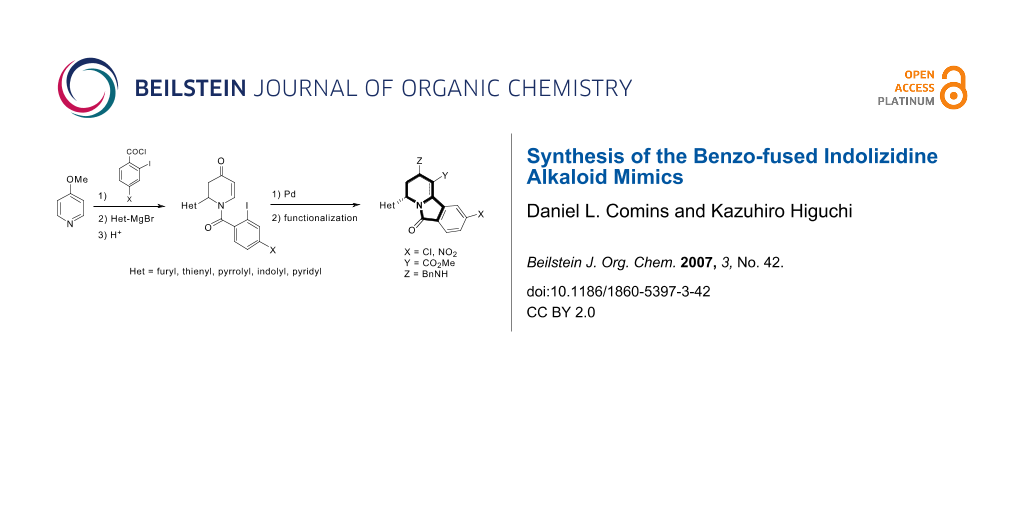
Abstract
A general synthesis of various benzo-fused indolizidine alkaloid mimics has been developed. The indolizidine derivatives 8 were prepared via heteroaryl Grignard addition to N-acylpyridinium salts followed by an intramolecular Heck cyclization. Further substitution reactions were developed to demonstrate that heterocycles 8 are good scaffolds for chemical library preparation.
Graphical Abstract
Background
As part of a program directed at studying the synthesis and synthetic utility of N-acyldihydropyridones, the heterocycles 1 were developed as useful building blocks for alkaloid synthesis (Figure 1). [1,2] Biologically active indolizidine alkaloids [3] such as (+)-allopumiliotoxin 267A (2) [4], (±)-indolizidine 209B (3) [5], (+)-indolizidine 209D (4) [6], and (±)-tylophorine (5) [7] were prepared in racemic or enantiopure form using these dihydropyridone intermediates. Herein we demonstrate the utility of this chemistry for preparing diverse benzo-fused indolizidine compounds.
Figure 1: N-Acyldihydropyridone 1 and indolizidine alkaloids.
Figure 1: N-Acyldihydropyridone 1 and indolizidine alkaloids.
Results and Discussion
The reaction of various kinds of heteroaryl Grignard reagents with the N-acylpyridinium salt prepared from 4-methoxypyridine (6) and 2-iodobenzoylchloride (7a) was studied (Table 1). The addition of 2-furyl [8], 2-thienyl [9] and 2-pyrrolyl [10,11] Grignard reagents gave N-acyldihydropyridones 1a-c in good yields (entries 1–3). In addition, the N-methyl-2-indolyl [11] Grignard reagent gave 1d in moderate yield (entry 4). In spite of trying various methods of preparing the 2-pyridyl [12-15] Grignard reagent, 1e was obtained in only 15% yield (entry 5). Encouraged by these results, the reaction of 3-heteroaryl Grignard reagents was also examined (entries 6–9). The 3-furyl [16] and 3-thienyl [17] Grignard reagents were prepared from the corresponding 3-bromo compounds and gave 1f and 1g in moderate yields (entries 6,7). The compounds 1h and 1i were prepared in good yield from N-TIPS-3-bromopyrrole [18] and N-TIPS-3-bromoindole (entries 8,9).
Next, the intramolecular reductive Heck cyclization with N-acyl-2,3-dihydropyridones 1a-i was investigated (Table 2). A short synthesis of indolizidine alkaloids of type 8 by using Heck or anionic cyclization methods was developed. [6,19] In this reaction, only the trans diastereomer was obtained as determined by analysis of the 1H-NMR spectrum of the crude product. This methodology is useful for the synthesis of various types of indolizidine alkaloids and their mimics. Treatment of 1a-i with 5 mol% of palladium catalyst, 2 equiv of formic acid and 4 equiv of triethylamine at 80°C in DMF provided 8a-i in good yields. THF could also be used as a solvent in this reaction. In the case of 1h and 1i, the N-TIPS group was cleaved under the reaction conditions (entries 8,9).
