Abstract
Dimeric forms of norbornadiene and benzonorbornadiene were synthesized starting with known monobromide derivatives. The Diels–Alder cycloaddition reaction of dimers with TCNE and PTAD was investigated and new norbornenoid polycyclics were obtained. All compounds were characterized properly using NMR spectroscopy.
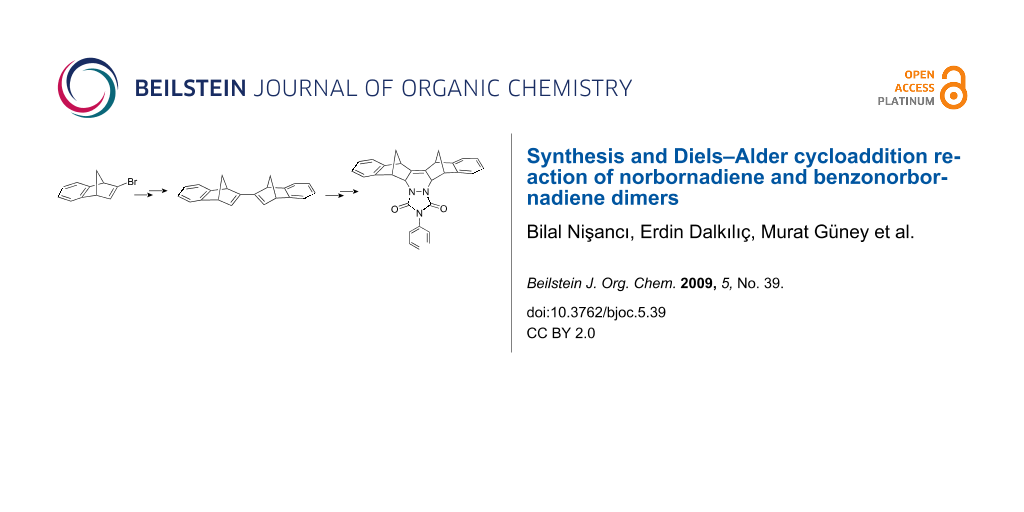
Graphical Abstract
Introduction
Norbornadiene (1) and related compounds are of great scientific interest because of their unusual geometry and high reactivity. For example, these compounds exhibit a unique behavior in the cationic Wagner–Meerwein rearrangement [1-10], in the solvolytic reactivity [11], in the photochemical di-π-methane rearrangement [12-15], as well as in other instances [16-22]. Therefore, functionalizations of these compounds are important. In this study, we investigated the synthesis and Diels–Alder cycloaddition reaction of norbornadiene and benzonorbornadiene dimers.
Results and Discussion
One of the starting materials, 2-bromobenzonorbornadiene 4 was synthesized using a procedure described in the literature [15,23] (Scheme 1). Photochemical bromination of benzonorbornadiene 2 with 1,2-dibromotetrachloroethane gave isomeric dibromides 3 in high yield. Dehydrobromination reaction of dibromides 3 with potassium tert-butoxide resulted in the formation of monobromide 4. The other starting material 5 was obtained using the reported procedures based on the use of potassium tert-butoxide/n-butyllithium super-base by starting with commercially available norbornadiene [24-27].
Scheme 1: Synthesis of starting materials 4 and 5.
Scheme 1: Synthesis of starting materials 4 and 5.
When 2-bromobenzonorbornadiene 4 was treated with n-BuLi at −78 °C and the resulting anion was quenched with trimethyltin chloride, a single trimethyltin derivative 6 was observed in the crude reaction mixture and was finally isolated in 91% yield. Copper salts have been successfully employed for Stille-type hetero-coupling between unsaturated halides and stannanes [28,29]. Treatment of 6 with Cu(NO3)2·3H2O in dry THF at r.t. afforded the first synthesis of the expected dimers 7 and 8 in 25% yield in a 3:4 ratio, respectively, besides benzonorbornadiene 2 after column chromatography. The Diels–Alder cycloaddition of dimers 7 and 8 with PTAD (9) and TCNE (10) resulted in the formation of the corresponding products 11–14 in high yields (Scheme 2).
Scheme 2: Synthesis and Diels–Alder cycloaddition reactions of dimers 7 and 8.
Scheme 2: Synthesis and Diels–Alder cycloaddition reactions of dimers 7 and 8.
Similarly, tin compound 15 was synthesized by the reaction of monobromide 5 with n-BuLi followed by reaction with trimethyltin chloride. Reaction of 15 with Cu(NO3)2·3H2O resulted in the formation of dimers 16 and 17 [30]. This reaction offered an alternative synthetic route to norbornadiene dimers 16 and 17. The isomers 16 and 17 could not be separated, but after cycloaddition reaction of the mixture, the corresponding addition products 18–21 were isolated by chromatographic methods (Scheme 3).
Scheme 3: Synthesis and Diels–Alder cycloaddition reactions of dimers 16 and 17.
Scheme 3: Synthesis and Diels–Alder cycloaddition reactions of dimers 16 and 17.
