Abstract
With 3,3′-bi[benzo[b]thiophenyl] as starting material, dibenzo[d,d′]benzo[1,2-b:4,3-b′]dithiophene, a [5]heterohelicene, was synthesized efficiently in 60% yield via formylation and McMurry reaction. Cyclopenta[1,2-b:4,3-b′]bis(benzo[d]thiophen)-6-one, another interesting helical ketone, was also prepared in 79% yield via deprotonation and ketonization of 3,3′-bi[benzo[b]thiophenyl]. In addition, the single-crystal structure of dibenzo[d,d′]benzo[1,2-b:4,3-b′]dithiophene and UV–vis spectra of both title compounds are described.
Graphical Abstract
Introduction
With considerable environmental stability and flexibility in synthesis, π-extended heteroarenes containing thiophene rings within an polyaromatic ring system are currently of great interest because they can potentially be used to fabricate organic field-effect transistors (OFETs) [1-7], light emitting diodes (LEDs) [8,9], and photovoltaic cells [10]. Various novel π-extended heteroarenes, e.g. dibenzo[d,d′]thieno[3,2-b:4,5-b′]dithiophene [11], pentathienoacene [12], and benzo[1,2-b:4,5-b′]bis[b]benzothiophene [13], have been developed and tested as active semiconducting channels in OFET devices due to their structural resemblance to pentacene [14] which possesses very high field-effect mobility (~3.0 cm2 V−1 s−1) for OFET devices. However, one of the possible analogs, dibenzo[d,d′]benzo[1,2-b:4,3-b′]dithiophene (1), an interesting [5]heterohelicene, has not received much attention during the last decade because of a lack of efficient synthesis, which limits its application in material science.
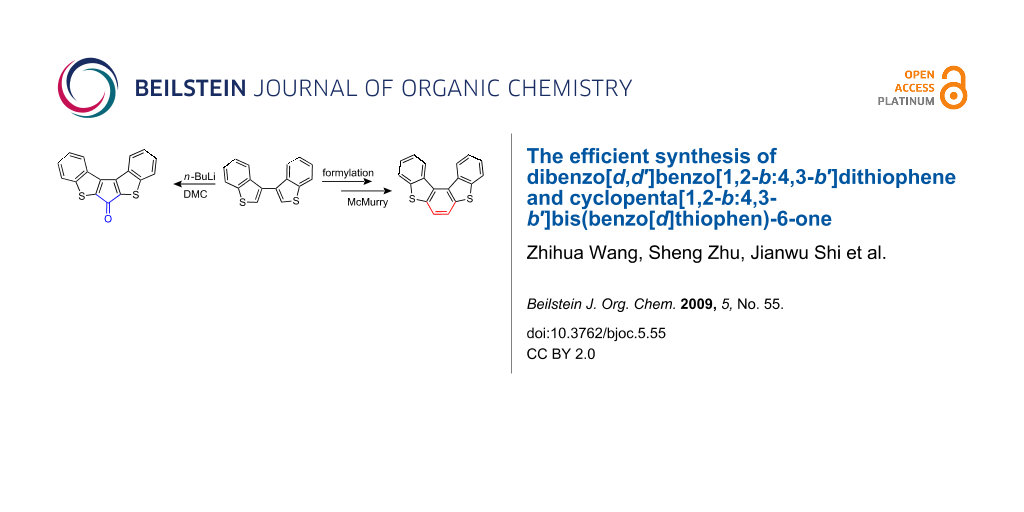
Photocyclization is normally employed for preparing heterohelicenes with 1,2-diaryl ethenes as precursors. Wynberg et al. [15] reported the synthesis of 1 in 57% yield by the photocyclization of 1,2-di(2-benzo[b]thienyl)ethene in benzene. Kudo et al. [16] improved the preparative yield of 1 up to 85% with the same precursor in the same way. Sugimoto et al. [17] reported the preparation of 1 in 19% yield via the photolysis of RCH(SPh)2 (R = benzo[b]thiophene). However, a non-photochemical method for preparing 1 has, to the best of our knowledge, not been reported. In our work, with 3,3′-bis[benzo[b]thiophenyl] (3) [18] as starting material, 1 (see Figure 1) was synthesized efficiently via formylation and McMurry reaction and its crystal structure was determined. In addition, cyclopenta[1,2-b:4,3-b′]bis(benzo[d]thiophen)-6-one (2), an interesting helical ketone, was also prepared via deprotonation and ketonization of 3.