Table 2: Intramolecular reductive Heck cyclization
|
|
|||||
| entry | 1 | yield of 8 | entry | 1 | yield of 8 |
|---|---|---|---|---|---|
| 1 | 1a | 8a 82% | 6 | 1f | 8f 78% |
| 2 | 1b | 8b 81% | 7 | 1g | 8g 79% |
| 3 | 1c | 8c 74% | 8 | 1h | 8h 57%a |
| 4 | 1d | 8d 48% | 9 | 1i | 8i 82%a |
| 5 | 1e | 8e 31% | |||
a TIPS group was cleaved.
To add more points of diversity, the preparation of derivatives containing functionality in the benzene ring was examined. The chloro-substituted compound 1j was prepared from 6 and 4-chloro-2-iodobenzoylchloride (7b). [20] The reductive Heck cyclization of 1j proceeded without difficulty to provide compound 8j in 82% yield (Scheme 1).
Scheme 1: Preparation of chloro-substituted compound 8j.
Scheme 1: Preparation of chloro-substituted compound 8j.
Next, the nitro-substituted compound 1k was prepared from 4-methoxypyridine (6) and 2-iodo-4-nitrobenzoyl chloride (7c) (Scheme 2). [21] Although the reductive Heck cyclization of 1k gave the desired compound 8k in 17% yield, the non-reductive cyclized product 9 and uncyclized compound 10 were isolated in 26% and 17%, respectively (entry 1). The reaction in THF with 10 mol% of palladium catalyst at a lower reaction temperature gave 8k in 67% yield (entry 3). [22]
Scheme 2: Preparation of nitro-substituted compound 8k.
Scheme 2: Preparation of nitro-substituted compound 8k.
Scheme 3 shows a method for substitution at the α-position of N-acyldihydropyridone 11. Our laboratories have reported C-5 substitution of 5-iodo-1,2-dihydropyridones via palladium mediated cross-coupling and carboalkoxylation. [23] Initially, non-reductive Heck cyclization of 1l [24] was carried out in the presence of Pd(OAc)2 and AgNO3 in CH3CN. [22] Treatment of the product 11 with ICl in CH2Cl2 at 0°C gave the iodinated dihydropyridone 12 in 86% yield. Palladium-catalyzed carboalkoxylation reaction of 12 gave the α-methoxycarbonyl dihydropyridone 13 in 82% yield.
The addition and modification of functional groups on 8a were investigated (Scheme 4). The protection of the C-4 carbonyl of 8a as a ketal followed by Vilsmeier-Haack formylation [25] furnished 14 in 22% yield. The furan ring of 8a was converted to a carboxylic acid by ozonolysis to afford 15. The reductive amination of 8a with benzylamine provided 16α and 16β in good yield. The stereochemistry of these compounds was determined by NOESY NMR analysis. These functional groups, such as carboxylic acid and secondary amine, provide diversity which could be important for the development of biologically active derivatives.
Scheme 4: Modification of functional groups within 8a.
Scheme 4: Modification of functional groups within 8a.
Conclusion
The synthesis and chemistry of indolizidine derivatives 8 was investigated with the goal of providing access to diverse heterocyclic compounds of potential biological activity. The various kinds of N-acyldihydropyridones 1 were conveniently prepared from heteroaryl Grignard reagents and N-acylpyridinium salts. Subsequently, dihydropyridones 1 were converted to 8 by use of an intramolecular Heck cyclization. The chloro- and nitro-substituted acyl chlorides 7 were also used to provide compounds with additional synthetic handles. The α-position of dihydropyridone 11 was halogenated and carbonylated to provide ester 13. Compound 8a was also converted to furylaldehyde 14, carboxylic acid 15 and secondary amines 16. Indolizidine alkaloids such as type 8 are readily synthesized in 2 steps from commercially available compounds. We have demonstrated that compound 8 can be substituted with functional groups, and provide useful scaffolds for the preparation of indolizidine alkaloid mimics.
Supporting Information
| Supporting Information File 1: Experimental Section. Experimental details and full spectroscopic data for new compounds | ||
| Format: DOC | Size: 92.0 KB | Download |
Acknowledgements
We express appreciation to SCYNEXIS Chemistry & Automation, Inc. for financial support of this research. The NMR and mass spectra were obtained at NCSU instrumentation laboratories, which were established by grants from the North Carolina Biotechnology Center and the National Science Foundation (Grant CHE-0078253).
References
-
Comins, D. L. J. Heterocycl. Chem. 1999, 36, 1491–1500.
Return to citation in text: [1] -
Joseph, S.; Comins, D. L. Curr. Opin. Drug Discovery Dev. 2002, 5, 870–880.
