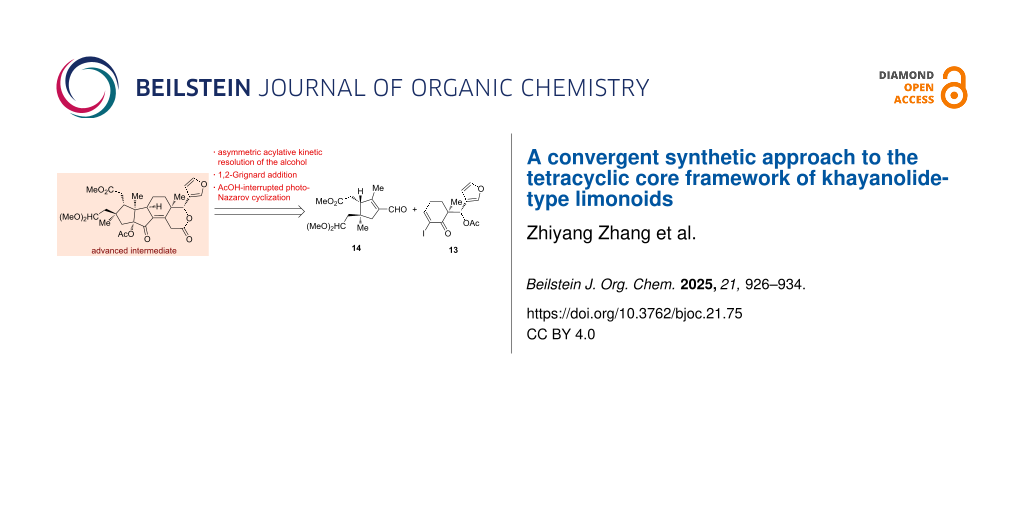
Abstract
A convergent approach for the enantioselective construction of an advanced intermediate containing the [5,5,6,6]-tetracyclic core framework of the khayanolide-type limonoids was described. The strategy features an acylative kinetic resolution of the benzylic alcohol, a 1,2-Grignard addition and an AcOH-interrupted Nazarov cyclization.
Graphical Abstract
Introduction
Limonoids, a class of tetranortriterpenoids derived biosynthetically from oxidative truncation of apotirucallane or apoeuphane precursors coupled with subsequent β-furan annulation [1-6], constitute an architecturally sophisticated family of natural products. Based on their specific skeletal rearrangements, limonoids can be systematically categorized into four subfamilies: ring intact limonoids, ring-seco limonoids, rearranged limonoids and N-containing limonoids (Figure 1). Moreover, these molecules exhibit a remarkable pharmacological portfolio encompassing anticancer, antimicrobial, anti-inflammatory, and insect antifeedant activities [7-9], positioning them as compelling targets for both therapeutic development and agrochemical innovation. Furthermore, limonoids are also renowned for their extraordinary structural complexity. For instance, the phragmalin-type limonoids represent a significant category of highly oxygenated rearranged limonoids, which contain a distinctive octahydro-1H-2,4-methanoindene cage. The fascinating architectures and remarkable biological profiles of these compounds have attracted widespread attention from the synthetic community. In 1989, Corey and Hahl made a seminal contribution [10] to the field by completing the total synthesis of azadiradione (1). Following this, Ley and his team successfully synthesized azadirachtin (2) in 2007, a limonoid extensively utilized in organic agriculture [11]. In recent years, remarkable progress has been made on the total syntheses of various limonoids, with notable contributions from researchers such as Williams [12], Yamashita [13], Hao/Yang/Shen [14], Gong/Hao/Yang [15], Newhouse [16-19], Yang/Chen [20], Renata [21], Qin/Yu [22], Ma [23], Watanabe [24], and Li [25]. Their groundbreaking work has provided valuable insights and inspired further research in synthetic chemistry involving limonoids.
Figure 1: Representative limonoid triterpenes.
Figure 1: Representative limonoid triterpenes.
Krishnolides A and C (7 and 8, respectively; Scheme 1A) were identified by Wu and co-workers from the seeds of a Krishna mangrove Xylocarpus moluccensis [26]. These two molecules belong to khayanolides, a class of rearranged phragmalin limonoids characterized by a structurally intricate tricyclo[4.2.110,30.11,4]decane ring system. Additionally, krishnolides A and C contain 9–11 stereogenic centers and exhibit diverse oxidation patterns. Their relative and absolute configurations were determined through NMR, HRESIMS and ECD experiments, as well as single crystal X-ray diffraction analysis. A preliminary investigation revealed that krishnolide A (7) exhibited unique anti-human immunodeficiency virus (HIV) activity, representing the first report of anti-HIV activity in khayanolide-type limonoids. However, the highly oxygenated and polycyclic scaffolds pose substantial challenges toward their total synthesis. Two synthetic studies were disclosed successively by Sarpong [27] and Jirgensons [28], both focusing on the construction of the unique methanoindene cage structure (A1A2B ring system). Building upon our previous syntheses of phragmalin-type limonoids [29], we herein disclose a convergent approach leveraging an AcOH-interrupted Nazarov cyclization to establish the [5,5,6,6]-tetracyclic scaffold with precise stereochemical fidelity.