Structural Analyses
The determination of the structures of dimers 7, 8 and dimers 16, 17 by spectroscopic methods was not simple because the Cs symmetry of the syn dimers and the C2 symmetry of the anti dimers and the free rotation around the central σ bond make them indistinguishable. To determine which is which, cycloaddition reactions of dimers are more informative. Dimers 7 and 16 give symmetric addition products 11, 12 and 18, 19, whereas the reaction of dimers 8 and 17 resulted in the formation of unsymmetrical products 13, 14 and 20, 21.
For the symmetric addition products 11, 12, 18 and 19, there are two possibilities: exo adduct or endo adduct (Figure 1). The coupling constants between the relevant protons in the norbornene unit are very informative to assign the correct configuration of the substituents [9,10]. The high value of J34 and J3′4′ (2.5–3.5 Hz) in the Diels–Alder addition products is uniquely accommodated by the exo orientation of the protons (endo orientation of -A-A- ring) at C3 and C3′ carbon atoms. For example, though there is coupling between the protons H3 and H4, there is no measurable coupling between the protons H3′ and H4′ in anti structures (Figure 1). On the other hand, the absence of any coupling between the related protons confirms the endo orientation of protons at C3 and C3′, which in turn proves the exo-orientation of the rings in adduct 11, 12, 18 and 19. The coupling between the protons H3 (H3′) and H7syn (H7′syn) (M or W orientation) also confirms the exo structures for 11, 12, 18 and 19 (Figure 1).
Figure 1: Numbering of carbon atoms and description of possible structures for dimers 11–14 and 18–21.
Figure 1: Numbering of carbon atoms and description of possible structures for dimers 11–14 and 18–21.
In summary, the synthesis and cycloaddition reaction of norbornadiene and benzonorbornadiene dimers was investigated and new norbornanoid polycyclic compounds, which open up several synthetic and mechanical investigations, were obtained.
Experimental
General: Melting points are uncorrected. Infrared spectra were obtained from solution in 0.1 mm cells or KBr pellets on a regular instrument. The 1H and 13C NMR spectra were recorded on 400 (100) and 200 (50) MHz spectrometers. Apparent splitting is given in all cases. Column chromatography was performed on silica gel (60-mesh, Merck) TLC was carried out on Merck 0.2 mm silica gel 60 F254 analytical aluminum plates. All substances reported in this paper are meso-compounds or racemates.
Synthesis of (1,4-dihydro-1,4-methano-naphthalen-2-yl)trimethylstannane (6): A solution of n-BuLi in n-hexane (2.7 M, 3.41 mL, 9.19 mmol) was added dropwise to a solution of monobromobenzonorbornadiene 4 (2.03 g, 9.19 mmol) in dry THF (20 mL) at −78 °C and the resulting mixture was stirred for 40 min. Trimethyltin chloride (1.83 g, 9.19 mmol) was added portionwise and then left to warm to room temperature. The mixture was stirred overnight at room temperature. The crude product was washed with water (15 mL) and extracted with Et2O (2 × 50 mL) and then the combined ethereal extracts were dried over MgSO4 and concentrated in vacuo. (1,4-dihydro-1,4-methano-naphthalen-2-yl)trimethylstannane (6) was obtained as yellow liquid (2.55 g, 91%). 1H NMR (400 MHz, CDCl3): δ 7.26–7.20 (m, 2H, aryl), 7.06 (d, J3,4 = 2.9 Hz, 1H, H3), 6.98–6.94 (m, 2H, aryl), 4.08 (m, 1H, H4), 3.98 (m, 1H, H1), 2.24 (m, 2H, H9syn and H9anti), 0.18 (s, 9H, 3 × CH3). 13C NMR (100 MHz, CDCl3): δ 155.61, 153.22, 151.95, 151.92, 124.36, 124.18, 121.62, 121.58, 69.65, 55,77, 52.05, −9.70.
