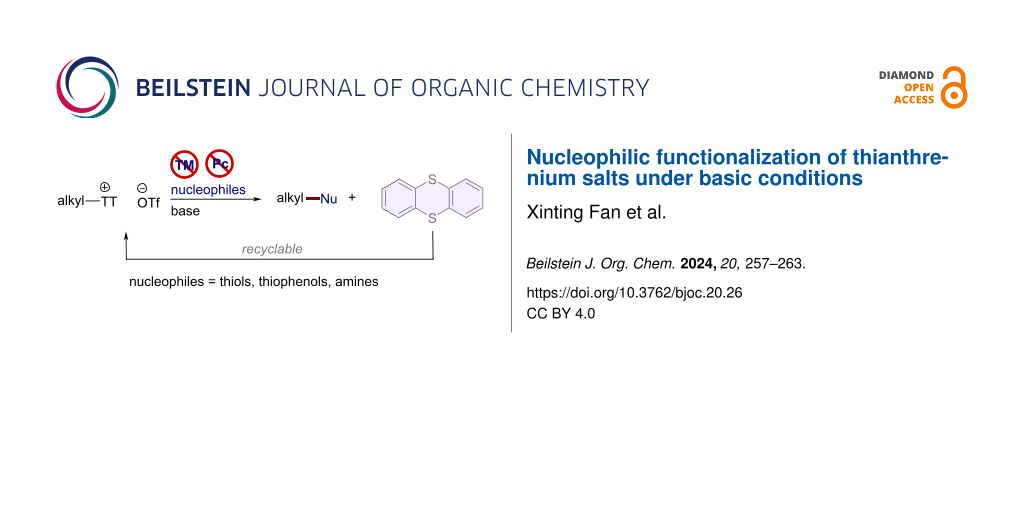
Abstract
In recent years, S-(alkyl)thianthrenium salts have become an important means of functionalizing alcohol compounds. However, additional transition metal catalysts and/or visible light are required. Herein, a direct thioetherification/amination reaction of thianthrenium salts is realized under metal-free conditions. This strategy exhibits good functional-group tolerance, operational simplicity, and an extensive range of compatible substrates.
Graphical Abstract
Introduction
Sulfonium salts [1-10] have been extensively utilized as readily accessible synthetic building blocks in organic synthesis, particularly in the ipso-functionalization of C–S bonds. Of the sulfonium salts, organothianthrenium salts exhibit distinct structural properties and reactivities, thereby offering further potential in organic synthesis. Despite establishing a few preliminary methods for preparing organothianthrenium salts, their application potential is historically thwarted by harsh synthetic conditions and the poor durability of the resulting products [7]. Recently, Ritter and co-workers have introduced a pioneering method for synthesizing air- and moisture-stable arylthianthrenium salts [11]. This novel approach involves the utilization of tetra-fluorothianthrene sulfoxide (TFT=O) or thianthrene sulfoxide (TT=O), which react with arenes under mild conditions, exhibiting exclusive regioselectivity. Significant advancements in the synthesis of arylthianthrenium salts have prompted a growing interest in their utilization as versatile precursors for the conversion of C–H bonds in arenes into C–C/X bonds through transition-metal-catalyzed cross-coupling processes [12-20]. On the other hand, sulfonium salts have emerged as appealing sources of aryl radicals for a wide range of transformations aimed at creating novel chemical bonds driven by their distinctive structural attributes and chemical tendencies (Scheme 1a) [9,21-26]. In addition to late-stage C–H functionalization of arenes, Wickens’s group has introduced an oxidative alkene aziridination strategy that relies on thianthrenation of an alkene under electrochemical conditions [27]. Subsequently, cyclopropanation, [28] aziridination, [29] allylic C–H functionalization, [30,31] transition-metal-catalyzed cross-coupling [32,33] and aminofunctionalization [34] of alkenes were achieved, benefiting from the unique reactivity of organothianthrenium species that are generated through the reaction of alkenes and thianthrene sulfoxide (TT=O) or thianthrene (TT) (Scheme 1b).
Scheme 1: Synthetic application of thianthrenium salts.