Figure 1: Chemical structures of 1 and 2.
Figure 1: Chemical structures of 1 and 2.
Results and Discussion
Synthesis
Scheme 1 shows the synthetic route to 1 and 2. With 3 as starting material, formylation and McMurry reaction were employed for preparing 1. The formylation step involves sequential deprotonation of 3 and treatment of the intermediate dilithiated species with N,N-dimethylformamide (DMF). Attempted generation of dilithiated 3 with either lithium diisopropylamide (LDA) or n-BuLi at −78 °C failed. However, under refluxing conditions, direct deprotonation of 3 with 2.3 equiv n-BuLi in anhydrous Et2O was successful. After quenching with excess DMF, [3,3′]bi[benzo[b]thiophenyl]-2,2′-dicarbaldehyde (4) was obtained in high yield (91%). The next step is the intramolecular McMurry reaction of 4. We found that the reactivity of elemental titanium is crucial in this step and that the Ti(0) must be freshly prepared from zinc dust, pyridine and TiCl4 in THF. By following standard procedures [19-21], the title compound 1 was obtained in 66% yield. The overall yield of 1 from 3 is about 60%. When dilithiated 3 was treated with N,N-dimethylcarbamoyl chloride (DMC) at low temperature (−50 °C), a new helical ketone, cyclopenta[1,2-b:4,3-b′]bis(benzo[d]thiophen)-6-one (2), was obtained in 79% yield.
Scheme 1: Synthetic route to 1 and 2. Reagents and conditions: (a) (i) n-BuLi (2.3 equiv), Et2O, reflux; (ii) −78 °C, DMF (4.0 equiv); (b) TiCl4 (3.0 equiv), Zn (6.0 equiv), pyridine (3.0 equiv), THF, reflux; (c) (i) n-BuLi (2.3 equiv), Et2O, reflux; (ii) Me2NCOCl (1.0 equiv), −50 °C to RT.
Scheme 1: Synthetic route to 1 and 2. Reagents and conditions: (a) (i) n-BuLi (2.3 equiv), Et2O, reflux; (ii)...
X-ray structural analysis
A single crystal of 1 was obtained by the slow evaporation of a solution of 1 in CHCl3/CH3OH (5/1, v/v). The crystal structure of 1 was confirmed by single-crystal X-ray analysis (Figure 2 and Figure 3). 1 has non-planar π-extended frameworks, and its molecule is compressed and dominated by a helical structure (Figure 2). The distance between the two H atoms H(5)A…H(18)A is 2.062 Å and these H atoms point away from each other. The repulsion of the facing terminal benzene rings causes an interplanar angle of 31.7° between the terminal benzene rings. The angles between the least-squares planes of neighboring rings are between 7.5° and 9.5°. Using the middle benzene ring as a reference, the inner (C(5), C(6), C(7), C(12), C(13), and C(18)) helix increases by 1.46 Å and turns in-plane by 237.0° [22,23].
Figure 2: Molecular structure and conformation of 1.
Figure 2: Molecular structure and conformation of 1.
The crystal packing structure of 1 (Figure 3) is based on π-stacking along the a-axis in which the plane-to-plane distance is ca. 3.857 Å. The lack of contact between the π-stacks indicates that the crystal has one-dimensional (1D) electronic structure [13].
Figure 3: Crystal packing structure of 1.
Figure 3: Crystal packing structure of 1.
UV–vis spectra of 1 and 2
The UV–vis spectra of 1 and 2 in chloroform are shown in Figure 4. 1 shows the main peaks at 323 nm, 335 nm, and 372 nm, and 2 gives two broad peaks at 339 nm and 380 nm. Both 1 and 2 possess molecular connectivities of cross-conjugated π-system that shows π-electron delocalization with helical distortion.
Figure 4: UV–vis spectra of 1 and 2 in chloroform ([C] = 1 × 10−5 M).
Figure 4: UV–vis spectra of 1 and 2 in chloroform ([C] = 1 × 10−5 M).
Conclusion
Derivatives of benzothiophene, thienothiophene, and thienodithiophene with good characteristics for OFETs were recently reported [5-7,11,24,25]. Because of higher π-electron delocalization, compounds 1 and 2 could be used in OFET and/or conducting polymers. From 3 as starting material, 1 and 2 have been efficiently obtained in a total yield of 60% and 79%, respectively, in our work. The efficient synthesis of 1 and 2 will facilitate the synthetic approaches to various organic functional materials, by using 1 and 2 as building blocks, and/or versatile intermediates. The measurement of the hole/electron mobilities of compounds 1 and 2 is in progress.
