Abstract
Copper-catalyzed, thermal or microwave promoted 1,3-dipolar cycloaddition (Click Reaction) of 2-propynyl and 3-butynyl 2,3,4-tri-O-acetyl-6-azido-6-deoxy-glycopyranosides in the D-gluco, D-galacto and D-manno series afford the corresponding dimeric cycloaddition products.
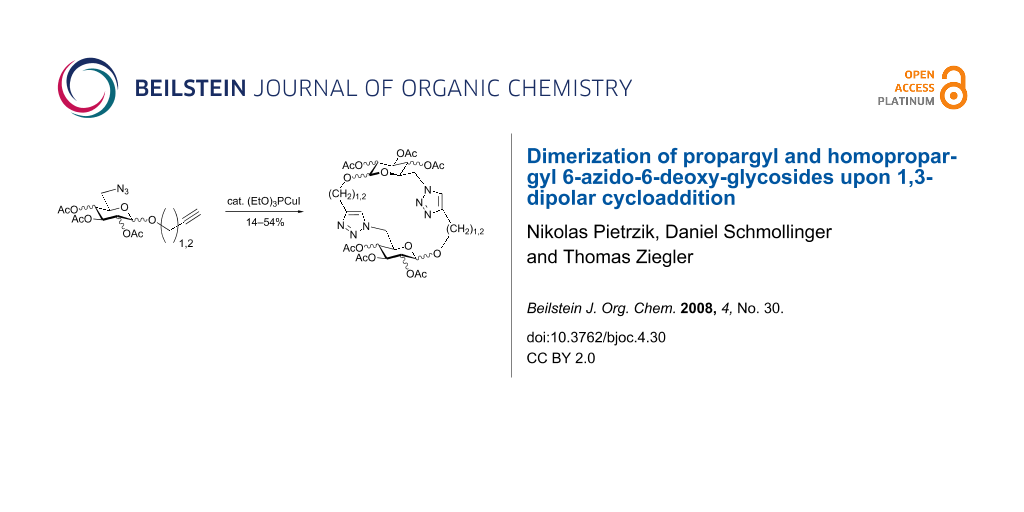
Graphical Abstract
Introduction
Our ongoing interest in constructing combinatorial libraries of highly glycosylated beta-peptides that can mimic specific oligosaccharide-protein interactions prompted us to further search for efficient routes toward glycosylated amino acid building blocks derived from asparaginic acid in which the glycon is bound to C-1 of the asparaginic acid through variable spacers (Figure 1). Previously, we have prepared a series of glycosylated asparaginic acid building blocks containing as spacers either simple alkyl chains [1], or amino alcohols [2,3]. Such building blocks have been shown to be well suited for combinatorial solid phase or spot synthesis of libraries of highly glycosylated peptides, some members of which were indeed shown to behave like oligosaccharide mimics capable to specifically bind lectins [1,4].
Figure 1: Schematic representation of glycosylated building blocks for the combinatorial synthesis of glycopeptides.
Figure 1: Schematic representation of glycosylated building blocks for the combinatorial synthesis of glycope...
In order to increase the structural diversity of the aforementioned building blocks, we contemplated using as the spacer entity 1,2,3-triazoles which are known to be easily generated through a copper-catalyzed 1,3-dipolar cycloaddition of an organic azide and an alkynyl derivative (Click Reaction) [5-7]. For review articles on copper-catalyzed Click Reactions see references [8-11]. Recently, we applied this approach to a series of 1,2,3-triazole containing per-O-acetyl-glycosides which were prepared by copper-catalyzed 1,3-dipolar cycloaddition either between fully acetylated propargyl 1-thio-glycosides and t-butyl (S)-4-azido-3-fluorenylmethyloxycarbamido-butyrate or between Fmoc-L-Asp(OtBu)-propargyl amide and 2,3,4,6-tetra-O-acetyl-glycosyl azides and ethyl 2,3,4-tri-O-acetyl-6-azido-6-deoxy-1-thio-glycosides, respectively [12]. In order to increase the structural diversity of glycosyl amino acid building blocks containing 1,2,3-triazole spacers even more, we next looked at the possibility to use glycosides bearing both, azido and alkynyl groups in copper-catalyzed 1,3-cycloadditions. The results are presented here.