Scheme 1: Synthetic application of thianthrenium salts.
Alcohols are widely accessible and have significant importance in the pharmaceutical industry, positioning them as appealing candidates for C(sp3) coupling due to their availability as a common chemical feedstock. However, due to the high bond dissociation energy of the C–O bond and the poor leaving ability of the hydroxy group [35], it is still a great challenge to transform alcohols into valuable chemicals [36-38]. A recent study by Shi and co-workers has successfully converted alcohols into thianthrenium salts, enabling the transformation of the hydroxy (OH) group into various functional groups via the photoassisted generation of alkyl radicals [39]. After that, a series of methods for the modification of alkylthianthrenium salts have been developed, including the transition-metal-catalyzed cross-coupling with terminal alkynes [40], sulfonylation with DABCO·(SO2)2 [41-43], or alkylation of active alkenes [44,45]. Recently, Ritter and co-workers reported that alkylthianthrenium salts can be employed to undergo reactions with N/O-nucleophiles under photocatalytic conditions [46]. Nevertheless, additional transition metal catalysts, visible light, or electrochemical devices are required for the reported works. Therefore, developing a green method to functionalize alkylthianthrenium salts is still highly desirable. Considering the highly polarized C(sp3)–S bond in alkylthianthrenium salts, alkylthianthrenium salts have the potential to serve as alkyl electrophiles to react with nucleophiles directly in the absence of a metal catalyst [46].
Results and Discussion
With these considerations in mind, we investigated the possibility of the thioetherification between alkylthianthrenium salts and thiophenols. After extensive screening of the reaction parameters, the desired thioetherification product 3aa was obtained in 88% yield under the following optimal conditions: S-(alkyl)thianthrenium salt 1a and 4-bromothiophenol (2a) as the model substrates, N,N-diisopropylethylamine (DIPEA) as the base, were added to a vessel in air; after stirring for 16 hours at room temperature, the corresponding product was isolated by chromatographic purification in 88% yield (Table 1, entry 1). Reducing the amount of DIPEA gave diminished yield (Table 1, entry 2). Adding more base was unnecessary to get a higher yield (Table 1, entry 3). Subsequently, the use of other bases was not appropriate for promoting the generation of the thioetherification product 3aa (Table 1, entries 4–7). Furthermore, the yields significantly varied (63−79%) depending on the different solvents tested (Table 1, entries 8–11).
Table 1: Optimization of the thioetherification of S-(alkyl)thianthrenium salt.a
|
|
||
| Entry | Deviation from "standard conditions" | Yield of 2a (%)b |
| 1 | none | 88 |
| 2 | 1.5 equiv of DIPEA | 73 |
| 3 | 3.0 equiv of DIPEA | 85 |
| 4 | NEt3 instead of DIPEA | 76 |
| 5 | Na2CO3 instead of DIPEA | 74 |
| 6 | LiOt-Bu instead of DIPEA | 67 |
| 7 | NaOH instead of DIPEA | 71 |
| 8 | toluene as solvent | 63 |
| 9 | DMF as solvent | 67 |
| 10 | THF as solvent | 72 |
| 11 | DCM as solvent | 79 |
aReaction conditions: all reactions were carried out using 1a (0.3 mmol), 2a (0.2 mmol), and DIPEA (0.4 mmol) in 2.0 mL of MeCN at room temperature for 16 h under an air atmosphere. bIsolated yields after purification by column chromatography.
