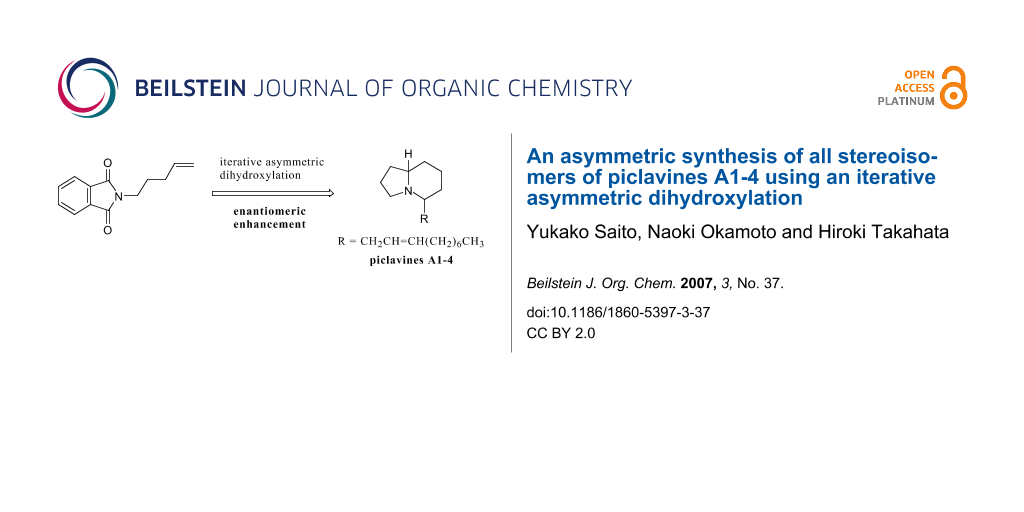
Abstract
The asymmetric synthesis of both enantiomers of piclavines A1, A2, A3, and A4 has been achieved using an iterative asymmetric dihydroxylation with enantiomeric enhancement.
Graphical Abstract
Background
Indolizidine units are frequently found in many natural products and designed bioactive molecules. [1] Among these alkaloids, piclavines A1-4 (Figure 1), extracted from the tunicate Clavelina picta and the first indolizidine alkaloids to be found in the marine biosphere, exhibit interesting antimicrobial activities. [2] However, very little effort has been made to synthesize the piclavines. So far, among the four isomers shown in Figure 1, the synthesis of piclavine A4 [3] and a mixture of piclavines A1 and A2 [4] has been reported, but the synthesis of all four isomers has never been reported. In addition, their biological activities have been evaluated as a mixture of piclavines A1-4. [2] Therefore, we were inspired to develop a comprehensive synthetic program for these alkaloids.
The asymmetric synthesis of an indolizidine ring remains a great challenge. Our interest in this field has been focused on potential strategies based on the enantiomeric enhancement caused by the twofold or more application of the Sharpless asymmetric dihydroxylation (AD) [5,6] or Brown's asymmetric allylboration[7] reactions. In general, the enantiomeric excesses (ees) obtained for AD of terminal olefins are lower than for trans disubstituted olefins. However, it is expected that iterative AD terminal olefins will give products with high ees based on the following consideration. The first AD (AD-mix-β) of 5 produces major and minor enantiomers, 6 and ent-6, which are elaborated by introduction of terminal olefins to afford 7 and ent-7, respectively. The second AD of a mixture of 7 and ent-7 provides four products 8, 9, ent-9, and ent-8. The relationship between 8 and 9 is diastereomeric. Since very little of the mirror image compound ent-8 is prepared, the ee of the major product 8 will be very high. On the other hand, the ee of the minor diastereomer 9 or ent-9 will be a low (Scheme 1). In most cases, when the products prepared by the iterative AD are acyclic and their asymmetric centers are remote, it is difficult to separate two diastereomers. Since transformation of acyclic compounds to cyclic derivatives provides rigid conformation and causes close proximity between two chiral centers, it is expected to greatly facilitate separation of two diastereomers. In this line, we report a full paper describing a new synthesis of all stereoisomers of piclavines A1-4 with high enantiomeric purity (amplification) for the major diastereomer via iterative AD reaction of terminal olefins. [8]
Scheme 1: Enantiomeric enhancement by iterative AD.
Scheme 1: Enantiomeric enhancement by iterative AD.