Results and Discussion
First, 2-propynyl 6-azido-6-deoxy-2,3,4-tri-O-acetyl-β-D-glucopyranoside (4a) was prepared by the following sequence. 2-Propynyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside (1a) [13] was Zemplén-deacetylated with a catalytic amount of sodium methanolate in methanol. Next, thus obtained crude 2-propynyl β-D-glucopyranoside was regioselectively tosylated at position 6 [14] followed by chromatographic purification to afford 6-O-p-tolylsulfonyl-glucoside 2a in 66% yield. Acetylation of the latter with acetic anhydride in pyridine gave crude tri-O-acetyl-6-O-p-tolylsulfonyl-glucoside 3a which was sufficiently pure for the next step. Treatment of 3a with NaN3 in DMF finally afforded 6-azido-6-deoxy-glucoside 4a in 38% yield. When glucoside 4a was reacted with asparaginic propargyl amide derivative 5 [12] in the presence of (EtO)3PCuI as catalyst and with or without microwave irradiation [15], the induced 1,3-dipolar cycloaddition between the alkynyl and azide moieties (Click Reaction) afforded compound 6 in variable medium yields of approximately 60%. The yield depended on the reaction conditions under which the cycloaddition was carried out. Several byproducts were formed during this cycloaddition reaction which, however, could not be separated and characterized. The amount of these byproducts increased at higher reaction temperatures or upon irradiation with microwave. It was anticipated that the byproducts which lowered the yield of compound 6 might be decomposition products of the starting material 4a. Therefore, the more stable benzoylated glucoside 3a' was prepared from 2a, and converted into the azide 4a' in 89% and 84% yield, respectively. Treatment of 4a' with 5 under Cu(I)-catalysis, however, only resulted in a complex mixture of reaction products from which no uniform product could be isolated. Therefore, it was concluded that 4a and 4a' may have reacted with themselves resulting in products of oligomerization. Indeed, when 4a was treated with a catalytic amount of (EtO)3PCuI , TLC (ethyl acetate/n-hexane 1:1) revealed the formation of one faster moving product along with a complex mixture of slower moving products with mobility similar to those previously observed. Careful inspection of the products revealed that dimerisation of 4a occurred, affording the dimeric glycoside 7a beside products of oligomerization (Scheme 1). The reaction proceeded significantly slower than the coupling of 4a and 5. A faster reaction occurred upon irradiation with microwave, which also gave a higher yield (54%) of 7a. Benzoylated glycoside 4a' did not give any product of dimerization though. Only oligomers 8 were observed in this case (for details see Supporting Information File 1).
Scheme 1: Synthesis and reaction of compounds 4a and 4a'.
Scheme 1: Synthesis and reaction of compounds 4a and 4a'.
At first, it was unclear whether 7a was formed by an intramolecular cyclization or a dimerization of 4a since its concentration-dependent ESI-MS and MALDI-TOF-MS spectra both showed peaks corresponding to the molecular mass of 4a and 7a, respectively. However, the dimeric structure of compound 7a was finally unambiguously assigned by NMR spectroscopy and field desorption (FD) mass spectrometry. The NMR spectra of 7a showed no conformative anomalies of the pyranose ring what would have been expected if 7a would have been the product of intramolecular 1,3-dipolar cycloaddition of the azido group and the 2-propynyl aglycon in the starting material 4a.
The oligomerization of glycosides containing both, an azido and an alkynyl group upon copper-catalyzed Click-Reaction had been observed previously in two instances. Gin and coworker recently found that 2,3,6-tri-O-benzyl-4-O-(2-propynyl)-α-D-mannopyranosyl azide affords a cyclic trimer upon 1,3-dipolar cycloaddition of its azido moiety to its propynyl moiety while the corresponding α-1,4-linked manno-disaccharide afforded a cyclic dimer similar to compound 7a [16]. Jarosz et al. also recently reported about the copper catalyzed reaction of 6-azido-1',2,3,3',4,4'-hexa-O-benzyl-6-deoxy-6'-propargyl-sucrose to afford either a product of intramolecular cyclization or a dimeric product, depending on the reaction conditions [17]. Likewise, Vasella reported the thermal intramolecular 1,3-dipolar cycloaddition of protected 2-azidoethyl 45-O-(2-propynyl)-malto-hexaoside, giving the corresponding isomeric macrocyclic derivatives [18]. In the light of Gin's and Jarosz's results and our own unexpected finding that 4a can form cyclic dimers upon copper-catalyzed Click-Reaction, we investigated several other 2-propynyl and 3-butynyl 6-azido-6-deoxy-glycosides 4 in order to probe their ability to form similar cyclic dimers 7.
