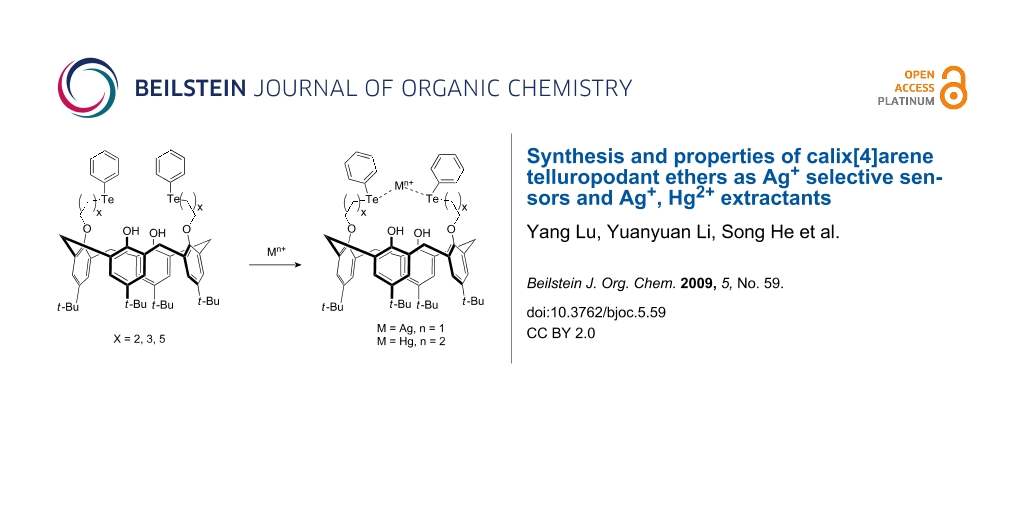
Abstract
Three novel phenyltelluroalkoxyl functionalized tweezer-like calix[4]arenes 6–8 and two monophenyltelluropropoxyl functionalized calix[4]arenes 10 (cone conformer) and 12 (partial cone conformer) were synthesized and characterized. They are good Ag+-selective ionophores in ion-selective electrodes evaluated by electromotive force measurements of polymer membrane electrodes. The tweezer-like ionophores 6–8 showed excellent extraction ability towards Ag+ and Hg2+.
Graphical Abstract
Introduction
There is much interest in the development of compounds that selectively respond to specific metal ions for use as ion sensors. As the third generation of supramolecule, benefiting from their three-dimensional structures and easy chemical modification both at the upper- and lower-rims as well as their potential receptor properties for cations, anions and neutral molecules, calixarenes have enjoyed widespread use in various areas of science and technology. One of their successful applications as sensors is in analytical chemistry. They are useful for separations, enrichment, and analyses of ionic and neutral molecular species [1-4]. In particular, the ion-selective electrode (ISE) is an important target in analytical applications [5-10]. To improve the ion selectivity of calixarenes, a great deal of effort has been devoted to the design and synthesis of novel functionalized calixarenes in recent years [1-4]. In fact, a large number of calixarene derivatives containing pendant ether, amide, ketonic, ester and crown ether groups have been employed in studies of ISEs sensitive to sodium ions [11-20], potassium ions [21-25], caesium ions [26-30], thallium ions [31], lead ions [32,33] and organic ammonium ions [34-36]. But only a few reports are concerned with calixarenes as carriers sensitive to transition metal ions in the ionophore-based ISEs [30,37]. Due to their large covalent radius and greater polarizability compared to oxygen, the coordinating chemistry of heavier Group 16 analogues (S, Se, Te), especially sulfur, has attracted considerable interest in the chemical community. A number of ligands including pendant thioethers and crown thioethers with different denticity have been synthesized and their metal ion chemistry studied, producing a diverse range of structures and unprecedented electronic and redox response [38-49]. However, the use of the tellurium atoms as soft donors to design sensors and investigation of their ion selective performances have not been described to date. Aiming to construct receptor molecules which are sensitive to transition metal ions, a large number of calix[4]arene derivatives containing N, S, Se and P(III) atoms as soft donors have been prepared and their sensor properties have also been evaluated by ion-selective electrodes (ISEs) in our group and by our co-workers [50-53]. To continue our interest in the development of new ionophores, herein we describe the design and synthesis of three novel tweezer-like 25,27-dihydroxy-26,28-bis(phenyltelluroalkoxy)calix[4]arenes 6–8 composed of two tellurium atoms on the lower rims linked via methylene groups respectively, and study their Ag+-selective behavior by electromotive force measurements of polymer membrane electrodes.