Results
Actually we developed a general access to 5-substituted indolizidines 10 (all four stereoisomers of indolizidine 209D) with high enantio-enhancement (92–98% ee) via a sequence of iterative AD reactions starting from an achiral N-pentenylphthalimide (11). [9] The two stereogenic centers in 10 were constructed with high enantio-enhancement via a sequence of twofold AD reactions as shown in Scheme 2.
Scheme 2: Asymmetric synthesis of indolidines 209D using an iterative AD.
Scheme 2: Asymmetric synthesis of indolidines 209D using an iterative AD.
We embarked on the synthesis of piclavines A1-4 using the four stereoisomers of the epoxides 15[9] derived from 11 according to our reported procedure as synthetic intermediates. Regioselective cleavage of the epoxide (2R-[4S])-15 rings with lithium acetylide generated from 1-nonyne with n-butyl lithium in combination with BF3-Et2O[10] gave the secondary alcohols (2R-[4S])-16 in 94%. It is impossible to utilize hydrogenolysis due to reactivity of the acetylene unit. Indeed, the N-protecting group exchange of benzyloxycarbonyl (Cbz) for 2,2,2-trichloroethoxycarbonyl (Troc) in 17 was examined. The use of basic reagents such as Ba(OH)2[11] and KOH[12] failed to afford clean deprotection of Cbz. However, treatment of (2R-[4S])-16 with iodotrimethylsilane (TMSI)[13] in CH3CN provided the amine (75%), which was treated with TrocCl/K2CO3 to afford the Troc carbamates (2R-[4S])-17. After mesylation of [2S-(4R)]-17, N-deprotection of the resulting mesylate with 10% Cd-Pb[14] gave the desired (5R,8aR)-18 {[α]25D +9.03 (c 0.32, CH3OH)} as a major product and (5R,8aS)-18 {[α]25D −22.7 (c 1.54, CH2Cl2)} as a minor product in a ratio of 2.3:1 in 25% overall yield. As expected, at this stage it was possible to separate the two diastereomers because transformation of monocyclic compounds (pyrrolidines) to bicyclic derivatives (indolizidines) provides rigid conformation and causes close proximity (a change from 1,5- to 1,3-relationship) between the two asymmetric centers. With indolizidine (5R, 8aR)-18 in hand, we examined partial-reduction of their triple bonds. First, treatment of (5R, 8aR)-18 with sodium in liquid ammonia gave the desired piclavine A1 (1) {[α]25D −5.6 (c 0.84, CH2Cl2)} containing a trans-olefin in 71% yield. Exposure of hydrogen to (5R, 8aR)-18 in the presence of Lindlar catalyst (Pd/CaCO3/Pb) or Rosenmund catalyst (5% Pd-BaSO4) was carried out in order to obtain a cis olefin product. However, hydrogenation using 10 g% catalysts scarcely proceeded, because the tertiary amine in indolizidine presumably works as a poison of the catalyst. Accordingly, the use of a large amount (50 g%) of 5% Pd-BaSO4 took place with semi-reduction of (5R,8aR)-18 to provide piclavine A2 (2) {[α]25D +4.03 (c 0.21 CH2Cl2)} in 53% yield (Scheme 3). The 1H and 13C NMR spectra of synthetic piclavines A1 and A2 are in good agreement with those of a mixture of natural piclavines A1 and A2. [2]
Scheme 3: Asymmetric synthesis of piclavines A1 and A2 from (2R-[4S])-15.
Scheme 3: Asymmetric synthesis of piclavines A1 and A2 from (2R-[4S])-15.
A similar sequence of the epoxide (2R-[4R])-15 prepared by (DHQD)2-PYR ligand-induced AD reaction of (R)-13 afforded the desired (5S,8aR)-18 {[α]25D −67.5 (c 1.11, CH2Cl2)} as a major product and (5S,8aS)-18 {[α]25D −3.11 (c 0.62, CH3OH)} as a minor product in a ratio of 3.6:1 in 46% overall yield from (2R-[4R])-15. Similar semi-reduction of (5S, 8aR)-18 with Na/NH3 and H2/10%Pd-BaSO4 gave piclavine A3 (3) (76%) {[α]25D −74.3 (c 1.30, CH2Cl2) and piclavine A4 (4) [α]25D −76.5 (c 0.63 CH2Cl2)} lit. [3] [α]20D −74.8 (c 0.5 CH2Cl2)} in 84% yield, respectively (Scheme 4). Spectral data of 4 were completely consistent with values reported. [3]
Scheme 4: Asymmetric synthesis of piclavines A3 and A4 from (2R-[4R])-15.
