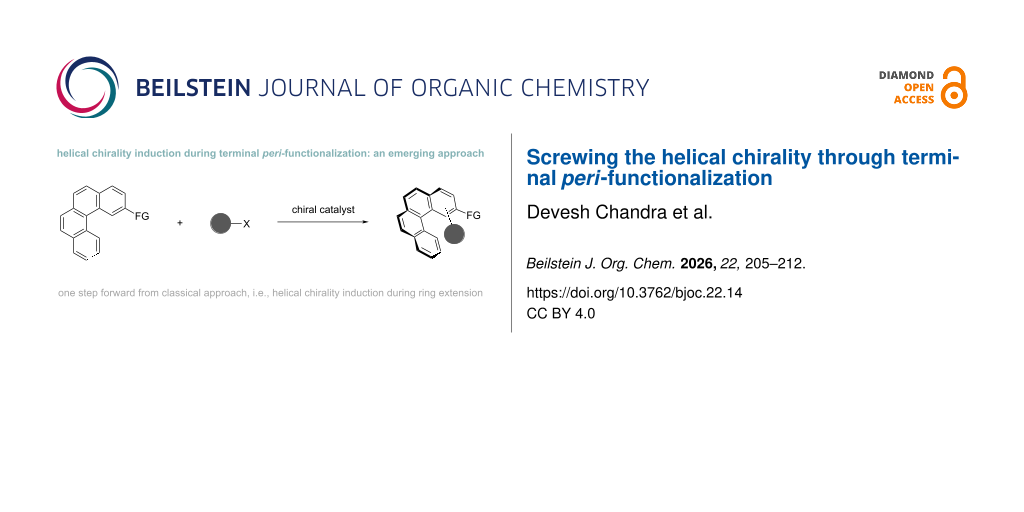
Abstract
Helical chirality is an important, but underrated form of chirality than other types of chirality. Molecules with helical chirality impart a crucial role in several biological phenomena as well as in modern materials applications. Classically, the generation of chiral helicity in organic molecules relies on a ring extension by means of cycloaddition and related reactions. Recently, the peri-functionalization approach paved a new pathway for the generation of chiral helical molecules. In this article, we highlight the key advancements in these parallel approaches for the generation of helical chiral architectures.
Graphical Abstract
Introduction
Nature utilizes helical chirality for the inception of life. The double-helical structure of DNA and the helical conformations of proteins are key examples of the helical chirality. Inspired by these natural systems, chemists have developed methods to construct and control helicity in synthetic systems. These methods aim to achieve precise enantioselectivity, enabling the synthesis of helical molecules with specific handedness. In modern day science, helical chirality is gaining more and more importance in various fields such as drug design, optoelectronics, spintronics, diagnosis tool, biosensors and other forms of the materials sciences continuing its relevance to cutting edge research [1,2]. However, precise control and manipulation of helical chirality in synthetic systems remain technically challenging. This complexity may have led to underestimation of its potential, as research frequently focused toward other types of chirality, like point chirality [3], axial chirality [4], and planar chirality [5-7], often overshadowing the potential of helical chirality. Point chirality has major applications in medicinal chemistry and several drug molecules contain point chirality centers in their core structure [8]. Point chirality is the simplest form of chirality, and it is relatively easy to control and characterize during the synthesis of organic molecules. Due to these facts point chirality holds a central position among the different types of chirality. Axial chirality is another important type of chirality, where the chirality is controlled by restricted rotation among an axis. These types of molecules play an important role as chiral ligands in organic synthesis, i.e., BINOL, BINAP, etc. [9-11]. Another form of chirality, where chirality depends on a constrained substitution pattern along the plane of symmetry, is known as planar chirality, as seen in metallocene and cyclophanes. Molecules containing planar chirality have greater relevance in materials science and stereoselective transformations [12,13].
The fourth branch of the chiral tree is helical chirality. A helical system can undergo molecular to macroscopic systems due to its three-dimensional nature. Thus, helical systems, require more precise control of chirality compared to other forms of chirality. Helical chiral molecules have recently been found to display functional advantages, i.e., chirality-induced spin selectivity. These functionalities have great potential to transform in key areas such as drug discovery and modern diagnostic tools, as well as in distinct emerging fields like spintronics and optoelectronics [14].