Reaction of (1,4-dihydro-1,4-methano-naphthalen-2-yl)trimethylstannane (6) with Cu(NO3)2·3H2O: Copper(II) nitrate trihydrate (345 mg, 1.4 mmol) was added portionwise to a solution of 6 (435 mg, 1.4 mmol) in THF (6 mL) at room temperature. The blue solution turned green within 1 h. The crude reaction mixture was diluted with Et2O (100 mL) and then washed with 5% NH3 (15 mL). The organic phase was dried over MgSO4 and concentrated in vacuo. The residue was chromatographed on neutral aluminum oxide (150 g) eluted with hexane. The first fraction was benzonorbornadiene (155 mg, 57%). The second fraction was anti isomer 8 (28 mg, 14%). 1H NMR (400 MHz, CDCl3): δ 7.15–6.79 (m, 8H, Haryl), 6.56 (d, J3,4 = J3′,4′ = 2.9 Hz, 2H, H3 and H3′), 3.92 (m, 2H, H4 and H4′), 3.86 (m, 2H, H1 and H1′), 2.40 (dt, A Part of AB system, J9syn,9anti = J9′syn,9′anti = 7.1 Hz, J9syn,1 = J9syn,4 = J9′syn,1′ = J9′syn,4′ = 1.5 Hz, 2H, H9syn and H9′syn), 2.25 (bd, B part of AB system, J9anti,9syn = J9′anti,9′syn = 7.1 Hz, 2H, H9anti,9′anti).13C NMR (100 MHz, CDCl3): δ 151.89, 151.46, 150.65, 133.96, 124.40, 124.32, 121.67, 120.97, 68.76, 52.12, 50.89. The third fraction was the syn-dimer 7 (23 mg, 11%). Colorless crystals from CH2Cl2/n-hexane (1:3). mp 152–154 °C. 1H NMR (400 MHz, CDCl3): δ 7.29–6.94 (m, 8H, Haryl), 6.61 (d, J3,4 = J3′,4′ = 2.9 Hz, 2H, H3 and H3′), 3.90 (m, 2H, H4 and H4′), 3.80 (m, 2H, H1 and H1′), 2.21 (dt, A Part of AB system, J9syn,9anti = J9′syn,9′anti = 7.3 Hz, J9syn,1 = J9syn,4 = J9′syn,1′ = J9′syn,4′ = 1.6 Hz, 2H, H9anti and H9′anti), 2.17 (bd, B Part of AB system, J9anti,9syn = J9′anti,9′syn = 7.3 Hz, 2H, H9anti and H9′anti). 13C NMR (100 MHz, CDCl3): δ 151.88, 151.55, 151.32, 134.38, 124.59, 124.41, 121.70, 121.15, 67.71, 51.59, 50.72. IR (KBr, cm−1): 3067, 2981, 2936, 2866, 1455, 1317, 1270, 1226, 1199, 1149, 1068, 1011, 909, 750, 735. MS (70 eV) m/z: 282.5 (M+, 32), 267.5 (21), 239.4 (5), 202.4 (2), 178.4 (5), 167.3 (26), 165.3 (32), 141.2 (28), 117.2 (71), 115.2 (56), 89.1 (6), 63.1 (3).
Cycloaddition reaction of the dimer 7 with PTAD (9): A solution of the syn dimer 7 (40 mg, 0.14 mmol) and PTAD (25 mg, 0.14 mmol) in 4 mL of CH2Cl2 was stirred at room temperature for 30 min. The solvent was removed under reduced pressure. The crude product was purified by crystallization from CH2Cl2/n-hexane (3:1) to give syn cycloadduct 11 (55 mg, 89%). Yellow crystals, mp 182–184 °C. 1H NMR (400 MHz, CDCl3): δ 7.60–7.12 (m, 13H), 4.66 (s, 2H), 4.24 (s, 2H), 3.75 (d, J = 1.5 Hz, 2H), 2.21 (dq, A Part of AB system, J = 9.4 Hz, J = 1.5 Hz, 2H), 2.14 (dt, B Part of AB system, J = 9.4 Hz, J = 1.4 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 151.74, 144.59, 144.54, 132.02, 131.84, 129.27, 128.28, 127.38, 127.05, 126.04, 123.09, 121.94, 59.25, 48.73, 48.20, 47.18. IR (KBr, cm−1): 3048, 2976, 2941, 1762, 1702, 1600, 1502, 1439, 1419, 1343, 1265, 1140. MS (70 eV) m/z: 458.4 (M+, 3), 344.0 (5), 282.0 (10), 280.8 (7), 165.6 (24), 119.4 (43), 116.4 (100), 91.3 (43).
Cycloaddition reaction of the dimer 7 with TCNE (10): A solution of the syn dimer 7 (50 mg, 0.17 mmol) and TCNE (10, 23 mg, 0.17 mmol) in 5 mL of CH2Cl2 was stirred at room temperature for overnight. The solvent was removed under reduced pressure. The crude product was purified by crystallization from CH2Cl2/n-hexane (3:1) to give syn cycloadduct 12 (68 mg, 93%). White crystals, mp 230–232 °C. 1H NMR (400 MHz, CDCl3): δ 7.54–7.17 (m, 8H, Haryl), 4.22 (s, 2H), 3.86 (m, 2H), 2.48 (bd, A Part of AB system, 2H, J = 10.3 Hz), 2.45 (m, 2H), 2.22 (d, B Part of AB system, 2H, J = 10.3 Hz). 13C NMR (100 MHz, CDCl3): δ 145.93, 144.40, 133.14, 127.76, 127.32, 122.17, 122.06, 112.08, 110.87, 50.01, 48.54, 47.81, 46.73, 45.90. IR (KBr, cm−1): 3050, 2955, 2872, 2306, 2254, 2217, 1463, 1318, 1265, 1120, 1153, 1013, 981, 785, 704. MS (70 eV) m/z: 410.1 (M+, 100), 394.1 (10), 370.1 (37), 345.1 (35), 319.1 (27), 295 (27), 267.1 (45), 265.0 (27), 229.0 (17), 205.0 (32), 176.9 (22), 164.9 (4), 152.9 (22), 151.9 (30).