Results and Discussion
Synthesis and conformations of calix[4]arene 6–8, 10 and 12
As shown in Scheme 1, the calix[4]arenes 6–8 were synthesized in yields between 92% and 97% by the reaction of the preorganized cone conformation calix[4]arene dibromides 3–5 [54,55] with the sodium salt of phenyltellurate, which was prepared in situ by the reaction of diphenylditelluride with sodium borohydride in the presence of NaOH at the reflux temperature of ethanol–benzene (1:1, v/v). The structure and conformation of compounds 6–8 could be conveniently determined by their proton magnetic resonance spectra. As seen from 1H NMR data of 6–8, the methylene bridge protons of the calix skeleton appeared as two doublets at nearly 4.25 ppm and 3.27 ppm and the signals of the calix aromatic protons and the upper-rim tert-butyl protons were separated as two singlet peaks in a 1/1 integration ratio, which all indicated that they are in cone conformation [56,57]. The methylene protons in the pendant region gave rise to different chemical shifts depending on the distance from O atoms or Te atoms, for example, those attached to the O atom appeared at 4.02–3.94 ppm and those close to the Te atom were found at 3.38–2.98 ppm. In addition, 13C NMR spectrum afforded some information about the influence of tellurium: the phenyl carbons attached to Te atoms were recorded at 111.79–111.57 ppm, while the signals of saturated carbons attached to Te atoms were detected at 8.60–4.88 ppm.
Scheme 1: Synthesis and structures of calix[4]arenes 6–8; 3, 6: n = 2; 4, 7: n = 3; 5, 8: n = 5.
Scheme 1: Synthesis and structures of calix[4]arenes 6–8; 3, 6: n = 2; 4, 7: n = 3; 5, 8: n = 5.
The cone and partial cone conformers of calix[4]arenes 10 (cone) and 12 (partial cone) were synthesized by the reaction of the cone and partial cone conformers of calix[4]arenes 9 and 11 with the sodium salt of phenyl tellurate in 93% and 71% yields, respectively (Scheme 2). Their structures were determined by 1H NMR and MS spectra. The phenyl carbon resonances attached to tellurium atoms were found at 112.08 ppm and 112.04 ppm, respectively. The signals of saturated carbons attached to tellurium were detected at 4.06 ppm and 3.54 ppm, respectively.
Scheme 2: Synthesis of calix[4]arene 10 and 12.
Scheme 2: Synthesis of calix[4]arene 10 and 12.
Ion selectivity
For assessment of Ag+ ion selective behaviour of phenyltelluroalkoxyl modified calix[4]arenes derivatives (6–8, 10 and 12), ISEs based on 6–8, 10 and 12 as ionophores were prepared and their selectivity coefficients for Ag+ cations were investigated against alkali metal, alkaline-earth metal, lead, ammonium ions and some transition metal ions using the fixed interference method (FIM) [54,55]. Firstly, the response characteristics of Ag+-ISEs based on different ionophores were tested. It was noted that the Nernstian slopes of 6–8, 10 and 12 based ISEs were between 49.4 ± 3.6 and 55.4 ± 1.3 mV·decade−1 to the activity of Ag+ ion within the activity range 10−5.3–10−2.0 M AgNO3, which indicated that all of calix[4]arenes 6–8, 10 and 12 were good ionophores for Ag+-ISEs.