Helicenes have found applications in chiral optoelectronics, i.e., as circularly polarized organic light emitting diodes (OLEDs), circularly polarized luminescence (CPL), and photonic devices, etc., due to their strong circular dichroism and circularly polarized luminescence [15,16]. Their well-defined three-dimensional chiral environment also makes helicenes useful in asymmetric synthesis, where they serve as chiral ligands and organocatalysts, delivering high enantioselectivities [17,18]. Their tunable HOMO–LUMO gaps and controlled π–π interactions, is key to the use of helicenes in molecular electronics and organic semiconductors as well. Helicenes function as active components in organic field effect transistors (OFETs), molecular wires, and chiral conductive materials [19-21]. Furthermore, helicenes are widely explored in chiroptical sensing, molecular recognition, and supramolecular self-assembly, enabling the construction of next generation responsive materials [22,23]. A very recent study also highlighted their potential application in biological interfaces [1], thus highlighting the versatility of helicenes in medicine along with next-generation materials and catalytic systems.
Due to the above-mentioned applications of chiral helicenes various synthetic approaches have been developed for the synthesis of chiral helical molecules and the most prevalent approaches include cycloaddition reactions [24], ring extensions [25], and related approaches [26,27]. However, some parallel and more sustainable approaches have emerged in recent years and C–H functionalizations are one of these approaches.
In the following section, we discuss the parallel approaches, i.e., peri-C–H functionalization, over the prevalent approach for the generation of helical chirality, which generally considers π extension. The peri-C–H functionalization approach provides direct access to a functional group installed in the terminal core of helicened with high level of stereocontrol, rather than merely increasing the number of fused rings in a helical system. The direct introduction of suitable functional groups at strategic positions can expand the structure diversity and enhance the utility of the parent helicene in diverse areas, thereby offering a more versatile alternative to classical helicene extension strategies.
Discussion
Only a handful attempts have been made for the asymmetric synthesis of helical molecules using π conjugation extension [28-30]. This prevalent method for the generation of helical chirality primarily involves cycloaddition and central/axial to helical chirality transfer reactions [31] (Figure 1A and B). In addition to this, a parallel strategy also offers a promising approach to achieving configurationally stable helicenes via terminal peri-functionalization, where a bulkier handle provides the required enantiomerization barrier to screw helical chirality (Figure 1C and D).
Figure 1: Representation of the general practices for the induction of helical chirality in organic scaffolds. A) Prelevant approach for the induction of helical chirality which majorly relies on the π extension of organic scaffolds via annulation/cycloaddition and B) central/axial to helical chirality conversion. C) Induction of helical chirality in [4]helicene via terminal peri-functionalization. D) Induction of helical chirality in [5]helicene via terminal peri-functionalization where a bulkier handle provides the required enantiomerization barrier to screw helicity.
Figure 1: Representation of the general practices for the induction of helical chirality in organic scaffolds...
A straight forward preparation of various carbohelicenes via an organocatalytic enantioselective hydroamination reaction of polyaromatic phenols with diazodicarboxamide derivatives through a catalytic kinetic resolution (KR) process was developed by Liu and co-workers [32]. The authors screened a range of organocatalysts, i.e., Takemoto’s thiourea catalyst, (S,S)-1,2-cyclohexanediamine-derived thioureas, cinchona alkaloid-derived thiourea, and squaramides to achieve the desired outcome of the reaction. After rigorous testing, (S,S)-1,2-cyclohexanediamine-derived catalyst L1 was found to be the most effective organocatalyst to induce both the helical chirality and remote axial chirality during the functionalization of the [4]- and [5]helicenes 3a–k with good yield and excellent enantioselectivity. Notably, [6]helicene 3k was also readily produced, via kinetic resolution (Scheme 1).
Scheme 1: Asymmetric synthesis of carbohelicenes via peri-C–H functionalization.
Scheme 1: Asymmetric synthesis of carbohelicenes via peri-C–H functionalization.
Computational studies suggested that hydrogen bonding and π interactions between the reactants and catalyst L1 controlled the stereochemical output of the products 3. The catalyst proximate both reactants to each other via hydrogen-bonding interaction, locking the relative orientation of the substrates producing the chiral [4]carbohelicene via peri-terminal functionalization (Figure 2).