Return to citation in text: [1] -
Michael, J. P. Nat. Prod. Rep. 2007, 24, 191–222. doi:10.1039/b509525p
Return to citation in text: [1] -
Comins, D. L.; Huang, S.; McArdle, C. L.; Ingalls, C. L. Org. Lett. 2001, 3, 469–471. doi:10.1021/ol0069709
Return to citation in text: [1] -
Comins, D. L.; Zeller, E. Tetrahedron Lett. 1991, 42, 5889–5892. doi:10.1016/S0040-4039(00)79418-4
Return to citation in text: [1] -
Comins, D. L.; Zhang, Y. M. J. Am. Chem. Soc. 1996, 118, 12248–12249. doi:10.1021/ja9626446
Return to citation in text: [1] [2] -
Comins, D. L.; Morgan, L. A. Tetrahedron Lett. 1991, 42, 5919–5922. doi:10.1016/S0040-4039(00)79426-3
Return to citation in text: [1] -
Focken, T.; Charette, A. B. Org. Lett. 2006, 8, 2985–2988. doi:10.1021/ol0609006
Return to citation in text: [1] -
Kuroda, M.; Nakayama, J.; Hoshino, M.; Furusho, N.; Kawata, T.; Ohba, S. Tetrahedron 1993, 49, 3735–3748. doi:10.1016/S0040-4020(01)90226-1
Return to citation in text: [1] -
Minato, A.; Suzuki, K.; Tamao, K.; Kumada, M. Tetrahedron Lett. 1984, 25, 83–86. doi:10.1016/S0040-4039(01)91154-2
Return to citation in text: [1] -
Kuethe, J. T.; Comins, D. L. J. Org. Chem. 2004, 69, 2863–2866. doi:10.1021/jo049943v
Return to citation in text: [1] [2] -
Iida, T.; Wada, T.; Tomimoto, K.; Mase, T. Tetrahedron Lett. 2001, 42, 4841–4844. doi:10.1016/S0040-4039(01)00861-9
Return to citation in text: [1] -
Inoue, A.; Kitagawa, K.; Shinokubo, H.; Oshima, K. J. Org. Chem. 2001, 66, 4333–4339. doi:10.1021/jo015597v
Return to citation in text: [1] -
Furukawa, N.; Shibutani, T.; Fujihara, H. Tetrahedron Lett. 1987, 28, 5845–5848. doi:10.1016/S0040-4039(01)81070-4
Return to citation in text: [1] -
Ozawa, K.; Ishii, S.; Hatanaka, M. Chem. Lett. 1985, 1803–1804. doi:10.1246/cl.1985.1803
Return to citation in text: [1] -
Haarmann, H.; Eberbach, W. Tetrahedron Lett. 1991, 32, 903–906. doi:10.1016/S0040-4039(00)92116-6
Return to citation in text: [1] -
Rieke, R. D.; Kim, S. H.; Wu, X. J. Org. Chem. 1997, 62, 6921–6927. doi:10.1021/jo970778b
Return to citation in text: [1] -
Bumagin, N. A.; Nikitina, A. F.; Beletskaya, I. P. Russ. J. Org. Chem. 1994, 30, 1619–1629.
Return to citation in text: [1] -
Comins, D. L.; Joseph, S. P.; Zhang, Y. Tetrahedron Lett. 1996, 37, 793–796. doi:10.1016/0040-4039(95)02297-X
Return to citation in text: [1] -
Pelz, K.; Ernest, I.; Adlerová, E.; Metysová, J.; Protiva, M. Collect. Czech. Chem. Commun. 1968, 33, 1852–1872.
Return to citation in text: [1] -
Protiva, J.; Krecek, V.; Máca, B.; Urban, J.; Budesínsky, M.; Procházka, M. Collect. Czech. Chem. Commun. 1989, 54, 1012–1018.
Return to citation in text: [1] -
Friestad, G. K.; Branchaud, B. P. Tetrahedron Lett. 1995, 36, 7047–7050. doi:10.1016/0040-4039(95)01460-Y
Return to citation in text: [1] [2] -
Comins, D. L.; Joseph, S. P.; Chen, X. Tetrahedron Lett. 1995, 36, 9141–9144. doi:10.1016/0040-4039(95)01958-K
Return to citation in text: [1] -
Beckwith, A. L. J.; Joseph, S. P.; Mayadunne, R. T. A. J. Org. Chem. 1993, 58, 4198–4199. doi:10.1021/jo00068a009
Return to citation in text: [1] -
Comins, D. L.; Herrick, J. J. Heterocycles 1987, 26, 2159–2164.
