anion. The lifetimes of the charge separation and charge recombination events were determined to be 2 and 165 ps, respectively, via multiwavelength analyses. The excitation at 640 nm, a wavelength proximate to the CT band, failed to induce any discernible electron or energy transfer from the DEA moiety to the C60 core. When compound 77 underwent photoexcitation at 640 nm, the CT band exhibited bleaching along with an increase in the absorption intensity at 523 nm and a decrease in the NIR region, similar to the trend observed for 80. Notably, no other distinctive changes were observed. The photoexcitation of 78 at 420 nm caused CT band excitation, leading to charge separation. This was exemplified by the emergence of a maximum absorption peak at 1,024 nm, indicating the one-electron reduced form of C60. Although the observation of the oxidation process of the ferrocene unit was obstructed by the more substantial absorption changes associated with fullerene reduction, the lifetimes of the formation and decay of the CS state, OEF•+–C60•−, were determined to be 13 and 82 ps, respectively. For 79, the excitation of C60 at 387 nm was essential for the development of the CS state, similar to that observed for 77. In this case, the lifetimes of the charge separation and recombination events were determined to be 2 and 58 ps, respectively. The observed longer lifetimes attributed to DEA substitution could be due to the larger distance and the Marcus inverted region [143] character, compared with the results obtained for OEF substitution.
Figure 8: Structures of the fullerene–TCBD and DCNQ conjugates 76–79 and their reference compounds 80–83.
Figure 8: Structures of the fullerene–TCBD and DCNQ conjugates 76–79 and their reference compounds 80–83.
Guldi et al. synthesized zinc phthalocyanine (ZnPC) covalently linked with anilino-TCBD molecules, denoted as 84 and 85, and investigated their optical properties (Figure 9). They observed robust low-energy ICT absorption peaks at 752/753 nm, along with the distinctive high-energy ICT absorption peaks at 470 nm, which are characteristic of anilino-TCBD. Furthermore, they identified B-band (355/361 nm) and Q-band (660/685 nm) transitions, which are hallmarks of phthalocyanines in benzonitrile, and elicit panchromatic optical absorption properties [144]. In 84 and 85, the two DCV structures comprising TCBD are primarily contorted and exhibit limited conjugation. Consequently, these two structures could independently contribute to the emergence of two distinct and robust ICT bands in close proximity to those of their neighboring electron-rich ZnPc and DMA groups. Notably, in the transient absorption spectra of 84, acquired upon excitation at 775 nm (an excitation wavelength corresponding to the low-energy CT absorption), the direct formation of a CS state, characterized as ZnPc•+–TCBD•−, can be observed.
Figure 9: Structures of the ZnPc–TCBD–aniline conjugates 84 and 85.
Figure 9: Structures of the ZnPc–TCBD–aniline conjugates 84 and 85.
SubPc–TCBD–aniline conjugates 59 and 60 exhibited distinct physicochemical properties, depending on whether the peripheral substituents introduced into the SubPcs consisted of hydrogen (as in 59) or fluorine (as in 60) atoms [131]. For 59, the emergence of a metastable radical ion-pair state (H12SubPc•+–TCBD•−–aniline) was realized after the generation of the H12SubPc singlet excited state via 550 nm and 458 nm excitations, corresponding to the Q-band of H12SubPc and the ICT band, respectively. This indicates that similar ultrafast charge-separation and charge-recombination dynamics will also be observed in toluene. For 60, the emergence of new maxima at 500, 660, and 742 nm and minima at 460 nm accompanied the decay of the excited singlet state of F12SubPc, which was first generated by 550 nm excitation in toluene. The transients at 500 and 742 nm were considered to result from the F12SubPc•− radical anion, that at 660 nm was considered to result from the TCBD•− radical anion, and the minimum at 460 nm corresponded to the bleaching of the ICT absorption band. This suggests that exciplexes ([(F12SubPc–TCBD)δ−–anilineδ+]*) are generated where the negative charge is delocalized over F12SubPc and TCBD. The radiative exciplex formation was also confirmed by time-resolved fluorescence measurements. In fact, in toluene, 60 showed maximum emission at 675 nm and a shoulder emission at 612 nm. When the solvent was changed from toluene to benzonitrile and the polarity of the solvent increased, the emission at 612 nm remained almost unchanged, while the emission at 675 nm significantly quenched with a red shift of 35 nm. In the transient absorption spectra of 60 in benzonitrile, the formation of an exciplex was not observed and it was considered that the CS state ((F12SubPc–TCBD)•−–aniline•+) was formed from the singlet excited state of F12SubPc before returning to the singlet ground state. The aforementioned phenomenon, characterized by the switch in the exciplex or the CS-state formation for 60 depending on the solvent, was also observed for excitation at 458 nm corresponding to the ICT band.
A series of multicomponent systems consisting of anilino-substituted TCBD or PCBD coupled with zinc porphyrins (ZnPs) were synthesized, and their photophysical properties were investigated by Diederich et al. [145]. The representative molecules among these are shown in Figure 10. In 86, wherein ZnP and anilino-PCBD are linked using a spacer, the excitation of the porphyrin chromophore (at 560 nm, corresponding to the Q-band) generated an excited singlet state of ZnP (1ZnP*) with a lifetime of 71 ps in toluene. Meanwhile, the absorption at 640 nm, corresponding to the ZnP radical cation, was not observed. The results obtained from Rehm–Weller’s equation [146] suggested that photoinduced electron transfer was thermodynamically permitted in 86. However, ultrafast energy transfer from 1ZnP* to the PCBD moiety was considered to have occurred. In 87, the energy transfer from 1ZnP* to the TCBD moiety was not thermodynamically allowed. Conversely, the CS state (ZnP•+-S-TCBD•−) was estimated to be approximately isoenergetic. The lifetime of 1ZnP* was estimated to be 1,080 ps. However, the emergence of the weak absorption band of the ZnP radical cation at 640 nm was hindered by the overwhelming absorption intensity of the residual porphyrin. The transient absorption spectra of 87 were obtained in benzonitrile solvent, which was expected to stabilize the CS state. Absorption corresponding to the ZnP radical cation was clearly observed with a lifetime of 2.3 μs. The occurrence of such a long-living CS state can be rationally associated with the Marcus-inverted-region [143] behavior of the charge-recombination process. For 88, which has no spacer between ZnP and TCBD, as opposed to the case for 87, the excitation of the porphyrin chromophore in toluene led to the formation of 1ZnP* with a lifetime of 6.6 ps. This was followed by the emergence of the CS state and subsequent charge recombination within 20 ps. Thus, the results indicate that a separating bridge is essential for guaranteeing long-lived CS states.
Figure 10: Structures of the ZnP–PCBD and TCBD conjugates 86–88.
Figure 10: Structures of the ZnP–PCBD and TCBD conjugates 86–88.
Guldi et al. synthesized 16 distinct donor–acceptor conjugated molecules, denoted as 89–104 (Figure 11). These molecules comprised ZnP as an electron donor and push–pull chromophores with varying reduction potentials as electron acceptors interconnected by various rigid spacers [147,148]. Comprehensive analyses and characterizations of the photoinduced electron-transfer processes in these conjugates were conducted. In these molecular designs, the center-to-center distances of the electron donor and acceptor ranged from 13.9 Å to 25.1 Å. Marcus curves were obtained by measuring the charge-separation and charge-recombination lifetimes of these molecules by laser-flash photolysis (excitation wavelength = 420 nm) and the resulting electron-transfer rates were plotted. In the measurements in toluene, the lifetimes of the CS states and charge-recombination events differed significantly depending on the type of spacer and acceptor moiety, with the CS-state lifetimes ranging from 3 (for 98) to 931 ps (for 103) and the charge-recombination lifetimes ranging from 12 (for 98) to 24,000 ps (for 101). The obtained plot is consistent with the Marcus curves obtained using the semi-empirical approach, which includes the nuclear factor, particularly the electron-vibration coupling. This indicates that the consideration of quantum chemical vibrational effects is important in explaining the electron-transfer processes in these conjugates. For these molecules, the dynamics of the charge-recombination processes were found to be located in the Marcus inverted region. The total reorganization energies and electronic coupling matrix elements estimated from the fitting of the Marcus curve were determined to be 0.66–0.79 eV and 13.9–30.9 cm−1, respectively. Using a molecular design similar to that of 102, the rectification property of a compound wherein TTF and DCNQ are linked by a rigid spacer has also been reported [149].
Figure 11: Structures of the porphyrin-based donor–acceptor conjugates (89–104).
Figure 11: Structures of the porphyrin-based donor–acceptor conjugates (89–104).
Misra et al. designed and synthesized a series of chromophore molecules 105–112 featuring porphyrin moieties and investigated their photophysical properties. They found that the introduction of the TCBD structures switched the role of porphyrins in photoevents (Figure 12) [150]. In molecules 105, 106, 109, and 110 without the TCBD structure, when the metalloporphyrin moiety (MP) was subjected to Soret-band excitation (426 nm) in benzonitrile, the emergence of the S2 state was observed; the subsequent internal conversion of S2 into the S1 state was also observed, from which the CS state (MP•−–D•+) emerged. Here, since the triplet state 3MP* is energetically lower than the CS state, 3MP* can partially emerge at the intersystem crossing from the S1 state; a transition from the CS state to 3MP* is also possible. In contrast, for molecules 107, 108, 111, and 112 bearing the TCBD structure, the S2 state emerges due to Soret-band excitation (426 nm) in benzonitrile. Further, after the transition from S2 to S1 by internal conversion, a CT state emerges: MPδ+–TCBDδ−–donor, where donor = phenothiazine (PTZ) or DMA. From this CT state, electron transfer occurs to form a CS state (MP•+–TCBD•−–donor), which returns to the ground state by charge recombination. These results indicate that MP functions as an electron acceptor in molecules without the TCBD structure, while the presence of the TCBD structure causes the porphyrin molecule to behave as an electron donor.
Figure 12: Structures of the porphyrin–PTZ or DMA conjugates 105–112.
Figure 12: Structures of the porphyrin–PTZ or DMA conjugates 105–112.
Misra et al. synthesized a series of TCBD and DCNQ derivatives with BF2-chelated BODIPYs and reported their photophysical properties [1,151]. Notably, for the CS state to emerge, it must have energy lower than the excited triplet state of BODIPY. Conjugates 113 and 114 have molecular structures wherein the TCBD or DCNQ moieties are inserted between the BODIPY and triphenylamine (TPA) moieties, as shown in Figure 13. The energy-level diagrams estimated from the Rehm–Weller equation suggested that at least two CS states were possible in the conjugates (BODIPY•+–acceptor•−–TPA and BODIPY–acceptor•−–TPA•+ (acceptor = TCBD or DCNQ)). In fact, when the transient absorption spectra of 113 and 114 were obtained using an excitation wavelength of 400 nm (the wavelength at which BODIPY is mainly excited), transient corresponding to the BODIPY•+–acceptor•−–TPA and BODIPY–acceptor•−–TPA•+ states were observed, highlighting ultrafast CS and charge-recombination events. In compounds 115 and 116 containing TCBD or DCNQ structures incorporated between BODIPY and PTZ, CS states, namely BODIPY–TCBD•−–PTZ•+ and BODIPY-DCNQ•−–PTZ•+, with a time constant of approximately 4 ps were observed after the formation of the excited singlet state of BODIPY by excitation at 511–512 nm in benzonitrile.
Figure 13: Structures of the BODIPY–Acceptor–TPA or PTZ conjugates 113–116.
Figure 13: Structures of the BODIPY–Acceptor–TPA or PTZ conjugates 113–116.
Sankar et al. designed and synthesized a conjugate consisting of TCBD coupled with a metal corrole to develop a system that can achieve an efficient population of the triplet states through the excited CT state [64]. TCBD derivatives containing copper or silver corrole complexes, denoted as 117 and 118, respectively, were synthesized in a single step from their corresponding alkynyl precursors (Figure 14). The corresponding DCNQ derivatives were not successfully synthesized because of degradation during column purification. For these systems, the computationally calculated energy of the CT state (ECT) was lower than the excitation energies at the Soret (i.e., S2) and visible peak positions. Furthermore, the ECT was higher than the triplet-state energies of the metal corroles. When 117 was subjected to the Soret-band (426 nm) excitation in benzonitrile, a short-lived, vibrationally hot S1 state emerged, along with a broad absorption peak at ≈592 nm, which is characteristic of the triplet state; the decay time constant of this state was 4.98 ns. Although the expected spectral features of the CT state were not observed in the transient absorption spectra because they were overwhelmed by the absorption band of the hot S1 state, the results suggested that the hot S1 state formed from the internal conversion of the initial S2 state promoted the formation of the CT state. Conversely, no transient absorption corresponding to the triplet state was observed when 117 was subjected to the visible band excitation (S1). In contrast, for 118, a triplet state was observed for both S2 and S1 excitations, with time constants of 3.5 ns and 86 ps, respectively.
Figure 14: Structures of the corrole–TCBD conjugates 117 and 118.
Figure 14: Structures of the corrole–TCBD conjugates 117 and 118.
Material applications
Due to their high molecular conversion efficiency, [2 + 2] CA–RE reactions have been employed to develop dendritic systems [55,63,152-155]. He et al. reported the synthesis of 119, which contains multiple dendritic TCBD motifs that form porous molecular crystals (Figure 15) [156]. The rotational freedom of the covalent linkage within 119 is postulated to be substantially restricted due to its pronounced steric hindrance. This tenacious microporous crystalline structure exhibited robust thermal stability (up to 200 °C), as confirmed by powder X-ray diffraction studies. Gas-sorption studies were also performed on the solid samples post activation at 120 °C, revealing CO2 adsorption with pronounced hysteresis. This may be attributed to the presence of narrow, slit-like pores and voids, as seen in the crystal lattice of 119. The Langmuir surface area of the compound was determined to be 317 m2/g. Furthermore, the compound exhibits easy fabrication of its thin films due to exceptional crystalline integrity when deposited on glass plates, highlighting its good reproducibility in solution-based processes. Li et al. analyzed the aggregation tendencies of push–pull chromophores involving TCBD or DCNQ moieties in the benzothiazole framework. Their findings revealed potential for the generation of hollow microstructures characterized by spherical or tubular morphologies [157].
Figure 15: Structure of the dendritic TCBD 119.
Figure 15: Structure of the dendritic TCBD 119.