![[1860-5397-22-14-2]](/bjoc/content/figures/1860-5397-22-14-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Stereomodel for peri-terminal functionalization of carbohelicene. (Figure 2 was reproduced from [32] (© 2024 X. Liu et al., published by Springer Nature, distributed under the terms of the Creative Commons Attribution 4.0 International License, https://creativecommons.org/licenses/by/4.0).
Figure 2: Stereomodel for peri-terminal functionalization of carbohelicene. (Figure 2 was reproduced from [32] (© 2024 X....
To examine the utility of the developed protocol, the reaction was scaled up to a 2 mmol scale and the product was further transformed into representative derivatives. Notably, the reaction performed well in the scale-up demonstration and the post-synthetic transformations proceeded smoothly without hampering the enantiomeric excess (Scheme 2).
Scheme 2: Scale-up synthesis and post-synthetic application of chiral helical product 3.
Scheme 2: Scale-up synthesis and post-synthetic application of chiral helical product 3.
Another report applying peri-C–H functionalization for the synthesis of helical chiral entities was reported by Wang and co-workers. They reported an organocatalyzed asymmetric synthesis of phosphorus-containing chiral helicenes enabled by dynamic kinetic resolution using copper and peptide-mimetic phosphonium salts, i.e. amino acid-derived phosphonium iodide and bromide as catalysts [33]. A domino reaction was developed, beginning with a PPS L2-catalyzed Michael addition of phosphine oxides 7 to nitro-substituted oxa[5]helicenes 6, followed by a copper-promoted aromatization. This sequence efficiently produced phosphorus-containing oxa[5]helicene derivatives 8 in high yields and with excellent enantioselectivities. The catalytic system operated under mild conditions within a practical timeframe and demonstrated broad substrate tolerance, providing a range of chiral helicenes. Notably, unsymmetrical phosphine oxides also underwent smooth Michael addition, with excellent enantiomeric excess and diastereomeric ratio (Scheme 3).
Scheme 3: Asymmetric peri-C–H functionalization of nitro-substituted oxa[5]helicenes.
Scheme 3: Asymmetric peri-C–H functionalization of nitro-substituted oxa[5]helicenes.
The developed protocol was also tested for up-scale synthesis and post-synthetic transformations. Notably, the reaction worked well at a 4.0 mmol scale providing product 8g in quantitative yield and >99% ee. This product was further reduced to the corresponding amine followed by N-tosylation, providing product 10 in 63% yield without altering the enantiomeric excess (Scheme 4).
Scheme 4: Scale-up synthesis and post-synthetic application of chiral helical product 8g.
Scheme 4: Scale-up synthesis and post-synthetic application of chiral helical product 8g.
Additionally, product 8g was converted into the chiral phosphine ligand 12 while fully preserving its helical chirality and successfully applied in palladium-catalyzed nucleophilic substitution reactions (Scheme 5).
Scheme 5: Post-synthetic transformation of product 8g to chiral phosphine ligand 12 and its application in Pd-catalyzed reactions.
Scheme 5: Post-synthetic transformation of product 8g to chiral phosphine ligand 12 and its application in Pd...
A stereochemical model was proposed for the phospha-Michael addition, indicating that enantioselectivity was primarily influenced by steric effects arising from hydrogen bonding and ion pairing between the peptide mimetic phosphonium salt (PPS)-activated phosphine oxide and the nitro-functionalized oxa[5]helicene. This ion-pairing interaction was also studied using NMR titration and Job’s plot analysis. The transition state depicted in Figure 3 was found to be most favorable providing the product with M-configuration.
![[1860-5397-22-14-3]](/bjoc/content/figures/1860-5397-22-14-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Stereomodel for the asymmetric peri-C–H functionalization of oxa[5]helicene. (Figure 3 was reproduced from [33], J.-H. Wu et al., Angew. Chem., Int. Ed., with permission from John Wiley and Sons. Copyright © 2023 Wiley-VCH GmbH. This content is not subject to CC BY 4.0)
Figure 3: Stereomodel for the asymmetric peri-C–H functionalization of oxa[5]helicene. (Figure 3 was reproduced from [33]...
Looking ahead, these strategies may also be applicable to other polyaromatic frameworks beyond specific helicenes for the construction of helically chiral structures.