First, an alternative route to 2-propynyl 6-azido-6-deoxy-glucoside 4a was attempted (Scheme 2). Compound 1a was deacetylated and treated with N-bromosuccinimide and triphenylphosphine in DMF according to Hanessian's procedure [19] followed by reacetylation of the OH-groups with acetic anhydride in pyridine to afford 2-propynyl 6-bromo-6-deoxy-2,3,4-tri-O-acetyl-β-D-glucopyranoside (3a'') in 60% yield. Next, the latter was stirred with NaN3 in DMF (48 h, 65 °C) to afford 4a in 44% yield. The preparation of compound 4a via the corresponding tosylate 3a was somewhat more convenient than the synthesis via the 6-bromo-6-deoxy counterpart 3a'' and resulted in a similar overall yield. Therefore, all other 6-azido-6-deoxy-glycosides 4 were prepared via the corresponding tosylates 3 as described above. Scheme 2 summarizes the yields for the preparation of the tosylates 3 and 6-azido-6-deoxy-glycosides 4. Starting materials 1 were prepared following known procedures for 1a [13,20], 1b [21], 1d [13], 1f [20,22] and 1g [21]. 2-Propynyl 2,3,4,6-tetra-O-acetyl-α-D-glycopyranosides 1c and 1e have not been described previously. They were prepared from D-glucose and D-galactose in 20% and 22% yield, respectively via classical Fischer-Glycosylation in 2-propynol as the solvent under acidic conditions followed by acetylation of the intermediate glycosides and chromatographic separation of the anomeric acetates.
Next, glycosides 4a–g were submitted to dimerization by 1,3-dipolar cycloaddition reaction. As the catalyst, 10 mol% (EtO)3PCuI was applied and used along with three equivalents diisopropyl ethylamine in toluene [15]. Microwave irradiation [23] reduced the reaction time significantly but also resulted in decomposition of the starting material in some cases. Table 1 summarizes the results for the dimerization of 4a–g to 7a–g.
Table 1: Dimerization of Glycosides 4a–g under Cu-Catalysis.
| Entry | Glycoside 4 | Product 7 | Conditions | Yield |
|---|---|---|---|---|
| 1 |
4a |
|
12 h rt
1 h 80 °C, 20 W MW |
54%
20% |
| 2 |
4b |
|
12 h rt
1 h 80 °C, 20 W MW |
-
32% |
| 3 |
4c |
|
12 h rt
1 h 80 °C, 20 W MW |
14%
- |
| 4 |
4d |
|
12 h rt
1 h 80 °C, 20 W MW |
28%
- |
| 5 |
4e |
|
12 h rt
1 h 80 °C, 20 W MW |
-
- |
| 6 |
4f |
|
12 h rt
1 h 80 °C, 20 W MW |
-
30% |
| 7 |
4g |
|
12 h rt
1 h 80 °C, 20 W MW |
-
53% |
Yields of the copper-catalyzed dimerizations were low to medium (14–54%) and depended on the sugar moiety, the anomeric configuration and the ring size which was formed during the Click-Reaction. In general, no other cyclization product could be isolated from the reaction mixtures although significant amounts of byproducts were formed. These byproducts were slower moving compounds on TLC (ethyl acetate/n-hexane 1:1) and appeared to be products of oligomerization of the starting material 4. In the case of benzoylated glycoside 4a' where no cyclic dimer could be isolated from the complex reaction mixture, FAB MS of the purified mixture indeed revealed the presence of linear dimers, trimers and tetramers.