Results and Discussion
Our retrosynthetic analysis toward krishnolides A (7) and C (8) is delineated in Scheme 1B. We hypothesized that these two molecules could be synthesized from diol 9 through a late-stage modification involving adjustment of the oxidation state and regioselective acylation. The formation of 9 was envisioned to proceed via an intramolecular pinacol coupling [30,31] of [5,5,6,6]-tetracycle 10, which forges the A2 ring while simultaneously installing the hydroxy group at C30. The latter intermediate could in turn be derived from dienone 11 by an AcOH-interrupted Nazarov cyclization [32-34], thereby establishing the B ring with the desired all-cis stereochemical configuration, including the quaternary carbon at C10 and the essential tertiary alcohol at C1. The β-hydroxylactone moiety (D ring) in 11 could be introduced through an intramolecular aldol condensation [35] of acetate 12. Ultimately, the preparation of 12 could be traced back to aldehyde 14 through 1,2-Grignard addition with an organomagnesium reagent [36] prepared from α-iodoenone 13.
Scheme 1: Structures and retrosynthetic analysis of krishnolides A (7) and C (8).
Scheme 1: Structures and retrosynthetic analysis of krishnolides A (7) and C (8).
Our synthesis began with the preparation of α-iodoenone 13 (Scheme 2). The α-monomethylation of cyclohexenone 15 was efficiently carried out with LDA, HMPA, and MeI [37], producing enone 16 in 78% yield. Subsequently, a diastereoselective aldol reaction between 16 and 3-furaldehyde promoted by LiHMDS gave alcohol 17 with high regioselectivity (14:1 dr at C17) after an extensive screening of bases (LDA, NaHMDS, KHMDS, etc.). Drawing inspiration from the pioneering work of Birman [38], as well as Newhouse’s applications [18,19], an acylative kinetic resolution of the alcohol was achieved by using (R)-BTM (19), furnishing acetate 18 with satisfactory efficiency and enantioselectivity (37% yield, 85% ee). Finally, iodination of 18 employing Johnsen’s protocol (I2, pyridine) [39] provided α-iodoenone 13 in 89% yield.
Scheme 2: Construction of α-iodoenone 13.
Scheme 2: Construction of α-iodoenone 13.
On the other hand, the synthesis of acetal aldehyde 14 commenced with bicyclic ketone 21, which was prepared from (+)-Hajos–Parrish ketone in 49% yield over two steps (Scheme 3) [40-43]. Ensuring silyl enol etherification of the ketone at C29 coupled with IBX-mediated Nicolaou oxidation [44] furnished the corresponding enone in 72% yield (90% brsm). The methyl group at C10 was then introduced via a Michael addition (MeMgBr, CuI) to afford 22 in a yield of 65% (4:1 dr at C10). Initial attempts on the carbonyl 1,2-transposition protocol reported by Dong and co-workers were ineffective [45], leading to premature hydride termination and the formation of alkene 23. As an alternative solution, by treating 22 with KHMDS and PhNTf2, enol triflation took place successfully. The resultant triflate was coupled with n-Bu3SnH to afford Δ1,29-alkene 23 in 83% yield over two steps. Subsequent hydroboration–oxidation by employing BH3·THF proceeded smoothly, providing a 4.4:1 mixture of regioisomeric alcohols with the desired isomer being the major component, presumably due to the considerable steric hindrance from the quaternary carbon at C4. However, decagram-scale separation of these two isomers by chromatography proved troublesome. Fortunately, the distinct reactivity of those two alcohols toward oxidation allowed for the selective conversion of desired alcohol to ketone 24 using PCC, producing a 50% overall yield, while the recovered undesired alcohol could be reverted to 21 by Swern oxidation.
