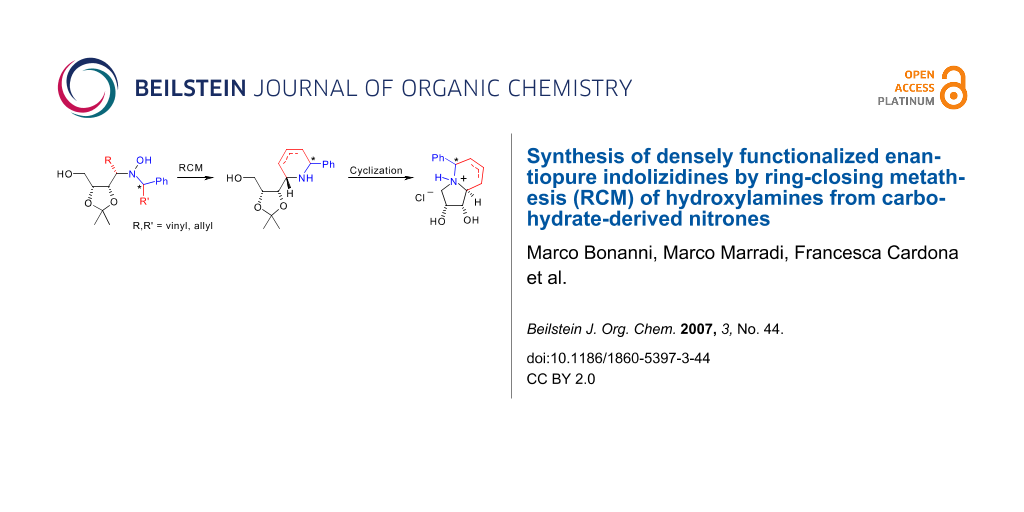
Abstract
Background
Indolizidine alkaloids widely occur in nature and display interesting biological activity. This is the reason for which their total synthesis as well as the synthesis of non-natural analogues still attracts the attention of many research groups. To establish new straightforward accesses to these molecules is therefore highly desirable.
Results
The ring closing metathesis (RCM) of enantiopure hydroxylamines bearing suitable unsaturated groups cleanly afforded piperidine derivatives in good yields. Further cyclization and deprotection of the hydroxy groups gave novel highly functionalized indolizidines. The synthesis of a pyrroloazepine analogue is also described.
Conclusion
We have developed a new straightforward methodology for the synthesis of densely functionalized indolizidines and pyrroloazepine analogues in 6 steps and 30–60% overall yields from enantiopure hydroxylamines obtained straightforwardly from carbohydrate-derived nitrones.
Graphical Abstract
Background
Indolizidine alkaloids have widespread occurrence in nature. They can be found in widely different organisms such as bacteria, fungi, higher plants, invertebrates and vertebrates.[1] For instance, the plant-derived polyhydroxylated indolizidines are well known as potent glycosidases inhibitors, and for this reason they are potential therapeutic agents. [2-4] A great deal of research is still devoted to the structural elucidation of these alkaloids as well as to their total syntheses. [5-18]
We accomplished the total syntheses of some indolizidine alkaloids and of several non-natural analogues employing chiral nitrones as key intermediates, either as dipolarophiles in 1,3-dipolar cycloaddition chemistry [19,20] or as electrophiles in the addition of organometallic reagents. [21,22] Recently, we developed a general protocol for the synthesis of α,α'-disubstituted enantiopure hydroxylamines 1 through the stereoselective double addition of an excess of a Grignard reagent to C-phenyl-N-erythrosylnitrone 2 (Scheme 1).[23] With this methodology, several symmetrically α,α'-disubstituted hydroxylamines 1 were afforded.
Scheme 1: Synthesis of symmetrically α,α'-disubstituted hydroxylamines 1.
Scheme 1: Synthesis of symmetrically α,α'-disubstituted hydroxylamines 1.
An alternative protocol for the synthesis of unsymmetrically α,α'-disubstituted hydroxylamines 3, resulting from the sequential addition of two different Grignard reagents, was also developed in a stepwise process, based on an addition-oxidation-addition sequence starting from N-glycosylhydroxylamine 4 (Scheme 2).[24]
Scheme 2: Synthesis of unsymmetrically α,α'-disubstituted hydroxylamines 3.
Scheme 2: Synthesis of unsymmetrically α,α'-disubstituted hydroxylamines 3.