TCBDs and related push–pull chromophores have attracted attention as organic nonlinear optical (NLO) materials because of their highly polarizable π-electron systems [158]. Thus far, second- and third-order NLO responses of several TCBD compounds have been evaluated [16,61,82,89,135,159-163]. Yang and Si theoretically calculated the second-order polarizability values that relate the second-order NLO responses of TCBD derivatives with different substituents and showed that the NLO properties can be tuned by changing the substituents [164]. Furthermore, Biaggio conducted a detailed study of the third-order NLO properties of TCBD derivatives – an overview is reported in a recent paper [165]. They experimentally determined the orientational average of the off-resonant third-order polarizability value (γrot) of 120 (a benchmark compound) to be 12 ± 2 × 10−48 m5 V−2 (Figure 16). Furthermore, the γrot values of a series of compounds containing acetylene spacers of different lengths between the anilino group and TCBD moiety (121–126) have been determined, and a correlation between the length of the acetylene spacer and the γrot value was established: longer linking distances of the acetylene spacer result in larger the γrot values [160]. The appeal of 120 is its ability to undergo sublimation without decomposition and produce high-density, high-quality homogeneous molecular assemblies via molecular beam deposition [166]. Freude et al. fabricated a silicon–organic-hybrid slot waveguide by depositing 120 between two silicon ribs via molecular beam deposition and successfully demonstrated the all-optical demultiplexing of a 170.8 Gb s−1 telecommunication signal to 42.7 Gb s−1 [167].
Figure 16: Structures of the TCBDs 120–126.
Figure 16: Structures of the TCBDs 120–126.
The two-photon absorption (2PA) properties of the TCBD derivatives have also been studied [135,168]. For example, the 2PA cross-section (σ2) of 128 measured by the Z-scan method was estimated to be 390 GM (2PA wavelength = 1,050 nm) (Figure 17) [135]. Although this value is smaller than that of the precursor (127; 540 GM; 2PA wavelength = 700 nm), 128 is expected to be used as a two-photon absorber in the NIR region. The branched TCBD derivatives 129 and 130 bearing the triarylamino core have also been synthesized and their 2PA properties have been evaluated. The σ2 values for 129 were 80 GM at 1,150 nm and 275 GM at 900 nm, and those of 130 were 90 GM at 1,150 nm and 350 GM at 900 nm, indicating that both compounds have relatively high 2PA cross-sections.
Figure 17: Structures of the precursor 127 and TCBDs 128–130.
Figure 17: Structures of the precursor 127 and TCBDs 128–130.
TCBDs and their related push–pull chromophores have also been studied as candidate materials for applications in photovoltaic devices because of their exceptional redox properties. Notably, push–pull chromophores, such as TCBD and DCNQ, exhibit flexible highest occupied molecular orbital/lowest unoccupied molecular orbital levels that can be tuned by the appropriate selection of electron donor and acceptor [41,44-46,169,170]. To date, various bulk heterojunction organic solar cells (BHJ OSCs) based on TCBD derivatives have been developed and their photoelectric conversion properties have been evaluated. A comprehensive overview of the advances in this regard can be found in the work of Butenschön et al. [8]. While fullerene derivatives, such as [6,6]-phenyl-C61-butyric acid methyl ester ([60]PCBM) and [6,6]-phenyl-C71-butyric acid methyl ester ([70]PCBM) [171], have been widely used as electron-acceptor materials in BHJ OSCs, TCBD derivatives are considered as promising nonfullerene electron acceptors [26,172-176]. The power conversion efficiency of the BHJ OSCs fabricated using the TCBD derivative containing carbazole and diketopyrrolopyrrole structures, denoted as 131, as the electron acceptor reached 7.19% (Figure 18) [173]. Some researchers have also focused on the introduction of electron-donating substituents into the TCBD structure and studied the use of TCBD as an electron donor in BHJ OSCs [24,25,177-181]. Praveen et al. fabricated BHJ OSCs with a 1:1 blending ratio of the DCNQ derivative 132 as an electron donor and [70]PCBM as an electron acceptor and reported that the power conversion efficiency reached 7.79% [25]. Dye-sensitized solar cells using 133, a compound with two ferrocenyl TCBD structures introduced into the TPA structure, as a sensitizer have also been fabricated, achieving a power conversion efficiency of 4.96% [182]. Further, a power conversion efficiency was 3.65% was obtained when the precursor compound 134 was used. Therefore, the light-harvesting capability of the TCBD derivative is expected to contribute to the conversion efficiency.
Figure 18: Structures of 131–134 utilized for BHJ OSCs.
Figure 18: Structures of 131–134 utilized for BHJ OSCs.
Conclusion
In summary, recent research advancements have revealed that [2 + 2] CA–RE reactions can not only yield nonplanar push–pull chromophores characterized by TCBD and DCNQ structures but also an array of π-electron compounds featuring attractive electronic and optical properties. These push–pull chromophores have been integrated into various molecules, including CPP, rotaxane, and fullerenes. Furthermore, studies have elucidated the linear and nonlinear optical characteristics of these compounds along with the dynamics of their excited states. Substantial progress has been made in the research on their utilization in photoelectric conversion and NLO materials. Future studies on these compounds should focus on improving their physical properties and functions to surpass those of the existing molecules.
References
-
Bureš, F. RSC Adv. 2014, 4, 58826–58851. doi:10.1039/c4ra11264d
Return to citation in text: [1] [2] -
Levi, L.; Müller, T. J. J. Chem. Soc. Rev. 2016, 45, 2825–2846. doi:10.1039/c5cs00805k
Return to citation in text: [1] -
Bruce, M. I.; Rodgers, J. R.; Snow, M. R.; Swincer, A. G. J. Chem. Soc., Chem. Commun. 1981, 271–272. doi:10.1039/c39810000271
Return to citation in text: [1] -
Kivala, M.; Diederich, F. Acc. Chem. Res. 2009, 42, 235–248. doi:10.1021/ar8001238
Return to citation in text: [1] [2] -
Kato, S.-i.; Diederich, F. Chem. Commun. 2010, 46, 1994–2006. doi:10.1039/b926601a
Return to citation in text: [1] [2] -
Bruce, M. I. J. Organomet. Chem. 2013, 730, 3–19. doi:10.1016/j.jorganchem.2012.11.004
Return to citation in text: [1] -
Michinobu, T.; Diederich, F. Angew. Chem., Int. Ed. 2018, 57, 3552–3577. doi:10.1002/anie.201711605
Return to citation in text: [1] [2] [3] [4] -
Patil, Y.; Butenschön, H.; Misra, R. Chem. Rec. 2023, 23, e202200208. doi:10.1002/tcr.202200208
Return to citation in text: [1] [2] -
Michinobu, T. Pure Appl. Chem. 2010, 82, 1001–1009. doi:10.1351/pac-con-09-09-09
Return to citation in text: [1] -
Michinobu, T. Chem. Soc. Rev. 2011, 40, 2306–2316. doi:10.1039/c0cs00205d
Return to citation in text: [1] -
Kivala, M.; Stanoeva, T.; Michinobu, T.; Frank, B.; Gescheidt, G.; Diederich, F. Chem. – Eur. J. 2008, 14, 7638–7647. doi:10.1002/chem.200800716
Return to citation in text: [1] -
Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Diederich, F. Chem. Commun. 2007, 4731–4733. doi:10.1039/b713683h
Return to citation in text: [1] -
Bruce, M. I.; Burgun, A.; Grelaud, G.; Lapinte, C.; Parker, C. R.; Roisnel, T.; Skelton, B. W.; Zaitseva, N. N. Organometallics 2012, 31, 6623–6634. doi:10.1021/om3006728
Return to citation in text: [1] -
García, R.; Calbo, J.; Viruela, R.; Herranz, M. Á.; Ortí, E.; Martín, N. ChemPlusChem 2018, 83, 300–307. doi:10.1002/cplu.201700553
Return to citation in text: [1] -
Michinobu, T.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Frank, B.; Moonen, N. N. P.; Gross, M.; Diederich, F. Chem. – Eur. J. 2006, 12, 1889–1905. doi:10.1002/chem.200501113
Return to citation in text: [1] [2] [3] -
Michinobu, T.; May, J. C.; Lim, J. H.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Biaggio, I.; Diederich, F. Chem. Commun. 2005, 737–739. doi:10.1039/b417393g
Return to citation in text: [1] [2] -
Reutenauer, P.; Kivala, M.; Jarowski, P. D.; Boudon, C.; Gisselbrecht, J.-P.; Gross, M.; Diederich, F. Chem. Commun. 2007, 4898–4900. doi:10.1039/b714731g
Return to citation in text: [1] -
Dar, A. H.; Gowri, V.; Gopal, A.; Muthukrishnan, A.; Bajaj, A.; Sartaliya, S.; Selim, A.; Ali, M. E.; Jayamurugan, G. J. Org. Chem. 2019, 84, 8941–8947. doi:10.1021/acs.joc.9b00841
Return to citation in text: [1] [2] -
Gowri, V.; Jalwal, S.; Dar, A. H.; Gopal, A.; Muthukrishnan, A.; Bajaj, A.; Ali, M. E.; Jayamurugan, G. J. Photochem. Photobiol., A 2021, 410, 113163. doi:10.1016/j.jphotochem.2021.113163
Return to citation in text: [1] [2] -
Kato, S.-i.; Noguchi, H.; Jin, S.; Nakamura, Y. Asian J. Org. Chem. 2016, 5, 246–256. doi:10.1002/ajoc.201500431
Return to citation in text: [1] -
Okuno, T.; Iwahashi, H. Acta Crystallogr., Sect. E: Struct. Rep. Online 2013, 69, o665. doi:10.1107/s1600536813008799
Return to citation in text: [1] -
Yadav, I. S.; Misra, R. New J. Chem. 2022, 46, 15999–16006. doi:10.1039/d2nj03089f
Return to citation in text: [1] -
Pappenfus, T. M.; Schneiderman, D. K.; Casado, J.; López Navarrete, J. T.; Ruiz Delgado, M. C.; Zotti, G.; Vercelli, B.; Lovander, M. D.; Hinkle, L. M.; Bohnsack, J. N.; Mann, K. R. Chem. Mater. 2011, 23, 823–831. doi:10.1021/cm102128g
Return to citation in text: [1] -
Simón Marqués, P.; Castán, J. M. A.; Raul, B. A. L.; Londi, G.; Ramirez, I.; Pshenichnikov, M. S.; Beljonne, D.; Walzer, K.; Blais, M.; Allain, M.; Cabanetos, C.; Blanchard, P. Chem. – Eur. J. 2020, 26, 16422–16433. doi:10.1002/chem.202002810
Return to citation in text: [1] [2] -
Raheem, A. A.; Kumar, C.; Shanmugam, R.; Murugan, P.; Praveen, C. J. Mater. Chem. C 2021, 9, 4562–4575. doi:10.1039/d1tc00708d
Return to citation in text: [1] [2] [3] -
Raheem, A. A.; Murugan, P.; Shanmugam, R.; Praveen, C. ChemPlusChem 2021, 86, 1451–1460. doi:10.1002/cplu.202100392
Return to citation in text: [1] [2] -
Kato, S.-i.; Kivala, M.; Schweizer, W. B.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Chem. – Eur. J. 2009, 15, 8687–8691. doi:10.1002/chem.200901630
Return to citation in text: [1] [2] -
García, R.; Herranz, M. Á.; Torres, M. R.; Bouit, P.-A.; Delgado, J. L.; Calbo, J.; Viruela, P. M.; Ortí, E.; Martín, N. J. Org. Chem. 2012, 77, 10707–10717. doi:10.1021/jo302047m
Return to citation in text: [1] -
Mochida, T.; Yamazaki, S. J. Chem. Soc., Dalton Trans. 2002, 3559–3564. doi:10.1039/b204168e
Return to citation in text: [1] -