Direct enol triflation (Et3N/Tf2O, NaH/PhNTf2, DTBMP/Tf2O, etc.) of 24 led only to epimerization at C10 or slow decomposition of the starting material (see Supporting Information File 1, Table S1). Pleasingly, treating 24 with HMDS and TMSI regioselectively generated the expected TMS enol ether [46], which underwent a Li/Si exchange in the presence of MeLi followed by interception of the lithium enolate with PhNTf2 to give enol triflate 25 in 59% overall yield. The following palladium-catalyzed methoxycarbonylation produced methyl ester 26 in a satisfactory yield of 84%. TIPS-protected allylic alcohol 28 was selected as the appropriate precursor for the α,β-unsaturated aldehyde and synthesized from 26 via a four-step transformation sequence, including deketalization, reduction, silylation of the primary alcohol and oxidation of the secondary alcohol. For the disconnection of the C2–C7 bond, a two-step protocol involving Rubottom oxidation and PIDA-promoted oxidative cleavage [47] was applied to deliver aldehyde 29. Finally, a one-pot acetalization and desilylation effectively afforded the acetal alcohol, which was then oxidized with the aid of TPAP, furnishing acetal aldehyde 14 in 67% yield over two steps.
With the two fragments 13 and 14 in hand, the next stage was set for the construction of the target skeleton through a 1,2-addition (Scheme 4). Preliminary trials to generate organometallic species via Li/I exchange under various conditions (n-BuLi, t-BuLi, or t-BuLi in combination with CeCl3 or MgBr2) led to rapid decomposition, likely due to inherent instability of α-iodoenone 13. Inspired by the influential studies by Knochel [36] and Baran [48], we discovered that Mg/I exchange of 13 could be accomplished with iPrMgCl·LiCl at −78 °C. The resulting Grignard reagent reacted smoothly with aldehyde 14, affording the corresponding adduct 12 in 58% yield (92% brsm). Since the newly created configuration at C30 was inconsequential, an intramolecular aldol reaction was directly carried out by treatment with LiHMDS to furnish β-hydroxylactone, which could be converted to dienone 11 through TPAP oxidation.
Scheme 4: Synthesis of the advanced intermediate 10 (in the X ray structure of 10 solvent molecule is omitted for clarity).
Scheme 4: Synthesis of the advanced intermediate 10 (in the X ray structure of 10 solvent molecule is omitted...
Having secured 11, we proceeded to evaluate the pivotal Nazarov cyclization under a variety of conditions (Table 1). Initial trials under acid-mediated Nazarov conditions (AlCl3, BF3·Et2O and Me2AlCl) led to complete decomposition, while the exposure to AcOH resulted in recovery of the starting material (Table 1, entries 1–4). Recognizing the limitations of these approaches, we then turned to the milder photo-Nazarov cyclization, in which the UV-light sources were found to be critical. While irradiation at 365 or 313 nm failed to induce cyclization and only allowed recovery of the starting material (Table 1, entries 6 and 7), the disrotatory cyclization of 11 in the presence of AcOH by exposure to UV-light at 254 nm occurred exclusively to provide an inseparable mixture of 32 and 33 (Table 1, entry 5). Subsequent dehydration of the resultant mixture with SOCl2 and pyridine yielded separable enones 34 (51%) and 10 (9%) over two steps. The structure of 10 was unequivocally determined through X-ray crystallographic analysis (ORTEP drawing, Scheme 4). Further optimization by elevating the reaction temperature did not noticeably alter the ratio of 32 and 33 (Table 1, entries 8 and 9). Moreover, attempts to apply interrupted Nazarov cyclization with H2O under neutral conditions merely resulted in decomposition (Table 1, entry 10). To our delight, photoirradiation of the corresponding methyl ether 31 at 254 nm in the presence of AcOH at 20 °C led to a smooth cyclization, followed by spontaneous elimination, which produced the desired 10 as the single product, with no detection of 34 (Table 1, entry 11). This result presumably arises from the minimization of dipole–dipole repulsions between the carbonyl of the dienone moiety and the C14-OMe within the desired transition state (more details were discussed in our group’s previous work [29]).
Table 1: Optimization of the interrupted Nazarov cyclization.a
|
|
|||
| Entry | Conditions | Yield (%)b | |
| 34 | 10 | ||
| 1c | 11, AlCl3, DCE, 60 °C | 0 | 0 |
| 2c | 11, BF3·Et2O, DCE, 60 °C | 0 | 0 |
| 3c | 11, Me2AlCl, toluene, 100 °C | 0 | 0 |
| 4 | 11, AcOH, DCE, 70 °C | 0 | 0 |
| 5 | 11, 254 nm hν, AcOH, DCM, 20 °C | 57 | 7 |
| 6d | 11, 313 nm hν, AcOH, DCM, 20 °C | 0 | 0 |
| 7d | 11, 365 nm hν, AcOH, DCM, 20 °C | 0 | 0 |
| 8 | 11, 254 nm hν, AcOH, DCE, 40 °C | 54 | 8 |
| 9 | 11, 254 nm hν, AcOH, DCE, 70 °C | 51 | 9 |
| 10c | 11, 254 nm hν, H2O, DCM, 20 °C | 0 | 0 |
| 11 | 31, 254 nm hν, AcOH, DCM, 20 °C | 0e | 73e |
aReaction conditions: substrate (0.015 mmol), acid (2.0 equiv), solvent (5.0 mL). bIsolated yields over two steps involving Nazarov cyclization and dehydration. cDecomposition. dNo reaction. eIsolated yields over Nazarov cyclization/elimination cascade.