Furthermore, the Ag+ selectivity of phenyltelluride functionalized calix[4]arene 6–8, 10 and 12 was evaluated by the potentiometric selectivity coefficients (logK) (Table 1). As shown in Table 1, the polymer membranes containing calix[4]arenes 6–8, 10 and 12 as ionophores gave excellent logK
values (≤−3.3) against most of the interfering cations examined (i.e., Na+, K+, NH4+, Mg2+, Ca2+, Ni2+, Zn2+, Cu2+, Cd2+ and Pb2+), except that Hg2+ exhibited relatively lower discrimination (logK
≤−1.4) compared with other interfering cations being examined. It is known that the smaller the logK
value, the greater the electrode preference for the primary ion over the interfering ions [54]. In other words, the lower value of selectivity coefficient obtained in present 6–8-, 10- and 12-based ISEs illustrated their high Ag+ selectivity and only weakly response to the above interfering ions. The strong Hg2+ interference, once observed in some of ionophore-based ISEs [54] and traditional Ag2S-based [58,59] ISE is largely eliminated in the present of ISEs (logK
≤−1.4). It is reasoned that those ions with high hydration energies, such as Na+, K+, NH4+, Ca2+, Mg2+, Pb2+ and most of divalent transition metal ions, can not strongly interact with tellurium donors in the ionophores, while less heavily hydrated soft Ag+ ion could coordinate to soft tellurium donors selectively. Another interesting observation was that for 12-based ISEs, the discrimination of Mg2+, Ca2+, Ni2+, Zn2+, Cu2+, Cd2+ and Pb2+ is almost one order of magnitude larger than those of 6–8- and 10-based ISEs. The weak binding ability of 12-based ISEs towards Mg2+, Ca2+, Ni2+, Zn2+, Cu2+, Cd2+ and Pb2+ maybe explained by the fact that the monophenyltelluropropoxyl substituted group was inverted to the upper rim of 12 due to its partial cone conformation (Scheme 2), which made phenolic oxygen (once cooperating with tellurium atom to bind metal ions) difficult to interact with cations simultaneously. Although the calix aromatic π system was recognized as an assistant donor instead of phenolic oxygen atoms to bind cations [60-64], their binding ability towards metal ions unavoidably decreased in this experiment. Therefore, calix[4]arene 12 exhibited higher Ag+ selectivity due to its lower binding ability mainly towards Mg2+, Ca2+, Ni2+, Zn2+, Cu2+, Cd2+ and Pb2+.
Table 1:
Selectivity coefficients (logK) of the electrodes based on ionophores 6–8, 10 and 12.a
| ion |
logK |
||||
|---|---|---|---|---|---|
| 6 | 7 | 8 | 10 | 12 | |
| Ag+ | 0 | 0 | 0 | 0 | 0 |
| Na+ | −4.0 | −4.0 | −3.7 | −3.8 | −4.2 |
| K+ | −3.9 | −3.6 | −3.5 | −3.8 | −4.1 |
| NH4+ | −3.7 | −4.1 | −4.2 | −3.5 | −4.3 |
| Mg2+ | −4.5 | −4.4 | −4.3 | −4.2 | −5.2 |
| Ca2+ | −4.4 | −4.2 | −4.5 | −4.5 | −5.2 |
| Zn2+ | −4.1 | −4.1 | −4.1 | −3.6 | −5.1 |
| Cu2+ | −3.8 | −3.9 | −3.8 | −3.3 | −5.3 |
| Ni2+ | −4.1 | −3.8 | −4.0 | −3.8 | −5.3 |
| Cd2+ | −4.4 | −3.9 | −4.0 | −3.8 | −5.0 |
| Pb2+ | −4.0 | −3.9 | −4.0 | −3.7 | −4.9 |
| Hg2+ | −1.5 | −1.8 | −1.5 | −1.4 | −1.8 |
aThe representative electrochemical cell for the EMF measurement was as follows: Ag·AgCl | int. soln. (0.01 M KCl) | PVC membrane | sample | salt bridge (3 M KCl) | saturated KCl | Hg2Cl2·Hg.