Jordan, M.; Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Seiler, P.; Diederich, F. Chem. – Asian J. 2011, 6, 396–401. doi:10.1002/asia.201000539
Return to citation in text: [1] -
Shoji, T.; Ito, S.; Okujima, T.; Morita, N. Eur. J. Org. Chem. 2011, 5134–5140. doi:10.1002/ejoc.201100650
Return to citation in text: [1] -
Shoji, T.; Ito, S.; Okujima, T.; Morita, N. Chem. – Eur. J. 2013, 19, 5721–5730. doi:10.1002/chem.201202257
Return to citation in text: [1] [2] -
Misra, R.; Sharma, R.; Mobin, S. M. Dalton Trans. 2014, 43, 6891–6896. doi:10.1039/c4dt00210e
Return to citation in text: [1] -
Gautam, P.; Maragani, R.; Misra, R. Tetrahedron Lett. 2014, 55, 6827–6830. doi:10.1016/j.tetlet.2014.10.094
Return to citation in text: [1] -
Maragani, R.; Misra, R. Tetrahedron 2014, 70, 3390–3399. doi:10.1016/j.tet.2014.03.096
Return to citation in text: [1] -
Shoji, T.; Maruyama, A.; Yaku, C.; Kamata, N.; Ito, S.; Okujima, T.; Toyota, K. Chem. – Eur. J. 2015, 21, 402–409. doi:10.1002/chem.201405004
Return to citation in text: [1] -
Shoji, T.; Kamata, N.; Maruyama, A.; Ito, S.; Okujima, T. Bull. Chem. Soc. Jpn. 2015, 88, 1338–1346. doi:10.1246/bcsj.20150165
Return to citation in text: [1] -
Misra, R.; Gautam, P.; Maragani, R. Tetrahedron Lett. 2015, 56, 1664–1666. doi:10.1016/j.tetlet.2015.02.031
Return to citation in text: [1] -
Dhokale, B.; Jadhav, T.; Mobin, S. M.; Misra, R. RSC Adv. 2015, 5, 57692–57699. doi:10.1039/c5ra11433k
Return to citation in text: [1] -
Krauße, N.; Kielmann, M.; Ma, J.; Butenschön, H. Eur. J. Org. Chem. 2015, 2622–2631. doi:10.1002/ejoc.201500105
Return to citation in text: [1] -
Dhokale, B.; Jadhav, T.; Mobin, S. M.; Misra, R. Dalton Trans. 2016, 45, 1476–1483. doi:10.1039/c5dt04037j
Return to citation in text: [1] [2] [3] -
Patil, Y.; Jadhav, T.; Dhokale, B.; Misra, R. Eur. J. Org. Chem. 2016, 733–738. doi:10.1002/ejoc.201501123
Return to citation in text: [1] -
Patil, Y.; Misra, R. J. Organomet. Chem. 2017, 840, 23–29. doi:10.1016/j.jorganchem.2017.03.048
Return to citation in text: [1] -
Patil, Y.; Popli, C.; Misra, R. New J. Chem. 2018, 42, 3892–3899. doi:10.1039/c7nj05162j
Return to citation in text: [1] [2] -
Rout, Y.; Mobin, S. M.; Misra, R. New J. Chem. 2019, 43, 12299–12307. doi:10.1039/c9nj01887e
Return to citation in text: [1] [2] -
Rout, Y.; Misra, R. New J. Chem. 2021, 45, 9838–9845. doi:10.1039/d1nj00919b
Return to citation in text: [1] [2] -
Shoji, T.; Ito, S.; Toyota, K.; Yasunami, M.; Morita, N. Chem. – Eur. J. 2008, 14, 8398–8408. doi:10.1002/chem.200701981
Return to citation in text: [1] -
Shoji, T.; Ito, S.; Toyota, K.; Iwamoto, T.; Yasunami, M.; Morita, N. Eur. J. Org. Chem. 2009, 4316–4324. doi:10.1002/ejoc.200900539
Return to citation in text: [1] -
Shoji, T.; Maruyama, M.; Ito, S.; Morita, N. Bull. Chem. Soc. Jpn. 2012, 85, 761–773. doi:10.1246/bcsj.20120037
Return to citation in text: [1] -
Shoji, T.; Maruyama, M.; Shimomura, E.; Maruyama, A.; Ito, S.; Okujima, T.; Toyota, K.; Morita, N. J. Org. Chem. 2013, 78, 12513–12524. doi:10.1021/jo402104n
Return to citation in text: [1] -
Shoji, T.; Shimomura, E.; Maruyama, M.; Maruyama, A.; Ito, S.; Okujima, T.; Toyota, K.; Morita, N. Eur. J. Org. Chem. 2013, 7785–7799. doi:10.1002/ejoc.201301006
Return to citation in text: [1] -
Shoji, T.; Maruyama, M.; Maruyama, A.; Ito, S.; Okujima, T.; Toyota, K. Chem. – Eur. J. 2014, 20, 11903–11912. doi:10.1002/chem.201402939
Return to citation in text: [1] -
Shoji, T.; Maruyama, A.; Shimomura, E.; Nagai, D.; Ito, S.; Okujima, T.; Toyota, K. Eur. J. Org. Chem. 2015, 1979–1990. doi:10.1002/ejoc.201403556
Return to citation in text: [1] -
Shoji, T.; Maruyama, A.; Tanaka, M.; Nagai, D.; Shimomura, E.; Fujimori, K.; Ito, S.; Okujima, T.; Toyota, K.; Yasunami, M. ChemistrySelect 2016, 1, 49–57. doi:10.1002/slct.201500036
Return to citation in text: [1] -
Shoji, T.; Ito, S. Chem. – Eur. J. 2017, 23, 16696–16709. doi:10.1002/chem.201702806
Return to citation in text: [1] [2] -
Shoji, T.; Miura, K.; Araki, T.; Maruyama, A.; Ohta, A.; Sekiguchi, R.; Ito, S.; Okujima, T. J. Org. Chem. 2018, 83, 6690–6705. doi:10.1021/acs.joc.8b01067
Return to citation in text: [1] -
Shoji, T.; Higashi, J.; Ito, S.; Okujima, T.; Yasunami, M.; Morita, N. Chem. – Eur. J. 2011, 17, 5116–5129. doi:10.1002/chem.201003628
Return to citation in text: [1] -
Shoji, T.; Higashi, J.; Ito, S.; Okujima, T.; Yasunami, M.; Morita, N. Org. Biomol. Chem. 2012, 10, 2431–2438. doi:10.1039/c2ob06931h
Return to citation in text: [1] -
Niu, S.; Ulrich, G.; Retailleau, P.; Ziessel, R. Org. Lett. 2011, 13, 4996–4999. doi:10.1021/ol201600s
Return to citation in text: [1] -
Niu, S.; Ulrich, G.; Retailleau, P.; Ziessel, R. Tetrahedron Lett. 2011, 52, 4848–4853. doi:10.1016/j.tetlet.2011.07.028
Return to citation in text: [1] -
Ulrich, G.; Barsella, A.; Boeglin, A.; Niu, S.; Ziessel, R. ChemPhysChem 2014, 15, 2693–2700. doi:10.1002/cphc.201402123
Return to citation in text: [1] [2] -
Koszelewski, D.; Nowak‐Król, A.; Gryko, D. T. Chem. – Asian J. 2012, 7, 1887–1894. doi:10.1002/asia.201200179
Return to citation in text: [1] -
Pengxia, L.; Du, Z.; Wang, D.; Yang, Z.; Sheng, H.; Liang, S.; Cao, H.; He, W.; Yang, H. ChemPhysChem 2014, 15, 3523–3529. doi:10.1002/cphc.201402401
Return to citation in text: [1] [2] -
Yadav, I.; Sharma, J. K.; Sankar, M.; D'Souza, F. Chem. – Eur. J. 2023, 29, e202301806. doi:10.1002/chem.202301806
Return to citation in text: [1] [2] -
Sasaki, S.-i.; Mizutani, K.; Kunieda, M.; Tamiaki, H. Tetrahedron 2013, 69, 9772–9778. doi:10.1016/j.tet.2013.09.007
Return to citation in text: [1] -
Tamiaki, H.; Mizutani, K.; Sasaki, S.-i.; Tatebe, T. Tetrahedron 2016, 72, 6626–6633. doi:10.1016/j.tet.2016.08.079
Return to citation in text: [1] -
Perrin, F. G.; Kiefer, G.; Jeanbourquin, L.; Racine, S.; Perrotta, D.; Waser, J.; Scopelliti, R.; Severin, K. Angew. Chem., Int. Ed. 2015, 54, 13393–13396. doi:10.1002/anie.201507033
Return to citation in text: [1] -
Mammadova, F.; Ozsinan, S.; Okutan, M.; Dengiz, C. J. Mol. Struct. 2020, 1220, 128726. doi:10.1016/j.molstruc.2020.128726
Return to citation in text: [1] -
Betou, M.; Kerisit, N.; Meledje, E.; Leroux, Y. R.; Katan, C.; Halet, J.-F.; Guillemin, J.-C.; Trolez, Y. Chem. – Eur. J. 2014, 20, 9553–9557. doi:10.1002/chem.201402653
Return to citation in text: [1] -
Betou, M.; Durand, R. J.; Sallustrau, D. A.; Gousset, C.; Le Coz, E.; Leroux, Y. R.; Toupet, D. L.; Trzop, E.; Roisnel, T.; Trolez, Y. Chem. – Asian J. 2017, 12, 1338–1346. doi:10.1002/asia.201700353
Return to citation in text: [1] -
Philippe, C.; Bui, A. T.; Batsongo-Boulingui, S.; Pokladek, Z.; Matczyszyn, K.; Mongin, O.; Lemiègre, L.; Paul, F.; Hamlin, T. A.; Trolez, Y. Org. Lett. 2021, 23, 2007–2012. doi:10.1021/acs.orglett.1c00136
Return to citation in text: [1] [2] -
Huang, S.; Ma, J.; Yi, Y.; Li, M.; Cai, P.; Wu, N. Org. Biomol. Chem. 2022, 20, 4081–4085. doi:10.1039/d2ob00677d
Return to citation in text: [1] -
Erden, K.; Dengiz, C. J. Org. Chem. 2022, 87, 4385–4399. doi:10.1021/acs.joc.2c00067
Return to citation in text: [1] -
Philippe, C.; Melan, J.; Barsella, A.; Vives, T.; Leroux, Y. R.; Robin-Le Guen, F.; Lemiègre, L.; Jacquemin, D.; Gauthier, S.; Trolez, Y. Tetrahedron Chem 2023, 5, 100036. doi:10.1016/j.tchem.2023.100036
Return to citation in text: [1] -
Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Enko, B.; Seiler, P.; Müller, I. B.; Langer, N.; Jarowski, P. D.; Gescheidt, G.; Diederich, F. Chem. – Eur. J. 2009, 15, 4111–4123. doi:10.1002/chem.200802563
Return to citation in text: [1] -
Jarowski, P. D.; Wu, Y.-L.; Boudon, C.; Gisselbrecht, J.-P.; Gross, M.; Schweizer, W. B.; Diederich, F. Org. Biomol. Chem. 2009, 7, 1312–1322. doi:10.1039/b821230a
Return to citation in text: [1] -
Wu, Y.-L.; Jarowski, P. D.; Schweizer, W. B.; Diederich, F. Chem. – Eur. J. 2010, 16, 202–211. doi:10.1002/chem.200902645
Return to citation in text: [1] [2] -
Rijkers, D. T. S.; de Prada López, F.; Liskamp, R. M. J.; Diederich, F. Tetrahedron Lett. 2011, 52, 6963–6967. doi:10.1016/j.tetlet.2011.10.084
Return to citation in text: [1] -
Silvestri, F.; Jordan, M.; Howes, K.; Kivala, M.; Rivera‐Fuentes, P.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Seiler, P.; Chiu, M.; Diederich, F. Chem. – Eur. J. 2011, 17, 6088–6097. doi:10.1002/chem.201003672
Return to citation in text: [1] [2] -
Tancini, F.; Wu, Y.-L.; Schweizer, W. B.; Gisselbrecht, J.-P.; Boudon, C.; Jarowski, P. D.; Beels, M. T.; Biaggio, I.; Diederich, F. Eur. J. Org. Chem. 2012, 2756–2765. doi:10.1002/ejoc.201200111
Return to citation in text: [1] [2] -
Galán, E.; Andreu, R.; Garín, J.; Orduna, J.; Villacampa, B.; Diosdado, B. E. Org. Biomol. Chem. 2012, 10, 8684–8691. doi:10.1039/c2ob26515j
Return to citation in text: [1] -
Chiu, M.; Jaun, B.; Beels, M. T. R.; Biaggio, I.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Kivala, M.; Diederich, F. Org. Lett. 2012, 14, 54–57. doi:10.1021/ol202815q
Return to citation in text: [1] [2] -
Finke, A. D.; Dumele, O.; Zalibera, M.; Confortin, D.; Cias, P.; Jayamurugan, G.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Gescheidt, G.; Diederich, F. J. Am. Chem. Soc. 2012, 134, 18139–18146. doi:10.1021/ja309141r
Return to citation in text: [1] [2] -
Finke, A. D.; Diederich, F. Chem. Rec. 2015, 15, 19–30. doi:10.1002/tcr.201402060
Return to citation in text: [1] [2] -
Fesser, P.; Iacovita, C.; Wäckerlin, C.; Vijayaraghavan, S.; Ballav, N.; Howes, K.; Gisselbrecht, J.-P.; Crobu, M.; Boudon, C.; Stöhr, M.; Jung, T. A.; Diederich, F. Chem. – Eur. J. 2011, 17, 5246–5250. doi:10.1002/chem.201100733
Return to citation in text: [1] -
Lacy, A. R.; Vogt, A.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Diederich, F. Eur. J. Org. Chem. 2013, 869–879. doi:10.1002/ejoc.201201371
Return to citation in text: [1] -
Breiten, B.; Wu, Y.-L.; Jarowski, P. D.; Gisselbrecht, J.-P.; Boudon, C.; Griesser, M.; Onitsch, C.; Gescheidt, G.; Schweizer, W. B.; Langer, N.; Lennartz, C.; Diederich, F. Chem. Sci. 2011, 2, 88–93. doi:10.1039/c0sc00387e
Return to citation in text: [1] -
Breiten, B.; Jordan, M.; Taura, D.; Zalibera, M.; Griesser, M.; Confortin, D.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Gescheidt, G.; Diederich, F. J. Org. Chem. 2013, 78, 1760–1767. doi:10.1021/jo301194y
Return to citation in text: [1] -
Kato, S.-i.; Beels, M. T. R.; La Porta, P.; Schweizer, W. B.; Boudon, C.; Gisselbrecht, J.-P.; Biaggio, I.; Diederich, F. Angew. Chem., Int. Ed. 2010, 49, 6207–6211. doi:10.1002/anie.201002236
Return to citation in text: [1] [2] -
Dengiz, C.; Dumele, O.; Kato, S.-i.; Zalibera, M.; Cias, P.; Schweizer, W. B.; Boudon, C.; Gisselbrecht, J.-P.; Gescheidt, G.; Diederich, F. Chem. – Eur. J. 2014, 20, 1279–1286. doi:10.1002/chem.201303533