Cycloaddition reaction of the dimer 8 with PTAD (9): A solution of the anti dimer (40 mg, 0.14 mmol) and PTAD (25 mg, 0.14 mmol) in 4 mL of CH2Cl2 was stirred at room temperature for 30 min. The solvent was removed under reduced pressure. The crude product was purified by crystallization from ether/n-hexane (2:1) to give anti cycloadduct 13 (58 mg, 90%). Yellow crystals, mp 168–170 °C. 1H NMR (400 MHz, CDCl3): δ 7.57–7.00 (m, 13H, Haryl), 4.94 (m, 1H), 4.52 (d, J = 2.3 Hz, 1H), 4.38 (m, 1H), 4.09 (m, 1H), 3.34 (m, 1H), 2.40 (dt, A part of AB system, J = 7.7 Hz, J = 1.5 Hz, 1H), 2.36 (dt, B part of AB system, J = 7.7 Hz, J = 1.5 Hz, 1H), 1.43 (bd, A part of AB system, J = 10.7 Hz, 1H), 1.25 (m, 1H), 0.46 (bd, B part of AB system, J = 10.7 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 155.20, 154.38, 150.70, 147.28, 147.18, 145.68, 142.93, 129.50, 129.35, 128.80, 128.53, 127.91, 127.53, 125.93, 125.85, 125.62, 125.41, 123.17, 122.43, 121.92, 69.32, 63.91, 62.90, 50.90, 49.64, 49.53, 49.27, 45.43. IR (KBr, cm−1): 3065, 2961, 2923, 2851, 1718, 1497, 1412, 1262, 1135, 1091, 1023, 801. MS (70 eV) m/z: 410.1 (M+, 100), 394.1 (10), 370.1 (33), 345.1 (31), 319.1 (26), 267.1 (45), 205.0 (33), 164.9 (44), 151.9 (32).
Cycloaddition reaction of the dimer 8 with TCNE (10): A solution of the anti dimer 8 (40 mg, 0.14 mmol) and TCNE (10, 18 mg, 0.14 mmol) in 5 mL of CH2Cl2 was stirred at room temperature for overnight. The solvent was removed under reduced pressure. The crude product was purified by crystallization from CH2Cl2/n-hexane (3:1) to give anti cycloadduct 14 (53 mg, 91%). White crystals, mp 240 °C (dec). 1H NMR (400 MHz, CDCl3): δ 7.55–7.17 (m, 8H), 4.15 (m, 1H), 4.13 (m, 1H), 3.92 (dd, J = 3.5 Hz, J = 1.5 Hz, 1H), 3.74 (m, 1H), 3.41 (dd, J = 3.5 Hz, J = 1.5 Hz, 1H), 2.52 (m, 1H), 2.31 (dt, A part of AB system, J = 9.5 Hz, J = 1.5 Hz, 1H), 2.11 (dt, A part of AB system, J = 10.3 Hz, J = 1.5 Hz, 1H), 2.06 (dt, B part of AB system, J = 9.5 Hz, J = 1.5 Hz, 1H), 2.02 (bd, B part of AB system, J = 10.3 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 146.29, 145.79, 144.63, 139.70, 132.89, 132.55, 128.80, 127.61, 127.33, 127.30, 126.60, 122.18, 121.98, 120.73, 112.40, 112.33, 109.23, 108.83, 52.93, 50.29, 49.19, 47.86, 47.71, 47.49, 47.43, 46.79, 45.12, 44.55. IR (KBr, cm−1): 3049, 2989, 2956, 2923, 2851, 2241, 1906, 1459, 1366, 1262, 1012, 984. MS (70 eV) m/z: 410.1 (M+, 40), 345.1 (13), 295.1 (10), 252.0 (7), 205.0 (13), 164.9 (12), 127.9 (8), 117.0 (30), 114.9 (100).
Synthesis of (bicyclo[2.2.1]hepta-2,5-dien-2-yl)trimethylstannane (15): A solution of n-BuLi in n-hexane (2.5 M, 1.2 ml, 2.9 mmol) was added dropwise to a solution of 2-bromobicyclo[2.2.1]hepta-2,5-diene (5, 0.50 g, 2.9 mmol) in dry THF (5 mL) at −78 °C and the resulting mixture was stirred for 1 h. Trimethyltin chloride (582 mg, 2.9 mmol) was added portionwise and then left to warm to room temperature. The mixture was stirred over night at room temperature. The crude product was washed with water (50 mL) and extracted with Et2O (2 × 50 mL) and then the combined ethereal extracts were dried over MgSO4 and concentrated in vacuo. (Bicyclo[2.2.1]hepta-2,5-dien-2-yl)trimethylstannane (15) was obtained in the form of a yellow liquid (700 mg, 95%). 1H NMR (400 MHz, CDCl3): δ 7.02 (bd, J3,4 = 2.9 Hz, 1H, H3), 6.70 (m, 1H, H5 or H6), 6.65 (m, 1H, H5 or H6), 3.76 (m, 1H, H1 or H4) 3.63 (m, 1H, H1 or H4), 1.91 (m, 1H, H7syn or H7anti), 1.88 (m, 1H, H7syn or H7anti), 0.12 (s, 9H, 3 × CH3).13C NMR (100 MHz, CDCl3): δ 155.46, 154.28, 143.12, 143.07, 74.70, 55.77, 52.15, −9.90.