Addition of unsaturated Grignard reagents afforded synthetically useful hydroxylamine intermediates, which may serve as substrates for nitrogen ring forming reactions. We report in this article a straightforward access to indolizidine derivatives and a pyrroloazepine analogue through a key ring closing metathesis (RCM) of sugar derived hydroxylamines 1 and 3 bearing suitable unsaturated substituents at the α and α' positions.
Results and discussion
Unsymetrically α,α'-disubstituted hydroxylamines 5 and 6 (Scheme 3) were synthesized according to our recently reported procedure based on the addition-oxidation-addition sequence starting from N-glycosylhydroxylamine 4 (Scheme 2),[24] while hydroxylamine 7 was obtained using an excess of allylmagnesium bromide in the addition to C-phenyl-N-erythrosylnitrone 2.[23] It should be noted that the stepwise process furnishes configurationally diversified stereoisomers at the benzylic position (e. g. 5 and 6), due to a high stereoselectivity in the first addition step but a poor one in the second.[24] Specifically, 5 was isolated as the major isomer from a ca 2:1 diastereomeric mixture, while 6 was obtained from an equimolecular mixture with its diastereoisomer.[24] Assignment of configuration has been secured by comparison with the double adducts of the one-pot process and by careful NMR studies of the final cyclic products after RCM. The scarce stereoselectivity of the second addition in the stepwise process, giving rise to two diastereoisomers, opens the way to the synthesis of diastereomeric indolizidines.
Scheme 3: Synthesis of piperidines 15–16 and azepine 17. Reagents and conditions: a) Ac2O, THF, 1 h, rt for 8 and 9, reflux for 10; b) 2nd generation Grubbs' catalyst 11 (5 mol%), CH2Cl2, reflux, 5.5 h; c) KHCO3, MeOH, rt, 12 h; d) Zn, AcOH, rt, 2 h.
Scheme 3: Synthesis of piperidines 15–16 and azepine 17. Reagents and conditions: a) Ac2O, THF, 1 h, rt for 8...
The RCM reaction has been successfully employed for the synthesis of polyfunctional indolizidines. [25-29] In order to accomplish successfully the key RCM reactions, preliminary protection of the hydroxylamine OH group was required. Selective acetylation of hydroxylamines 5–6 was achieved with acetic anhydride in THF at room temperature, while for hydroxylamine 7 it was necessary to heat the mixture at reflux. No acetylation of the primary alcohol was observed under these conditions.
Ring-closing metathesis (RCM) of O-acetylhydroxylamines 8–10 using the second generation Grubbs' catalyst 11 [30] in refluxing CH2Cl2 afforded compounds 12–14 in nearly quantitative yields (Scheme 3). However, compounds 12 and 13 suffered from low stability and for this reason the crude reaction mixtures were directly employed in the following steps.
Identity of compounds 12 and 13 was firmly established after their transformation into the corresponding amines and further elaboration. After deacetylation with KHCO3, in situ reduction of the N-O bond with zinc dust afforded tetrahydropyridines 15 and 16 (Scheme 3). Analogously, deprotection of 14 and in situ reduction with zinc dust gave tetrahydroazepine 17 (Scheme 3).
Cyclization to give the fused 5-membered ring was achieved by treatment of compounds 15–17 with trifluoromethanesulfonic anhydride in pyridine at room temperature (Scheme 4). The structure of protected indolizidines 18–19 and of pyrroloazepine 20 (and therefore of compounds 12–14) was unambiguously determined by spectral data, including 2D COSY and 1D NOESY experiments (See Experimental).
Scheme 4: Synthesis of indolizidines 21–22 and pyrroloazepine 23. Reaction conditions: a) Tf2O, Py, rt, 2 h; b) Conc. HCl, MeOH, rt, 2 h.
Scheme 4: Synthesis of indolizidines 21–22 and pyrroloazepine 23. Reaction conditions: a) Tf2O, Py, rt, 2 h; ...
Final deprotection of 18–20 with an acidic solution of MeOH afforded protonated indolizidines 21–22 in good yields (Scheme 4). Analogously, deprotection of 20 gave pyrrolazepine 23, which displayed good inhibition of α-glucosidase from yeast (90% at 1 mM).[23] Compounds 17 and 23, containing an azepane moiety, might be of biological interest as shown recently. [31-35] Indolizidines 21–22 differ in the absolute configuration at C5 and in the position of the double bond, illustrating the structural diversity attainable with this strategy. It should be noted that similar dihydroxyhexahydroindolizines maintained glucosidase inhibition activity in analogy to the completely unsaturated compounds.[22] Moreover, it has been recently proved that dihydroxypyrrolidines bearing aromatic rings have interesting antitumor activities. [36,37] Work is underway to evaluate the biological activity of the newly synthesized compounds. In addition, the presence of a double bond should allow the introduction of additional hydroxy groups or other functionalities by appropriate elaboration.