Conclusion
In conclusion, we have developed a convergent approach for the enantioselective assembly of an advanced intermediate en route to krishnolides A and C. Key steps of our strategy entail an acylative kinetic resolution of the alcohol, a 1,2-Grignard addition and an AcOH-interrupted Nazarov cyclization. Further elaboration of intermediate 10 to krishnolides A and C, as well as other khayanolide-type limonoids is currently ongoing, and the results will be disclosed in future reports.
Supporting Information
Deposition number 2406738 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via the joint Cambridge Crystallographic Data Centre (CCDC) and Fachinformationszentrum Karlsruhe Access Structures service.
| Supporting Information File 1: Experimental procedures, NMR spectra and other characterization data for all new compounds. | ||
| Format: PDF | Size: 6.9 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Heasley, B. Eur. J. Org. Chem. 2011, 19–46. doi:10.1002/ejoc.201001218
Return to citation in text: [1] -
Tan, Q.-G.; Luo, X.-D. Chem. Rev. 2011, 111, 7437–7522. doi:10.1021/cr9004023
Return to citation in text: [1] -
Gualdani, R.; Cavalluzzi, M. M.; Lentini, G.; Habtemariam, S. Molecules 2016, 21, 1530. doi:10.3390/molecules21111530
Return to citation in text: [1] -
Zhang, Y.; Xu, H. RSC Adv. 2017, 7, 35191–35220. doi:10.1039/c7ra04715k
Return to citation in text: [1] -
Fu, S.; Liu, B. Org. Chem. Front. 2020, 7, 1903–1947. doi:10.1039/d0qo00203h
Return to citation in text: [1] -
Luo, J.; Sun, Y.; Li, Q.; Kong, L. Nat. Prod. Rep. 2022, 39, 1325–1365. doi:10.1039/d2np00015f
Return to citation in text: [1] -
Champagne, D. E.; Koul, O.; Isman, M. B.; Scudder, G. G. E.; Towers, G. H. N. Phytochemistry 1992, 31, 377–394. doi:10.1016/0031-9422(92)90003-9
Return to citation in text: [1] -
Jain, D. C.; Tripathi, A. K. Phytother. Res. 1993, 7, 327–334. doi:10.1002/ptr.2650070502
Return to citation in text: [1] -
Roy, A.; Saraf, S. Biol. Pharm. Bull. 2006, 29, 191–201. doi:10.1248/bpb.29.191
Return to citation in text: [1] -
Corey, E. J.; Hahl, R. W. Tetrahedron Lett. 1989, 30, 3023–3026. doi:10.1016/s0040-4039(00)99392-4
Return to citation in text: [1] -
Veitch, G. E.; Beckmann, E.; Burke, B. J.; Boyer, A.; Maslen, S. L.; Ley, S. V. Angew. Chem., Int. Ed. 2007, 46, 7629–7632. doi:10.1002/anie.200703027
Return to citation in text: [1] -
Faber, J. M.; Eger, W. A.; Williams, C. M. J. Org. Chem. 2012, 77, 8913–8921. doi:10.1021/jo301182f
Return to citation in text: [1] -
Yamashita, S.; Naruko, A.; Nakazawa, Y.; Zhao, L.; Hayashi, Y.; Hirama, M. Angew. Chem., Int. Ed. 2015, 54, 8538–8541. doi:10.1002/anie.201503794
Return to citation in text: [1] -
Lv, C.; Yan, X.; Tu, Q.; Di, Y.; Yuan, C.; Fang, X.; Ben‐David, Y.; Xia, L.; Gong, J.; Shen, Y.; Yang, Z.; Hao, X. Angew. Chem., Int. Ed. 2016, 55, 7539–7543. doi:10.1002/anie.201602783
Return to citation in text: [1] -