Having established the optimized reaction conditions, we assessed the range of substrates suitable for this method. First, the scope of sulfonium salts was examined, as summarized in Scheme 2a. Alkylsulfonium salts substituted with a halide (F, Cl, or Br) or isocyano group at the para-position of the aryl ring (1b–e) were successfully converted into the carbon–sulfur bond formation products (3ba–fa) in moderate to good yields. Even when sulfonium salt 1g bearing a C(sp3)–Br bond is susceptible to nucleophilic attack, the desired product 3ga can still be obtained in good yield. Furthermore, substrate 1h featuring two sulfonium salt motifs could undergo dual thioetherification at both reaction sites, resulting in the target product 3ha in good yield. Next, we investigated the compatibility of various thiophenols with thianthrenium salt 1a (Scheme 2b). When simple thiophenol 2b was used as the substrate, a good yield of the target product 3ab was obtained smoothly. To our satisfaction, both electron-donating groups (Me, t-Bu, OMe; 2c–e) and electron-withdrawing groups (Cl and CF3; 2f, and 2g) at the para-position of the aryl ring of thiophenols were well tolerated, furnishing the desired products (3ac–ag) in good yields. The reaction yield remains unaffected by the position of halogen substituents (2h–j), and the resulting products (3ah–aj) can also be obtained with high efficiency. This underscores the viability of integrating this metal-free thioetherification method with other traditional cross-coupling reactions. Sterically hindered ortho-disubstituted thiophenol 2k is also compatible with this reaction system, yielding product 3ak in good yield. Furthermore, heteroaromatic rings, such as pyridine (2l), thiophene (2m), benzoxazole (2n), and benzimidazole (2o) all afforded the desired products (3al–ao) in satisfactory yields.
Scheme 2: Substrate scope. Reaction conditions: alkylthianthrenium salts 1 (0.3 mmol), thiophenols 2 (0.2 mmol), DIPEA (0.4 mmol) in 2.0 mL of MeCN at room temperature for 16 h under air atmosphere. Isolated yields.
Scheme 2: Substrate scope. Reaction conditions: alkylthianthrenium salts 1 (0.3 mmol), thiophenols 2 (0.2 mmo...
Subsequently, we investigated the substrate scope of amines, which is outlined in Scheme 3. To our delight, various amines (2p–r), including aniline, N-methylaniline, and naphthylmethylamine are also compatible under the optimal conditions to give the corresponding amination products (3ap–ar) in moderate to high yields. For this amination method, it was necessary to investigate simple alkylamines (2s and 2t) as the substrates. In doing so, we could not isolate the corresponding amination products 3as and 3at.
Scheme 3: Substrate scope of amines. Reaction conditions: alkylthianthrenium salts 1 (0.3 mmol), amines 2 (0.2 mmol), DIPEA (0.4 mmol) in 2.0 mL of MeCN at 80 °C for 16 h under air atmosphere. Isolated yields.
Scheme 3: Substrate scope of amines. Reaction conditions: alkylthianthrenium salts 1 (0.3 mmol), amines 2 (0....
To showcase the practical utility of our metal-free thioether formation process, we conducted a 5.0 mmol scale reaction and obtained the target product 3aa in 69% yield (Scheme 4). This operationally simple protocol enables the rapid development of novel thioetherification reactions using bench-stable alkylthianthrenium salts as the electrophiles. As is well known, alkyl trifluoromethanesulfonate (alkyl-OTf), serving as a potent electrophilic reagent, can also engage in reactions with electrophilic reagents like thiophenol or amines under alkaline conditions, facilitating the formation of respective C–N/O bonds. The synthesis of alkylthianthrenium salts requires alkyl trifluoromethanesulfonate as a precursor, which can also act as an electrophile. However, alkyl-OTf is prone to decomposition and poses challenges for storage at ambient temperature (for details, see Supporting Information File 1).