Return to citation in text: [1] -
Shoji, T.; Higashi, J.; Ito, S.; Yasunami, M.; Moria, N. Heterocycles 2011, 83, 2271–2274. doi:10.3987/com-11-12303
Return to citation in text: [1] -
Shoji, T.; Maruyama, M.; Shimomura, E.; Maruyama, A.; Ito, S.; Yasunami, M.; Higashi, J.; Toyota, K.; Morita, N. Heterocycles 2014, 88, 319–329. doi:10.3987/com-13-s(s)24
Return to citation in text: [1] -
Dengiz, C.; Prange, C.; Gawel, P.; Trapp, N.; Ruhlmann, L.; Boudon, C.; Diederich, F. Tetrahedron 2016, 72, 1213–1224. doi:10.1016/j.tet.2016.01.017
Return to citation in text: [1] -
Erden, K.; Savaş, İ.; Dengiz, C. Tetrahedron Lett. 2019, 60, 1982–1985. doi:10.1016/j.tetlet.2019.06.046
Return to citation in text: [1] -
Reekie, T. A.; Donckele, E. J.; Ruhlmann, L.; Boudon, C.; Trapp, N.; Diederich, F. Eur. J. Org. Chem. 2015, 7264–7275. doi:10.1002/ejoc.201501085
Return to citation in text: [1] -
Januszewski, J. A.; Hampel, F.; Neiss, C.; Görling, A.; Tykwinski, R. R. Angew. Chem., Int. Ed. 2014, 53, 3743–3747. doi:10.1002/anie.201309355
Return to citation in text: [1] -
Gawel, P.; Dengiz, C.; Finke, A. D.; Trapp, N.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Angew. Chem., Int. Ed. 2014, 53, 4341–4345. doi:10.1002/anie.201402299
Return to citation in text: [1] -
Wu, Y.-L.; Tancini, F.; Schweizer, W. B.; Paunescu, D.; Boudon, C.; Gisselbrecht, J.-P.; Jarowski, P. D.; Dalcanale, E.; Diederich, F. Chem. – Asian J. 2012, 7, 1185–1190. doi:10.1002/asia.201100997
Return to citation in text: [1] -
Gawel, P.; Wu, Y.-L.; Finke, A. D.; Trapp, N.; Zalibera, M.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Gescheidt, G.; Diederich, F. Chem. – Eur. J. 2015, 21, 6215–6225. doi:10.1002/chem.201406583
Return to citation in text: [1] -
Dar, A. H.; Gowri, V.; Neethu, K. M.; Jayamurugan, G. ChemistrySelect 2020, 5, 12437–12441. doi:10.1002/slct.202003179
Return to citation in text: [1] -
Jayamurugan, G.; Finke, A. D.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Diederich, F. J. Org. Chem. 2014, 79, 426–431. doi:10.1021/jo402440m
Return to citation in text: [1] [2] -
Jayamurugan, G.; Gisselbrecht, J.-P.; Boudon, C.; Schoenebeck, F.; Schweizer, W. B.; Bernet, B.; Diederich, F. Chem. Commun. 2011, 47, 4520–4522. doi:10.1039/c1cc10247h
Return to citation in text: [1] -
Jayamurugan, G.; Dumele, O.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Bernet, B.; Diederich, F. J. Am. Chem. Soc. 2013, 135, 3599–3606. doi:10.1021/ja312084s
Return to citation in text: [1] -
Hansen, J. K. S.; Tortzen, C. G.; Sørensen, P. G.; Brøndsted Nielsen, M. Chem. – Eur. J. 2023, 29, e202202833. doi:10.1002/chem.202202833
Return to citation in text: [1] -
Nielsen, M. B. ChemPhysChem 2023, 24, e202300236. doi:10.1002/cphc.202300236
Return to citation in text: [1] -
Lemiègre, L.; Trolez, Y. Asian J. Org. Chem. 2023, 12, e202300321. doi:10.1002/ajoc.202300321
Return to citation in text: [1] -
Sletten, E. M.; Bertozzi, C. R. Angew. Chem., Int. Ed. 2009, 48, 6974–6998. doi:10.1002/anie.200900942
Return to citation in text: [1] -
Sletten, E. M.; Bertozzi, C. R. Acc. Chem. Res. 2011, 44, 666–676. doi:10.1021/ar200148z
Return to citation in text: [1] -
Debets, M. F.; van Berkel, S. S.; Dommerholt, J.; Dirks, A. T. J.; Rutjes, F. P. J. T.; van Delft, F. L. Acc. Chem. Res. 2011, 44, 805–815. doi:10.1021/ar200059z
Return to citation in text: [1] -
Chiu, M.; Tchitchanov, B. H.; Zimmerli, D.; Sanhueza, I. A.; Schoenebeck, F.; Trapp, N.; Schweizer, W. B.; Diederich, F. Angew. Chem., Int. Ed. 2015, 54, 349–354. doi:10.1002/anie.201409289
Return to citation in text: [1] -
Schaub, T. A.; Margraf, J. T.; Zakharov, L.; Reuter, K.; Jasti, R. Angew. Chem., Int. Ed. 2018, 57, 16348–16353. doi:10.1002/anie.201808611
Return to citation in text: [1] -
Yamada, M.; Schweizer, W. B.; Schoenebeck, F.; Diederich, F. Chem. Commun. 2010, 46, 5334–5336. doi:10.1039/c0cc00881h
Return to citation in text: [1] -
Reisinger, C. M.; Rivera‐Fuentes, P.; Lampart, S.; Schweizer, W. B.; Diederich, F. Chem. – Eur. J. 2011, 17, 12906–12911. doi:10.1002/chem.201102852
Return to citation in text: [1] -
Kerisit, N.; Finke, A. D.; Trapp, N.; Leroux, Y. R.; Guillemin, J.-C.; Trolez, Y.; Diederich, F. Tetrahedron 2015, 71, 4393–4399. doi:10.1016/j.tet.2015.03.081
Return to citation in text: [1] -
Donckele, E. J.; Finke, A. D.; Ruhlmann, L.; Boudon, C.; Trapp, N.; Diederich, F. Org. Lett. 2015, 17, 3506–3509. doi:10.1021/acs.orglett.5b01598
Return to citation in text: [1] -
Shoji, T.; Nagai, D.; Tanaka, M.; Araki, T.; Ohta, A.; Sekiguchi, R.; Ito, S.; Mori, S.; Okujima, T. Chem. – Eur. J. 2017, 23, 5126–5136. doi:10.1002/chem.201700121
Return to citation in text: [1] [2] -
Neethu, K. M.; Nag, K.; Dar, A. H.; Bajaj, A.; Gopal, S. A.; Gowri, V.; Nagpure, M.; Sartaliya, S.; Sharma, R.; Solanki, A. K.; Ehesan Ali, M.; Muthukrishnan, A.; Jayamurugan, G. Org. Biomol. Chem. 2023, 21, 2922–2929. doi:10.1039/d3ob00053b
Return to citation in text: [1] -
Bruce, M. I.; Burgun, A.; Jevric, M.; Morris, J. C.; Nicholson, B. K.; Parker, C. R.; Scoleri, N.; Skelton, B. W.; Zaitseva, N. N. J. Organomet. Chem. 2014, 756, 68–78. doi:10.1016/j.jorganchem.2013.12.038
Return to citation in text: [1] -
Mateo, L. M.; Sagresti, L.; Luo, Y.; Guldi, D. M.; Torres, T.; Brancato, G.; Bottari, G. Chem. – Eur. J. 2021, 27, 16049–16055. doi:10.1002/chem.202103079
Return to citation in text: [1] -
Zhou, W.; Xu, J.; Zheng, H.; Liu, H.; Li, Y.; Zhu, D. J. Org. Chem. 2008, 73, 7702–7709. doi:10.1021/jo8014566
Return to citation in text: [1] -
Zhou, W.; Xu, J.; Zheng, H.; Yin, X.; Zuo, Z.; Liu, H.; Li, Y. Adv. Funct. Mater. 2009, 19, 141–149. doi:10.1002/adfm.200801149
Return to citation in text: [1] -
Li, Y.; Liu, T.; Liu, H.; Tian, M.-Z.; Li, Y. Acc. Chem. Res. 2014, 47, 1186–1198. doi:10.1021/ar400264e
Return to citation in text: [1] -
Trolez, Y.; Finke, A. D.; Silvestri, F.; Monti, F.; Ventura, B.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Sauvage, J.-P.; Armaroli, N.; Diederich, F. Chem. – Eur. J. 2018, 24, 10422–10433. doi:10.1002/chem.201801161
Return to citation in text: [1] [2] -
Alonso‐Gómez, J. L.; Schanen, P.; Rivera‐Fuentes, P.; Seiler, P.; Diederich, F. Chem. – Eur. J. 2008, 14, 10564–10568. doi:10.1002/chem.200801456
Return to citation in text: [1] -
Alonso‐Gómez, J. L.; Petrovic, A. G.; Harada, N.; Rivera‐Fuentes, P.; Berova, N.; Diederich, F. Chem. – Eur. J. 2009, 15, 8396–8400. doi:10.1002/chem.200900103
Return to citation in text: [1] -
Frank, B. B.; Camafort Blanco, B.; Jakob, S.; Ferroni, F.; Pieraccini, S.; Ferrarini, A.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Spada, G. P.; Diederich, F. Chem. – Eur. J. 2009, 15, 9005–9016. doi:10.1002/chem.200900913
Return to citation in text: [1] -
Frank, B. B.; Kivala, M.; Camafort Blanco, B.; Breiten, B.; Schweizer, W. B.; Laporta, P. R.; Biaggio, I.; Jahnke, E.; Tykwinski, R. R.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Eur. J. Org. Chem. 2010, 2487–2503. doi:10.1002/ejoc.201000030
Return to citation in text: [1] -
Ozcelik, A.; Pereira‐Cameselle, R.; Peña‐Gallego, Á.; Alonso‐Gómez, J. L. Eur. J. Org. Chem. 2022, e202101333. doi:10.1002/ejoc.202101333
Return to citation in text: [1] -
Bouvier, R.; Durand, R.; Favereau, L.; Srebro‐Hooper, M.; Dorcet, V.; Roisnel, T.; Vanthuyne, N.; Vesga, Y.; Donnelly, J.; Hernandez, F.; Autschbach, J.; Trolez, Y.; Crassous, J. Chem. – Eur. J. 2018, 24, 14484–14494. doi:10.1002/chem.201802763
Return to citation in text: [1] [2] -
Yamada, M.; Rivera‐Fuentes, P.; Schweizer, W. B.; Diederich, F. Angew. Chem., Int. Ed. 2010, 49, 3532–3535. doi:10.1002/anie.200906853
Return to citation in text: [1] -
Winterfeld, K. A.; Lavarda, G.; Guilleme, J.; Sekita, M.; Guldi, D. M.; Torres, T.; Bottari, G. J. Am. Chem. Soc. 2017, 139, 5520–5529. doi:10.1021/jacs.7b01460
Return to citation in text: [1] [2] -
Lavarda, G.; Bhattacharjee, N.; Brancato, G.; Torres, T.; Bottari, G. Angew. Chem., Int. Ed. 2020, 59, 21224–21229. doi:10.1002/anie.202010133
Return to citation in text: [1] -
Winterfeld, K. A.; Lavarda, G.; Yoshida, K.; Bayerlein, M. J.; Kise, K.; Tanaka, T.; Osuka, A.; Guldi, D. M.; Torres, T.; Bottari, G. J. Am. Chem. Soc. 2020, 142, 7920–7929. doi:10.1021/jacs.0c01646
Return to citation in text: [1] -
Monti, F.; Venturini, A.; Nenov, A.; Tancini, F.; Finke, A. D.; Diederich, F.; Armaroli, N. J. Phys. Chem. A 2015, 119, 10677–10683. doi:10.1021/acs.jpca.5b09291
Return to citation in text: [1] [2] -
Pokladek, Z.; Ripoche, N.; Betou, M.; Trolez, Y.; Mongin, O.; Olesiak‐Banska, J.; Matczyszyn, K.; Samoc, M.; Humphrey, M. G.; Blanchard‐Desce, M.; Paul, F. Chem. – Eur. J. 2016, 22, 10155–10167. doi:10.1002/chem.201600897
Return to citation in text: [1] [2] [3] [4] -
Gautam, P.; Misra, R.; Thomas, M. B.; D'Souza, F. Chem. – Eur. J. 2017, 23, 9192–9200. doi:10.1002/chem.201701604
Return to citation in text: [1] -
Xu, J.; Liu, X.; Lv, J.; Zhu, M.; Huang, C.; Zhou, W.; Yin, X.; Liu, H.; Li, Y.; Ye, J. Langmuir 2008, 24, 4231–4237. doi:10.1021/la703662w
Return to citation in text: [1] -
Bui, A. T.; Philippe, C.; Beau, M.; Richy, N.; Cordier, M.; Roisnel, T.; Lemiègre, L.; Mongin, O.; Paul, F.; Trolez, Y. Chem. Commun. 2020, 56, 3571–3574. doi:10.1039/c9cc09560h
Return to citation in text: [1] -
Philippe, C.; Bui, A. T.; Beau, M.; Bloux, H.; Riobé, F.; Mongin, O.; Roisnel, T.; Cordier, M.; Paul, F.; Lemiègre, L.; Trolez, Y. Chem. – Eur. J. 2022, 28, e202200025. doi:10.1002/chem.202200025
Return to citation in text: [1] -
Philippe, C.; Coste, M.; Bretonnière, Y.; Lemiègre, L.; Ulrich, S.; Trolez, Y. Eur. J. Org. Chem. 2022, 10.1002/ejoc.202200049. doi:10.1002/ejoc.202200049
Return to citation in text: [1] -
Dar, A. H.; Gowri, V.; Mishra, R. K.; Khan, R.; Jayamurugan, G. Langmuir 2022, 38, 430–438. doi:10.1021/acs.langmuir.1c02797
Return to citation in text: [1] -
Yamada, M.; Tancini, F.; Sekita, M.; Guldi, D. M.; Boudon, C.; Gisselbrecht, J.-P.; Alberti, M. N.; Schweizer, W. B.; Diederich, F. Fullerenes, Nanotubes, Carbon Nanostruct. 2014, 22, 99–127. doi:10.1080/1536383x.2013.794342
Return to citation in text: [1] -
Marcus, R. A. J. Chem. Phys. 1956, 24, 966–978. doi:10.1063/1.1742723
Return to citation in text: [1] [2] -
Sekita, M.; Ballesteros, B.; Diederich, F.; Guldi, D. M.; Bottari, G.; Torres, T. Angew. Chem., Int. Ed. 2016, 55, 5560–5564. doi:10.1002/anie.201601258
Return to citation in text: [1] -
Tancini, F.; Monti, F.; Howes, K.; Belbakra, A.; Listorti, A.; Schweizer, W. B.; Reutenauer, P.; Alonso‐Gómez, J.-L.; Chiorboli, C.; Urner, L. M.; Gisselbrecht, J.-P.; Boudon, C.; Armaroli, N.; Diederich, F. Chem. – Eur. J. 2014, 20, 202–216. doi:10.1002/chem.201303284