α-Galactoside 4e did not give any isolable dimer 7e at all (cf. Table 1, entry 5). Similarly, α-glucoside 4c resulted in a lower yield of the corresponding dimer compared to β-glucoside 4a (cf. Table 1, entries 1 and 3). This may be attributed to a significant ring-strain in the α-linked dimers. For example, the 1H NMR of compound 7c showed an unusually small coupling constant between H-1 and H-2 (<1.0 Hz) and H-2 and H-3 (3.1 Hz) which is indicative that the sugar moieties in 7c are no longer in a chair conformation (see Table 1 in the Supporting Information File 1). No such effects were observed in the manno series though (cf. Table 1, entries 6 and 7). Here, the corresponding dimers 7f and 7g showed regular coupling constants in their NMR spectra.
The effect of microwave irradiation on the outcome of the dimerization is somewhat confusing. In general, microwave irradiation resulted in a faster reaction, i.e. faster disappearance of the starting material (cf. Table 1, entry 1). Similar accelerations of Click-Reactions upon microwave irradiation had been observed previously as well [15]. However, the higher temperature associated with the microwave irradiation also resulted in a more pronounced decomposition of the starting material, and thus resulted in a lower yield of the dimers (cf. Table 1, entries 1, 3 and 4) while heating of the reaction mixture alone resulted in complex product mixtures from which no dimerization products could be isolated. In the case of compounds 4b and 4e–g, no reaction occurred at room temperature (cf. Table 1, entries 2 and 5–7).
Conclusion
We describe for the first time the copper-catalyzed dimerization of simple acetylated 2-propynyl and 3-butynyl 6-azido-6-deoxy-glycosides in the gluco, galacto and manno series leading to macrocyclic rings containing two sugar moieties and two 1,2,3-triazole moieties. For instance, such compounds may function as novel ligands for the preparation of metal complexes [24]. Further examples for cyclizations of other azido-alkynyl-glycosides are under investigation.
Supporting Information
| Supporting Information File 1: Experimental Data | ||
| Format: DOC | Size: 565.5 KB | Download |
References
-
Ziegler, T.; Röseling, D.; Subramanian, L. R. Tetrahedron: Asymmetry 2002, 13, 911–914. doi:10.1016/S0957-4166(02)00212-4
Return to citation in text: [1] [2] -
Schips, C.; Ziegler, T. J. Carbohydr. Chem. 2005, 24, 773–788. doi:10.1080/07328300500326859
Return to citation in text: [1] -
Ziegler, T.; Schips, C. Umsetzung von Aminoalkoholen mit sauren, organischen Substraten nach Art einer Mitsunobu-Reaktion. German Patent 102004046010B3, Dec 8, 2005.
Chem. Abstr. 2005, 144, 1282957.
Return to citation in text: [1] -
Ziegler, T.; Schips, C. Nat. Protoc. 2006, 1, 1987–1994. doi:10.1038/nprot.2006.307
Return to citation in text: [1] -
Huisgen, R.; Knorr, R.; Möbius, L.; Szeimies, G. Chem. Ber. 1965, 98, 4014–4021. doi:10.1002/cber.19650981228
Return to citation in text: [1] -
Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
Return to citation in text: [1] -
Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j
Return to citation in text: [1] -
Bock, V. D.; Hiemstra, H.; van Maarseveen, J. H. Eur. J. Org. Chem. 2006, 51–68. doi:10.1002/ejoc.200500483