Outlook
Despite its potential, this strategy has faced significant challenges in the context of catalytic enantioselective synthesis of functionalized helicenes and their heteroanalogues. These challenges include: (1) the limited availability of suitable substrates, (2) substantial steric hindrance from the terminal ring, making it difficult to introduce substituents at the required positions onto helical precursors, and (3) the complex nature of controlling helical chirality, a nanoscale phenomenon that requires the design of advanced catalysts to achieve high enantioselectivity. Although the parallel approach is still in its infancy, it holds immense promise. With the continued and widespread efforts of synthetic chemists, we anticipate that the existing challenges will be surmounted, paving the way for the development of innovative approaches and new materials. Such advancements are expected to unlock the full potential of chiral helicenes.
Data Availability Statement
Data sharing is not applicable as no new data was generated or analyzed in this study.
References
-
Bouz, G.; Žádný, J.; Storch, J.; Vacek, J. Biotechnol. Adv. 2025, 79, 108513. doi:10.1016/j.biotechadv.2024.108513
Return to citation in text: [1] [2] -
Lee, H.; Kim, D.; Cho, J. H.; Lim, J. A. Acc. Mater. Res. 2025, 6, 434–446. doi:10.1021/accountsmr.4c00374
Return to citation in text: [1] -
Kagan, H. B. Chiral Ligands for Asymmtric Catalysis. In Asymmetric Synthesis; Morrison, J. D., Ed.; Academic Press: San Diego, CA, USA, 1985. doi:10.1016/b978-0-08-092493-9.50006-2
Return to citation in text: [1] -
Kozlowski, M. C.; Miller, S. J.; Perreault, S. Acc. Chem. Res. 2023, 56, 187–188. doi:10.1021/acs.accounts.2c00765
Return to citation in text: [1] -
Colacot, T. J. Chem. Rev. 2003, 103, 3101–3118. doi:10.1021/cr000427o
Return to citation in text: [1] -
López, R.; Palomo, C. Angew. Chem., Int. Ed. 2022, 61, e202113504. doi:10.1002/anie.202113504
Return to citation in text: [1] -
Chandra, D.; Sharma, T.; Sarthi; Sachin; Sharma, U. J. Catal. 2024, 439, 115756. doi:10.1016/j.jcat.2024.115756
Return to citation in text: [1] -
Brooks, H.; Guida, W. C.; Daniel, K. G. Curr. Top. Med. Chem. 2011, 11, 760–770. doi:10.2174/156802611795165098
Return to citation in text: [1] -
Trost, B. M. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5348–5355. doi:10.1073/pnas.0306715101
Return to citation in text: [1] -
Sumit; Chandra, D.; Sharma, U. Org. Biomol. Chem. 2021, 19, 4014–4026. doi:10.1039/d1ob00232e
Return to citation in text: [1] -
Sumit; Chandra, D.; Sharma, U. Chem. Commun. 2023, 59, 9288–9300. doi:10.1039/d3cc02293e
Return to citation in text: [1] -
Patra, M.; Gasser, G. Nat. Rev. Chem. 2017, 1, 0066. doi:10.1038/s41570-017-0066
Return to citation in text: [1] -
Dai, L.-X.; Hou, X.-L., Eds. Chiral Ferrocenes in Asymmetric Catalysis; Wiley-VCH: Weinheim, Germany, 2010. doi:10.1002/9783527628841