Supporting Information
| Supporting Information File 1: Synthesis of densely functionalized enantiopure indolizidines by ring-closing metathesis (RCM) of hydroxylamines from carbohydrate-derived nitrones. Experimental Sections. Experimental procedures, characterization of new compounds. | ||
| Format: PDF | Size: 109.3 KB | Download |
References
-
Michael, J. P. Simple indolizidine and quinolizidine alkaloids. In The Alkaloids; Cordell, G., Ed.; Academic Press: San Diego, 2001; Vol. 55, pp 91–258. doi:10.1016/S0099-9598(01)55004-X
Return to citation in text: [1] -
Watson, A. A.; Fleet, G. W. J.; Asano, N.; Molyneux, R. J.; Nash, R. J. Phytochemistry 2001, 56, 265–295. doi:10.1016/S0031-9422(00)00451-9
Return to citation in text: [1] -
Asano, N. Glycobiology 2003, 13, 93R–104R. doi:10.1093/glycob/cwg090
Return to citation in text: [1] -
Robina, I.; Moreno-Vargas, A. J.; Carmona, A. T.; Vogely, P. Curr. Drug Metab. 2004, 5, 329–361. doi:10.2174/1389200043335513
Return to citation in text: [1] -
Michael, J. P. Nat. Prod. Rep. 2007, 24, 191–222. doi:10.1039/b509525p
and previous review in the series of annual reviews on indolizidine and quinolizidine alkaloids.
Return to citation in text: [1] -
Torres-Sanchez, M. I.; Borrachero, P.; Cabrera-Escribano, F.; Gomez-Guillen, M.; Angulo-Alvarez, M.; Alvarez, E.; Favre, S.; Vogel, P. Tetrahedron: Asymmetry 2007, 18, 1809–1827. doi:10.1016/j.tetasy.2007.08.001
Return to citation in text: [1] -
Carmona, A. T.; Fuentes, G.; Robina, I.; Rodriguez Garcia, E.; Demange, R.; Vogel, P.; Winters, A. L. J. Org. Chem. 2003, 68, 3874–3883. doi:10.1021/jo026688a
Return to citation in text: [1] -
Reymond, J. L.; Pinkerton, A.; Vogel, P. J. Org. Chem. 1991, 56, 2128–2135. doi:10.1021/jo00006a031
Return to citation in text: [1] -
Hanessian, S.; Therrien, E.; Warrier, J. S.; Charron, G. Heterocycles 2006, 70, 461–476.
Return to citation in text: [1] -
Langlois, N.; Le Nguyen, B. K.; Retailleau, P.; Tarnus, C.; Salomon, E. Tetrahedron: Asymmetry 2006, 17, 53–60. doi:10.1016/j.tetasy.2005.11.017
Return to citation in text: [1] -
Socha, D.; Jurczak, M.; Chmielewski, M. Carbohydr. Res. 2001, 336, 315–318. doi:10.1016/S0008-6215(01)00265-8
Return to citation in text: [1] -
Izquierdo, I.; Plaza, M. T.; Robles, R.; Mota, A. J. Eur. J. Org. Chem. 2000, 2071–2078. doi:10.1002/1099-0690(200006)2000:11<2071::AID-EJOC2071>3.0.CO;2-A
Return to citation in text: [1] -
Vyavahare, V. P.; Chakraborty, C.; Maity, B.; Chattopadhyay, S.; Puranik, V. G.; Dhavale, D. D. J. Med. Chem. 2007, 48, 5519–5523. doi:10.1021/jm070660f
Return to citation in text: [1] -
Chen, M.-J.; Tsai, Y.-M. Tetrahedron Lett. 2007, 48, 6271–6274. doi:10.1016/j.tetlet.2007.07.021
Return to citation in text: [1] -
Pandey, G.; Dumbre, S. G.; Pal, S.; Khan, M. I.; Shabab, M. Tetrahedron 2007, 63, 4756–4761. doi:10.1016/j.tet.2007.03.085
Return to citation in text: [1] -
Karanjule, N. S.; Markad, S. D.; Dhavale, D. D. J. Org. Chem. 2006, 71, 6273–6276. doi:10.1021/jo060823s
Return to citation in text: [1] -
Song, L.; Duesler, E. N.; Mariano, P. S. J. Org. Chem. 2004, 69, 7284–7293. doi:10.1021/jo040226a
Return to citation in text: [1] -
Sletten, E. M.; Liotta, L. J. J. Org. Chem. 2006, 71, 1335–1343. doi:10.1021/jo051792o
Return to citation in text: [1] -
Brandi, A.; Cardona, F.; Cicchi, S.; Cordero, F. M.; Goti, A. Curr. Trends. Org. Synth. 1999, 213–220.