Lv, C.; Tu, Q.; Gong, J.; Hao, X.; Yang, Z. Tetrahedron 2017, 73, 3612–3621. doi:10.1016/j.tet.2017.03.072
Return to citation in text: [1] -
Schuppe, A. W.; Newhouse, T. R. J. Am. Chem. Soc. 2017, 139, 631–634. doi:10.1021/jacs.6b12268
Return to citation in text: [1] -
Schuppe, A. W.; Huang, D.; Chen, Y.; Newhouse, T. R. J. Am. Chem. Soc. 2018, 140, 2062–2066. doi:10.1021/jacs.7b13189
Return to citation in text: [1] -
Schuppe, A. W.; Zhao, Y.; Liu, Y.; Newhouse, T. R. J. Am. Chem. Soc. 2019, 141, 9191–9196. doi:10.1021/jacs.9b04508
Return to citation in text: [1] [2] -
Schuppe, A. W.; Liu, Y.; Gonzalez-Hurtado, E.; Zhao, Y.; Jiang, X.; Ibarraran, S.; Huang, D.; Wang, X.; Lee, J.; Loria, J. P.; Dixit, V. D.; Li, X.; Newhouse, T. R. Chem 2022, 8, 2856–2887. doi:10.1016/j.chempr.2022.09.012
Return to citation in text: [1] [2] -
Zhang, W.; Zhang, Z.; Tang, J.-C.; Che, J.-T.; Zhang, H.-Y.; Chen, J.-H.; Yang, Z. J. Am. Chem. Soc. 2020, 142, 19487–19492. doi:10.1021/jacs.0c10122
Return to citation in text: [1] -
Li, J.; Chen, F.; Renata, H. J. Am. Chem. Soc. 2022, 144, 19238–19242. doi:10.1021/jacs.2c09048
Return to citation in text: [1] -
Deng, H.; Deng, H.; Kim, C.; Li, P.; Wang, X.; Yu, Y.; Qin, T. Nat. Synth. 2023, 3, 378–385. doi:10.1038/s44160-023-00437-w
Return to citation in text: [1] -
Jin, S. C.; Ma, D. W. ChemRxiv 2024. doi:10.26434/chemrxiv-2024-dscn9
Return to citation in text: [1] -
Mori, N.; Kitahara, T.; Mori, K.; Watanabe, H. Angew. Chem., Int. Ed. 2015, 54, 14920–14923. doi:10.1002/anie.201507935
Return to citation in text: [1] -
Liu, X.; Xu, Y.; Li, L.; Li, J. J. Am. Chem. Soc. 2024, 146, 26243–26250. doi:10.1021/jacs.4c07956
Return to citation in text: [1] -
Zhang, Q.; Satyanandamurty, T.; Shen, L.; Wu, J. Mar. Drugs 2017, 15, 333. doi:10.3390/md15110333
Return to citation in text: [1] -
Lebold, T. P.; Gallego, G. M.; Marth, C. J.; Sarpong, R. Org. Lett. 2012, 14, 2110–2113. doi:10.1021/ol300647k
Return to citation in text: [1] -
Becica, J.; Ra̅ciņš, O.; Ivanova, M.; Jirgensons, A. J. Org. Chem. 2023, 88, 10306–10309. doi:10.1021/acs.joc.3c00952
Return to citation in text: [1] -
Rao, P.; Tang, D.; Xia, Q.; Hu, J.; Lin, X.; Xuan, J.; Ding, H. J. Am. Chem. Soc. 2025, 147, 3003–3009. doi:10.1021/jacs.4c16265
Return to citation in text: [1] [2] -
Hovey, M. T.; Cohen, D. T.; Walden, D. M.; Cheong, P. H.-Y.; Scheidt, K. A. Angew. Chem., Int. Ed. 2017, 56, 9864–9867. doi:10.1002/anie.201705308
Return to citation in text: [1] -
Sasano, Y.; Koyama, J.; Yoshikawa, K.; Kanoh, N.; Kwon, E.; Iwabuchi, Y. Org. Lett. 2018, 20, 3053–3056. doi:10.1021/acs.orglett.8b01087
Return to citation in text: [1] -
Grant, T. N.; Rieder, C. J.; West, F. G. Chem. Commun. 2009, 5676–5688. doi:10.1039/b908515g
Return to citation in text: [1] -
Yadykov, A. V.; Shirinian, V. Z. Adv. Synth. Catal. 2020, 362, 702–723. doi:10.1002/adsc.201901001
Return to citation in text: [1] -