Experimental
Synthesis of [3,3′]bi[benzo[b]thiophenyl]-2,2′-dicarbaldehyde (4)
To a solution of 3 (0.2986 g, 1.12 mmol) in anhydrous Et2O (30 mL), n-BuLi (1.0 mL, 2.58 mmol, 2.3 equiv) was added dropwise at −78 °C. The resulting reaction mixture was slowly warmed to ambient temperature, then heated to 50 °C, refluxed for 2 h, and quenched with dry DMF (0.35 mL, 4.48 mmol, 4.0 equiv) at −78 °C. The reaction mixture was warmed slowly to ambient temperature overnight. The reaction mixture was quenched with H2O, extracted with Et2O (3 × 15 mL), washed with H2O (2 × 30 mL), and dried over MgSO4. The residue was purified by column chromatography on silica gel with PE (60–90 °C)/ethyl acetate (8/1, v/v) as eluent to yield 4 (0.3284 g, 90.8%) as light yellow crystals. mp 143–144 °C; 1H NMR (400 MHz, CDCl3): δ 9.82 (s, 2H), 8.03 (d, J = 8.4 Hz, 2H), 7.64–7.54 (m, 4H), 7.42 (t, J = 7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 184.2, 141.9, 141.7, 139.6, 136.6, 129.1, 126.0, 125.0, 123.5. HRMS (TOF MS EI+) m/z: calcd for [C18H10O2S2] 322.0122, found 322.0126. IR (KBr): 2928, 2823 (C–H), 1668 (C=O) cm−1.
Synthesis of dibenzo[d,d′]benzo[1,2-b:4,3-b′]dithiophene (1)
TiCl4 (0.16 mL, 1.5 mmol, 3.0 equiv) was carefully added to dry THF (20 mL) at 0 °C, after keeping at 0 °C for 10 min, zinc dust (0.20 g, 3.0 mmol, 6.0 equiv) was added, and the mixture refluxed for 2 h. Pyridine (0.12 mL, 1.5 mmol, 3.0 equiv) was added and the mixture was heated under reflux for further 1 h. After cooling to ambient temperature, a solution of 4 (0.1607 g, 0.5 mmol) in dry THF (10 mL) was added and the reaction mixture was heated under reflux overnight. The reaction mixture was quenched with 18% HCl (20 mL) at 0 °C, extracted with CHCl3 (4 × 30 mL), washed with H2O (3 × 30 mL), and dried over MgSO4. After the removal of the solvent in vacuo, the crude product was purified by column chromatography on silica gel with PE (60–90 °C)/ethyl acetate (8/1, v/v) as eluent to yield 1 (0.0958 g, 66.2%) as a white solid. mp 182–183 °C (lit. mp 183–185 °C [15], 184–185 °C [16]); 1H NMR (400 MHz, CDCl3): δ 9.05–8.99 (m, 2H), 8.02–7.95 (m, 2H), 7.91 (s, 2H), 7.56–7.50 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 139.9, 137.3, 135.2, 131.2, 126.3, 125.0, 123.5, 123.1, 121.1. HRMS (TOF MS EI+) m/z: calcd for [C18H10S2] 290.0224, found 290.0226. IR: 3054, 2922, 2852 (C–H) cm−1.
Synthesis of cyclopenta[1,2-b:4,3-b′]bis(benzo[d]thiophen)-6-one (2)
To a solution of 3 (0.1556 g, 0.58 mmol) in anhydrous Et2O (40 mL), n-BuLi (0.4 mL, 1.23 mmol, 2.1 equiv) was added dropwise at −78 °C. The resulting reaction mixture was slowly heated to 50 °C and refluxed for 2 h. After being cooled to −50 °C, DMC (0.05 mL, 0.58 mmol, 1.0 equiv) was added dropwise. The reaction mixture was warmed slowly to ambient temperature overnight. The reaction mixture was quenched with saturated NH4Cl (20 mL) at 0 °C, extracted with Et2O (3 × 20 mL), washed with H2O (3 × 30 mL), and dried over MgSO4. The residue was purified by column chromatography on silica gel with CHCl3 as eluent to yield 2 (0.1356 g, 79.4%) as orange crystals. mp 180 °C (decomposed); 1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 8.0 Hz, 2H), 7.84 (d, J = 8.0 Hz, 2H), 7.51 (td, J = 7.6, 1.2 Hz, 2H), 7.40 (td, J = 7.6, 1.2 Hz, 2H). HRMS (TOF MS EI+) m/z: calcd for [C17H8OS2] 290.0017, found 290.0020. IR (KBr): 3053, 2927 (C–H), 1709 (C=O) cm−1.