Reaction of (bicyclo[2.2.1]hepta-2,5-dien-2-yl)trimethylstannane (15) with Cu(NO3)2·3H2O: Copper(II) nitrate trihydrate (1.13 g, 4.69 mmol) was added portionwise to a solution of 15 (1.2 g, 4.69 mmol) in THF (10 mL) at room temperature. The blue solution turned green within 40 min. The crude reaction mixture was diluted with Et2O (100 mL) and then washed with 5% NH3 (15 mL). The organic phase was dried over MgSO4 and concentrated in vacuo. The syn-dimer 16 and anti-dimer 17 (in a 46:54 ratio) were obtained as a mixture (130 mg, 30%). The isomeric dimers 16 [30] and 17 [30] could not be separated and were used as the mixture for the following step.
Cycloaddition reaction of syn-16 and anti-17 mixture with PTAD: A solution of mixture of syn-16 and anti-17 (120 mg, 0,66 mmol) and PTAD (116 mg, 0,66 mmol) in 10 mL of CH2Cl2 was stirred at room temperature for 30 min. The solvent was removed under reduced pressure. The residue was chromatographed on silica gel (30 g) column eluted with EtOAc/n-hexane (1:9).
The first fraction was anti-cycloadduct 20 (89 mg, 70% based on anti dimer 17). Yellowish crystals from CH2Cl2/n-hexane (2:1), mp: 174–176 °C. 1H NMR (400 MHz, CDCl3): δ 7.53–7.25 (m, 5H, H), 6.33–6.27 (m, 3H), 6.06 (dd, J = 5.5 Hz, J = 2.9 Hz, 1H), 4.33 (d, J = 3.6 Hz, 1H), 4.09 (m, 1H), 3.93 (m, 1H), 3.68 (d, J = 1.7, 1H), 3.54 (m, 2H), 1.88 (dt, A part of AB system, J = 9.2 Hz, J = 1.7 Hz, 1H), 1.77 (bd, A part of AB system, J = 8.8 Hz, 1H), 1.68 (bd, B part of AB system, J = 9.2 Hz, 1H), 1.54 (bd, B part of AB system, J = 8.8 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 151.07, 149.85, 136.64, 136.55, 135.80, 133.12, 131.90, 131.61, 131.34, 129.20, 128.05, 125.87, 58.26, 56.54, 48.04, 47.57, 46.72, 46.37, 45.59, 45.38. IR (KBr, cm−1): 3060, 2925, 2852, 1760, 1698, 1502, 1419, 1130, 1028, 721. MS (70 eV) m/z: 357.3 (M+, 19), 315.8 (16), 291.2 (93), 250.9 (21), 239.2 (41), 195.1 (22), 182.1 (82), 118.8 (90), 91.0 (77), 77.0 (44). The second fraction was syn-cycloadduct 18 (75 mg, 69% based on syn dimer 16) Yellowish crystals from CH2Cl2/n-hexane (2:1), mp: 194–196 °C. 1H NMR (400 MHz, CDCl3): δ 7.54–7.34 (m, 5H, Haryl), 6.28 (m, 4H), 4.10 (m 2H), 3.73 (m, 2H), 3.64 (m, 2H), 1.84 (bd, A Part of AB system, J = 9.0 Hz, 2H), 1.69 (bd, B part of AB system, J = 9.0 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 151.47, 136.83, 135.82, 131.83, 131.05, 129.25, 128.19, 126.06, 57.94, 48.10, 46.20, 45.66. IR (KBr, cm−1): 3060, 2962, 2929, 2863, 1760, 1700, 1502, 1422, 1279, 1139, 761, 729. MS (70 eV) m/z: 357.4 (M+, 4), 316.4 (4), 291.3 (54), 280.1 (3), 252.0 (4), 239.0 (7), 210.3 (9), 182.1 (25), 165.1 (35), 144.0 (71), 120.0 (16), 115.0 (40), 102.0 (9), 90.7 (65), 66.1 (23).