Return to citation in text: [1] -
Binder, W. H.; Kluger, C. Curr. Org. Chem. 2006, 10, 1791–1815. doi:10.2174/138527206778249838
Return to citation in text: [1] -
Dedola, S.; Nepogodiev, S. A.; Field, R. A. Org. Biomol. Chem. 2007, 5, 1006–1017. doi:10.1039/b618048p
Return to citation in text: [1] -
Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674–1689. doi:10.1039/b701444a
Return to citation in text: [1] -
Pietrzik, N.; Schips, C.; Ziegler, T. Synthesis 2008, 519–526. doi:10.1055/s-2008-1032150
Return to citation in text: [1] [2] -
Mereyala, H. B.; Gurrala, S. R. Carbohydr. Res. 1998, 307, 351–354. doi:10.1016/S0008-6215(97)10104-5
Return to citation in text: [1] [2] [3] -
Cramer, F.; Otterbach, H.; Springmann, H. Chem. Ber. 1959, 92, 384–391. doi:10.1002/cber.19590920221
Return to citation in text: [1] -
Pérez-Balderas, F.; Ortega-Muñoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F. G.; Calvo-Asín, J. A.; Isac-García, J.; Santoyo-González, F. Org. Lett. 2003, 5, 1951–1954. doi:10.1021/ol034534r
Return to citation in text: [1] [2] [3] -
Bodine, K. D.; Gin, D. Y.; Gin, M. S. Org. Lett. 2005, 7, 4479–4482. doi:10.1021/ol051818y
Return to citation in text: [1] -
Jarosz, S.; Lewandowski, B.; Listkowski, A. Synthesis 2008, 913–916. doi:10.1055/s-2008-1032198
Return to citation in text: [1] -
Hoffmann, B.; Bernet, B.; Vasella, A. Helv. Chim. Acta 2002, 85, 265–287. doi:10.1002/1522-2675(200201)85:1<265::AID-HLCA265>3.0.CO;2-1
Return to citation in text: [1] -
Hanessian, S.; Ponpipom, M. M.; Lavallee, P. Carbohydr. Res. 1972, 24, 45–56. doi:10.1016/S0008-6215(00)82258-2
Return to citation in text: [1] -
Kaufman, R. J.; Sidhu, R. S. J. Org. Chem. 1982, 47, 4941–4947. doi:10.1021/jo00146a023
Return to citation in text: [1] [2] -
Tietze, L. F.; Bothe, U. Chem.–Eur. J. 1998, 4, 1179–1183. doi:10.1002/(SICI)1521-3765(19980710)4:7<1179::AID-CHEM1179>3.0.CO;2-F
Return to citation in text: [1] [2] -
Fernandez-Megia, E.; Correa, J.; Rodríguez-Meizoso, I.; Riguera, R. Macromolecules 2006, 39, 2113–2120. doi:10.1021/ma052448w
Return to citation in text: [1] -
Savin, K. A.; Robertson, M.; Gernert, D.; Green, S.; Hembre, E. J.; Bishop, J. Mol. Diversity 2003, 7, 171–174. doi:10.1023/B:MODI.0000006801.27748.3b
Return to citation in text: [1] -
Ziegler, T.; Hermann, C. Tetrahedron Lett. 2008, 49, 2166–2169. doi:10.1016/j.tetlet.2008.01.081
Return to citation in text: [1]
| 21. | Tietze, L. F.; Bothe, U. Chem.–Eur. J. 1998, 4, 1179–1183. doi:10.1002/(SICI)1521-3765(19980710)4:7<1179::AID-CHEM1179>3.0.CO;2-F |
| 13. | Mereyala, H. B.; Gurrala, S. R. Carbohydr. Res. 1998, 307, 351–354. doi:10.1016/S0008-6215(97)10104-5 |
| 20. | Kaufman, R. J.; Sidhu, R. S. J. Org. Chem. 1982, 47, 4941–4947. doi:10.1021/jo00146a023 |
| 22. | Fernandez-Megia, E.; Correa, J.; Rodríguez-Meizoso, I.; Riguera, R. Macromolecules 2006, 39, 2113–2120. doi:10.1021/ma052448w |
| 1. | Ziegler, T.; Röseling, D.; Subramanian, L. R. Tetrahedron: Asymmetry 2002, 13, 911–914. doi:10.1016/S0957-4166(02)00212-4 |
| 8. | Bock, V. D.; Hiemstra, H.; van Maarseveen, J. H. Eur. J. Org. Chem. 2006, 51–68. doi:10.1002/ejoc.200500483 |
| 9. | Binder, W. H.; Kluger, C. Curr. Org. Chem. 2006, 10, 1791–1815. doi:10.2174/138527206778249838 |