Return to citation in text: [1] -
Rehm, D.; Weller, A. Isr. J. Chem. 1970, 8, 259–271. doi:10.1002/ijch.197000029
Return to citation in text: [1] -
Urner, L. M.; Sekita, M.; Trapp, N.; Schweizer, W. B.; Wörle, M.; Gisselbrecht, J.-P.; Boudon, C.; Guldi, D. M.; Diederich, F. Eur. J. Org. Chem. 2015, 91–108. doi:10.1002/ejoc.201403252
Return to citation in text: [1] -
Reekie, T. A.; Sekita, M.; Urner, L. M.; Bauroth, S.; Ruhlmann, L.; Gisselbrecht, J.-P.; Boudon, C.; Trapp, N.; Clark, T.; Guldi, D. M.; Diederich, F. Chem. – Eur. J. 2017, 23, 6357–6369. doi:10.1002/chem.201700043
Return to citation in text: [1] -
Jayamurugan, G.; Gowri, V.; Hernández, D.; Martin, S.; González‐Orive, A.; Dengiz, C.; Dumele, O.; Pérez‐Murano, F.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Breiten, B.; Finke, A. D.; Jeschke, G.; Bernet, B.; Ruhlmann, L.; Cea, P.; Diederich, F. Chem. – Eur. J. 2016, 22, 10539–10547. doi:10.1002/chem.201505216
Return to citation in text: [1] -
Sekaran, B.; Dawson, A.; Jang, Y.; MohanSingh, K. V.; Misra, R.; D'Souza, F. Chem. – Eur. J. 2021, 27, 14335–14344. doi:10.1002/chem.202102865
Return to citation in text: [1] -
Poddar, M.; Jang, Y.; Misra, R.; D'Souza, F. Chem. – Eur. J. 2020, 26, 6869–6878. doi:10.1002/chem.202000346
Return to citation in text: [1] -
Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Diederich, F. Angew. Chem., Int. Ed. 2007, 46, 6357–6360. doi:10.1002/anie.200701733
Return to citation in text: [1] -
Wu, Y.-L.; Stuparu, M. C.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Baldridge, K. K.; Siegel, J. S.; Diederich, F. J. Org. Chem. 2012, 77, 11014–11026. doi:10.1021/jo302217n
Return to citation in text: [1] -
Dengiz, C.; Breiten, B.; Gisselbrecht, J.-P.; Boudon, C.; Trapp, N.; Schweizer, W. B.; Diederich, F. J. Org. Chem. 2015, 80, 882–896. doi:10.1021/jo502367h
Return to citation in text: [1] -
Sharma, R.; Maragani, R.; Misra, R. New J. Chem. 2018, 42, 882–890. doi:10.1039/c7nj03934d
Return to citation in text: [1] -
Cheng, S.; Ma, X.; He, Y.; He, J.; Zeller, M.; Xu, Z. Cryst. Growth Des. 2019, 19, 7411–7419. doi:10.1021/acs.cgd.9b01488
Return to citation in text: [1] -
Chen, S.; Li, Y.; Liu, C.; Yang, W.; Li, Y. Eur. J. Org. Chem. 2011, 6445–6451. doi:10.1002/ejoc.201101009
Return to citation in text: [1] -
Tykwinski, R. R.; Gubler, U.; Martin, R. E.; Diederich, F.; Bosshard, C.; Günter, P. J. Phys. Chem. B 1998, 102, 4451–4465. doi:10.1021/jp980829o
Return to citation in text: [1] -
Frank, B. B.; Laporta, P. R.; Breiten, B.; Kuzyk, M. C.; Jarowski, P. D.; Schweizer, W. B.; Seiler, P.; Biaggio, I.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Eur. J. Org. Chem. 2011, 4307–4317. doi:10.1002/ejoc.201100378
Return to citation in text: [1] -
Štefko, M.; Tzirakis, M. D.; Breiten, B.; Ebert, M.-O.; Dumele, O.; Schweizer, W. B.; Gisselbrecht, J.-P.; Boudon, C.; Beels, M. T.; Biaggio, I.; Diederich, F. Chem. – Eur. J. 2013, 19, 12693–12704. doi:10.1002/chem.201301642
Return to citation in text: [1] [2] -
Han, P.; Yang, Z.; Cao, H.; He, W.; Wang, D.; Zhang, J.; Xing, Y.; Gao, H. Tetrahedron 2017, 73, 6210–6216. doi:10.1016/j.tet.2017.09.020
Return to citation in text: [1] -
Reekie, T. A.; Gisselbrecht, J.-P.; Boudon, C.; Trapp, N.; Diederich, F. Tetrahedron Lett. 2017, 58, 2414–2416. doi:10.1016/j.tetlet.2017.05.009
Return to citation in text: [1] -
Mammadova, F.; Inyurt, F. C.; Barsella, A.; Dengiz, C. Dyes Pigm. 2023, 209, 110894. doi:10.1016/j.dyepig.2022.110894
Return to citation in text: [1] -
Si, Y.; Yang, G. J. Phys. Chem. A 2014, 118, 1094–1102. doi:10.1021/jp4099717
Return to citation in text: [1] -
Biaggio, I. Chem. – Eur. J. 2022, 28, e202103168. doi:10.1002/chem.202103168
Return to citation in text: [1] -
Esembeson, B.; Scimeca, M. L.; Michinobu, T.; Diederich, F.; Biaggio, I. Adv. Mater. (Weinheim, Ger.) 2008, 20, 4584–4587. doi:10.1002/adma.200801552
Return to citation in text: [1] -
Koos, C.; Vorreau, P.; Vallaitis, T.; Dumon, P.; Bogaerts, W.; Baets, R.; Esembeson, B.; Biaggio, I.; Michinobu, T.; Diederich, F.; Freude, W.; Leuthold, J. Nat. Photonics 2009, 3, 216–219. doi:10.1038/nphoton.2009.25
Return to citation in text: [1] -
Ripoche, N.; Betou, M.; Philippe, C.; Trolez, Y.; Mongin, O.; Dudek, M.; Pokladek, Z.; Matczyszyn, K.; Samoc, M.; Sahnoune, H.; Halet, J.-F.; Roisnel, T.; Toupet, L.; Cordier, M.; Moxey, G. J.; Humphrey, M. G.; Paul, F. Phys. Chem. Chem. Phys. 2021, 23, 22283–22297. doi:10.1039/d1cp03346h
Return to citation in text: [1] -
Rout, Y.; Chauhan, V.; Misra, R. J. Org. Chem. 2020, 85, 4611–4618. doi:10.1021/acs.joc.9b03267
Return to citation in text: [1] -
Banziger, S. D.; Clendening, R. A.; Oxley, B. M.; Ren, T. J. Phys. Chem. B 2020, 124, 11901–11909. doi:10.1021/acs.jpcb.0c09450
Return to citation in text: [1] -
Yu, G.; Gao, J.; Hummelen, J. C.; Wudl, F.; Heeger, A. J. Science 1995, 270, 1789–1791. doi:10.1126/science.270.5243.1789
Return to citation in text: [1] -
Patil, Y.; Misra, R.; Keshtov, M. L.; Sharma, G. D. J. Phys. Chem. C 2016, 120, 6324–6335. doi:10.1021/acs.jpcc.5b12307
Return to citation in text: [1] -
Patil, Y.; Misra, R.; Keshtov, M. L.; Sharma, G. D. J. Mater. Chem. A 2017, 5, 3311–3319. doi:10.1039/c6ta09607g
Return to citation in text: [1] [2] -
Gautam, P.; Sharma, R.; Misra, R.; Keshtov, M. L.; Kuklin, S. A.; Sharma, G. D. Chem. Sci. 2017, 8, 2017–2024. doi:10.1039/c6sc04461a
Return to citation in text: [1] -
Patil, Y.; Misra, R.; Singhal, R.; Sharma, G. D. J. Mater. Chem. A 2017, 5, 13625–13633. doi:10.1039/c7ta03322b
Return to citation in text: [1] -
Raheem, A. A.; Kumar, C.; Murugan, P.; Praveen, C. ACS Appl. Energy Mater. 2021, 4, 11609–11623. doi:10.1021/acsaem.1c02353
Return to citation in text: [1] -
Leliège, A.; Blanchard, P.; Rousseau, T.; Roncali, J. Org. Lett. 2011, 13, 3098–3101. doi:10.1021/ol201002j
Return to citation in text: [1] -
Gautam, P.; Misra, R.; Koukaras, E. N.; Sharma, A.; Sharma, G. D. Org. Electron. 2015, 27, 72–83. doi:10.1016/j.orgel.2015.09.006
Return to citation in text: [1] -
Gautam, P.; Misra, R.; Siddiqui, S. A.; Sharma, G. D. ACS Appl. Mater. Interfaces 2015, 7, 10283–10292. doi:10.1021/acsami.5b02250
Return to citation in text: [1] -
Gautam, P.; Misra, R.; Sharma, G. D. Phys. Chem. Chem. Phys. 2016, 18, 7235–7241. doi:10.1039/c6cp00243a
Return to citation in text: [1] -
Raheem, A. A.; Kamaraj, S.; Sannasi, V.; Praveen, C. Org. Chem. Front. 2018, 5, 777–787. doi:10.1039/c7qo00920h
Return to citation in text: [1] -
Misra, R.; Maragani, R.; Patel, K. R.; Sharma, G. D. RSC Adv. 2014, 4, 34904–34911. doi:10.1039/c4ra03088e
Return to citation in text: [1]
| 15. | Michinobu, T.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Frank, B.; Moonen, N. N. P.; Gross, M.; Diederich, F. Chem. – Eur. J. 2006, 12, 1889–1905. doi:10.1002/chem.200501113 |
| 134. | Monti, F.; Venturini, A.; Nenov, A.; Tancini, F.; Finke, A. D.; Diederich, F.; Armaroli, N. J. Phys. Chem. A 2015, 119, 10677–10683. doi:10.1021/acs.jpca.5b09291 |
| 135. | Pokladek, Z.; Ripoche, N.; Betou, M.; Trolez, Y.; Mongin, O.; Olesiak‐Banska, J.; Matczyszyn, K.; Samoc, M.; Humphrey, M. G.; Blanchard‐Desce, M.; Paul, F. Chem. – Eur. J. 2016, 22, 10155–10167. doi:10.1002/chem.201600897 |
| 136. | Gautam, P.; Misra, R.; Thomas, M. B.; D'Souza, F. Chem. – Eur. J. 2017, 23, 9192–9200. doi:10.1002/chem.201701604 |
| 134. | Monti, F.; Venturini, A.; Nenov, A.; Tancini, F.; Finke, A. D.; Diederich, F.; Armaroli, N. J. Phys. Chem. A 2015, 119, 10677–10683. doi:10.1021/acs.jpca.5b09291 |
| 133. | Winterfeld, K. A.; Lavarda, G.; Yoshida, K.; Bayerlein, M. J.; Kise, K.; Tanaka, T.; Osuka, A.; Guldi, D. M.; Torres, T.; Bottari, G. J. Am. Chem. Soc. 2020, 142, 7920–7929. doi:10.1021/jacs.0c01646 |
| 15. | Michinobu, T.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Frank, B.; Moonen, N. N. P.; Gross, M.; Diederich, F. Chem. – Eur. J. 2006, 12, 1889–1905. doi:10.1002/chem.200501113 |
| 132. | Lavarda, G.; Bhattacharjee, N.; Brancato, G.; Torres, T.; Bottari, G. Angew. Chem., Int. Ed. 2020, 59, 21224–21229. doi:10.1002/anie.202010133 |
| 139. | Philippe, C.; Bui, A. T.; Beau, M.; Bloux, H.; Riobé, F.; Mongin, O.; Roisnel, T.; Cordier, M.; Paul, F.; Lemiègre, L.; Trolez, Y. Chem. – Eur. J. 2022, 28, e202200025. doi:10.1002/chem.202200025 |
| 129. | Bouvier, R.; Durand, R.; Favereau, L.; Srebro‐Hooper, M.; Dorcet, V.; Roisnel, T.; Vanthuyne, N.; Vesga, Y.; Donnelly, J.; Hernandez, F.; Autschbach, J.; Trolez, Y.; Crassous, J. Chem. – Eur. J. 2018, 24, 14484–14494. doi:10.1002/chem.201802763 |
| 123. | Trolez, Y.; Finke, A. D.; Silvestri, F.; Monti, F.; Ventura, B.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Sauvage, J.-P.; Armaroli, N.; Diederich, F. Chem. – Eur. J. 2018, 24, 10422–10433. doi:10.1002/chem.201801161 |
| 137. | Xu, J.; Liu, X.; Lv, J.; Zhu, M.; Huang, C.; Zhou, W.; Yin, X.; Liu, H.; Li, Y.; Ye, J. Langmuir 2008, 24, 4231–4237. doi:10.1021/la703662w |
| 138. | Bui, A. T.; Philippe, C.; Beau, M.; Richy, N.; Cordier, M.; Roisnel, T.; Lemiègre, L.; Mongin, O.; Paul, F.; Trolez, Y. Chem. Commun. 2020, 56, 3571–3574. doi:10.1039/c9cc09560h |
| 135. | Pokladek, Z.; Ripoche, N.; Betou, M.; Trolez, Y.; Mongin, O.; Olesiak‐Banska, J.; Matczyszyn, K.; Samoc, M.; Humphrey, M. G.; Blanchard‐Desce, M.; Paul, F. Chem. – Eur. J. 2016, 22, 10155–10167. doi:10.1002/chem.201600897 |
| 144. | Sekita, M.; Ballesteros, B.; Diederich, F.; Guldi, D. M.; Bottari, G.; Torres, T. Angew. Chem., Int. Ed. 2016, 55, 5560–5564. doi:10.1002/anie.201601258 |
| 135. | Pokladek, Z.; Ripoche, N.; Betou, M.; Trolez, Y.; Mongin, O.; Olesiak‐Banska, J.; Matczyszyn, K.; Samoc, M.; Humphrey, M. G.; Blanchard‐Desce, M.; Paul, F. Chem. – Eur. J. 2016, 22, 10155–10167. doi:10.1002/chem.201600897 |
| 168. | Ripoche, N.; Betou, M.; Philippe, C.; Trolez, Y.; Mongin, O.; Dudek, M.; Pokladek, Z.; Matczyszyn, K.; Samoc, M.; Sahnoune, H.; Halet, J.-F.; Roisnel, T.; Toupet, L.; Cordier, M.; Moxey, G. J.; Humphrey, M. G.; Paul, F. Phys. Chem. Chem. Phys. 2021, 23, 22283–22297. doi:10.1039/d1cp03346h |
| 141. | Dar, A. H.; Gowri, V.; Mishra, R. K.; Khan, R.; Jayamurugan, G. Langmuir 2022, 38, 430–438. doi:10.1021/acs.langmuir.1c02797 |
| 8. | Patil, Y.; Butenschön, H.; Misra, R. Chem. Rec. 2023, 23, e202200208. doi:10.1002/tcr.202200208 |
| 142. | Yamada, M.; Tancini, F.; Sekita, M.; Guldi, D. M.; Boudon, C.; Gisselbrecht, J.-P.; Alberti, M. N.; Schweizer, W. B.; Diederich, F. Fullerenes, Nanotubes, Carbon Nanostruct. 2014, 22, 99–127. doi:10.1080/1536383x.2013.794342 |