Extraction behaviors
The extraction ability of tweezer-like receptors 6–8 for alkali, alkaline earth metals, lead, ammonium and some of transition metal cations was measured by Pederson’s method. The data are summarized in Table 2. For comparison, the cone and partial cone conformers of calix[4]arenes 10 and 12 were examined under the same conditions. As can be seen from Table 2, most of the receptors showed very weak extraction ability towards Li+, Na+, K+, Ca2+, which meant that these ionophores had weak affinity for these main Group cations. It was noteworthy that the soft Ag+ and Hg2+ ions were almost quantitatively extracted by calix[4]arenes 6–8. The cone conformer 10 exhibited strong extraction ability towards Hg2+ and moderate extraction ability towards Ag+. However, the partial cone conformer 12 just gave moderate extraction ability towards Hg2+ and weak extraction ability towards Ag+. Thus, the extraction ability of the receptor 6–8, 10 and 12 towards Ag+ and Hg2+ was given in the following order: Te2 (6–8) > Te (10, cone conformer) > Te (12, partial cone conformer). This sequence revealed that the soft tellurium donors play a key role in the binding with the soft Ag+ and Hg2+ cations and the phenolic oxygen atoms on the lower-rim may involve in the complexation with Ag+ and Hg2+ as assistant donors. These observations further proved that the cone conformation of calix[4]arene was much more favorable molecular framework for binding metal ions than corresponding partial cone conformation. In other transition metal ions cases such as Ni2+, Zn2+, Mn2+, Cu2+ and Cd2+, the receptors 6–8 and cone conformer 10 showed weak to moderate extraction ability (extraction% = 16.8–43.7%). But the extraction ability of partial cone conformer 12 towards these ions is much weaker than those of receptors 6–8 and 10. Based on the better performance of receptor 10 compared with corresponding analogue 12, it was further confirmed that the binding ability of phenolic oxygen donors on the lower rim of ligand 10 towards Ni2+, Zn2+, Mn2+, Cu2+ and Cd2+ was much stronger than those of the calix aromatic π system of ionophore 12, which is in accordance with the complexation behavior in the ISEs though the solvent systems are significantly different.
Table 2: The percentage of metal picrates extracted from the aqueous to the organic phase by calixarenes 6–8, 10, and 12.
| ion | Extraction percentage (%) | ||||
|---|---|---|---|---|---|
| 6 | 7 | 8 | 10 | 12 | |
| Li+ | 12.8 | 11.8 | 1.7 | 1.1 | 2.3 |
| Na+ | 0.9 | 0.9 | 0.7 | 0.5 | 5.4 |
| K+ | 0 | 1.0 | 0 | 0.5 | 0.5 |
| Ca2+ | 5.8 | 3.9 | 4.1 | 0.8 | 1.2 |
| Cu2+ | 25.1 | 18.6 | 19.0 | 16.6 | 11.8 |
| Mn2+ | 29.9 | 34.7 | 20.1 | 19.5 | 7.8 |
| Cd2+ | 23.7 | 16.8 | 20.0 | 34.6 | 7.9 |
| Ni2+ | 33.3 | 43.5 | 30.8 | 28.7 | 20.6 |
| Zn2+ | 42.9 | 39.3 | 35.5 | 27.5 | 17.0 |
| Pb2+ | 25.7 | 18.4 | 28.6 | 8.7 | 9.2 |
| Hg2+ | 100 | 89.8 | 98.6 | 86.4 | 51.8 |
| Ag+ | 100 | 100 | 100 | 68.3 | 21.2 |
Conclusion
In summary, three novel phenyltelluroalkoxyl functionalized tweezers-like calix[4]arenes 6–8 and two monophenyltelluropropoxyl functionalized calix[4]arenes 10 (cone conformer) and 12 (partial cone conformer) were synthesized and characterized. Potentiometric selectivity evaluation showed that they are good Ag+-selective ionophores in ISEs. The tweezer-like ionophores 6–8 showed excellent extraction ability towards Ag+ and Hg2+. Their structure–selectivity relationships and comparative experiments with other ionophores containing S and Se donors are now being investigated and will be reported in due course.