| 10. | Dedola, S.; Nepogodiev, S. A.; Field, R. A. Org. Biomol. Chem. 2007, 5, 1006–1017. doi:10.1039/b618048p |
| 11. | Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674–1689. doi:10.1039/b701444a |
| 13. | Mereyala, H. B.; Gurrala, S. R. Carbohydr. Res. 1998, 307, 351–354. doi:10.1016/S0008-6215(97)10104-5 |
| 20. | Kaufman, R. J.; Sidhu, R. S. J. Org. Chem. 1982, 47, 4941–4947. doi:10.1021/jo00146a023 |
| 5. | Huisgen, R.; Knorr, R.; Möbius, L.; Szeimies, G. Chem. Ber. 1965, 98, 4014–4021. doi:10.1002/cber.19650981228 |
| 6. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 7. | Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j |
| 21. | Tietze, L. F.; Bothe, U. Chem.–Eur. J. 1998, 4, 1179–1183. doi:10.1002/(SICI)1521-3765(19980710)4:7<1179::AID-CHEM1179>3.0.CO;2-F |
| 1. | Ziegler, T.; Röseling, D.; Subramanian, L. R. Tetrahedron: Asymmetry 2002, 13, 911–914. doi:10.1016/S0957-4166(02)00212-4 |
| 4. | Ziegler, T.; Schips, C. Nat. Protoc. 2006, 1, 1987–1994. doi:10.1038/nprot.2006.307 |
| 18. | Hoffmann, B.; Bernet, B.; Vasella, A. Helv. Chim. Acta 2002, 85, 265–287. doi:10.1002/1522-2675(200201)85:1<265::AID-HLCA265>3.0.CO;2-1 |
| 2. | Schips, C.; Ziegler, T. J. Carbohydr. Chem. 2005, 24, 773–788. doi:10.1080/07328300500326859 |
| 3. |
Ziegler, T.; Schips, C. Umsetzung von Aminoalkoholen mit sauren, organischen Substraten nach Art einer Mitsunobu-Reaktion. German Patent 102004046010B3, Dec 8, 2005.
Chem. Abstr. 2005, 144, 1282957. |
| 19. | Hanessian, S.; Ponpipom, M. M.; Lavallee, P. Carbohydr. Res. 1972, 24, 45–56. doi:10.1016/S0008-6215(00)82258-2 |
| 12. | Pietrzik, N.; Schips, C.; Ziegler, T. Synthesis 2008, 519–526. doi:10.1055/s-2008-1032150 |
| 16. | Bodine, K. D.; Gin, D. Y.; Gin, M. S. Org. Lett. 2005, 7, 4479–4482. doi:10.1021/ol051818y |
| 15. | Pérez-Balderas, F.; Ortega-Muñoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F. G.; Calvo-Asín, J. A.; Isac-García, J.; Santoyo-González, F. Org. Lett. 2003, 5, 1951–1954. doi:10.1021/ol034534r |
| 14. | Cramer, F.; Otterbach, H.; Springmann, H. Chem. Ber. 1959, 92, 384–391. doi:10.1002/cber.19590920221 |
| 17. | Jarosz, S.; Lewandowski, B.; Listkowski, A. Synthesis 2008, 913–916. doi:10.1055/s-2008-1032198 |
| 24. | Ziegler, T.; Hermann, C. Tetrahedron Lett. 2008, 49, 2166–2169. doi:10.1016/j.tetlet.2008.01.081 |
| 13. | Mereyala, H. B.; Gurrala, S. R. Carbohydr. Res. 1998, 307, 351–354. doi:10.1016/S0008-6215(97)10104-5 |
| 15. | Pérez-Balderas, F.; Ortega-Muñoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F. G.; Calvo-Asín, J. A.; Isac-García, J.; Santoyo-González, F. Org. Lett. 2003, 5, 1951–1954. doi:10.1021/ol034534r |
| 12. | Pietrzik, N.; Schips, C.; Ziegler, T. Synthesis 2008, 519–526. doi:10.1055/s-2008-1032150 |
| 15. | Pérez-Balderas, F.; Ortega-Muñoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F. G.; Calvo-Asín, J. A.; Isac-García, J.; Santoyo-González, F. Org. Lett. 2003, 5, 1951–1954. doi:10.1021/ol034534r |
| 23. | Savin, K. A.; Robertson, M.; Gernert, D.; Green, S.; Hembre, E. J.; Bishop, J. Mol. Diversity 2003, 7, 171–174. doi:10.1023/B:MODI.0000006801.27748.3b |
© 2008 Pietrzik et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)