| 41. | Dhokale, B.; Jadhav, T.; Mobin, S. M.; Misra, R. Dalton Trans. 2016, 45, 1476–1483. doi:10.1039/c5dt04037j |
| 44. | Patil, Y.; Popli, C.; Misra, R. New J. Chem. 2018, 42, 3892–3899. doi:10.1039/c7nj05162j |
| 45. | Rout, Y.; Mobin, S. M.; Misra, R. New J. Chem. 2019, 43, 12299–12307. doi:10.1039/c9nj01887e |
| 46. | Rout, Y.; Misra, R. New J. Chem. 2021, 45, 9838–9845. doi:10.1039/d1nj00919b |
| 169. | Rout, Y.; Chauhan, V.; Misra, R. J. Org. Chem. 2020, 85, 4611–4618. doi:10.1021/acs.joc.9b03267 |
| 170. | Banziger, S. D.; Clendening, R. A.; Oxley, B. M.; Ren, T. J. Phys. Chem. B 2020, 124, 11901–11909. doi:10.1021/acs.jpcb.0c09450 |
| 140. | Philippe, C.; Coste, M.; Bretonnière, Y.; Lemiègre, L.; Ulrich, S.; Trolez, Y. Eur. J. Org. Chem. 2022, 10.1002/ejoc.202200049. doi:10.1002/ejoc.202200049 |
| 160. | Štefko, M.; Tzirakis, M. D.; Breiten, B.; Ebert, M.-O.; Dumele, O.; Schweizer, W. B.; Gisselbrecht, J.-P.; Boudon, C.; Beels, M. T.; Biaggio, I.; Diederich, F. Chem. – Eur. J. 2013, 19, 12693–12704. doi:10.1002/chem.201301642 |
| 18. | Dar, A. H.; Gowri, V.; Gopal, A.; Muthukrishnan, A.; Bajaj, A.; Sartaliya, S.; Selim, A.; Ali, M. E.; Jayamurugan, G. J. Org. Chem. 2019, 84, 8941–8947. doi:10.1021/acs.joc.9b00841 |
| 19. | Gowri, V.; Jalwal, S.; Dar, A. H.; Gopal, A.; Muthukrishnan, A.; Bajaj, A.; Ali, M. E.; Jayamurugan, G. J. Photochem. Photobiol., A 2021, 410, 113163. doi:10.1016/j.jphotochem.2021.113163 |
| 165. | Biaggio, I. Chem. – Eur. J. 2022, 28, e202103168. doi:10.1002/chem.202103168 |
| 167. | Koos, C.; Vorreau, P.; Vallaitis, T.; Dumon, P.; Bogaerts, W.; Baets, R.; Esembeson, B.; Biaggio, I.; Michinobu, T.; Diederich, F.; Freude, W.; Leuthold, J. Nat. Photonics 2009, 3, 216–219. doi:10.1038/nphoton.2009.25 |
| 166. | Esembeson, B.; Scimeca, M. L.; Michinobu, T.; Diederich, F.; Biaggio, I. Adv. Mater. (Weinheim, Ger.) 2008, 20, 4584–4587. doi:10.1002/adma.200801552 |
| 164. | Si, Y.; Yang, G. J. Phys. Chem. A 2014, 118, 1094–1102. doi:10.1021/jp4099717 |
| 146. | Rehm, D.; Weller, A. Isr. J. Chem. 1970, 8, 259–271. doi:10.1002/ijch.197000029 |
| 131. | Winterfeld, K. A.; Lavarda, G.; Guilleme, J.; Sekita, M.; Guldi, D. M.; Torres, T.; Bottari, G. J. Am. Chem. Soc. 2017, 139, 5520–5529. doi:10.1021/jacs.7b01460 |
| 145. | Tancini, F.; Monti, F.; Howes, K.; Belbakra, A.; Listorti, A.; Schweizer, W. B.; Reutenauer, P.; Alonso‐Gómez, J.-L.; Chiorboli, C.; Urner, L. M.; Gisselbrecht, J.-P.; Boudon, C.; Armaroli, N.; Diederich, F. Chem. – Eur. J. 2014, 20, 202–216. doi:10.1002/chem.201303284 |
| 171. | Yu, G.; Gao, J.; Hummelen, J. C.; Wudl, F.; Heeger, A. J. Science 1995, 270, 1789–1791. doi:10.1126/science.270.5243.1789 |
| 71. | Philippe, C.; Bui, A. T.; Batsongo-Boulingui, S.; Pokladek, Z.; Matczyszyn, K.; Mongin, O.; Lemiègre, L.; Paul, F.; Hamlin, T. A.; Trolez, Y. Org. Lett. 2021, 23, 2007–2012. doi:10.1021/acs.orglett.1c00136 |
| 107. | Sletten, E. M.; Bertozzi, C. R. Angew. Chem., Int. Ed. 2009, 48, 6974–6998. doi:10.1002/anie.200900942 |
| 108. | Sletten, E. M.; Bertozzi, C. R. Acc. Chem. Res. 2011, 44, 666–676. doi:10.1021/ar200148z |
| 109. | Debets, M. F.; van Berkel, S. S.; Dommerholt, J.; Dirks, A. T. J.; Rutjes, F. P. J. T.; van Delft, F. L. Acc. Chem. Res. 2011, 44, 805–815. doi:10.1021/ar200059z |
| 104. | Hansen, J. K. S.; Tortzen, C. G.; Sørensen, P. G.; Brøndsted Nielsen, M. Chem. – Eur. J. 2023, 29, e202202833. doi:10.1002/chem.202202833 |
| 105. | Nielsen, M. B. ChemPhysChem 2023, 24, e202300236. doi:10.1002/cphc.202300236 |
| 106. | Lemiègre, L.; Trolez, Y. Asian J. Org. Chem. 2023, 12, e202300321. doi:10.1002/ajoc.202300321 |
| 1. | Bureš, F. RSC Adv. 2014, 4, 58826–58851. doi:10.1039/c4ra11264d |
| 2. | Levi, L.; Müller, T. J. J. Chem. Soc. Rev. 2016, 45, 2825–2846. doi:10.1039/c5cs00805k |
| 25. | Raheem, A. A.; Kumar, C.; Shanmugam, R.; Murugan, P.; Praveen, C. J. Mater. Chem. C 2021, 9, 4562–4575. doi:10.1039/d1tc00708d |
| 24. | Simón Marqués, P.; Castán, J. M. A.; Raul, B. A. L.; Londi, G.; Ramirez, I.; Pshenichnikov, M. S.; Beljonne, D.; Walzer, K.; Blais, M.; Allain, M.; Cabanetos, C.; Blanchard, P. Chem. – Eur. J. 2020, 26, 16422–16433. doi:10.1002/chem.202002810 |
| 25. | Raheem, A. A.; Kumar, C.; Shanmugam, R.; Murugan, P.; Praveen, C. J. Mater. Chem. C 2021, 9, 4562–4575. doi:10.1039/d1tc00708d |
| 177. | Leliège, A.; Blanchard, P.; Rousseau, T.; Roncali, J. Org. Lett. 2011, 13, 3098–3101. doi:10.1021/ol201002j |
| 178. | Gautam, P.; Misra, R.; Koukaras, E. N.; Sharma, A.; Sharma, G. D. Org. Electron. 2015, 27, 72–83. doi:10.1016/j.orgel.2015.09.006 |
| 179. | Gautam, P.; Misra, R.; Siddiqui, S. A.; Sharma, G. D. ACS Appl. Mater. Interfaces 2015, 7, 10283–10292. doi:10.1021/acsami.5b02250 |
| 180. | Gautam, P.; Misra, R.; Sharma, G. D. Phys. Chem. Chem. Phys. 2016, 18, 7235–7241. doi:10.1039/c6cp00243a |
| 181. | Raheem, A. A.; Kamaraj, S.; Sannasi, V.; Praveen, C. Org. Chem. Front. 2018, 5, 777–787. doi:10.1039/c7qo00920h |
| 182. | Misra, R.; Maragani, R.; Patel, K. R.; Sharma, G. D. RSC Adv. 2014, 4, 34904–34911. doi:10.1039/c4ra03088e |
| 8. | Patil, Y.; Butenschön, H.; Misra, R. Chem. Rec. 2023, 23, e202200208. doi:10.1002/tcr.202200208 |
| 118. | Bruce, M. I.; Burgun, A.; Jevric, M.; Morris, J. C.; Nicholson, B. K.; Parker, C. R.; Scoleri, N.; Skelton, B. W.; Zaitseva, N. N. J. Organomet. Chem. 2014, 756, 68–78. doi:10.1016/j.jorganchem.2013.12.038 |
| 7. | Michinobu, T.; Diederich, F. Angew. Chem., Int. Ed. 2018, 57, 3552–3577. doi:10.1002/anie.201711605 |
| 4. | Kivala, M.; Diederich, F. Acc. Chem. Res. 2009, 42, 235–248. doi:10.1021/ar8001238 |
| 5. | Kato, S.-i.; Diederich, F. Chem. Commun. 2010, 46, 1994–2006. doi:10.1039/b926601a |
| 6. | Bruce, M. I. J. Organomet. Chem. 2013, 730, 3–19. doi:10.1016/j.jorganchem.2012.11.004 |
| 116. | Shoji, T.; Nagai, D.; Tanaka, M.; Araki, T.; Ohta, A.; Sekiguchi, R.; Ito, S.; Mori, S.; Okujima, T. Chem. – Eur. J. 2017, 23, 5126–5136. doi:10.1002/chem.201700121 |
| 173. | Patil, Y.; Misra, R.; Keshtov, M. L.; Sharma, G. D. J. Mater. Chem. A 2017, 5, 3311–3319. doi:10.1039/c6ta09607g |
| 3. | Bruce, M. I.; Rodgers, J. R.; Snow, M. R.; Swincer, A. G. J. Chem. Soc., Chem. Commun. 1981, 271–272. doi:10.1039/c39810000271 |
| 117. | Neethu, K. M.; Nag, K.; Dar, A. H.; Bajaj, A.; Gopal, S. A.; Gowri, V.; Nagpure, M.; Sartaliya, S.; Sharma, R.; Solanki, A. K.; Ehesan Ali, M.; Muthukrishnan, A.; Jayamurugan, G. Org. Biomol. Chem. 2023, 21, 2922–2929. doi:10.1039/d3ob00053b |
| 26. | Raheem, A. A.; Murugan, P.; Shanmugam, R.; Praveen, C. ChemPlusChem 2021, 86, 1451–1460. doi:10.1002/cplu.202100392 |
| 172. | Patil, Y.; Misra, R.; Keshtov, M. L.; Sharma, G. D. J. Phys. Chem. C 2016, 120, 6324–6335. doi:10.1021/acs.jpcc.5b12307 |
| 173. | Patil, Y.; Misra, R.; Keshtov, M. L.; Sharma, G. D. J. Mater. Chem. A 2017, 5, 3311–3319. doi:10.1039/c6ta09607g |
| 174. | Gautam, P.; Sharma, R.; Misra, R.; Keshtov, M. L.; Kuklin, S. A.; Sharma, G. D. Chem. Sci. 2017, 8, 2017–2024. doi:10.1039/c6sc04461a |
| 175. | Patil, Y.; Misra, R.; Singhal, R.; Sharma, G. D. J. Mater. Chem. A 2017, 5, 13625–13633. doi:10.1039/c7ta03322b |
| 176. | Raheem, A. A.; Kumar, C.; Murugan, P.; Praveen, C. ACS Appl. Energy Mater. 2021, 4, 11609–11623. doi:10.1021/acsaem.1c02353 |
| 12. | Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Diederich, F. Chem. Commun. 2007, 4731–4733. doi:10.1039/b713683h |
| 13. | Bruce, M. I.; Burgun, A.; Grelaud, G.; Lapinte, C.; Parker, C. R.; Roisnel, T.; Skelton, B. W.; Zaitseva, N. N. Organometallics 2012, 31, 6623–6634. doi:10.1021/om3006728 |
| 112. | Yamada, M.; Schweizer, W. B.; Schoenebeck, F.; Diederich, F. Chem. Commun. 2010, 46, 5334–5336. doi:10.1039/c0cc00881h |
| 11. | Kivala, M.; Stanoeva, T.; Michinobu, T.; Frank, B.; Gescheidt, G.; Diederich, F. Chem. – Eur. J. 2008, 14, 7638–7647. doi:10.1002/chem.200800716 |
| 113. | Reisinger, C. M.; Rivera‐Fuentes, P.; Lampart, S.; Schweizer, W. B.; Diederich, F. Chem. – Eur. J. 2011, 17, 12906–12911. doi:10.1002/chem.201102852 |
| 114. | Kerisit, N.; Finke, A. D.; Trapp, N.; Leroux, Y. R.; Guillemin, J.-C.; Trolez, Y.; Diederich, F. Tetrahedron 2015, 71, 4393–4399. doi:10.1016/j.tet.2015.03.081 |
| 115. | Donckele, E. J.; Finke, A. D.; Ruhlmann, L.; Boudon, C.; Trapp, N.; Diederich, F. Org. Lett. 2015, 17, 3506–3509. doi:10.1021/acs.orglett.5b01598 |
| 116. | Shoji, T.; Nagai, D.; Tanaka, M.; Araki, T.; Ohta, A.; Sekiguchi, R.; Ito, S.; Mori, S.; Okujima, T. Chem. – Eur. J. 2017, 23, 5126–5136. doi:10.1002/chem.201700121 |
| 7. | Michinobu, T.; Diederich, F. Angew. Chem., Int. Ed. 2018, 57, 3552–3577. doi:10.1002/anie.201711605 |
| 110. | Chiu, M.; Tchitchanov, B. H.; Zimmerli, D.; Sanhueza, I. A.; Schoenebeck, F.; Trapp, N.; Schweizer, W. B.; Diederich, F. Angew. Chem., Int. Ed. 2015, 54, 349–354. doi:10.1002/anie.201409289 |
| 9. | Michinobu, T. Pure Appl. Chem. 2010, 82, 1001–1009. doi:10.1351/pac-con-09-09-09 |
| 10. | Michinobu, T. Chem. Soc. Rev. 2011, 40, 2306–2316. doi:10.1039/c0cs00205d |
| 111. | Schaub, T. A.; Margraf, J. T.; Zakharov, L.; Reuter, K.; Jasti, R. Angew. Chem., Int. Ed. 2018, 57, 16348–16353. doi:10.1002/anie.201808611 |
| 121. | Zhou, W.; Xu, J.; Zheng, H.; Yin, X.; Zuo, Z.; Liu, H.; Li, Y. Adv. Funct. Mater. 2009, 19, 141–149. doi:10.1002/adfm.200801149 |
| 122. | Li, Y.; Liu, T.; Liu, H.; Tian, M.-Z.; Li, Y. Acc. Chem. Res. 2014, 47, 1186–1198. doi:10.1021/ar400264e |
| 123. | Trolez, Y.; Finke, A. D.; Silvestri, F.; Monti, F.; Ventura, B.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Sauvage, J.-P.; Armaroli, N.; Diederich, F. Chem. – Eur. J. 2018, 24, 10422–10433. doi:10.1002/chem.201801161 |
| 119. | Mateo, L. M.; Sagresti, L.; Luo, Y.; Guldi, D. M.; Torres, T.; Brancato, G.; Bottari, G. Chem. – Eur. J. 2021, 27, 16049–16055. doi:10.1002/chem.202103079 |
| 120. | Zhou, W.; Xu, J.; Zheng, H.; Liu, H.; Li, Y.; Zhu, D. J. Org. Chem. 2008, 73, 7702–7709. doi:10.1021/jo8014566 |
| 130. | Yamada, M.; Rivera‐Fuentes, P.; Schweizer, W. B.; Diederich, F. Angew. Chem., Int. Ed. 2010, 49, 3532–3535. doi:10.1002/anie.200906853 |
| 131. | Winterfeld, K. A.; Lavarda, G.; Guilleme, J.; Sekita, M.; Guldi, D. M.; Torres, T.; Bottari, G. J. Am. Chem. Soc. 2017, 139, 5520–5529. doi:10.1021/jacs.7b01460 |
| 128. | Ozcelik, A.; Pereira‐Cameselle, R.; Peña‐Gallego, Á.; Alonso‐Gómez, J. L. Eur. J. Org. Chem. 2022, e202101333. doi:10.1002/ejoc.202101333 |
| 129. | Bouvier, R.; Durand, R.; Favereau, L.; Srebro‐Hooper, M.; Dorcet, V.; Roisnel, T.; Vanthuyne, N.; Vesga, Y.; Donnelly, J.; Hernandez, F.; Autschbach, J.; Trolez, Y.; Crassous, J. Chem. – Eur. J. 2018, 24, 14484–14494. doi:10.1002/chem.201802763 |