Return to citation in text: [1] -
Bloom, B. P.; Paltiel, Y.; Naaman, R.; Waldeck, D. H. Chem. Rev. 2024, 124, 1950–1991. doi:10.1021/acs.chemrev.3c00661
Return to citation in text: [1] -
Qiu, M.; Du, J.; Yao, N.-T.; Wang, X.-Y.; Gong, H.-Y. Beilstein J. Org. Chem. 2025, 21, 1422–1453. doi:10.3762/bjoc.21.106
Return to citation in text: [1] -
Meng, D.; Fu, H.; Xiao, C.; Meng, X.; Winands, T.; Ma, W.; Wei, W.; Fan, B.; Huo, L.; Doltsinis, N. L.; Li, Y.; Sun, Y.; Wang, Z. J. Am. Chem. Soc. 2016, 138, 10184–10190. doi:10.1021/jacs.6b04368
Return to citation in text: [1] -
Peng, Z.; Takenaka, N. Chem. Rec. 2013, 13, 28–42. doi:10.1002/tcr.201200010
Return to citation in text: [1] -
Chen, C.-F.; Shen, Y. Helicenes in Catalysis. Helicene Chemistry; Springer: Berlin, Heidelberg, Germany, 2017; pp 187–199. doi:10.1007/978-3-662-53168-6_9
Return to citation in text: [1] -
de Ara, T.; Hsu, C.; Martinez-Garcia, A.; Baciu, B. C.; Bronk, P. J.; Ornago, L.; van der Poel, S.; Lombardi, E. B.; Guijarro, A.; Sabater, C.; Untiedt, C.; van der Zant, H. S. J. J. Phys. Chem. Lett. 2024, 15, 8343–8350. doi:10.1021/acs.jpclett.4c01425
Return to citation in text: [1] -
He, P.; Ye, J.; Zhang, J.; Lu, T.; Cui, W.; Liu, J.; Shen, C.; Hong, W.; Liu, X. Angew. Chem., Int. Ed. 2025, 64, e202416319. doi:10.1002/anie.202416319
Return to citation in text: [1] -
Yang, Y.; da Costa, R. C.; Fuchter, M. J.; Campbell, A. J. Nat. Photonics 2013, 7, 634–638. doi:10.1038/nphoton.2013.176
Return to citation in text: [1] -
Kanao, E.; Murata, Y.; Miwa, S.; Takikawa, H.; Takasu, K.; Ishihama, Y.; Kubo, T. Anal. Chem. (Washington, DC, U. S.) 2025, 97, 12690–12698. doi:10.1021/acs.analchem.5c01385
Return to citation in text: [1] -
Yang, D.-C.; Li, M.; Chen, C.-F. Chem. Commun. 2017, 53, 9336–9339. doi:10.1039/c7cc03519e
Return to citation in text: [1] -
Stará, I. G.; Starý, I. Acc. Chem. Res. 2020, 53, 144–158. doi:10.1021/acs.accounts.9b00364
Return to citation in text: [1] -
Kötzner, L.; Webber, M. J.; Martínez, A.; De Fusco, C.; List, B. Angew. Chem., Int. Ed. 2014, 53, 5202–5205. doi:10.1002/anie.201400474
Return to citation in text: [1] -
Liu, P.; Bao, X.; Naubron, J.-V.; Chentouf, S.; Humbel, S.; Vanthuyne, N.; Jean, M.; Giordano, L.; Rodriguez, J.; Bonne, D. J. Am. Chem. Soc. 2020, 142, 16199–16204. doi:10.1021/jacs.0c07995
Return to citation in text: [1] -
Sako, M.; Takeuchi, Y.; Tsujihara, T.; Kodera, J.; Kawano, T.; Takizawa, S.; Sasai, H. J. Am. Chem. Soc. 2016, 138, 11481–11484. doi:10.1021/jacs.6b07424
Return to citation in text: [1] -
Wang, Y.; Wu, Z.-G.; Shi, F. Chem Catal. 2022, 2, 3077–3111. doi:10.1016/j.checat.2022.10.011
Return to citation in text: [1] -
Liu, P.; Bao, X.; Naubron, J.-V.; Chentouf, S.; Humbel, S.; Vanthuyne, N.; Jean, M.; Giordano, L.; Rodriguez, J.; Bonne, D. J. Am. Chem. Soc. 2020, 142, 16199–16204. doi:10.1021/jacs.0c07995
Return to citation in text: [1] -