Cycloaddition reaction of syn-16 and anti-17 mixture with TCNE (10): A solution of mixture of syn-16 and anti-17 (103 mg, 0.56 mmol) and TCNE (10, 72 mg, 0.56 mmol) in 10 mL of CH2Cl2 was stirred at room temperature overnight. The solvent was removed under reduced pressure. The residue was chromatographed on silica gel (30 g) eluted with EtOAc/n-hexane (1:32). The first fraction was anti-1,4,5,8,8a,10a-hexahydro-1,4:5,8-dimethanophenanthrene-9,9,10,10-tetracarbonitrile (21) (82 mg, 87% based on anti dimer 17). Yellowish crystals from CH2Cl2/n-hexane (3:1), mp:160–162 °C. 1H NMR (400 MHz, CDCl3): δ 6.46–6.35 (m, 4H), 3.61 (m, 2H), 3.49 (m, 1H), 3.36–3.33 (m, 2H), 2.36 (m, 1H), 2.16 (d, A part of AB system, J = 9.9 Hz, 1H), 1.95 (d, B Part of AB system, J = 9.9 Hz, 1H), 1.74–1.71 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 138.63, 138.37, 138.27, 132.47, 131.96, 130.76, 113.25, 112.03, 110.50, 110.47, 51.40, 51.32, 48.01, 46.74, 46.47, 46.43, 45.63, 45.62, 44.79, 44.75. IR (KBr, cm−1): 2934, 2896, 2868, 2352, 2093, 1457, 1235, 1043, 960. MS (70 eV) m/z: 310.1 (M+, 83), 295.0 (17), 282.1 (70), 268.0 (57), 243.0 (73), 229.0 (55), 218.1 (80), 203.0 (48), 190.0 (52), 179.0 (40), 167.0 (100), 151.9 (53). The second fraction was syn-1,4,5,8,8a,10a-hexahydro-1,4:5,8-dimethanophenanthrene-9,9,10,10-tetracarbonitrile (19) (75 mg, 93% based on syn dimer 16). Colorless crystals from CH2Cl2/n-hexane (2:1), mp: 136–138 °C. 1H NMR (400 MHz, CDCl3): δ 6.43 (dd, J = 5.5 Hz, J = 3.3 Hz, 2H), 6.30 (dd, J = 5,5 Hz, J = 2.9 Hz, 2H), 3.66 (s, 2H), 3.36 (d, J = 1.3 Hz, 2H), 2.48 (d, J = 1.3 Hz, 2H), 2.10 (d, A Part of AB system, Ji = 9.5 Hz, 2H), 1.89 (d, B Part of AB system, J = 9.5 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 138.25, 137.78, 132.26, 112.39, 110.79, 49.49, 47.90, 46.52, 45.95, 45.30. IR (KBr, cm−1): 3066, 2995, 2951, 2874, 2247, 1454, 1317, 1007, 727. MS (70 eV) m/z: 310.1 (M+, 35), 282.1 (24), 268.1 (25), 242.1 (27), 228.1 (25), 217.1 (35), 204.1 (18), 189.0 (24), 178.1 (19), 167.1 (38), 126.9 (20), 115.2 (30), 101.3 (14), 91.0 (18), 88.1 (22), 76.1 (18), 65.5 (100).
References
-
Gassman, P. G.; Gennick, I. J. Org. Chem. 1980, 45, 5211–5213. doi:10.1021/jo01313a039
Return to citation in text: [1] -
Horasan (Kishali), N.; Kara, Y.; Azizoğlu, A.; Balci, M. Tetrahedron 2003, 59, 3691–3699. doi:10.1016/S0040-4020(03)00549-0
Return to citation in text: [1] -
Tutar, A.; Taşkesenligil, Y.; Çakmak, O.; Abbasoğlu, R.; Balci, M. J. Org. Chem. 1996, 61, 8297–8300. doi:10.1021/jo960225l
Return to citation in text: [1] -
Winstein, S. J. Am. Chem. Soc. 1961, 83, 1516–1517. doi:10.1021/ja01467a058
Return to citation in text: [1] -
Dastan, A. Tetrahedron 2001, 57, 8725–8732. doi:10.1016/S0040-4020(01)00851-1
Return to citation in text: [1] -
Daştan, A.; Demir, U.; Balci, M. J. Org. Chem. 1994, 59, 6534–6538. doi:10.1021/jo00101a011
Return to citation in text: [1] -
Cristol, S. J.; Nachtigall, G. W. J. Org. Chem. 1967, 32, 3727–3737. doi:10.1021/jo01287a001
Return to citation in text: [1] -
Wilt, J. W.; Chenier, P. J. J. Org. Chem. 1970, 35, 1562–1570. doi:10.1021/jo00830a065
Return to citation in text: [1] -
Daştan, A.; Balci, M. Tetrahedron 2005, 61, 5481–5488. doi:10.1016/j.tet.2005.03.132
Return to citation in text: [1] [2] -
Gültekin, D. D.; Taşkesenligil, Y.; Daştan, A.; Balcı, M. Tetrahedron 2008, 64, 4377–4383. doi:10.1016/j.tet.2008.02.067
Return to citation in text: [1] [2] -
Barkhash, V. A. Top. Curr. Chem. 1984, 116–117, 1–265. doi:10.1007/3-540-12555-8_1
Return to citation in text: [1] -
Zimmerman, H. E. In Rearrangements in Ground and Excited States; de Mayo, P., Ed.; Academic Press: New York, 1980; Vol. 3, Essay 16, pp 131–164.