| 124. | Alonso‐Gómez, J. L.; Schanen, P.; Rivera‐Fuentes, P.; Seiler, P.; Diederich, F. Chem. – Eur. J. 2008, 14, 10564–10568. doi:10.1002/chem.200801456 |
| 125. | Alonso‐Gómez, J. L.; Petrovic, A. G.; Harada, N.; Rivera‐Fuentes, P.; Berova, N.; Diederich, F. Chem. – Eur. J. 2009, 15, 8396–8400. doi:10.1002/chem.200900103 |
| 126. | Frank, B. B.; Camafort Blanco, B.; Jakob, S.; Ferroni, F.; Pieraccini, S.; Ferrarini, A.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Spada, G. P.; Diederich, F. Chem. – Eur. J. 2009, 15, 9005–9016. doi:10.1002/chem.200900913 |
| 127. | Frank, B. B.; Kivala, M.; Camafort Blanco, B.; Breiten, B.; Schweizer, W. B.; Laporta, P. R.; Biaggio, I.; Jahnke, E.; Tykwinski, R. R.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Eur. J. Org. Chem. 2010, 2487–2503. doi:10.1002/ejoc.201000030 |
| 73. | Erden, K.; Dengiz, C. J. Org. Chem. 2022, 87, 4385–4399. doi:10.1021/acs.joc.2c00067 |
| 74. | Philippe, C.; Melan, J.; Barsella, A.; Vives, T.; Leroux, Y. R.; Robin-Le Guen, F.; Lemiègre, L.; Jacquemin, D.; Gauthier, S.; Trolez, Y. Tetrahedron Chem 2023, 5, 100036. doi:10.1016/j.tchem.2023.100036 |
| 75. | Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Enko, B.; Seiler, P.; Müller, I. B.; Langer, N.; Jarowski, P. D.; Gescheidt, G.; Diederich, F. Chem. – Eur. J. 2009, 15, 4111–4123. doi:10.1002/chem.200802563 |
| 86. | Lacy, A. R.; Vogt, A.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Diederich, F. Eur. J. Org. Chem. 2013, 869–879. doi:10.1002/ejoc.201201371 |
| 87. | Breiten, B.; Wu, Y.-L.; Jarowski, P. D.; Gisselbrecht, J.-P.; Boudon, C.; Griesser, M.; Onitsch, C.; Gescheidt, G.; Schweizer, W. B.; Langer, N.; Lennartz, C.; Diederich, F. Chem. Sci. 2011, 2, 88–93. doi:10.1039/c0sc00387e |
| 88. | Breiten, B.; Jordan, M.; Taura, D.; Zalibera, M.; Griesser, M.; Confortin, D.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Gescheidt, G.; Diederich, F. J. Org. Chem. 2013, 78, 1760–1767. doi:10.1021/jo301194y |
| 83. | Finke, A. D.; Dumele, O.; Zalibera, M.; Confortin, D.; Cias, P.; Jayamurugan, G.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Gescheidt, G.; Diederich, F. J. Am. Chem. Soc. 2012, 134, 18139–18146. doi:10.1021/ja309141r |
| 84. | Finke, A. D.; Diederich, F. Chem. Rec. 2015, 15, 19–30. doi:10.1002/tcr.201402060 |
| 85. | Fesser, P.; Iacovita, C.; Wäckerlin, C.; Vijayaraghavan, S.; Ballav, N.; Howes, K.; Gisselbrecht, J.-P.; Crobu, M.; Boudon, C.; Stöhr, M.; Jung, T. A.; Diederich, F. Chem. – Eur. J. 2011, 17, 5246–5250. doi:10.1002/chem.201100733 |
| 81. | Galán, E.; Andreu, R.; Garín, J.; Orduna, J.; Villacampa, B.; Diosdado, B. E. Org. Biomol. Chem. 2012, 10, 8684–8691. doi:10.1039/c2ob26515j |
| 82. | Chiu, M.; Jaun, B.; Beels, M. T. R.; Biaggio, I.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Kivala, M.; Diederich, F. Org. Lett. 2012, 14, 54–57. doi:10.1021/ol202815q |
| 76. | Jarowski, P. D.; Wu, Y.-L.; Boudon, C.; Gisselbrecht, J.-P.; Gross, M.; Schweizer, W. B.; Diederich, F. Org. Biomol. Chem. 2009, 7, 1312–1322. doi:10.1039/b821230a |
| 77. | Wu, Y.-L.; Jarowski, P. D.; Schweizer, W. B.; Diederich, F. Chem. – Eur. J. 2010, 16, 202–211. doi:10.1002/chem.200902645 |
| 78. | Rijkers, D. T. S.; de Prada López, F.; Liskamp, R. M. J.; Diederich, F. Tetrahedron Lett. 2011, 52, 6963–6967. doi:10.1016/j.tetlet.2011.10.084 |
| 79. | Silvestri, F.; Jordan, M.; Howes, K.; Kivala, M.; Rivera‐Fuentes, P.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Seiler, P.; Chiu, M.; Diederich, F. Chem. – Eur. J. 2011, 17, 6088–6097. doi:10.1002/chem.201003672 |
| 80. | Tancini, F.; Wu, Y.-L.; Schweizer, W. B.; Gisselbrecht, J.-P.; Boudon, C.; Jarowski, P. D.; Beels, M. T.; Biaggio, I.; Diederich, F. Eur. J. Org. Chem. 2012, 2756–2765. doi:10.1002/ejoc.201200111 |
| 79. | Silvestri, F.; Jordan, M.; Howes, K.; Kivala, M.; Rivera‐Fuentes, P.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Seiler, P.; Chiu, M.; Diederich, F. Chem. – Eur. J. 2011, 17, 6088–6097. doi:10.1002/chem.201003672 |
| 80. | Tancini, F.; Wu, Y.-L.; Schweizer, W. B.; Gisselbrecht, J.-P.; Boudon, C.; Jarowski, P. D.; Beels, M. T.; Biaggio, I.; Diederich, F. Eur. J. Org. Chem. 2012, 2756–2765. doi:10.1002/ejoc.201200111 |
| 89. | Kato, S.-i.; Beels, M. T. R.; La Porta, P.; Schweizer, W. B.; Boudon, C.; Gisselbrecht, J.-P.; Biaggio, I.; Diederich, F. Angew. Chem., Int. Ed. 2010, 49, 6207–6211. doi:10.1002/anie.201002236 |
| 90. | Dengiz, C.; Dumele, O.; Kato, S.-i.; Zalibera, M.; Cias, P.; Schweizer, W. B.; Boudon, C.; Gisselbrecht, J.-P.; Gescheidt, G.; Diederich, F. Chem. – Eur. J. 2014, 20, 1279–1286. doi:10.1002/chem.201303533 |
| 91. | Shoji, T.; Higashi, J.; Ito, S.; Yasunami, M.; Moria, N. Heterocycles 2011, 83, 2271–2274. doi:10.3987/com-11-12303 |
| 92. | Shoji, T.; Maruyama, M.; Shimomura, E.; Maruyama, A.; Ito, S.; Yasunami, M.; Higashi, J.; Toyota, K.; Morita, N. Heterocycles 2014, 88, 319–329. doi:10.3987/com-13-s(s)24 |
| 93. | Dengiz, C.; Prange, C.; Gawel, P.; Trapp, N.; Ruhlmann, L.; Boudon, C.; Diederich, F. Tetrahedron 2016, 72, 1213–1224. doi:10.1016/j.tet.2016.01.017 |
| 94. | Erden, K.; Savaş, İ.; Dengiz, C. Tetrahedron Lett. 2019, 60, 1982–1985. doi:10.1016/j.tetlet.2019.06.046 |
| 95. | Reekie, T. A.; Donckele, E. J.; Ruhlmann, L.; Boudon, C.; Trapp, N.; Diederich, F. Eur. J. Org. Chem. 2015, 7264–7275. doi:10.1002/ejoc.201501085 |
| 96. | Januszewski, J. A.; Hampel, F.; Neiss, C.; Görling, A.; Tykwinski, R. R. Angew. Chem., Int. Ed. 2014, 53, 3743–3747. doi:10.1002/anie.201309355 |
| 97. | Gawel, P.; Dengiz, C.; Finke, A. D.; Trapp, N.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Angew. Chem., Int. Ed. 2014, 53, 4341–4345. doi:10.1002/anie.201402299 |
| 98. | Wu, Y.-L.; Tancini, F.; Schweizer, W. B.; Paunescu, D.; Boudon, C.; Gisselbrecht, J.-P.; Jarowski, P. D.; Dalcanale, E.; Diederich, F. Chem. – Asian J. 2012, 7, 1185–1190. doi:10.1002/asia.201100997 |
| 99. | Gawel, P.; Wu, Y.-L.; Finke, A. D.; Trapp, N.; Zalibera, M.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Gescheidt, G.; Diederich, F. Chem. – Eur. J. 2015, 21, 6215–6225. doi:10.1002/chem.201406583 |
| 101. | Jayamurugan, G.; Finke, A. D.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Diederich, F. J. Org. Chem. 2014, 79, 426–431. doi:10.1021/jo402440m |
| 77. | Wu, Y.-L.; Jarowski, P. D.; Schweizer, W. B.; Diederich, F. Chem. – Eur. J. 2010, 16, 202–211. doi:10.1002/chem.200902645 |
| 102. | Jayamurugan, G.; Gisselbrecht, J.-P.; Boudon, C.; Schoenebeck, F.; Schweizer, W. B.; Bernet, B.; Diederich, F. Chem. Commun. 2011, 47, 4520–4522. doi:10.1039/c1cc10247h |
| 103. | Jayamurugan, G.; Dumele, O.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Bernet, B.; Diederich, F. J. Am. Chem. Soc. 2013, 135, 3599–3606. doi:10.1021/ja312084s |
| 83. | Finke, A. D.; Dumele, O.; Zalibera, M.; Confortin, D.; Cias, P.; Jayamurugan, G.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Gescheidt, G.; Diederich, F. J. Am. Chem. Soc. 2012, 134, 18139–18146. doi:10.1021/ja309141r |
| 84. | Finke, A. D.; Diederich, F. Chem. Rec. 2015, 15, 19–30. doi:10.1002/tcr.201402060 |
| 101. | Jayamurugan, G.; Finke, A. D.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Diederich, F. J. Org. Chem. 2014, 79, 426–431. doi:10.1021/jo402440m |
| 7. | Michinobu, T.; Diederich, F. Angew. Chem., Int. Ed. 2018, 57, 3552–3577. doi:10.1002/anie.201711605 |
| 100. | Dar, A. H.; Gowri, V.; Neethu, K. M.; Jayamurugan, G. ChemistrySelect 2020, 5, 12437–12441. doi:10.1002/slct.202003179 |
| 55. | Shoji, T.; Ito, S. Chem. – Eur. J. 2017, 23, 16696–16709. doi:10.1002/chem.201702806 |
| 63. | Pengxia, L.; Du, Z.; Wang, D.; Yang, Z.; Sheng, H.; Liang, S.; Cao, H.; He, W.; Yang, H. ChemPhysChem 2014, 15, 3523–3529. doi:10.1002/cphc.201402401 |
| 152. | Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Diederich, F. Angew. Chem., Int. Ed. 2007, 46, 6357–6360. doi:10.1002/anie.200701733 |
| 153. | Wu, Y.-L.; Stuparu, M. C.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Baldridge, K. K.; Siegel, J. S.; Diederich, F. J. Org. Chem. 2012, 77, 11014–11026. doi:10.1021/jo302217n |
| 154. | Dengiz, C.; Breiten, B.; Gisselbrecht, J.-P.; Boudon, C.; Trapp, N.; Schweizer, W. B.; Diederich, F. J. Org. Chem. 2015, 80, 882–896. doi:10.1021/jo502367h |
| 155. | Sharma, R.; Maragani, R.; Misra, R. New J. Chem. 2018, 42, 882–890. doi:10.1039/c7nj03934d |
| 156. | Cheng, S.; Ma, X.; He, Y.; He, J.; Zeller, M.; Xu, Z. Cryst. Growth Des. 2019, 19, 7411–7419. doi:10.1021/acs.cgd.9b01488 |
| 1. | Bureš, F. RSC Adv. 2014, 4, 58826–58851. doi:10.1039/c4ra11264d |
| 151. | Poddar, M.; Jang, Y.; Misra, R.; D'Souza, F. Chem. – Eur. J. 2020, 26, 6869–6878. doi:10.1002/chem.202000346 |
| 64. | Yadav, I.; Sharma, J. K.; Sankar, M.; D'Souza, F. Chem. – Eur. J. 2023, 29, e202301806. doi:10.1002/chem.202301806 |
| 149. | Jayamurugan, G.; Gowri, V.; Hernández, D.; Martin, S.; González‐Orive, A.; Dengiz, C.; Dumele, O.; Pérez‐Murano, F.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Breiten, B.; Finke, A. D.; Jeschke, G.; Bernet, B.; Ruhlmann, L.; Cea, P.; Diederich, F. Chem. – Eur. J. 2016, 22, 10539–10547. doi:10.1002/chem.201505216 |
| 150. | Sekaran, B.; Dawson, A.; Jang, Y.; MohanSingh, K. V.; Misra, R.; D'Souza, F. Chem. – Eur. J. 2021, 27, 14335–14344. doi:10.1002/chem.202102865 |
| 147. | Urner, L. M.; Sekita, M.; Trapp, N.; Schweizer, W. B.; Wörle, M.; Gisselbrecht, J.-P.; Boudon, C.; Guldi, D. M.; Diederich, F. Eur. J. Org. Chem. 2015, 91–108. doi:10.1002/ejoc.201403252 |
| 148. | Reekie, T. A.; Sekita, M.; Urner, L. M.; Bauroth, S.; Ruhlmann, L.; Gisselbrecht, J.-P.; Boudon, C.; Trapp, N.; Clark, T.; Guldi, D. M.; Diederich, F. Chem. – Eur. J. 2017, 23, 6357–6369. doi:10.1002/chem.201700043 |
| 20. | Kato, S.-i.; Noguchi, H.; Jin, S.; Nakamura, Y. Asian J. Org. Chem. 2016, 5, 246–256. doi:10.1002/ajoc.201500431 |
| 21. | Okuno, T.; Iwahashi, H. Acta Crystallogr., Sect. E: Struct. Rep. Online 2013, 69, o665. doi:10.1107/s1600536813008799 |
| 22. | Yadav, I. S.; Misra, R. New J. Chem. 2022, 46, 15999–16006. doi:10.1039/d2nj03089f |
| 17. | Reutenauer, P.; Kivala, M.; Jarowski, P. D.; Boudon, C.; Gisselbrecht, J.-P.; Gross, M.; Diederich, F. Chem. Commun. 2007, 4898–4900. doi:10.1039/b714731g |
| 18. | Dar, A. H.; Gowri, V.; Gopal, A.; Muthukrishnan, A.; Bajaj, A.; Sartaliya, S.; Selim, A.; Ali, M. E.; Jayamurugan, G. J. Org. Chem. 2019, 84, 8941–8947. doi:10.1021/acs.joc.9b00841 |