Return to citation in text: [1]
| 23. | Comins, D. L.; Joseph, S. P.; Chen, X. Tetrahedron Lett. 1995, 36, 9141–9144. doi:10.1016/0040-4039(95)01958-K |
| 21. | Protiva, J.; Krecek, V.; Máca, B.; Urban, J.; Budesínsky, M.; Procházka, M. Collect. Czech. Chem. Commun. 1989, 54, 1012–1018. |
| 22. | Friestad, G. K.; Branchaud, B. P. Tetrahedron Lett. 1995, 36, 7047–7050. doi:10.1016/0040-4039(95)01460-Y |
| 1. | Comins, D. L. J. Heterocycl. Chem. 1999, 36, 1491–1500. |
| 2. | Joseph, S.; Comins, D. L. Curr. Opin. Drug Discovery Dev. 2002, 5, 870–880. |
| 6. | Comins, D. L.; Zhang, Y. M. J. Am. Chem. Soc. 1996, 118, 12248–12249. doi:10.1021/ja9626446 |
| 6. | Comins, D. L.; Zhang, Y. M. J. Am. Chem. Soc. 1996, 118, 12248–12249. doi:10.1021/ja9626446 |
| 19. | Comins, D. L.; Joseph, S. P.; Zhang, Y. Tetrahedron Lett. 1996, 37, 793–796. doi:10.1016/0040-4039(95)02297-X |
| 5. | Comins, D. L.; Zeller, E. Tetrahedron Lett. 1991, 42, 5889–5892. doi:10.1016/S0040-4039(00)79418-4 |
| 20. | Pelz, K.; Ernest, I.; Adlerová, E.; Metysová, J.; Protiva, M. Collect. Czech. Chem. Commun. 1968, 33, 1852–1872. |
| 4. | Comins, D. L.; Huang, S.; McArdle, C. L.; Ingalls, C. L. Org. Lett. 2001, 3, 469–471. doi:10.1021/ol0069709 |
| 17. | Rieke, R. D.; Kim, S. H.; Wu, X. J. Org. Chem. 1997, 62, 6921–6927. doi:10.1021/jo970778b |
| 18. | Bumagin, N. A.; Nikitina, A. F.; Beletskaya, I. P. Russ. J. Org. Chem. 1994, 30, 1619–1629. |
| 10. | Minato, A.; Suzuki, K.; Tamao, K.; Kumada, M. Tetrahedron Lett. 1984, 25, 83–86. doi:10.1016/S0040-4039(01)91154-2 |
| 11. | Kuethe, J. T.; Comins, D. L. J. Org. Chem. 2004, 69, 2863–2866. doi:10.1021/jo049943v |
| 12. | Iida, T.; Wada, T.; Tomimoto, K.; Mase, T. Tetrahedron Lett. 2001, 42, 4841–4844. doi:10.1016/S0040-4039(01)00861-9 |
| 13. | Inoue, A.; Kitagawa, K.; Shinokubo, H.; Oshima, K. J. Org. Chem. 2001, 66, 4333–4339. doi:10.1021/jo015597v |
| 14. | Furukawa, N.; Shibutani, T.; Fujihara, H. Tetrahedron Lett. 1987, 28, 5845–5848. doi:10.1016/S0040-4039(01)81070-4 |
| 15. | Ozawa, K.; Ishii, S.; Hatanaka, M. Chem. Lett. 1985, 1803–1804. doi:10.1246/cl.1985.1803 |
| 9. | Kuroda, M.; Nakayama, J.; Hoshino, M.; Furusho, N.; Kawata, T.; Ohba, S. Tetrahedron 1993, 49, 3735–3748. doi:10.1016/S0040-4020(01)90226-1 |
| 16. | Haarmann, H.; Eberbach, W. Tetrahedron Lett. 1991, 32, 903–906. doi:10.1016/S0040-4039(00)92116-6 |
| 8. | Focken, T.; Charette, A. B. Org. Lett. 2006, 8, 2985–2988. doi:10.1021/ol0609006 |
| 24. | Beckwith, A. L. J.; Joseph, S. P.; Mayadunne, R. T. A. J. Org. Chem. 1993, 58, 4198–4199. doi:10.1021/jo00068a009 |
| 7. | Comins, D. L.; Morgan, L. A. Tetrahedron Lett. 1991, 42, 5919–5922. doi:10.1016/S0040-4039(00)79426-3 |
| 11. | Kuethe, J. T.; Comins, D. L. J. Org. Chem. 2004, 69, 2863–2866. doi:10.1021/jo049943v |
| 22. | Friestad, G. K.; Branchaud, B. P. Tetrahedron Lett. 1995, 36, 7047–7050. doi:10.1016/0040-4039(95)01460-Y |
© 2007 Comins and Higuchi; licencee Beilstein-Institut
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)