Experimental
General Remarks
Melting points were determined with a Boetius Block apparatus. 1H NMR spectra were recorded on a Bruker AC-P200 spectrometer at 200 MHz in CDCl3 solution, using tetramethylsilane as an internal standard. 13C NMR spectra were recorded on a Bruker AC-P200 spectrometer at 50 MHz in CDCl3 solution. Elemental analyses were performed on a Perkin-Elmer 2400C instrument. Mass spectra were recorded on a VG ZAB-HS spectrometer. Compounds 3–5 [54,55] and 9 and 11 [50-53] were prepared according to literature procedures.
25,27-Dihydroxy-26,28-bis(phenyltelluropropoxy)-5,11,17,23-tetra-tert-butylcalix[4]arene 6
Diphenyl ditelluride (512 mg, 1.25 mmol), prepared from phenyl Grignard reagent with tellurium powder in 78% yield, was dissolved in ethanol (30 ml) and benzene (30 ml) in a 100 ml round-bottomed flask. Under an atmosphere of nitrogen, solid sodium borohydride (228 mg, 6 mmol) was added in small portions to the solution until the orange color of the diphenyl ditelluride disappeared. The rest of the sodium borohydride was added in one portion. The colorless solution was heated to reflux. A solution of calix[4]arene dibromide 3 (1 mmol) in benzene (20 ml) was added. The reaction mixture was heated at reflux for 1 h, cooled to room temperature, and poured into water (100 ml). The mixture was extracted three times with chloroform. The combined extracts were washed thoroughly with water and dried over anhydrous sodium sulfate. The dry solution was filtered. The filtrate was evaporated to dryness under vacuum. The oily residue was purified by chromatography on silica gel with (CH2Cl2/petroleum ether v/v, 1/3) to give 6 as a white powder in 97% yield. FAB+-MS m/z 1139.9 (M+, Calcd, 1139.8). 1H NMR: 7.75 (d, 4H, J = 6.6 Hz, Te–Ph–H), 7.70 (s, 2H, OH), 7.23–7.16 (m, 6H, Te–Ph–H), 7.02 (s, 4H, Ar–H), 6.82 (s, 4H, Ar–H), 4.24 (d, 4H, J = 12.8 Hz, ArCH2Ar), 4.02 (t, 4H, J = 5.7 Hz, OCH2CH2), 3.38 (t, 4H, J = 6.4 Hz, TeCH2CH2), 3.29 (d, 4H, J = 12.8 Hz, ArCH2Ar), 2.34 (m, 4H, CH2), 1.26 (s, 18H, t-Bu–H), 0.98 (s, 18H, t-Bu–H). 13C NMR: 150.66, 149.49, 146.91, 141.38, 138.30, 132.63, 129.12, 127.59, 127.47, 125.53, 125.07, 111.62, 76.82, 33.87, 33.77, 31.97, 31.69, 31.00, 4.88. Anal. Calcd. for C62H76O4Te2: C, 65.30; H, 6.72. Found: C, 65.22; H, 6.79.
25,27-Dihydroxy-26,28-bis(phenyltellurobutoxy)-5,11,17,23-tetra-tert-butylcalix[4]arene 7
Compound 7 was synthesized as yellowish oil in 96% yield. FAB+-MS m/z 1167.6 (M+, Calcd, 1167.8). 1H NMR: 7.75 (d, 4H, J = 6.2 Hz, Te–Ph–H), 7.56 (s, 2H, OH), 7.23–7.11 (m, 6H, Te–Ph–H) 7.02 (s, 4H, Ar–H), 6.79 (s, 4H, Ar–H), 4.21 (d, 4H, J = 13.0 Hz, ArCH2Ar), 3.94 (t, 4H, J = 5.7 Hz, OCH2CH2), 3.27 (d, 4H, J = 13.0 Hz, ArCH2Ar), 3.07 (t, 4H, J = 6.8 Hz, TeCH2CH2), 2.07 (m, 8H, CH2CH2), 1.27 (s, 18H, t-Bu–H), 0.96 (s, 18H, t-Bu–H). 13C NMR: 150.60, 149.73, 146.59, 141.16, 138.28, 132.44, 128.99, 127.59, 127.34, 125.32, 124.88, 111.57, 75.47, 33.74, 32.03, 31.60, 30.89, 28.32, 8.30. Calcd. for C64H80O4Te2: C, 65.78; H, 6.90. Found: C, 66.01; H, 6.79.