Conclusion
In summary, using the presented operational simple and metal-free method, we have synthesized thioethers and amines from bench stable and readily available alkylthianthrenium salts. Given the importance of alkylthianthrenium salts in synthetic chemistry as alkyl reagents and the distinctive reactivities observed under photo/electrochemical conditions, we foresee significant opportunities emerging in the functionalization of alkylthianthrenium salts using nucleophiles directly, without the need for an external metal catalyst.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data for all new compounds, and NMR spectra of products. | ||
| Format: PDF | Size: 3.7 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Chem. Rev. 2019, 119, 8701–8780. doi:10.1021/acs.chemrev.9b00111
Return to citation in text: [1] -
Péter, Á.; Perry, G. J. P.; Procter, D. J. Adv. Synth. Catal. 2020, 362, 2135–2142. doi:10.1002/adsc.202000220
Return to citation in text: [1] -
Lou, J.; Wang, Q.; Wu, P.; Wang, H.; Zhou, Y.-G.; Yu, Z. Chem. Soc. Rev. 2020, 49, 4307–4359. doi:10.1039/c9cs00837c
Return to citation in text: [1] -
Gao, J.; Feng, J.; Du, D. Chem. – Asian J. 2020, 15, 3637–3659. doi:10.1002/asia.202000905
Return to citation in text: [1] -
Kozhushkov, S. I.; Alcarazo, M. Eur. J. Inorg. Chem. 2020, 2486–2500. doi:10.1002/ejic.202000249
Return to citation in text: [1] -
Tian, Z.-Y.; Ma, Y.; Zhang, C.-P. Synthesis 2022, 54, 1478–1502. doi:10.1055/a-1677-5971
Return to citation in text: [1] -
Meng, H.; Liu, M.-S.; Shu, W. Chem. Sci. 2022, 13, 13690–13707. doi:10.1039/d2sc04507a
Return to citation in text: [1] [2] -
van Dalsen, L.; Brown, R. E.; Rossi-Ashton, J. A.; Procter, D. J. Angew. Chem., Int. Ed. 2023, 62, e202303104. doi:10.1002/anie.202303104
Return to citation in text: [1] -
Wu, X.; Gao, P.; Chen, F. Eur. J. Org. Chem. 2023, 26, e202300864. doi:10.1002/ejoc.202300864
Return to citation in text: [1] [2] -
Paul, S.; Filippini, D.; Silvi, M. J. Am. Chem. Soc. 2023, 145, 2773–2778. doi:10.1021/jacs.2c12699
Return to citation in text: [1] -
Berger, F.; Plutschack, M. B.; Riegger, J.; Yu, W.; Speicher, S.; Ho, M.; Frank, N.; Ritter, T. Nature 2019, 567, 223–228. doi:10.1038/s41586-019-0982-0
Return to citation in text: [1] -
Wu, J.; Wang, Z.; Chen, X.-Y.; Wu, Y.; Wang, D.; Peng, Q.; Wang, P. Sci. China: Chem. 2020, 63, 336–340. doi:10.1007/s11426-019-9652-x
Return to citation in text: [1] -
Nie, X.-X.; Huang, Y.-H.; Wang, P. Org. Lett. 2020, 22, 7716–7720. doi:10.1021/acs.orglett.0c02913
Return to citation in text: [1] -
Selmani, A.; Gevondian, A. G.; Schoenebeck, F. Org. Lett. 2020, 22, 4802–4805. doi:10.1021/acs.orglett.0c01609
Return to citation in text: [1] -
Zhao, D.; Petzold, R.; Yan, J.; Muri, D.; Ritter, T. Nature 2021, 600, 444–449. doi:10.1038/s41586-021-04007-y
Return to citation in text: [1] -
Ye, Y.; Zhu, J.; Huang, Y. Org. Lett. 2021, 23, 2386–2391. doi:10.1021/acs.orglett.1c00748