Return to citation in text: [1] -
Hemetsberger, H.; Nispel, F. Tetrahedron 1990, 46, 3823–3840. doi:10.1016/S0040-4020(01)90518-6
Return to citation in text: [1] -
Johnson, R. P.; Schlimgen Davis, D. Tetrahedron Lett. 1981, 22, 3171–3174. doi:10.1016/S0040-4039(01)81855-4
Return to citation in text: [1] -
Altundas, R.; Dastan, A.; Ünaldi, N. S.; Güven, K.; Uzun, O.; Balci, M. Eur. J. Org. Chem. 2002, 526–533. doi:10.1002/1099-0690(20022)2002:3<526::AID-EJOC526>3.0.CO;2-N
Return to citation in text: [1] [2] -
Tang, H.; Dong, Z.; Merican, Z.; Margetić, D.; Marinić, Ž.; Gunter, M. J.; Officer, D.; Butler, D. N.; Warrener, R. N. Tetrahedron Lett. 2009, 50, 667–670. doi:10.1016/j.tetlet.2008.11.109
Return to citation in text: [1] -
Lledó, A.; Solà, J.; Verdaguer, X.; Riera, A.; Maestro, M. A. Adv. Synth. Catal. 2007, 349, 2121–2128. doi:10.1002/adsc.200700145
Return to citation in text: [1] -
Altuna-Urquijo, M.; Gehre, A.; Stanforth, S. P.; Tarbit, B. Tetrahedron 2009, 65, 975–984. doi:10.1016/j.tet.2008.11.090
Return to citation in text: [1] -
Tseng, N.-W.; Lautens, M. J. Org. Chem. 2009, 74, 1809–1811. doi:10.1021/jo802622d
Return to citation in text: [1] -
Zhou, J. (S.); Hartwig, J. F. J. Am. Chem. Soc. 2008, 130, 12220–12221. doi:10.1021/ja803523z
Return to citation in text: [1] -
Rendler, S.; Froehlich, R.; Keller, M.; Oestreich, M. Eur. J. Org. Chem. 2008, 2582–2591. doi:10.1002/ejoc.200800107
Return to citation in text: [1] -
Dalkılıç, E.; Güney, M.; Daştan, A.; Saracoglu, N.; De Lucchi, O.; Fabris, F. Tetrahedron Lett. 2009, 50, 1989–1991. doi:10.1016/j.tetlet.2009.02.070
Return to citation in text: [1] -
Zonta, C.; Cossu, S.; De Lucchi, O. Eur. J. Org. Chem. 2000, 1965–1971. doi:10.1002/(SICI)1099-0690(200005)2000:10<1965::AID-EJOC1965>3.0.CO;2-C
Return to citation in text: [1] -
Borsato, G.; Linden, A.; De Lucchi, O.; Lucchini, V.; Wolstenholme, D.; Zambon, A. J. Org. Chem. 2007, 72, 4272–4275. doi:10.1021/jo070222g
Return to citation in text: [1] -
Peluso, P.; Greco, C.; De Lucchi, O.; Cossu, S. Eur. J. Org. Chem. 2002, 4024–4031. doi:10.1002/1099-0690(200212)2002:23<4024::AID-EJOC4024>3.0.CO;2-N
Return to citation in text: [1] -
Tranmer, G. K.; Yip, C.; Handerson, S.; Jordan, R. W.; Tam, W. Can. J. Chem. 2000, 78, 527–535. doi:10.1139/cjc-78-5-527
Return to citation in text: [1] -
Kenndoff, J.; Polborn, K.; Szeimies, G. J. Am. Chem. Soc. 1990, 112, 6117–6118. doi:10.1021/ja00172a031
Return to citation in text: [1] -
Stille, J. K. Angew. Chem., Int. Ed. Engl. 1986, 25, 508–524. doi:10.1002/anie.198605081
Return to citation in text: [1] -
Fabris, F.; Leoni, L.; De Lucchi, O. Tetrahedron Lett. 1999, 40, 1223–1226. doi:10.1016/S0040-4039(98)02571-4
Return to citation in text: [1] -
Baumgärtel, O.; Szeimies, G. Chem. Ber. 1983, 116, 2180–2204. doi:10.1002/cber.19831160612
Return to citation in text: [1] [2] [3]
| 1. | Gassman, P. G.; Gennick, I. J. Org. Chem. 1980, 45, 5211–5213. doi:10.1021/jo01313a039 |
| 2. | Horasan (Kishali), N.; Kara, Y.; Azizoğlu, A.; Balci, M. Tetrahedron 2003, 59, 3691–3699. doi:10.1016/S0040-4020(03)00549-0 |
| 3. | Tutar, A.; Taşkesenligil, Y.; Çakmak, O.; Abbasoğlu, R.; Balci, M. J. Org. Chem. 1996, 61, 8297–8300. doi:10.1021/jo960225l |
| 4. | Winstein, S. J. Am. Chem. Soc. 1961, 83, 1516–1517. doi:10.1021/ja01467a058 |
| 5. | Dastan, A. Tetrahedron 2001, 57, 8725–8732. doi:10.1016/S0040-4020(01)00851-1 |
| 6. | Daştan, A.; Demir, U.; Balci, M. J. Org. Chem. 1994, 59, 6534–6538. doi:10.1021/jo00101a011 |
| 7. | Cristol, S. J.; Nachtigall, G. W. J. Org. Chem. 1967, 32, 3727–3737. doi:10.1021/jo01287a001 |