Gaucherand, A.; Yen-Pon, E.; García-López, D.; Naubron, J.-V.; Chentouf, S.; Giorgi, M.; Humbel, S.; Jean, M.; Rodriguez, J.; Bonne, D. Chem. Sci. 2024, 15, 7300–7307. doi:10.1039/d4sc00745j
Return to citation in text: [1] -
Huang, Q.; Tang, Y.-P.; Zhang, C.-G.; Wang, Z.; Dai, L. ACS Catal. 2024, 14, 16256–16265. doi:10.1021/acscatal.4c05345
Return to citation in text: [1] -
Liu, X.; Zhu, B.; Zhang, X.; Zhu, H.; Zhang, J.; Chu, A.; Wang, F.; Wang, R. Nat. Commun. 2024, 15, 732. doi:10.1038/s41467-024-45049-w
Return to citation in text: [1] [2] -
Wu, J.-H.; Fang, S.; Zheng, X.; He, J.; Ma, Y.; Su, Z.; Wang, T. Angew. Chem., Int. Ed. 2023, 62, e202309515. doi:10.1002/anie.202309515
Return to citation in text: [1] [2]
| 32. | Liu, X.; Zhu, B.; Zhang, X.; Zhu, H.; Zhang, J.; Chu, A.; Wang, F.; Wang, R. Nat. Commun. 2024, 15, 732. doi:10.1038/s41467-024-45049-w |
| 28. | Wang, Y.; Wu, Z.-G.; Shi, F. Chem Catal. 2022, 2, 3077–3111. doi:10.1016/j.checat.2022.10.011 |
| 29. | Liu, P.; Bao, X.; Naubron, J.-V.; Chentouf, S.; Humbel, S.; Vanthuyne, N.; Jean, M.; Giordano, L.; Rodriguez, J.; Bonne, D. J. Am. Chem. Soc. 2020, 142, 16199–16204. doi:10.1021/jacs.0c07995 |
| 30. | Gaucherand, A.; Yen-Pon, E.; García-López, D.; Naubron, J.-V.; Chentouf, S.; Giorgi, M.; Humbel, S.; Jean, M.; Rodriguez, J.; Bonne, D. Chem. Sci. 2024, 15, 7300–7307. doi:10.1039/d4sc00745j |
| 31. | Huang, Q.; Tang, Y.-P.; Zhang, C.-G.; Wang, Z.; Dai, L. ACS Catal. 2024, 14, 16256–16265. doi:10.1021/acscatal.4c05345 |
| 1. | Bouz, G.; Žádný, J.; Storch, J.; Vacek, J. Biotechnol. Adv. 2025, 79, 108513. doi:10.1016/j.biotechadv.2024.108513 |
| 2. | Lee, H.; Kim, D.; Cho, J. H.; Lim, J. A. Acc. Mater. Res. 2025, 6, 434–446. doi:10.1021/accountsmr.4c00374 |
| 8. | Brooks, H.; Guida, W. C.; Daniel, K. G. Curr. Top. Med. Chem. 2011, 11, 760–770. doi:10.2174/156802611795165098 |
| 25. | Kötzner, L.; Webber, M. J.; Martínez, A.; De Fusco, C.; List, B. Angew. Chem., Int. Ed. 2014, 53, 5202–5205. doi:10.1002/anie.201400474 |
| 5. | Colacot, T. J. Chem. Rev. 2003, 103, 3101–3118. doi:10.1021/cr000427o |
| 6. | López, R.; Palomo, C. Angew. Chem., Int. Ed. 2022, 61, e202113504. doi:10.1002/anie.202113504 |
| 7. | Chandra, D.; Sharma, T.; Sarthi; Sachin; Sharma, U. J. Catal. 2024, 439, 115756. doi:10.1016/j.jcat.2024.115756 |
| 26. | Liu, P.; Bao, X.; Naubron, J.-V.; Chentouf, S.; Humbel, S.; Vanthuyne, N.; Jean, M.; Giordano, L.; Rodriguez, J.; Bonne, D. J. Am. Chem. Soc. 2020, 142, 16199–16204. doi:10.1021/jacs.0c07995 |
| 27. | Sako, M.; Takeuchi, Y.; Tsujihara, T.; Kodera, J.; Kawano, T.; Takizawa, S.; Sasai, H. J. Am. Chem. Soc. 2016, 138, 11481–11484. doi:10.1021/jacs.6b07424 |
| 4. | Kozlowski, M. C.; Miller, S. J.; Perreault, S. Acc. Chem. Res. 2023, 56, 187–188. doi:10.1021/acs.accounts.2c00765 |
| 1. | Bouz, G.; Žádný, J.; Storch, J.; Vacek, J. Biotechnol. Adv. 2025, 79, 108513. doi:10.1016/j.biotechadv.2024.108513 |