Return to citation in text: [1] -
Cordero, F. M.; Pisaneschi, F.; Gensini, M.; Goti, A.; Brandi, A. Eur. J. Org. Chem. 2002, 1941–1951. doi:10.1002/1099-0690(200206)2002:12<1941::AID-EJOC1941>3.0.CO;2-T
Return to citation in text: [1] -
Cardona, F.; Goti, A.; Brandi, A. Eur. J. Org. Chem. 2007, 1551–1565. doi:10.1002/ejoc.200600633
and references cited therein.
Return to citation in text: [1] -
Cardona, F.; Moreno, G.; Guarna, G.; Vogel, P.; Schuetz, C.; Merino, P.; Goti, A. J. Org. Chem. 2005, 70, 6552–6555. doi:10.1021/jo0509408
Return to citation in text: [1] [2] -
Bonanni, M.; Marradi, M.; Cicchi, S.; Faggi, C.; Goti, A. Org. Lett. 2005, 7, 319–322. doi:10.1021/ol047691e
Return to citation in text: [1] [2] [3] -
Bonanni, M.; Marradi, M.; Cicchi, S.; Goti, A. Synlett, in press.
Return to citation in text: [1] [2] [3] [4] -
Ovaa, H.; Stragies, R.; van der Marel, G. A. Chem. Commun. 2000, 1501–1502. doi:10.1039/b004106h
Return to citation in text: [1] -
Pandit, U. K.; Overkleeft, H. S.; Borer, B. C.; Bieräugel, H. Eur. J. Org. Chem. 1999, 959–968. doi:10.1002/(SICI)1099-0690(199905)1999:5<959::AID-EJOC959>3.0.CO;2-K
Return to citation in text: [1] -
Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872
Return to citation in text: [1] -
Wakamatsu, H.; Sato, Y.; Fujita, R.; Mori, M. Adv. Synth. Catal. 2007, 349, 1231–1246. doi:10.1002/adsc.200600539
Return to citation in text: [1] -
Murray, A. J.; Parsons, P. J.; Hitchcock, P. Tetrahedron 2007, 63, 6485–6492. doi:10.1016/j.tet.2007.03.103
Return to citation in text: [1] -
Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. Org. Lett. 1999, 1, 953–956. doi:10.1021/ol990909q
Return to citation in text: [1] -
Li, H.; Zhang, Y.; Vogel, P.; Sinay, P.; Bleriot, Y. Chem. Commun. 2007, 183–185. doi:10.1039/b610377d
Return to citation in text: [1] -
Li, H.; Schuetz, C.; Favre, S.; Zhang, Y.; Vogel, P.; Sinay, P.; Bleriot, Y. Org. Biomol. Chem. 2006, 4, 1653–1662. doi:10.1039/b518117h
Return to citation in text: [1] -
Bleriot, Y.; Mallet, J.-M.; Zhang, Y.; Rodriguez-Garcia, E.; Vogel, P.; Mari, S.; Jimenez-Barbero, J.; Sinay, P. Heterocycles 2004, 64, 65–74.
Return to citation in text: [1] -
Li, H.; Bleriot, Y.; Mallet, J.-M.; Rodriguez-Garcia, E.; Vogel, P.; Zhang, Y.; Sinay, P. Tetrahedron: Asymmetry 2005, 16, 313–319. doi:10.1016/j.tetasy.2004.12.005
Return to citation in text: [1] -
Lertpibulpanya, D.; Marsden, S. P. Org. Biomol. Chem. 2006, 4, 3498–3504. doi:10.1039/b608801e
Return to citation in text: [1] -
Fiaux, A.; Popowycz, F.; Favre, S.; Schütz, C.; Vogel, P.; Gerber-Lemaire, S.; Juillerat-Jeanneret, L. J. Med. Chem. 2005, 48, 4237–4246. doi:10.1021/jm0409019
Return to citation in text: [1] -
Favre, S.; Fiaux, H.; Schutz, C.; Vogel, P.; Juillerat-Jeanneret, L.; Gerber-Lemaire, S. Heterocycles 2006, 69, 179–192.