Scheme 4: Asymmetric synthesis of piclavines A3 and A4 from (2R-[4R])-15.
With two diastereomers (2S-[4R])- and (2S-[4S])-15 in hand, [9] the enantiomers of piclavines A1-4 were prepared according to the procedure described above (Scheme 5). However, the absolute configuration of natural products remains unassigned. [2]
Scheme 5: Asymmetric synthesis of enantiomers of piclavines (1–4).
Scheme 5: Asymmetric synthesis of enantiomers of piclavines (1–4).
Conclusion
In summary, we accomplished the asymmetric synthesis of both enantiomers of piclavines A1, A2, A3, and A4 with high enantio-enhancement via iterative AD reactions starting from an achiral N-pentenylphthalimide 11.
Supporting Information
| Supporting Information File 1: Synthetic details, spectral properties and HRMS data. Experimental details for an asymmetric synthesis of all stereoisomers of piclavines A1-4 using an iterative asymmetric dihydroxylation. Experimental data which includes experimental details on the spectral instruments. | ||
| Format: DOC | Size: 51.5 KB | Download |
References
-
Michael, J. P. Nat. Prod. Rep. 2007, 24, 191–222. doi:10.1039/b509525p
Return to citation in text: [1] -
Raub, M. F.; Cardellinia, J. H., II; Spande, T. F. Tetrahedron Lett. 1992, 33, 2257–2260. doi:10.1016/S0040-4039(00)74183-9
Return to citation in text: [1] [2] [3] [4] -
Jefford, C. W.; Sienkiewicz, K.; Thornton, S. R. Helv. Chim. Acta 1995, 78, 1511–1524. doi:10.1002/hlca.19950780610
Return to citation in text: [1] [2] [3] -
McAlonan, H.; Potts, D.; Stevenson, P. J.; Thompson, N. Tetrahedron Lett. 2000, 41, 5411–5414. doi:10.1016/S0040-4039(00)00868-6
Return to citation in text: [1] -
Takahata, H. J. Synth. Org. Chem., Jpn. 1999, 57, 835–844.
Return to citation in text: [1] -
Takahata, H. Trends Org. Chem. 2000, 8, 101–119.
Return to citation in text: [1] -
Takahata, H.; Saito, Y.; Ichinose, M. Org. Biomol. Chem. 2006, 4, 1587–1595. doi:10.1039/b601489e
Return to citation in text: [1] -
Takahata, H.; Okamoto, N. Bioorg. Med. Chem. Lett. 2000, 10, 1799–1801. doi:10.1016/S0960-894X(00)00341-3
Return to citation in text: [1] -
Takahata, H.; Kubota, M.; Ihara, K.; Okamoto, N.; Momose, T.; Azer, N.; Eldefrawi, A. T.; Eldefrawi, M. E. Tetrahedron: Asymmetry 1998, 9, 3289–3301. doi:10.1016/S0957-4166(98)00338-3
Return to citation in text: [1] [2] [3] -
Yamaguchi, M.; Nobayashi, Y.; Hirao, I. Tetrahedron Lett. 1983, 24, 5121–5122. doi:10.1016/S0040-4039(00)94057-7
Return to citation in text: [1] -
Overman, L. E.; Sharp, M. J. Tetrahedron Lett. 1988, 29, 901–904. doi:10.1016/S0040-4039(00)82477-6
Return to citation in text: [1] -
Angle, S. R.; Arnaiz, D. O. Tetrahedron Lett. 1989, 30, 515–518. doi:10.1016/S0040-4039(00)95241-9
Return to citation in text: [1] -
Ihara, M.; Taniguchi, N.; Noguchi, K.; Fukumoto, K.; Kametani, T. J. Chem. Soc., Perkin Trans. 1 1988, 1277–1281. doi:10.1039/p19880001277