Crystal data for 1
M = 290.38, C18H10S2, orthorhombic, space group P2(1)2(1)2(1), a = 3.9971(16) Å, b = 10.334(4) Å, c = 31.473(13) Å, α = 90°, β = 90°, γ = 90°, V = 1300.1(9) Å3, Z = 4, dcalcd = 1.484 g/cm3. A colorless crystal with a size of 0.29 mm × 0.15 mm × 0.10 mm was used for measurement at 296(2) K in ω scan mode (Bruker Smart APEX X-ray diffractometer, CCD detector, Mo Kα radiation (λ = 0.71073 Å)). The data were corrected for Lorentz and polarization effects and absorption corrections were performed using the SADABS [26] program. The crystal structures were solved using the SHELXTL [27] program and refined using full matrix least-squares. The positions of hydrogen atoms were calculated theoretically and included in the final cycles of refinement in a riding model along with attached carbons. The final cycle of full matrix least-squares refinement was based on 7988 independent reflections [I > 2σ(I)] and 181 variable parameters with R1 = 0.0335, wR2 = 0.0846.
Supporting Information
Supporting information features experimental procedures and spectroscopic analysis for compounds 1, 2, and 4.
| Supporting Information File 1: The efficient synthesis of dibenzo[d,d′]benzo[1,2-b:4,3-b′]dithiophene and cyclopenta[1,2-b:4,3-b′]bis(benzo[d]thiophen)-6-one. | ||
| Format: DOC | Size: 987.5 KB | Download |
| Supporting Information File 2: Crystal structure of compound 1 in cif format. | ||
| Format: CIF | Size: 14.4 KB | Download |
References
-
Li, X.-C.; Sirringhaus, H.; Garnier, F.; Holmes, A. B.; Moratti, S. C.; Feeder, N.; Clegg, W.; Teat, S. J.; Friend, R. H. J. Am. Chem. Soc. 1998, 120, 2206–2207. doi:10.1021/ja9735968
Return to citation in text: [1] -
Anthony, J. E. Chem. Rev. 2006, 106, 5028–5048. doi:10.1021/cr050966z
Return to citation in text: [1] -
Laquindanum, J. G.; Katz, H. E.; Lovinger, A. J.; Dodabalapur, A. Adv. Mater. 1997, 9, 36–39. doi:10.1002/adma.19970090106
Return to citation in text: [1] -
Takimiya, K.; Kunugi, Y.; Toyoshima, Y.; Otsubo, T. J. Am. Chem. Soc. 2005, 127, 3605–3612. doi:10.1021/ja043429p
Return to citation in text: [1] -
Sun, Y. M.; Ma, Y. Q.; Liu, Y. Q.; Lin, Y. Y.; Wang, Z. Y.; Wang, Y.; Di, C. A.; Xiao, K.; Chen, X. M.; Qiu, W. F.; Zhang, B.; Yu, G.; Hu, W. P.; Zhu, D. B. Adv. Funct. Mater. 2006, 16, 426–432. doi:10.1002/adfm.200500547
Return to citation in text: [1] [2] -
Zhang, L.; Tan, L.; Wang, Z. H.; Hu, W. P.; Zhu, D. B. Chem. Mater. 2009, 21, 1993–1999. doi:10.1021/cm900369s
Return to citation in text: [1] [2] -
Tan, L.; Zhang, L.; Jiang, X.; Yang, X. D.; Wang, L. G.; Wang, Z. H.; Li, L. Q.; Hu, W. P.; Shuai, Z. G.; Li, L.; Zhu, D. B. Adv. Funct. Mater. 2009, 19, 272–276. doi:10.1002/adfm.200800933
Return to citation in text: [1] [2] -
Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M.; Fattori, V.; Cocchi, M.; Cacialli, F.; Gigli, G.; Cingolani, R. Adv. Mater. 1999, 11, 1375–1379. doi:10.1002/(SICI)1521-4095(199911)11:16<1375::AID-ADMA1375>3.0.CO;2-D