Return to citation in text: [1]
| 36. | Fiaux, A.; Popowycz, F.; Favre, S.; Schütz, C.; Vogel, P.; Gerber-Lemaire, S.; Juillerat-Jeanneret, L. J. Med. Chem. 2005, 48, 4237–4246. doi:10.1021/jm0409019 |
| 37. | Favre, S.; Fiaux, H.; Schutz, C.; Vogel, P.; Juillerat-Jeanneret, L.; Gerber-Lemaire, S. Heterocycles 2006, 69, 179–192. |
| 1. | Michael, J. P. Simple indolizidine and quinolizidine alkaloids. In The Alkaloids; Cordell, G., Ed.; Academic Press: San Diego, 2001; Vol. 55, pp 91–258. doi:10.1016/S0099-9598(01)55004-X |
| 21. |
Cardona, F.; Goti, A.; Brandi, A. Eur. J. Org. Chem. 2007, 1551–1565. doi:10.1002/ejoc.200600633
and references cited therein. |
| 22. | Cardona, F.; Moreno, G.; Guarna, G.; Vogel, P.; Schuetz, C.; Merino, P.; Goti, A. J. Org. Chem. 2005, 70, 6552–6555. doi:10.1021/jo0509408 |
| 31. | Li, H.; Zhang, Y.; Vogel, P.; Sinay, P.; Bleriot, Y. Chem. Commun. 2007, 183–185. doi:10.1039/b610377d |
| 32. | Li, H.; Schuetz, C.; Favre, S.; Zhang, Y.; Vogel, P.; Sinay, P.; Bleriot, Y. Org. Biomol. Chem. 2006, 4, 1653–1662. doi:10.1039/b518117h |
| 33. | Bleriot, Y.; Mallet, J.-M.; Zhang, Y.; Rodriguez-Garcia, E.; Vogel, P.; Mari, S.; Jimenez-Barbero, J.; Sinay, P. Heterocycles 2004, 64, 65–74. |
| 34. | Li, H.; Bleriot, Y.; Mallet, J.-M.; Rodriguez-Garcia, E.; Vogel, P.; Zhang, Y.; Sinay, P. Tetrahedron: Asymmetry 2005, 16, 313–319. doi:10.1016/j.tetasy.2004.12.005 |
| 35. | Lertpibulpanya, D.; Marsden, S. P. Org. Biomol. Chem. 2006, 4, 3498–3504. doi:10.1039/b608801e |
| 19. | Brandi, A.; Cardona, F.; Cicchi, S.; Cordero, F. M.; Goti, A. Curr. Trends. Org. Synth. 1999, 213–220. |
| 20. | Cordero, F. M.; Pisaneschi, F.; Gensini, M.; Goti, A.; Brandi, A. Eur. J. Org. Chem. 2002, 1941–1951. doi:10.1002/1099-0690(200206)2002:12<1941::AID-EJOC1941>3.0.CO;2-T |
| 22. | Cardona, F.; Moreno, G.; Guarna, G.; Vogel, P.; Schuetz, C.; Merino, P.; Goti, A. J. Org. Chem. 2005, 70, 6552–6555. doi:10.1021/jo0509408 |
| 5. |
Michael, J. P. Nat. Prod. Rep. 2007, 24, 191–222. doi:10.1039/b509525p
and previous review in the series of annual reviews on indolizidine and quinolizidine alkaloids. |
| 6. | Torres-Sanchez, M. I.; Borrachero, P.; Cabrera-Escribano, F.; Gomez-Guillen, M.; Angulo-Alvarez, M.; Alvarez, E.; Favre, S.; Vogel, P. Tetrahedron: Asymmetry 2007, 18, 1809–1827. doi:10.1016/j.tetasy.2007.08.001 |
| 7. | Carmona, A. T.; Fuentes, G.; Robina, I.; Rodriguez Garcia, E.; Demange, R.; Vogel, P.; Winters, A. L. J. Org. Chem. 2003, 68, 3874–3883. doi:10.1021/jo026688a |
| 8. | Reymond, J. L.; Pinkerton, A.; Vogel, P. J. Org. Chem. 1991, 56, 2128–2135. doi:10.1021/jo00006a031 |
| 9. | Hanessian, S.; Therrien, E.; Warrier, J. S.; Charron, G. Heterocycles 2006, 70, 461–476. |