Trudel, V.; Tien, C.-H.; Trofimova, A.; Yudin, A. K. Nat. Rev. Chem. 2021, 5, 604–623. doi:10.1038/s41570-021-00304-2
Return to citation in text: [1] -
Fernández-Mateos, A.; Grande Benito, M.; Pascual Coca, G.; Rubio González, R.; Tapia Hernández, C. Tetrahedron 1995, 51, 7521–7526. doi:10.1016/0040-4020(95)00375-i
Return to citation in text: [1] -
Ren, H.; Krasovskiy, A.; Knochel, P. Org. Lett. 2004, 6, 4215–4217. doi:10.1021/ol048363h
Return to citation in text: [1] [2] -
Marques, F. A.; Lenz, C. A.; Simonelli, F.; Noronha Sales Maia, B. H. L.; Vellasco, A. P.; Eberlin, M. N. J. Nat. Prod. 2004, 67, 1939–1941. doi:10.1021/np049771x
Return to citation in text: [1] -
Birman, V. B.; Li, X. Org. Lett. 2006, 8, 1351–1354. doi:10.1021/ol060065s
Return to citation in text: [1] -
Johnson, C. R.; Adams, J. P.; Braun, M. P.; Senanayake, C. B. W.; Wovkulich, P. M.; Uskoković, M. R. Tetrahedron Lett. 1992, 33, 917–918. doi:10.1016/s0040-4039(00)91575-2
Return to citation in text: [1] -
Daniewski, A. R.; Kiegiel, J. Synth. Commun. 1988, 18, 115–118. doi:10.1080/00397918808057826
Return to citation in text: [1] -
Corey, E. J.; Huang, A. X. J. Am. Chem. Soc. 1999, 121, 710–714. doi:10.1021/ja983179a
Return to citation in text: [1] -
Daniewski, A. R.; Liu, W. J. Org. Chem. 2001, 66, 626–628. doi:10.1021/jo0014414
Return to citation in text: [1] -
Humphreys, D. J.; Newall, C. E.; Paskins, H. A.; Phillipps, G. H. J. Chem. Soc., Perkin Trans. 1 1978, 15–19. doi:10.1039/p19780000015
Return to citation in text: [1] -
Nicolaou, K. C.; Gray, D. L. F.; Montagnon, T.; Harrison, S. T. Angew. Chem., Int. Ed. 2002, 41, 996–1000. doi:10.1002/1521-3773(20020315)41:6<996::aid-anie996>3.0.co;2-i
Return to citation in text: [1] -
Wu, Z.; Xu, X.; Wang, J.; Dong, G. Science 2021, 374, 734–740. doi:10.1126/science.abl7854
Return to citation in text: [1] -
Moher, E. D.; Collins, J. L.; Grieco, P. A. J. Am. Chem. Soc. 1992, 114, 2764–2765. doi:10.1021/ja00033a087
Return to citation in text: [1] -
Ohno, M.; Oguri, I.; Eguchi, S. J. Org. Chem. 1999, 64, 8995–9000. doi:10.1021/jo990704v
Return to citation in text: [1] -
Cernijenko, A.; Risgaard, R.; Baran, P. S. J. Am. Chem. Soc. 2016, 138, 9425–9428. doi:10.1021/jacs.6b06623
Return to citation in text: [1]
| 40. | Daniewski, A. R.; Kiegiel, J. Synth. Commun. 1988, 18, 115–118. doi:10.1080/00397918808057826 |
| 41. | Corey, E. J.; Huang, A. X. J. Am. Chem. Soc. 1999, 121, 710–714. doi:10.1021/ja983179a |
| 42. | Daniewski, A. R.; Liu, W. J. Org. Chem. 2001, 66, 626–628. doi:10.1021/jo0014414 |
| 43. | Humphreys, D. J.; Newall, C. E.; Paskins, H. A.; Phillipps, G. H. J. Chem. Soc., Perkin Trans. 1 1978, 15–19. doi:10.1039/p19780000015 |
| 44. | Nicolaou, K. C.; Gray, D. L. F.; Montagnon, T.; Harrison, S. T. Angew. Chem., Int. Ed. 2002, 41, 996–1000. doi:10.1002/1521-3773(20020315)41:6<996::aid-anie996>3.0.co;2-i |