Return to citation in text: [1] -
Lansbergen, B.; Granatino, P.; Ritter, T. J. Am. Chem. Soc. 2021, 143, 7909–7914. doi:10.1021/jacs.1c03459
Return to citation in text: [1] -
Chen, X.-Y.; Huang, Y.-H.; Zhou, J.; Wang, P. Chin. J. Chem. 2020, 38, 1269–1272. doi:10.1002/cjoc.202000212
Return to citation in text: [1] -
Tian, Z.-Y.; Lin, Z.-H.; Zhang, C.-P. Org. Lett. 2021, 23, 4400–4405. doi:10.1021/acs.orglett.1c01322
Return to citation in text: [1] -
Wu, Y.; Huang, Y.-H.; Chen, X.-Y.; Wang, P. Org. Lett. 2020, 22, 6657–6661. doi:10.1021/acs.orglett.0c02458
Return to citation in text: [1] -
Aukland, M. H.; Šiaučiulis, M.; West, A.; Perry, G. J. P.; Procter, D. J. Nat. Catal. 2020, 3, 163–169. doi:10.1038/s41929-019-0415-3
Return to citation in text: [1] -
Dewanji, A.; van Dalsen, L.; Rossi-Ashton, J. A.; Gasson, E.; Crisenza, G. E. M.; Procter, D. J. Nat. Chem. 2023, 15, 43–52. doi:10.1038/s41557-022-01092-y
Return to citation in text: [1] -
Cai, Y.; Chatterjee, S.; Ritter, T. J. Am. Chem. Soc. 2023, 145, 13542–13548. doi:10.1021/jacs.3c04016
Return to citation in text: [1] -
Li, J.; Chen, J.; Sang, R.; Ham, W.-S.; Plutschack, M. B.; Berger, F.; Chabbra, S.; Schnegg, A.; Genicot, C.; Ritter, T. Nat. Chem. 2020, 12, 56–62. doi:10.1038/s41557-019-0353-3
Return to citation in text: [1] -
Mato, M.; Bruzzese, P. C.; Takahashi, F.; Leutzsch, M.; Reijerse, E. J.; Schnegg, A.; Cornella, J. J. Am. Chem. Soc. 2023, 145, 18742–18747. doi:10.1021/jacs.3c06651
Return to citation in text: [1] -
Xu, H.; Li, X.; Dong, Y.; Ji, S.; Zuo, J.; Lv, J.; Yang, D. Org. Lett. 2023, 25, 3784–3789. doi:10.1021/acs.orglett.3c01303
Return to citation in text: [1] -
Holst, D. E.; Wang, D. J.; Kim, M. J.; Guzei, I. A.; Wickens, Z. K. Nature 2021, 596, 74–79. doi:10.1038/s41586-021-03717-7
Return to citation in text: [1] -
Kim, M. J.; Wang, D. J.; Targos, K.; Garcia, U. A.; Harris, A. F.; Guzei, I. A.; Wickens, Z. K. Angew. Chem., Int. Ed. 2023, 62, e202303032. doi:10.1002/anie.202303032
Return to citation in text: [1] -
Liu, M.-S.; Du, H.-W.; Cui, J.-F.; Shu, W. Angew. Chem., Int. Ed. 2022, 61, e20220992. doi:10.1002/anie.202209929
Return to citation in text: [1] -
Wang, D. J.; Targos, K.; Wickens, Z. K. J. Am. Chem. Soc. 2021, 143, 21503–21510. doi:10.1021/jacs.1c11763
Return to citation in text: [1] -
Liu, M.-S.; Du, H.-W.; Shu, W. Chem. Sci. 2022, 13, 1003–1008. doi:10.1039/d1sc06577g
Return to citation in text: [1] -
Juliá, F.; Yan, J.; Paulus, F.; Ritter, T. J. Am. Chem. Soc. 2021, 143, 12992–12998. doi:10.1021/jacs.1c06632
Return to citation in text: [1] -
Zhu, J.; Ye, Y.; Huang, Y. Organometallics 2022, 41, 2342–2348. doi:10.1021/acs.organomet.2c00330
Return to citation in text: [1] -
Holst, D. E.; Dorval, C.; Winter, C. K.; Guzei, I. A.; Wickens, Z. K. J. Am. Chem. Soc. 2023, 145, 8299–8307. doi:10.1021/jacs.3c01137
Return to citation in text: [1] -
Luo, Y.-R., Ed. Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: Boca Raton, FL, USA, 2002.