| 8. | Wilt, J. W.; Chenier, P. J. J. Org. Chem. 1970, 35, 1562–1570. doi:10.1021/jo00830a065 |
| 9. | Daştan, A.; Balci, M. Tetrahedron 2005, 61, 5481–5488. doi:10.1016/j.tet.2005.03.132 |
| 10. | Gültekin, D. D.; Taşkesenligil, Y.; Daştan, A.; Balcı, M. Tetrahedron 2008, 64, 4377–4383. doi:10.1016/j.tet.2008.02.067 |
| 15. | Altundas, R.; Dastan, A.; Ünaldi, N. S.; Güven, K.; Uzun, O.; Balci, M. Eur. J. Org. Chem. 2002, 526–533. doi:10.1002/1099-0690(20022)2002:3<526::AID-EJOC526>3.0.CO;2-N |
| 23. | Zonta, C.; Cossu, S.; De Lucchi, O. Eur. J. Org. Chem. 2000, 1965–1971. doi:10.1002/(SICI)1099-0690(200005)2000:10<1965::AID-EJOC1965>3.0.CO;2-C |
| 16. | Tang, H.; Dong, Z.; Merican, Z.; Margetić, D.; Marinić, Ž.; Gunter, M. J.; Officer, D.; Butler, D. N.; Warrener, R. N. Tetrahedron Lett. 2009, 50, 667–670. doi:10.1016/j.tetlet.2008.11.109 |
| 17. | Lledó, A.; Solà, J.; Verdaguer, X.; Riera, A.; Maestro, M. A. Adv. Synth. Catal. 2007, 349, 2121–2128. doi:10.1002/adsc.200700145 |
| 18. | Altuna-Urquijo, M.; Gehre, A.; Stanforth, S. P.; Tarbit, B. Tetrahedron 2009, 65, 975–984. doi:10.1016/j.tet.2008.11.090 |
| 19. | Tseng, N.-W.; Lautens, M. J. Org. Chem. 2009, 74, 1809–1811. doi:10.1021/jo802622d |
| 20. | Zhou, J. (S.); Hartwig, J. F. J. Am. Chem. Soc. 2008, 130, 12220–12221. doi:10.1021/ja803523z |
| 21. | Rendler, S.; Froehlich, R.; Keller, M.; Oestreich, M. Eur. J. Org. Chem. 2008, 2582–2591. doi:10.1002/ejoc.200800107 |
| 22. | Dalkılıç, E.; Güney, M.; Daştan, A.; Saracoglu, N.; De Lucchi, O.; Fabris, F. Tetrahedron Lett. 2009, 50, 1989–1991. doi:10.1016/j.tetlet.2009.02.070 |
| 12. | Zimmerman, H. E. In Rearrangements in Ground and Excited States; de Mayo, P., Ed.; Academic Press: New York, 1980; Vol. 3, Essay 16, pp 131–164. |
| 13. | Hemetsberger, H.; Nispel, F. Tetrahedron 1990, 46, 3823–3840. doi:10.1016/S0040-4020(01)90518-6 |
| 14. | Johnson, R. P.; Schlimgen Davis, D. Tetrahedron Lett. 1981, 22, 3171–3174. doi:10.1016/S0040-4039(01)81855-4 |
| 15. | Altundas, R.; Dastan, A.; Ünaldi, N. S.; Güven, K.; Uzun, O.; Balci, M. Eur. J. Org. Chem. 2002, 526–533. doi:10.1002/1099-0690(20022)2002:3<526::AID-EJOC526>3.0.CO;2-N |
| 11. | Barkhash, V. A. Top. Curr. Chem. 1984, 116–117, 1–265. doi:10.1007/3-540-12555-8_1 |
| 9. | Daştan, A.; Balci, M. Tetrahedron 2005, 61, 5481–5488. doi:10.1016/j.tet.2005.03.132 |
| 10. | Gültekin, D. D.; Taşkesenligil, Y.; Daştan, A.; Balcı, M. Tetrahedron 2008, 64, 4377–4383. doi:10.1016/j.tet.2008.02.067 |
| 30. | Baumgärtel, O.; Szeimies, G. Chem. Ber. 1983, 116, 2180–2204. doi:10.1002/cber.19831160612 |
| 30. | Baumgärtel, O.; Szeimies, G. Chem. Ber. 1983, 116, 2180–2204. doi:10.1002/cber.19831160612 |
| 28. | Stille, J. K. Angew. Chem., Int. Ed. Engl. 1986, 25, 508–524. doi:10.1002/anie.198605081 |
| 29. | Fabris, F.; Leoni, L.; De Lucchi, O. Tetrahedron Lett. 1999, 40, 1223–1226. doi:10.1016/S0040-4039(98)02571-4 |
| 24. | Borsato, G.; Linden, A.; De Lucchi, O.; Lucchini, V.; Wolstenholme, D.; Zambon, A. J. Org. Chem. 2007, 72, 4272–4275. doi:10.1021/jo070222g |
| 25. | Peluso, P.; Greco, C.; De Lucchi, O.; Cossu, S. Eur. J. Org. Chem. 2002, 4024–4031. doi:10.1002/1099-0690(200212)2002:23<4024::AID-EJOC4024>3.0.CO;2-N |
| 26. | Tranmer, G. K.; Yip, C.; Handerson, S.; Jordan, R. W.; Tam, W. Can. J. Chem. 2000, 78, 527–535. doi:10.1139/cjc-78-5-527 |
| 27. | Kenndoff, J.; Polborn, K.; Szeimies, G. J. Am. Chem. Soc. 1990, 112, 6117–6118. doi:10.1021/ja00172a031 |
| 30. | Baumgärtel, O.; Szeimies, G. Chem. Ber. 1983, 116, 2180–2204. doi:10.1002/cber.19831160612 |
© 2009 Nişancı et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)