Return to citation in text: [1] -
Dong, Q.; Anderson, C. E.; Ciufolini, M. A. Tetrahedron Lett. 1995, 36, 5681–5682. doi:10.1016/0040-4039(95)01122-X
Return to citation in text: [1]
| 9. | Takahata, H.; Kubota, M.; Ihara, K.; Okamoto, N.; Momose, T.; Azer, N.; Eldefrawi, A. T.; Eldefrawi, M. E. Tetrahedron: Asymmetry 1998, 9, 3289–3301. doi:10.1016/S0957-4166(98)00338-3 |
| 3. | Jefford, C. W.; Sienkiewicz, K.; Thornton, S. R. Helv. Chim. Acta 1995, 78, 1511–1524. doi:10.1002/hlca.19950780610 |
| 3. | Jefford, C. W.; Sienkiewicz, K.; Thornton, S. R. Helv. Chim. Acta 1995, 78, 1511–1524. doi:10.1002/hlca.19950780610 |
| 2. | Raub, M. F.; Cardellinia, J. H., II; Spande, T. F. Tetrahedron Lett. 1992, 33, 2257–2260. doi:10.1016/S0040-4039(00)74183-9 |
| 14. | Dong, Q.; Anderson, C. E.; Ciufolini, M. A. Tetrahedron Lett. 1995, 36, 5681–5682. doi:10.1016/0040-4039(95)01122-X |
| 4. | McAlonan, H.; Potts, D.; Stevenson, P. J.; Thompson, N. Tetrahedron Lett. 2000, 41, 5411–5414. doi:10.1016/S0040-4039(00)00868-6 |
| 2. | Raub, M. F.; Cardellinia, J. H., II; Spande, T. F. Tetrahedron Lett. 1992, 33, 2257–2260. doi:10.1016/S0040-4039(00)74183-9 |
| 3. | Jefford, C. W.; Sienkiewicz, K.; Thornton, S. R. Helv. Chim. Acta 1995, 78, 1511–1524. doi:10.1002/hlca.19950780610 |
| 12. | Angle, S. R.; Arnaiz, D. O. Tetrahedron Lett. 1989, 30, 515–518. doi:10.1016/S0040-4039(00)95241-9 |
| 2. | Raub, M. F.; Cardellinia, J. H., II; Spande, T. F. Tetrahedron Lett. 1992, 33, 2257–2260. doi:10.1016/S0040-4039(00)74183-9 |
| 13. | Ihara, M.; Taniguchi, N.; Noguchi, K.; Fukumoto, K.; Kametani, T. J. Chem. Soc., Perkin Trans. 1 1988, 1277–1281. doi:10.1039/p19880001277 |
| 9. | Takahata, H.; Kubota, M.; Ihara, K.; Okamoto, N.; Momose, T.; Azer, N.; Eldefrawi, A. T.; Eldefrawi, M. E. Tetrahedron: Asymmetry 1998, 9, 3289–3301. doi:10.1016/S0957-4166(98)00338-3 |
| 10. | Yamaguchi, M.; Nobayashi, Y.; Hirao, I. Tetrahedron Lett. 1983, 24, 5121–5122. doi:10.1016/S0040-4039(00)94057-7 |
| 8. | Takahata, H.; Okamoto, N. Bioorg. Med. Chem. Lett. 2000, 10, 1799–1801. doi:10.1016/S0960-894X(00)00341-3 |
| 11. | Overman, L. E.; Sharp, M. J. Tetrahedron Lett. 1988, 29, 901–904. doi:10.1016/S0040-4039(00)82477-6 |
| 7. | Takahata, H.; Saito, Y.; Ichinose, M. Org. Biomol. Chem. 2006, 4, 1587–1595. doi:10.1039/b601489e |
| 2. | Raub, M. F.; Cardellinia, J. H., II; Spande, T. F. Tetrahedron Lett. 1992, 33, 2257–2260. doi:10.1016/S0040-4039(00)74183-9 |
| 5. | Takahata, H. J. Synth. Org. Chem., Jpn. 1999, 57, 835–844. |
| 6. | Takahata, H. Trends Org. Chem. 2000, 8, 101–119. |
| 9. | Takahata, H.; Kubota, M.; Ihara, K.; Okamoto, N.; Momose, T.; Azer, N.; Eldefrawi, A. T.; Eldefrawi, M. E. Tetrahedron: Asymmetry 1998, 9, 3289–3301. doi:10.1016/S0957-4166(98)00338-3 |
© 2007 Saito et al; licensee Beilstein-Institut
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)