Return to citation in text: [1] -
Lee, D.-H.; Kwon, K.-H.; Yi, C. S. Science 2011, 333, 1613–1616. doi:10.1126/science.1208839
Return to citation in text: [1] -
Lee, D.-H.; Kwon, K.-H.; Yi, C. S. J. Am. Chem. Soc. 2012, 134, 7325–7328. doi:10.1021/ja302710v
Return to citation in text: [1] -
Jin, J.; MacMillan, D. W. C. Nature 2015, 525, 87–90. doi:10.1038/nature14885
Return to citation in text: [1] -
Chen, C.; Wang, Z.-J.; Lu, H.; Zhao, Y.; Shi, Z. Nat. Commun. 2021, 12, 4526. doi:10.1038/s41467-021-24716-2
Return to citation in text: [1] -
Chen, C.; Wang, M.; Lu, H.; Zhao, B.; Shi, Z. Angew. Chem., Int. Ed. 2021, 60, 21756–21760. doi:10.1002/anie.202109723
Return to citation in text: [1] -
Ma, H.; Li, Y.; Wang, P.; Ye, J.; Zhang, J.; Liu, G.; Wu, J. Org. Chem. Front. 2023, 10, 866–871. doi:10.1039/d2qo01706g
Return to citation in text: [1] -
Kong, X.; Chen, Y.; Liu, Q.; Wang, W.; Zhang, S.; Zhang, Q.; Chen, X.; Xu, Y.-Q.; Cao, Z.-Y. Org. Lett. 2023, 25, 581–586. doi:10.1021/acs.orglett.2c03956
Return to citation in text: [1] -
Yuan, X.; Liu, J.; Qin, L.-Z.; Duan, X.; Wang, J.; Wu, M.-Y.; Qiu, J.-K.; Guo, K. Adv. Synth. Catal. 2023, 365, 555–567. doi:10.1002/adsc.202201294
Return to citation in text: [1] -
Li, X.; Si, W.; Liu, Z.; Qian, H.; Wang, T.; Leng, S.; Sun, J.; Jiao, Y.; Zhang, X. Org. Lett. 2022, 24, 4070–4074. doi:10.1021/acs.orglett.2c01525
Return to citation in text: [1] -
Wang, M.; Wang, C.; Xie, X.; Pan, D.; Liu, L.; Chen, Q.; Li, Z.; Zhang, Q.; Xu, Z. Chem. Commun. 2023, 59, 3305–3308. doi:10.1039/d2cc06653j
Return to citation in text: [1] -
Alvarez, E. M.; Bai, Z.; Pandit, S.; Frank, N.; Torkowski, L.; Ritter, T. Nat. Synth. 2023, 2, 548–556. doi:10.1038/s44160-023-00277-8
Return to citation in text: [1] [2]
| 46. | Alvarez, E. M.; Bai, Z.; Pandit, S.; Frank, N.; Torkowski, L.; Ritter, T. Nat. Synth. 2023, 2, 548–556. doi:10.1038/s44160-023-00277-8 |
| 44. | Li, X.; Si, W.; Liu, Z.; Qian, H.; Wang, T.; Leng, S.; Sun, J.; Jiao, Y.; Zhang, X. Org. Lett. 2022, 24, 4070–4074. doi:10.1021/acs.orglett.2c01525 |
| 45. | Wang, M.; Wang, C.; Xie, X.; Pan, D.; Liu, L.; Chen, Q.; Li, Z.; Zhang, Q.; Xu, Z. Chem. Commun. 2023, 59, 3305–3308. doi:10.1039/d2cc06653j |
| 46. | Alvarez, E. M.; Bai, Z.; Pandit, S.; Frank, N.; Torkowski, L.; Ritter, T. Nat. Synth. 2023, 2, 548–556. doi:10.1038/s44160-023-00277-8 |
| 1. | Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Chem. Rev. 2019, 119, 8701–8780. doi:10.1021/acs.chemrev.9b00111 |
| 2. | Péter, Á.; Perry, G. J. P.; Procter, D. J. Adv. Synth. Catal. 2020, 362, 2135–2142. doi:10.1002/adsc.202000220 |
| 3. | Lou, J.; Wang, Q.; Wu, P.; Wang, H.; Zhou, Y.-G.; Yu, Z. Chem. Soc. Rev. 2020, 49, 4307–4359. doi:10.1039/c9cs00837c |