| 45. | Wu, Z.; Xu, X.; Wang, J.; Dong, G. Science 2021, 374, 734–740. doi:10.1126/science.abl7854 |
| 1. | Heasley, B. Eur. J. Org. Chem. 2011, 19–46. doi:10.1002/ejoc.201001218 |
| 2. | Tan, Q.-G.; Luo, X.-D. Chem. Rev. 2011, 111, 7437–7522. doi:10.1021/cr9004023 |
| 3. | Gualdani, R.; Cavalluzzi, M. M.; Lentini, G.; Habtemariam, S. Molecules 2016, 21, 1530. doi:10.3390/molecules21111530 |
| 4. | Zhang, Y.; Xu, H. RSC Adv. 2017, 7, 35191–35220. doi:10.1039/c7ra04715k |
| 5. | Fu, S.; Liu, B. Org. Chem. Front. 2020, 7, 1903–1947. doi:10.1039/d0qo00203h |
| 6. | Luo, J.; Sun, Y.; Li, Q.; Kong, L. Nat. Prod. Rep. 2022, 39, 1325–1365. doi:10.1039/d2np00015f |
| 12. | Faber, J. M.; Eger, W. A.; Williams, C. M. J. Org. Chem. 2012, 77, 8913–8921. doi:10.1021/jo301182f |
| 25. | Liu, X.; Xu, Y.; Li, L.; Li, J. J. Am. Chem. Soc. 2024, 146, 26243–26250. doi:10.1021/jacs.4c07956 |
| 11. | Veitch, G. E.; Beckmann, E.; Burke, B. J.; Boyer, A.; Maslen, S. L.; Ley, S. V. Angew. Chem., Int. Ed. 2007, 46, 7629–7632. doi:10.1002/anie.200703027 |
| 26. | Zhang, Q.; Satyanandamurty, T.; Shen, L.; Wu, J. Mar. Drugs 2017, 15, 333. doi:10.3390/md15110333 |
| 10. | Corey, E. J.; Hahl, R. W. Tetrahedron Lett. 1989, 30, 3023–3026. doi:10.1016/s0040-4039(00)99392-4 |
| 29. | Rao, P.; Tang, D.; Xia, Q.; Hu, J.; Lin, X.; Xuan, J.; Ding, H. J. Am. Chem. Soc. 2025, 147, 3003–3009. doi:10.1021/jacs.4c16265 |
| 7. | Champagne, D. E.; Koul, O.; Isman, M. B.; Scudder, G. G. E.; Towers, G. H. N. Phytochemistry 1992, 31, 377–394. doi:10.1016/0031-9422(92)90003-9 |
| 8. | Jain, D. C.; Tripathi, A. K. Phytother. Res. 1993, 7, 327–334. doi:10.1002/ptr.2650070502 |
| 9. | Roy, A.; Saraf, S. Biol. Pharm. Bull. 2006, 29, 191–201. doi:10.1248/bpb.29.191 |
| 24. | Mori, N.; Kitahara, T.; Mori, K.; Watanabe, H. Angew. Chem., Int. Ed. 2015, 54, 14920–14923. doi:10.1002/anie.201507935 |
| 16. | Schuppe, A. W.; Newhouse, T. R. J. Am. Chem. Soc. 2017, 139, 631–634. doi:10.1021/jacs.6b12268 |
| 17. | Schuppe, A. W.; Huang, D.; Chen, Y.; Newhouse, T. R. J. Am. Chem. Soc. 2018, 140, 2062–2066. doi:10.1021/jacs.7b13189 |
| 18. | Schuppe, A. W.; Zhao, Y.; Liu, Y.; Newhouse, T. R. J. Am. Chem. Soc. 2019, 141, 9191–9196. doi:10.1021/jacs.9b04508 |
| 19. | Schuppe, A. W.; Liu, Y.; Gonzalez-Hurtado, E.; Zhao, Y.; Jiang, X.; Ibarraran, S.; Huang, D.; Wang, X.; Lee, J.; Loria, J. P.; Dixit, V. D.; Li, X.; Newhouse, T. R. Chem 2022, 8, 2856–2887. doi:10.1016/j.chempr.2022.09.012 |
| 21. | Li, J.; Chen, F.; Renata, H. J. Am. Chem. Soc. 2022, 144, 19238–19242. doi:10.1021/jacs.2c09048 |
| 36. | Ren, H.; Krasovskiy, A.; Knochel, P. Org. Lett. 2004, 6, 4215–4217. doi:10.1021/ol048363h |
| 15. | Lv, C.; Tu, Q.; Gong, J.; Hao, X.; Yang, Z. Tetrahedron 2017, 73, 3612–3621. doi:10.1016/j.tet.2017.03.072 |
| 22. | Deng, H.; Deng, H.; Kim, C.; Li, P.; Wang, X.; Yu, Y.; Qin, T. Nat. Synth. 2023, 3, 378–385. doi:10.1038/s44160-023-00437-w |