Return to citation in text: [1] -
Mazzeo, M.; Vitale, V.; Della Sala, F.; Anni, M.; Barbarella, G.; Favaretto, L.; Sotgiu, G.; Cingolani, R.; Gigli, G. Adv. Mater. 2005, 17, 34–39. doi:10.1002/adma.200400670
Return to citation in text: [1] -
Noma, N.; Tsuzuki, T.; Shirota, Y. Adv. Mater. 1995, 7, 647–648. doi:10.1002/adma.19950070709
Return to citation in text: [1] -
Gao, J. H.; Li, R. J.; Li, L. Q.; Meng, Q.; Jiang, H.; Li, H. X.; Hu, W. P. Adv. Mater. 2007, 19, 3008–3011. doi:10.1002/adma.200701167
Return to citation in text: [1] [2] -
Xiao, K.; Liu, Y. Q.; Qi, T.; Zhang, W.; Wang, F.; Gao, J. H.; Qiu, W. F.; Ma, Y. Q.; Cui, G. L.; Chen, S. Y.; Zhan, X. W.; Yu, G.; Qin, J. G.; Hu, W. P.; Zhu, D. B. J. Am. Chem. Soc. 2005, 127, 13281–13286. doi:10.1021/ja052816b
Return to citation in text: [1] -
Ebata, H.; Miyazaki, E.; Yamamoto, T.; Takimiya, K. Org. Lett. 2007, 9, 4499–4502. doi:10.1021/ol701815j
Return to citation in text: [1] [2] -
Lin, Y.-Y.; Gundlach, D. J.; Nelson, S. F.; Jackson, T. N. IEEE Electron Device Lett. 1997, 18, 606–608. doi:10.1109/55.644085
Return to citation in text: [1] -
Groen, M. B.; Schadenberg, H.; Wynberg, H. J. Org. Chem. 1971, 36, 2797–2809. doi:10.1021/jo00818a016
Return to citation in text: [1] [2] -
Kudo, H.; Tedjamulia, M. L.; Castle, R. N.; Lee, M. L. J. Heterocycl. Chem. 1984, 21, 185–192. doi:10.1002/jhet.5570210137
Return to citation in text: [1] [2] -
Sugimoto, A.; Okada, M.; Yoneda, S. Chem. Express 1987, 2, 425–428.
Return to citation in text: [1] -
Schroth, W.; Hintzsche, E.; Jordan, H.; Jende, T.; Spitzner, R.; Thondorf, I. Tetrahedron 1997, 53, 7509–7528. doi:10.1016/S0040-4020(97)00439-0
Return to citation in text: [1] -
Suzuki, T.; Shiohara, H.; Monobe, M.; Sakimura, T.; Tanaka, S.; Yamashita, Y.; Miyashi, T. Angew. Chem., Int. Ed. Engl. 1992, 31, 455–458. doi:10.1002/anie.199204551
Return to citation in text: [1] -
Fischer, E.; Larsen, J.; Christensen, J. B.; Fourmigué, M.; Madsen, H. G.; Harrit, N. J. Org. Chem. 1996, 61, 6997–7005. doi:10.1021/jo960022x
Return to citation in text: [1] -
Dai, W.-M.; Mak, W. L. Tetrahedron Lett. 2000, 41, 10277–10280. doi:10.1016/S0040-4039(00)01846-3
Return to citation in text: [1] -
Rajca, A.; Wang, H.; Pink, M.; Rajca, S. Angew. Chem., Int. Ed. 2000, 39, 4481–4483. doi:10.1002/1521-3773(20001215)39:24<4481::AID-ANIE4481>3.0.CO;2-G
Return to citation in text: [1] -
Li, C. L.; Shi, J. W.; Xu, L.; Wang, Y. G.; Cheng, Y. X.; Wang, H. J. Org. Chem. 2009, 74, 408–411. doi:10.1021/jo802080g
Return to citation in text: [1] -
Sirringhaus, H.; Friend, R. H.; Li, X. C.; Moratti, S. C.; Holmes, A. B.; Feeder, N. Appl. Phys. Lett. 1997, 71, 3871–3873. doi:10.1063/1.120529
Return to citation in text: [1] -
Takimiya, K.; Ebata, H.; Sakamoto, K.; Izawa, T.; Otsubo, T.; Kunugi, Y. J. Am. Chem. Soc. 2006, 128, 12604–12605. doi:10.1021/ja064052l
Return to citation in text: [1] -
Sheldrick, G. M. SADABS; University of Göttingen: Germany, 1996.