| 4. | Gao, J.; Feng, J.; Du, D. Chem. – Asian J. 2020, 15, 3637–3659. doi:10.1002/asia.202000905 |
| 5. | Kozhushkov, S. I.; Alcarazo, M. Eur. J. Inorg. Chem. 2020, 2486–2500. doi:10.1002/ejic.202000249 |
| 6. | Tian, Z.-Y.; Ma, Y.; Zhang, C.-P. Synthesis 2022, 54, 1478–1502. doi:10.1055/a-1677-5971 |
| 7. | Meng, H.; Liu, M.-S.; Shu, W. Chem. Sci. 2022, 13, 13690–13707. doi:10.1039/d2sc04507a |
| 8. | van Dalsen, L.; Brown, R. E.; Rossi-Ashton, J. A.; Procter, D. J. Angew. Chem., Int. Ed. 2023, 62, e202303104. doi:10.1002/anie.202303104 |
| 9. | Wu, X.; Gao, P.; Chen, F. Eur. J. Org. Chem. 2023, 26, e202300864. doi:10.1002/ejoc.202300864 |
| 10. | Paul, S.; Filippini, D.; Silvi, M. J. Am. Chem. Soc. 2023, 145, 2773–2778. doi:10.1021/jacs.2c12699 |
| 9. | Wu, X.; Gao, P.; Chen, F. Eur. J. Org. Chem. 2023, 26, e202300864. doi:10.1002/ejoc.202300864 |
| 21. | Aukland, M. H.; Šiaučiulis, M.; West, A.; Perry, G. J. P.; Procter, D. J. Nat. Catal. 2020, 3, 163–169. doi:10.1038/s41929-019-0415-3 |
| 22. | Dewanji, A.; van Dalsen, L.; Rossi-Ashton, J. A.; Gasson, E.; Crisenza, G. E. M.; Procter, D. J. Nat. Chem. 2023, 15, 43–52. doi:10.1038/s41557-022-01092-y |
| 23. | Cai, Y.; Chatterjee, S.; Ritter, T. J. Am. Chem. Soc. 2023, 145, 13542–13548. doi:10.1021/jacs.3c04016 |
| 24. | Li, J.; Chen, J.; Sang, R.; Ham, W.-S.; Plutschack, M. B.; Berger, F.; Chabbra, S.; Schnegg, A.; Genicot, C.; Ritter, T. Nat. Chem. 2020, 12, 56–62. doi:10.1038/s41557-019-0353-3 |
| 25. | Mato, M.; Bruzzese, P. C.; Takahashi, F.; Leutzsch, M.; Reijerse, E. J.; Schnegg, A.; Cornella, J. J. Am. Chem. Soc. 2023, 145, 18742–18747. doi:10.1021/jacs.3c06651 |
| 26. | Xu, H.; Li, X.; Dong, Y.; Ji, S.; Zuo, J.; Lv, J.; Yang, D. Org. Lett. 2023, 25, 3784–3789. doi:10.1021/acs.orglett.3c01303 |
| 40. | Chen, C.; Wang, M.; Lu, H.; Zhao, B.; Shi, Z. Angew. Chem., Int. Ed. 2021, 60, 21756–21760. doi:10.1002/anie.202109723 |
| 12. | Wu, J.; Wang, Z.; Chen, X.-Y.; Wu, Y.; Wang, D.; Peng, Q.; Wang, P. Sci. China: Chem. 2020, 63, 336–340. doi:10.1007/s11426-019-9652-x |
| 13. | Nie, X.-X.; Huang, Y.-H.; Wang, P. Org. Lett. 2020, 22, 7716–7720. doi:10.1021/acs.orglett.0c02913 |
| 14. | Selmani, A.; Gevondian, A. G.; Schoenebeck, F. Org. Lett. 2020, 22, 4802–4805. doi:10.1021/acs.orglett.0c01609 |
| 15. | Zhao, D.; Petzold, R.; Yan, J.; Muri, D.; Ritter, T. Nature 2021, 600, 444–449. doi:10.1038/s41586-021-04007-y |
| 16. | Ye, Y.; Zhu, J.; Huang, Y. Org. Lett. 2021, 23, 2386–2391. doi:10.1021/acs.orglett.1c00748 |
| 17. | Lansbergen, B.; Granatino, P.; Ritter, T. J. Am. Chem. Soc. 2021, 143, 7909–7914. doi:10.1021/jacs.1c03459 |