| 48. | Cernijenko, A.; Risgaard, R.; Baran, P. S. J. Am. Chem. Soc. 2016, 138, 9425–9428. doi:10.1021/jacs.6b06623 |
| 14. | Lv, C.; Yan, X.; Tu, Q.; Di, Y.; Yuan, C.; Fang, X.; Ben‐David, Y.; Xia, L.; Gong, J.; Shen, Y.; Yang, Z.; Hao, X. Angew. Chem., Int. Ed. 2016, 55, 7539–7543. doi:10.1002/anie.201602783 |
| 46. | Moher, E. D.; Collins, J. L.; Grieco, P. A. J. Am. Chem. Soc. 1992, 114, 2764–2765. doi:10.1021/ja00033a087 |
| 13. | Yamashita, S.; Naruko, A.; Nakazawa, Y.; Zhao, L.; Hayashi, Y.; Hirama, M. Angew. Chem., Int. Ed. 2015, 54, 8538–8541. doi:10.1002/anie.201503794 |
| 20. | Zhang, W.; Zhang, Z.; Tang, J.-C.; Che, J.-T.; Zhang, H.-Y.; Chen, J.-H.; Yang, Z. J. Am. Chem. Soc. 2020, 142, 19487–19492. doi:10.1021/jacs.0c10122 |
| 47. | Ohno, M.; Oguri, I.; Eguchi, S. J. Org. Chem. 1999, 64, 8995–9000. doi:10.1021/jo990704v |
| 29. | Rao, P.; Tang, D.; Xia, Q.; Hu, J.; Lin, X.; Xuan, J.; Ding, H. J. Am. Chem. Soc. 2025, 147, 3003–3009. doi:10.1021/jacs.4c16265 |
| 27. | Lebold, T. P.; Gallego, G. M.; Marth, C. J.; Sarpong, R. Org. Lett. 2012, 14, 2110–2113. doi:10.1021/ol300647k |
| 28. | Becica, J.; Ra̅ciņš, O.; Ivanova, M.; Jirgensons, A. J. Org. Chem. 2023, 88, 10306–10309. doi:10.1021/acs.joc.3c00952 |
| 18. | Schuppe, A. W.; Zhao, Y.; Liu, Y.; Newhouse, T. R. J. Am. Chem. Soc. 2019, 141, 9191–9196. doi:10.1021/jacs.9b04508 |
| 19. | Schuppe, A. W.; Liu, Y.; Gonzalez-Hurtado, E.; Zhao, Y.; Jiang, X.; Ibarraran, S.; Huang, D.; Wang, X.; Lee, J.; Loria, J. P.; Dixit, V. D.; Li, X.; Newhouse, T. R. Chem 2022, 8, 2856–2887. doi:10.1016/j.chempr.2022.09.012 |
| 39. | Johnson, C. R.; Adams, J. P.; Braun, M. P.; Senanayake, C. B. W.; Wovkulich, P. M.; Uskoković, M. R. Tetrahedron Lett. 1992, 33, 917–918. doi:10.1016/s0040-4039(00)91575-2 |
| 37. | Marques, F. A.; Lenz, C. A.; Simonelli, F.; Noronha Sales Maia, B. H. L.; Vellasco, A. P.; Eberlin, M. N. J. Nat. Prod. 2004, 67, 1939–1941. doi:10.1021/np049771x |
| 35. | Fernández-Mateos, A.; Grande Benito, M.; Pascual Coca, G.; Rubio González, R.; Tapia Hernández, C. Tetrahedron 1995, 51, 7521–7526. doi:10.1016/0040-4020(95)00375-i |
| 36. | Ren, H.; Krasovskiy, A.; Knochel, P. Org. Lett. 2004, 6, 4215–4217. doi:10.1021/ol048363h |
| 30. | Hovey, M. T.; Cohen, D. T.; Walden, D. M.; Cheong, P. H.-Y.; Scheidt, K. A. Angew. Chem., Int. Ed. 2017, 56, 9864–9867. doi:10.1002/anie.201705308 |
| 31. | Sasano, Y.; Koyama, J.; Yoshikawa, K.; Kanoh, N.; Kwon, E.; Iwabuchi, Y. Org. Lett. 2018, 20, 3053–3056. doi:10.1021/acs.orglett.8b01087 |
| 32. | Grant, T. N.; Rieder, C. J.; West, F. G. Chem. Commun. 2009, 5676–5688. doi:10.1039/b908515g |
| 33. | Yadykov, A. V.; Shirinian, V. Z. Adv. Synth. Catal. 2020, 362, 702–723. doi:10.1002/adsc.201901001 |
| 34. | Trudel, V.; Tien, C.-H.; Trofimova, A.; Yudin, A. K. Nat. Rev. Chem. 2021, 5, 604–623. doi:10.1038/s41570-021-00304-2 |
© 2025 Zhang et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.