Return to citation in text: [1] -
Sheldrick, G. M. SHELXTL, Version 5.1; Bruker Analytical X-ray Systems, Inc.: Madison, WI, 1997.
Return to citation in text: [1]
| 27. | Sheldrick, G. M. SHELXTL, Version 5.1; Bruker Analytical X-ray Systems, Inc.: Madison, WI, 1997. |
| 16. | Kudo, H.; Tedjamulia, M. L.; Castle, R. N.; Lee, M. L. J. Heterocycl. Chem. 1984, 21, 185–192. doi:10.1002/jhet.5570210137 |
| 1. | Li, X.-C.; Sirringhaus, H.; Garnier, F.; Holmes, A. B.; Moratti, S. C.; Feeder, N.; Clegg, W.; Teat, S. J.; Friend, R. H. J. Am. Chem. Soc. 1998, 120, 2206–2207. doi:10.1021/ja9735968 |
| 2. | Anthony, J. E. Chem. Rev. 2006, 106, 5028–5048. doi:10.1021/cr050966z |
| 3. | Laquindanum, J. G.; Katz, H. E.; Lovinger, A. J.; Dodabalapur, A. Adv. Mater. 1997, 9, 36–39. doi:10.1002/adma.19970090106 |
| 4. | Takimiya, K.; Kunugi, Y.; Toyoshima, Y.; Otsubo, T. J. Am. Chem. Soc. 2005, 127, 3605–3612. doi:10.1021/ja043429p |
| 5. | Sun, Y. M.; Ma, Y. Q.; Liu, Y. Q.; Lin, Y. Y.; Wang, Z. Y.; Wang, Y.; Di, C. A.; Xiao, K.; Chen, X. M.; Qiu, W. F.; Zhang, B.; Yu, G.; Hu, W. P.; Zhu, D. B. Adv. Funct. Mater. 2006, 16, 426–432. doi:10.1002/adfm.200500547 |
| 6. | Zhang, L.; Tan, L.; Wang, Z. H.; Hu, W. P.; Zhu, D. B. Chem. Mater. 2009, 21, 1993–1999. doi:10.1021/cm900369s |
| 7. | Tan, L.; Zhang, L.; Jiang, X.; Yang, X. D.; Wang, L. G.; Wang, Z. H.; Li, L. Q.; Hu, W. P.; Shuai, Z. G.; Li, L.; Zhu, D. B. Adv. Funct. Mater. 2009, 19, 272–276. doi:10.1002/adfm.200800933 |
| 12. | Xiao, K.; Liu, Y. Q.; Qi, T.; Zhang, W.; Wang, F.; Gao, J. H.; Qiu, W. F.; Ma, Y. Q.; Cui, G. L.; Chen, S. Y.; Zhan, X. W.; Yu, G.; Qin, J. G.; Hu, W. P.; Zhu, D. B. J. Am. Chem. Soc. 2005, 127, 13281–13286. doi:10.1021/ja052816b |
| 5. | Sun, Y. M.; Ma, Y. Q.; Liu, Y. Q.; Lin, Y. Y.; Wang, Z. Y.; Wang, Y.; Di, C. A.; Xiao, K.; Chen, X. M.; Qiu, W. F.; Zhang, B.; Yu, G.; Hu, W. P.; Zhu, D. B. Adv. Funct. Mater. 2006, 16, 426–432. doi:10.1002/adfm.200500547 |
| 6. | Zhang, L.; Tan, L.; Wang, Z. H.; Hu, W. P.; Zhu, D. B. Chem. Mater. 2009, 21, 1993–1999. doi:10.1021/cm900369s |
| 7. | Tan, L.; Zhang, L.; Jiang, X.; Yang, X. D.; Wang, L. G.; Wang, Z. H.; Li, L. Q.; Hu, W. P.; Shuai, Z. G.; Li, L.; Zhu, D. B. Adv. Funct. Mater. 2009, 19, 272–276. doi:10.1002/adfm.200800933 |
| 11. | Gao, J. H.; Li, R. J.; Li, L. Q.; Meng, Q.; Jiang, H.; Li, H. X.; Hu, W. P. Adv. Mater. 2007, 19, 3008–3011. doi:10.1002/adma.200701167 |
| 24. | Sirringhaus, H.; Friend, R. H.; Li, X. C.; Moratti, S. C.; Holmes, A. B.; Feeder, N. Appl. Phys. Lett. 1997, 71, 3871–3873. doi:10.1063/1.120529 |