| 10. | Langlois, N.; Le Nguyen, B. K.; Retailleau, P.; Tarnus, C.; Salomon, E. Tetrahedron: Asymmetry 2006, 17, 53–60. doi:10.1016/j.tetasy.2005.11.017 |
| 11. | Socha, D.; Jurczak, M.; Chmielewski, M. Carbohydr. Res. 2001, 336, 315–318. doi:10.1016/S0008-6215(01)00265-8 |
| 12. | Izquierdo, I.; Plaza, M. T.; Robles, R.; Mota, A. J. Eur. J. Org. Chem. 2000, 2071–2078. doi:10.1002/1099-0690(200006)2000:11<2071::AID-EJOC2071>3.0.CO;2-A |
| 13. | Vyavahare, V. P.; Chakraborty, C.; Maity, B.; Chattopadhyay, S.; Puranik, V. G.; Dhavale, D. D. J. Med. Chem. 2007, 48, 5519–5523. doi:10.1021/jm070660f |
| 14. | Chen, M.-J.; Tsai, Y.-M. Tetrahedron Lett. 2007, 48, 6271–6274. doi:10.1016/j.tetlet.2007.07.021 |
| 15. | Pandey, G.; Dumbre, S. G.; Pal, S.; Khan, M. I.; Shabab, M. Tetrahedron 2007, 63, 4756–4761. doi:10.1016/j.tet.2007.03.085 |
| 16. | Karanjule, N. S.; Markad, S. D.; Dhavale, D. D. J. Org. Chem. 2006, 71, 6273–6276. doi:10.1021/jo060823s |
| 17. | Song, L.; Duesler, E. N.; Mariano, P. S. J. Org. Chem. 2004, 69, 7284–7293. doi:10.1021/jo040226a |
| 18. | Sletten, E. M.; Liotta, L. J. J. Org. Chem. 2006, 71, 1335–1343. doi:10.1021/jo051792o |
| 30. | Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. Org. Lett. 1999, 1, 953–956. doi:10.1021/ol990909q |
| 2. | Watson, A. A.; Fleet, G. W. J.; Asano, N.; Molyneux, R. J.; Nash, R. J. Phytochemistry 2001, 56, 265–295. doi:10.1016/S0031-9422(00)00451-9 |
| 3. | Asano, N. Glycobiology 2003, 13, 93R–104R. doi:10.1093/glycob/cwg090 |
| 4. | Robina, I.; Moreno-Vargas, A. J.; Carmona, A. T.; Vogely, P. Curr. Drug Metab. 2004, 5, 329–361. doi:10.2174/1389200043335513 |
| 23. | Bonanni, M.; Marradi, M.; Cicchi, S.; Faggi, C.; Goti, A. Org. Lett. 2005, 7, 319–322. doi:10.1021/ol047691e |
| 23. | Bonanni, M.; Marradi, M.; Cicchi, S.; Faggi, C.; Goti, A. Org. Lett. 2005, 7, 319–322. doi:10.1021/ol047691e |
| 25. | Ovaa, H.; Stragies, R.; van der Marel, G. A. Chem. Commun. 2000, 1501–1502. doi:10.1039/b004106h |
| 26. | Pandit, U. K.; Overkleeft, H. S.; Borer, B. C.; Bieräugel, H. Eur. J. Org. Chem. 1999, 959–968. doi:10.1002/(SICI)1099-0690(199905)1999:5<959::AID-EJOC959>3.0.CO;2-K |
| 27. | Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872 |
| 28. | Wakamatsu, H.; Sato, Y.; Fujita, R.; Mori, M. Adv. Synth. Catal. 2007, 349, 1231–1246. doi:10.1002/adsc.200600539 |
| 29. | Murray, A. J.; Parsons, P. J.; Hitchcock, P. Tetrahedron 2007, 63, 6485–6492. doi:10.1016/j.tet.2007.03.103 |
| 23. | Bonanni, M.; Marradi, M.; Cicchi, S.; Faggi, C.; Goti, A. Org. Lett. 2005, 7, 319–322. doi:10.1021/ol047691e |
© 2007 Bonanni et al; licensee Beilstein-Institut
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)