| 3. | Kagan, H. B. Chiral Ligands for Asymmtric Catalysis. In Asymmetric Synthesis; Morrison, J. D., Ed.; Academic Press: San Diego, CA, USA, 1985. doi:10.1016/b978-0-08-092493-9.50006-2 |
| 24. | Stará, I. G.; Starý, I. Acc. Chem. Res. 2020, 53, 144–158. doi:10.1021/acs.accounts.9b00364 |
| 15. | Qiu, M.; Du, J.; Yao, N.-T.; Wang, X.-Y.; Gong, H.-Y. Beilstein J. Org. Chem. 2025, 21, 1422–1453. doi:10.3762/bjoc.21.106 |
| 16. | Meng, D.; Fu, H.; Xiao, C.; Meng, X.; Winands, T.; Ma, W.; Wei, W.; Fan, B.; Huo, L.; Doltsinis, N. L.; Li, Y.; Sun, Y.; Wang, Z. J. Am. Chem. Soc. 2016, 138, 10184–10190. doi:10.1021/jacs.6b04368 |
| 19. | de Ara, T.; Hsu, C.; Martinez-Garcia, A.; Baciu, B. C.; Bronk, P. J.; Ornago, L.; van der Poel, S.; Lombardi, E. B.; Guijarro, A.; Sabater, C.; Untiedt, C.; van der Zant, H. S. J. J. Phys. Chem. Lett. 2024, 15, 8343–8350. doi:10.1021/acs.jpclett.4c01425 |
| 20. | He, P.; Ye, J.; Zhang, J.; Lu, T.; Cui, W.; Liu, J.; Shen, C.; Hong, W.; Liu, X. Angew. Chem., Int. Ed. 2025, 64, e202416319. doi:10.1002/anie.202416319 |
| 21. | Yang, Y.; da Costa, R. C.; Fuchter, M. J.; Campbell, A. J. Nat. Photonics 2013, 7, 634–638. doi:10.1038/nphoton.2013.176 |
| 33. | Wu, J.-H.; Fang, S.; Zheng, X.; He, J.; Ma, Y.; Su, Z.; Wang, T. Angew. Chem., Int. Ed. 2023, 62, e202309515. doi:10.1002/anie.202309515 |
| 14. | Bloom, B. P.; Paltiel, Y.; Naaman, R.; Waldeck, D. H. Chem. Rev. 2024, 124, 1950–1991. doi:10.1021/acs.chemrev.3c00661 |
| 22. | Kanao, E.; Murata, Y.; Miwa, S.; Takikawa, H.; Takasu, K.; Ishihama, Y.; Kubo, T. Anal. Chem. (Washington, DC, U. S.) 2025, 97, 12690–12698. doi:10.1021/acs.analchem.5c01385 |
| 23. | Yang, D.-C.; Li, M.; Chen, C.-F. Chem. Commun. 2017, 53, 9336–9339. doi:10.1039/c7cc03519e |
| 12. | Patra, M.; Gasser, G. Nat. Rev. Chem. 2017, 1, 0066. doi:10.1038/s41570-017-0066 |
| 13. | Dai, L.-X.; Hou, X.-L., Eds. Chiral Ferrocenes in Asymmetric Catalysis; Wiley-VCH: Weinheim, Germany, 2010. doi:10.1002/9783527628841 |
| 32. | Liu, X.; Zhu, B.; Zhang, X.; Zhu, H.; Zhang, J.; Chu, A.; Wang, F.; Wang, R. Nat. Commun. 2024, 15, 732. doi:10.1038/s41467-024-45049-w |
| 9. | Trost, B. M. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5348–5355. doi:10.1073/pnas.0306715101 |
| 10. | Sumit; Chandra, D.; Sharma, U. Org. Biomol. Chem. 2021, 19, 4014–4026. doi:10.1039/d1ob00232e |
| 11. | Sumit; Chandra, D.; Sharma, U. Chem. Commun. 2023, 59, 9288–9300. doi:10.1039/d3cc02293e |
| 17. | Peng, Z.; Takenaka, N. Chem. Rec. 2013, 13, 28–42. doi:10.1002/tcr.201200010 |
| 18. | Chen, C.-F.; Shen, Y. Helicenes in Catalysis. Helicene Chemistry; Springer: Berlin, Heidelberg, Germany, 2017; pp 187–199. doi:10.1007/978-3-662-53168-6_9 |
| 33. | Wu, J.-H.; Fang, S.; Zheng, X.; He, J.; Ma, Y.; Su, Z.; Wang, T. Angew. Chem., Int. Ed. 2023, 62, e202309515. doi:10.1002/anie.202309515 |
© 2026 Chandra et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.