| 19. | Gowri, V.; Jalwal, S.; Dar, A. H.; Gopal, A.; Muthukrishnan, A.; Bajaj, A.; Ali, M. E.; Jayamurugan, G. J. Photochem. Photobiol., A 2021, 410, 113163. doi:10.1016/j.jphotochem.2021.113163 |
| 4. | Kivala, M.; Diederich, F. Acc. Chem. Res. 2009, 42, 235–248. doi:10.1021/ar8001238 |
| 5. | Kato, S.-i.; Diederich, F. Chem. Commun. 2010, 46, 1994–2006. doi:10.1039/b926601a |
| 7. | Michinobu, T.; Diederich, F. Angew. Chem., Int. Ed. 2018, 57, 3552–3577. doi:10.1002/anie.201711605 |
| 16. | Michinobu, T.; May, J. C.; Lim, J. H.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Biaggio, I.; Diederich, F. Chem. Commun. 2005, 737–739. doi:10.1039/b417393g |
| 61. | Ulrich, G.; Barsella, A.; Boeglin, A.; Niu, S.; Ziessel, R. ChemPhysChem 2014, 15, 2693–2700. doi:10.1002/cphc.201402123 |
| 82. | Chiu, M.; Jaun, B.; Beels, M. T. R.; Biaggio, I.; Gisselbrecht, J.-P.; Boudon, C.; Schweizer, W. B.; Kivala, M.; Diederich, F. Org. Lett. 2012, 14, 54–57. doi:10.1021/ol202815q |
| 89. | Kato, S.-i.; Beels, M. T. R.; La Porta, P.; Schweizer, W. B.; Boudon, C.; Gisselbrecht, J.-P.; Biaggio, I.; Diederich, F. Angew. Chem., Int. Ed. 2010, 49, 6207–6211. doi:10.1002/anie.201002236 |
| 135. | Pokladek, Z.; Ripoche, N.; Betou, M.; Trolez, Y.; Mongin, O.; Olesiak‐Banska, J.; Matczyszyn, K.; Samoc, M.; Humphrey, M. G.; Blanchard‐Desce, M.; Paul, F. Chem. – Eur. J. 2016, 22, 10155–10167. doi:10.1002/chem.201600897 |
| 159. | Frank, B. B.; Laporta, P. R.; Breiten, B.; Kuzyk, M. C.; Jarowski, P. D.; Schweizer, W. B.; Seiler, P.; Biaggio, I.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Eur. J. Org. Chem. 2011, 4307–4317. doi:10.1002/ejoc.201100378 |
| 160. | Štefko, M.; Tzirakis, M. D.; Breiten, B.; Ebert, M.-O.; Dumele, O.; Schweizer, W. B.; Gisselbrecht, J.-P.; Boudon, C.; Beels, M. T.; Biaggio, I.; Diederich, F. Chem. – Eur. J. 2013, 19, 12693–12704. doi:10.1002/chem.201301642 |
| 161. | Han, P.; Yang, Z.; Cao, H.; He, W.; Wang, D.; Zhang, J.; Xing, Y.; Gao, H. Tetrahedron 2017, 73, 6210–6216. doi:10.1016/j.tet.2017.09.020 |
| 162. | Reekie, T. A.; Gisselbrecht, J.-P.; Boudon, C.; Trapp, N.; Diederich, F. Tetrahedron Lett. 2017, 58, 2414–2416. doi:10.1016/j.tetlet.2017.05.009 |
| 163. | Mammadova, F.; Inyurt, F. C.; Barsella, A.; Dengiz, C. Dyes Pigm. 2023, 209, 110894. doi:10.1016/j.dyepig.2022.110894 |
| 15. | Michinobu, T.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Frank, B.; Moonen, N. N. P.; Gross, M.; Diederich, F. Chem. – Eur. J. 2006, 12, 1889–1905. doi:10.1002/chem.200501113 |
| 16. | Michinobu, T.; May, J. C.; Lim, J. H.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Biaggio, I.; Diederich, F. Chem. Commun. 2005, 737–739. doi:10.1039/b417393g |
| 157. | Chen, S.; Li, Y.; Liu, C.; Yang, W.; Li, Y. Eur. J. Org. Chem. 2011, 6445–6451. doi:10.1002/ejoc.201101009 |
| 14. | García, R.; Calbo, J.; Viruela, R.; Herranz, M. Á.; Ortí, E.; Martín, N. ChemPlusChem 2018, 83, 300–307. doi:10.1002/cplu.201700553 |
| 158. | Tykwinski, R. R.; Gubler, U.; Martin, R. E.; Diederich, F.; Bosshard, C.; Günter, P. J. Phys. Chem. B 1998, 102, 4451–4465. doi:10.1021/jp980829o |
| 28. | García, R.; Herranz, M. Á.; Torres, M. R.; Bouit, P.-A.; Delgado, J. L.; Calbo, J.; Viruela, P. M.; Ortí, E.; Martín, N. J. Org. Chem. 2012, 77, 10707–10717. doi:10.1021/jo302047m |
| 23. | Pappenfus, T. M.; Schneiderman, D. K.; Casado, J.; López Navarrete, J. T.; Ruiz Delgado, M. C.; Zotti, G.; Vercelli, B.; Lovander, M. D.; Hinkle, L. M.; Bohnsack, J. N.; Mann, K. R. Chem. Mater. 2011, 23, 823–831. doi:10.1021/cm102128g |
| 24. | Simón Marqués, P.; Castán, J. M. A.; Raul, B. A. L.; Londi, G.; Ramirez, I.; Pshenichnikov, M. S.; Beljonne, D.; Walzer, K.; Blais, M.; Allain, M.; Cabanetos, C.; Blanchard, P. Chem. – Eur. J. 2020, 26, 16422–16433. doi:10.1002/chem.202002810 |
| 25. | Raheem, A. A.; Kumar, C.; Shanmugam, R.; Murugan, P.; Praveen, C. J. Mater. Chem. C 2021, 9, 4562–4575. doi:10.1039/d1tc00708d |
| 26. | Raheem, A. A.; Murugan, P.; Shanmugam, R.; Praveen, C. ChemPlusChem 2021, 86, 1451–1460. doi:10.1002/cplu.202100392 |
| 27. | Kato, S.-i.; Kivala, M.; Schweizer, W. B.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Chem. – Eur. J. 2009, 15, 8687–8691. doi:10.1002/chem.200901630 |
| 69. | Betou, M.; Kerisit, N.; Meledje, E.; Leroux, Y. R.; Katan, C.; Halet, J.-F.; Guillemin, J.-C.; Trolez, Y. Chem. – Eur. J. 2014, 20, 9553–9557. doi:10.1002/chem.201402653 |
| 70. | Betou, M.; Durand, R. J.; Sallustrau, D. A.; Gousset, C.; Le Coz, E.; Leroux, Y. R.; Toupet, D. L.; Trzop, E.; Roisnel, T.; Trolez, Y. Chem. – Asian J. 2017, 12, 1338–1346. doi:10.1002/asia.201700353 |
| 71. | Philippe, C.; Bui, A. T.; Batsongo-Boulingui, S.; Pokladek, Z.; Matczyszyn, K.; Mongin, O.; Lemiègre, L.; Paul, F.; Hamlin, T. A.; Trolez, Y. Org. Lett. 2021, 23, 2007–2012. doi:10.1021/acs.orglett.1c00136 |
| 72. | Huang, S.; Ma, J.; Yi, Y.; Li, M.; Cai, P.; Wu, N. Org. Biomol. Chem. 2022, 20, 4081–4085. doi:10.1039/d2ob00677d |
| 65. | Sasaki, S.-i.; Mizutani, K.; Kunieda, M.; Tamiaki, H. Tetrahedron 2013, 69, 9772–9778. doi:10.1016/j.tet.2013.09.007 |
| 66. | Tamiaki, H.; Mizutani, K.; Sasaki, S.-i.; Tatebe, T. Tetrahedron 2016, 72, 6626–6633. doi:10.1016/j.tet.2016.08.079 |
| 67. | Perrin, F. G.; Kiefer, G.; Jeanbourquin, L.; Racine, S.; Perrotta, D.; Waser, J.; Scopelliti, R.; Severin, K. Angew. Chem., Int. Ed. 2015, 54, 13393–13396. doi:10.1002/anie.201507033 |
| 68. | Mammadova, F.; Ozsinan, S.; Okutan, M.; Dengiz, C. J. Mol. Struct. 2020, 1220, 128726. doi:10.1016/j.molstruc.2020.128726 |
| 41. | Dhokale, B.; Jadhav, T.; Mobin, S. M.; Misra, R. Dalton Trans. 2016, 45, 1476–1483. doi:10.1039/c5dt04037j |
| 59. | Niu, S.; Ulrich, G.; Retailleau, P.; Ziessel, R. Org. Lett. 2011, 13, 4996–4999. doi:10.1021/ol201600s |
| 60. | Niu, S.; Ulrich, G.; Retailleau, P.; Ziessel, R. Tetrahedron Lett. 2011, 52, 4848–4853. doi:10.1016/j.tetlet.2011.07.028 |
| 61. | Ulrich, G.; Barsella, A.; Boeglin, A.; Niu, S.; Ziessel, R. ChemPhysChem 2014, 15, 2693–2700. doi:10.1002/cphc.201402123 |
| 62. | Koszelewski, D.; Nowak‐Król, A.; Gryko, D. T. Chem. – Asian J. 2012, 7, 1887–1894. doi:10.1002/asia.201200179 |
| 63. | Pengxia, L.; Du, Z.; Wang, D.; Yang, Z.; Sheng, H.; Liang, S.; Cao, H.; He, W.; Yang, H. ChemPhysChem 2014, 15, 3523–3529. doi:10.1002/cphc.201402401 |
| 64. | Yadav, I.; Sharma, J. K.; Sankar, M.; D'Souza, F. Chem. – Eur. J. 2023, 29, e202301806. doi:10.1002/chem.202301806 |
| 27. | Kato, S.-i.; Kivala, M.; Schweizer, W. B.; Boudon, C.; Gisselbrecht, J.-P.; Diederich, F. Chem. – Eur. J. 2009, 15, 8687–8691. doi:10.1002/chem.200901630 |
| 29. | Mochida, T.; Yamazaki, S. J. Chem. Soc., Dalton Trans. 2002, 3559–3564. doi:10.1039/b204168e |
| 30. | Jordan, M.; Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Schweizer, W. B.; Seiler, P.; Diederich, F. Chem. – Asian J. 2011, 6, 396–401. doi:10.1002/asia.201000539 |
| 31. | Shoji, T.; Ito, S.; Okujima, T.; Morita, N. Eur. J. Org. Chem. 2011, 5134–5140. doi:10.1002/ejoc.201100650 |
| 32. | Shoji, T.; Ito, S.; Okujima, T.; Morita, N. Chem. – Eur. J. 2013, 19, 5721–5730. doi:10.1002/chem.201202257 |
| 33. | Misra, R.; Sharma, R.; Mobin, S. M. Dalton Trans. 2014, 43, 6891–6896. doi:10.1039/c4dt00210e |
| 34. | Gautam, P.; Maragani, R.; Misra, R. Tetrahedron Lett. 2014, 55, 6827–6830. doi:10.1016/j.tetlet.2014.10.094 |
| 35. | Maragani, R.; Misra, R. Tetrahedron 2014, 70, 3390–3399. doi:10.1016/j.tet.2014.03.096 |
| 36. | Shoji, T.; Maruyama, A.; Yaku, C.; Kamata, N.; Ito, S.; Okujima, T.; Toyota, K. Chem. – Eur. J. 2015, 21, 402–409. doi:10.1002/chem.201405004 |
| 37. | Shoji, T.; Kamata, N.; Maruyama, A.; Ito, S.; Okujima, T. Bull. Chem. Soc. Jpn. 2015, 88, 1338–1346. doi:10.1246/bcsj.20150165 |
| 38. | Misra, R.; Gautam, P.; Maragani, R. Tetrahedron Lett. 2015, 56, 1664–1666. doi:10.1016/j.tetlet.2015.02.031 |
| 39. | Dhokale, B.; Jadhav, T.; Mobin, S. M.; Misra, R. RSC Adv. 2015, 5, 57692–57699. doi:10.1039/c5ra11433k |
| 40. | Krauße, N.; Kielmann, M.; Ma, J.; Butenschön, H. Eur. J. Org. Chem. 2015, 2622–2631. doi:10.1002/ejoc.201500105 |
| 41. | Dhokale, B.; Jadhav, T.; Mobin, S. M.; Misra, R. Dalton Trans. 2016, 45, 1476–1483. doi:10.1039/c5dt04037j |
| 42. | Patil, Y.; Jadhav, T.; Dhokale, B.; Misra, R. Eur. J. Org. Chem. 2016, 733–738. doi:10.1002/ejoc.201501123 |
| 43. | Patil, Y.; Misra, R. J. Organomet. Chem. 2017, 840, 23–29. doi:10.1016/j.jorganchem.2017.03.048 |
| 44. | Patil, Y.; Popli, C.; Misra, R. New J. Chem. 2018, 42, 3892–3899. doi:10.1039/c7nj05162j |
| 45. | Rout, Y.; Mobin, S. M.; Misra, R. New J. Chem. 2019, 43, 12299–12307. doi:10.1039/c9nj01887e |
| 46. | Rout, Y.; Misra, R. New J. Chem. 2021, 45, 9838–9845. doi:10.1039/d1nj00919b |
| 32. | Shoji, T.; Ito, S.; Okujima, T.; Morita, N. Chem. – Eur. J. 2013, 19, 5721–5730. doi:10.1002/chem.201202257 |
| 47. | Shoji, T.; Ito, S.; Toyota, K.; Yasunami, M.; Morita, N. Chem. – Eur. J. 2008, 14, 8398–8408. doi:10.1002/chem.200701981 |
| 48. | Shoji, T.; Ito, S.; Toyota, K.; Iwamoto, T.; Yasunami, M.; Morita, N. Eur. J. Org. Chem. 2009, 4316–4324. doi:10.1002/ejoc.200900539 |
| 49. | Shoji, T.; Maruyama, M.; Ito, S.; Morita, N. Bull. Chem. Soc. Jpn. 2012, 85, 761–773. doi:10.1246/bcsj.20120037 |
| 50. | Shoji, T.; Maruyama, M.; Shimomura, E.; Maruyama, A.; Ito, S.; Okujima, T.; Toyota, K.; Morita, N. J. Org. Chem. 2013, 78, 12513–12524. doi:10.1021/jo402104n |
| 51. | Shoji, T.; Shimomura, E.; Maruyama, M.; Maruyama, A.; Ito, S.; Okujima, T.; Toyota, K.; Morita, N. Eur. J. Org. Chem. 2013, 7785–7799. doi:10.1002/ejoc.201301006 |
| 52. | Shoji, T.; Maruyama, M.; Maruyama, A.; Ito, S.; Okujima, T.; Toyota, K. Chem. – Eur. J. 2014, 20, 11903–11912. doi:10.1002/chem.201402939 |
| 53. | Shoji, T.; Maruyama, A.; Shimomura, E.; Nagai, D.; Ito, S.; Okujima, T.; Toyota, K. Eur. J. Org. Chem. 2015, 1979–1990. doi:10.1002/ejoc.201403556 |
| 54. | Shoji, T.; Maruyama, A.; Tanaka, M.; Nagai, D.; Shimomura, E.; Fujimori, K.; Ito, S.; Okujima, T.; Toyota, K.; Yasunami, M. ChemistrySelect 2016, 1, 49–57. doi:10.1002/slct.201500036 |
| 55. | Shoji, T.; Ito, S. Chem. – Eur. J. 2017, 23, 16696–16709. doi:10.1002/chem.201702806 |
| 56. | Shoji, T.; Miura, K.; Araki, T.; Maruyama, A.; Ohta, A.; Sekiguchi, R.; Ito, S.; Okujima, T. J. Org. Chem. 2018, 83, 6690–6705. doi:10.1021/acs.joc.8b01067 |
| 57. | Shoji, T.; Higashi, J.; Ito, S.; Okujima, T.; Yasunami, M.; Morita, N. Chem. – Eur. J. 2011, 17, 5116–5129. doi:10.1002/chem.201003628 |
| 58. | Shoji, T.; Higashi, J.; Ito, S.; Okujima, T.; Yasunami, M.; Morita, N. Org. Biomol. Chem. 2012, 10, 2431–2438. doi:10.1039/c2ob06931h |
© 2024 Yamada; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.