| 18. | Chen, X.-Y.; Huang, Y.-H.; Zhou, J.; Wang, P. Chin. J. Chem. 2020, 38, 1269–1272. doi:10.1002/cjoc.202000212 |
| 19. | Tian, Z.-Y.; Lin, Z.-H.; Zhang, C.-P. Org. Lett. 2021, 23, 4400–4405. doi:10.1021/acs.orglett.1c01322 |
| 20. | Wu, Y.; Huang, Y.-H.; Chen, X.-Y.; Wang, P. Org. Lett. 2020, 22, 6657–6661. doi:10.1021/acs.orglett.0c02458 |
| 41. | Ma, H.; Li, Y.; Wang, P.; Ye, J.; Zhang, J.; Liu, G.; Wu, J. Org. Chem. Front. 2023, 10, 866–871. doi:10.1039/d2qo01706g |
| 42. | Kong, X.; Chen, Y.; Liu, Q.; Wang, W.; Zhang, S.; Zhang, Q.; Chen, X.; Xu, Y.-Q.; Cao, Z.-Y. Org. Lett. 2023, 25, 581–586. doi:10.1021/acs.orglett.2c03956 |
| 43. | Yuan, X.; Liu, J.; Qin, L.-Z.; Duan, X.; Wang, J.; Wu, M.-Y.; Qiu, J.-K.; Guo, K. Adv. Synth. Catal. 2023, 365, 555–567. doi:10.1002/adsc.202201294 |
| 11. | Berger, F.; Plutschack, M. B.; Riegger, J.; Yu, W.; Speicher, S.; Ho, M.; Frank, N.; Ritter, T. Nature 2019, 567, 223–228. doi:10.1038/s41586-019-0982-0 |
| 36. | Lee, D.-H.; Kwon, K.-H.; Yi, C. S. Science 2011, 333, 1613–1616. doi:10.1126/science.1208839 |
| 37. | Lee, D.-H.; Kwon, K.-H.; Yi, C. S. J. Am. Chem. Soc. 2012, 134, 7325–7328. doi:10.1021/ja302710v |
| 38. | Jin, J.; MacMillan, D. W. C. Nature 2015, 525, 87–90. doi:10.1038/nature14885 |
| 7. | Meng, H.; Liu, M.-S.; Shu, W. Chem. Sci. 2022, 13, 13690–13707. doi:10.1039/d2sc04507a |
| 39. | Chen, C.; Wang, Z.-J.; Lu, H.; Zhao, Y.; Shi, Z. Nat. Commun. 2021, 12, 4526. doi:10.1038/s41467-021-24716-2 |
| 30. | Wang, D. J.; Targos, K.; Wickens, Z. K. J. Am. Chem. Soc. 2021, 143, 21503–21510. doi:10.1021/jacs.1c11763 |
| 31. | Liu, M.-S.; Du, H.-W.; Shu, W. Chem. Sci. 2022, 13, 1003–1008. doi:10.1039/d1sc06577g |
| 34. | Holst, D. E.; Dorval, C.; Winter, C. K.; Guzei, I. A.; Wickens, Z. K. J. Am. Chem. Soc. 2023, 145, 8299–8307. doi:10.1021/jacs.3c01137 |
| 29. | Liu, M.-S.; Du, H.-W.; Cui, J.-F.; Shu, W. Angew. Chem., Int. Ed. 2022, 61, e20220992. doi:10.1002/anie.202209929 |
| 35. | Luo, Y.-R., Ed. Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: Boca Raton, FL, USA, 2002. |
| 28. | Kim, M. J.; Wang, D. J.; Targos, K.; Garcia, U. A.; Harris, A. F.; Guzei, I. A.; Wickens, Z. K. Angew. Chem., Int. Ed. 2023, 62, e202303032. doi:10.1002/anie.202303032 |
| 27. | Holst, D. E.; Wang, D. J.; Kim, M. J.; Guzei, I. A.; Wickens, Z. K. Nature 2021, 596, 74–79. doi:10.1038/s41586-021-03717-7 |
| 32. | Juliá, F.; Yan, J.; Paulus, F.; Ritter, T. J. Am. Chem. Soc. 2021, 143, 12992–12998. doi:10.1021/jacs.1c06632 |
| 33. | Zhu, J.; Ye, Y.; Huang, Y. Organometallics 2022, 41, 2342–2348. doi:10.1021/acs.organomet.2c00330 |
© 2024 Fan et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.