| 25. | Takimiya, K.; Ebata, H.; Sakamoto, K.; Izawa, T.; Otsubo, T.; Kunugi, Y. J. Am. Chem. Soc. 2006, 128, 12604–12605. doi:10.1021/ja064052l |
| 11. | Gao, J. H.; Li, R. J.; Li, L. Q.; Meng, Q.; Jiang, H.; Li, H. X.; Hu, W. P. Adv. Mater. 2007, 19, 3008–3011. doi:10.1002/adma.200701167 |
| 15. | Groen, M. B.; Schadenberg, H.; Wynberg, H. J. Org. Chem. 1971, 36, 2797–2809. doi:10.1021/jo00818a016 |
| 10. | Noma, N.; Tsuzuki, T.; Shirota, Y. Adv. Mater. 1995, 7, 647–648. doi:10.1002/adma.19950070709 |
| 22. | Rajca, A.; Wang, H.; Pink, M.; Rajca, S. Angew. Chem., Int. Ed. 2000, 39, 4481–4483. doi:10.1002/1521-3773(20001215)39:24<4481::AID-ANIE4481>3.0.CO;2-G |
| 23. | Li, C. L.; Shi, J. W.; Xu, L.; Wang, Y. G.; Cheng, Y. X.; Wang, H. J. Org. Chem. 2009, 74, 408–411. doi:10.1021/jo802080g |
| 8. | Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M.; Fattori, V.; Cocchi, M.; Cacialli, F.; Gigli, G.; Cingolani, R. Adv. Mater. 1999, 11, 1375–1379. doi:10.1002/(SICI)1521-4095(199911)11:16<1375::AID-ADMA1375>3.0.CO;2-D |
| 9. | Mazzeo, M.; Vitale, V.; Della Sala, F.; Anni, M.; Barbarella, G.; Favaretto, L.; Sotgiu, G.; Cingolani, R.; Gigli, G. Adv. Mater. 2005, 17, 34–39. doi:10.1002/adma.200400670 |
| 13. | Ebata, H.; Miyazaki, E.; Yamamoto, T.; Takimiya, K. Org. Lett. 2007, 9, 4499–4502. doi:10.1021/ol701815j |
| 16. | Kudo, H.; Tedjamulia, M. L.; Castle, R. N.; Lee, M. L. J. Heterocycl. Chem. 1984, 21, 185–192. doi:10.1002/jhet.5570210137 |
| 18. | Schroth, W.; Hintzsche, E.; Jordan, H.; Jende, T.; Spitzner, R.; Thondorf, I. Tetrahedron 1997, 53, 7509–7528. doi:10.1016/S0040-4020(97)00439-0 |
| 15. | Groen, M. B.; Schadenberg, H.; Wynberg, H. J. Org. Chem. 1971, 36, 2797–2809. doi:10.1021/jo00818a016 |
| 19. | Suzuki, T.; Shiohara, H.; Monobe, M.; Sakimura, T.; Tanaka, S.; Yamashita, Y.; Miyashi, T. Angew. Chem., Int. Ed. Engl. 1992, 31, 455–458. doi:10.1002/anie.199204551 |
| 20. | Fischer, E.; Larsen, J.; Christensen, J. B.; Fourmigué, M.; Madsen, H. G.; Harrit, N. J. Org. Chem. 1996, 61, 6997–7005. doi:10.1021/jo960022x |
| 21. | Dai, W.-M.; Mak, W. L. Tetrahedron Lett. 2000, 41, 10277–10280. doi:10.1016/S0040-4039(00)01846-3 |
| 14. | Lin, Y.-Y.; Gundlach, D. J.; Nelson, S. F.; Jackson, T. N. IEEE Electron Device Lett. 1997, 18, 606–608. doi:10.1109/55.644085 |
| 13. | Ebata, H.; Miyazaki, E.; Yamamoto, T.; Takimiya, K. Org. Lett. 2007, 9, 4499–4502. doi:10.1021/ol701815j |
© 2009 Wang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)