25,27-Dihydroxy-26,28-bis(phenyltellurohexoxy)-5,11,17,23-tetra-tert-butylcalix[4]arene 8
Compound 8 was synthesized as yellowish oil in 92% yield. FAB+-MS m/z 1223.7 (M+, Calcd, 1223.9). 1H NMR: 8.10 (s, 2H, OH), 7.73 (d, 2H, J = 6.3 Hz, Te–Ph–H), 7.71 (d, 2H, J = 6.3 Hz, Te–Ph–H), 7.58 (m, 3H, Te–Ph–H), 7.18 (m, 3H, Te–Ph–H), 7.02 (s, 4H, Ar–H), 6.81 (s, 4H, Ar–H), 4.25 (d, 4H, J = 12.8 Hz, ArCH2Ar), 3.98 (t, 4H, J = 6.2 Hz, OCH2CH2), 3.27 (d, 4H, J = 12.8 Hz, ArCH2Ar), 2.98 (m, 4H, TeCH2CH2), 1.98–1.55 (m, 16H, –(CH2)4–), 1.31 (s, 18H, t-Bu–H), 0.97 (s, 18H, t-Bu–H). 13C NMR: 150.73, 149.67, 146.57, 141.19, 138.12, 132.61, 129.02, 127.76, 127.32, 125.39, 124.98, 111.79, 76.20, 33.77, 31.69, 30.99, 29.79, 25.23, 8.60. Calcd. for C68H88O4Te2: C, 66.69; H, 7.24. Found: C, 66.59; H, 7.28.
General procedure for the synthesis of ditellurocalix[4]arenes 10 (cone) and 12 (partial cone)
Diphenyl ditelluride (128 mg, 0.31 mmol) was dissolved in ethanol (10 ml) and benzene (20 ml) in a 50 ml round-bottomed flask. Under an atmosphere of nitrogen, solid sodium borohydride (57 mg, 1.5 mmol) was added in one portion. The orange solution was heated to reflux for one hour. Then, a solution of calix[4]arene monobromide (214 mg, 0.25 mmol) in benzene (5 ml) is added. The reaction mixture was heated at reflux for 1 h, cooled to room temperature, and poured into water (40 ml). The mixture was extracted with chloroform for three times. The combined extracts were washed thoroughly with water and then dried over anhydrous sodium sulfate. The dry solution was filtered. The filtrate was evaporated to dryness under vacuum. The oily residue was purified by column chromatography.
25-Hydroxy-26,28-dipropoxy-27-(3-phenyltelluropropoxy)-5,11,17,23-tetra-tert-butyl-calix[4]arene 10 (cone)
Calix[4]arene 10 is obtained as yellowish oil in 93% yield (228 mg). FAB+-MS m/z 978.0 (M+). 1H NMR: 7.45 (d, 2H, J = 7.2 Hz, Te–Ph–H), 7.25–7.18 (m, 3H, Te–Ph–H), 7.15 (s, 2H, ArH), 7.02 (s, 2H, ArH), 6.51 (s, 2H, ArH), 6.46 (s, 2H, ArH), 4.18 (d, 2H, J = 12.9 Hz, ArCH2Ar), 4.16 (d, 2H, J = 12.9 Hz, ArCH2Ar), 4.05–3.75 (m, 6H, OCH2), 3.20 (d, 2H, J = 12.9 Hz, ArCH2Ar), 3.17 (d, 2H, J = 12.9 Hz, ArCH2Ar), 1.89–1.78 (m, 4H, CH2CH2Te), 1.33 (s, 9H, t-Bu–H), 1.31 (s, 18H, t-Bu–H), 0.97 (t, 6H, J = 9.1 Hz, CH2CH3), 0.79 (s, 9H, t-Bu–H). 13C NMR: 153.76, 151.64, 150.79, 145.73, 145.03, 141.30, 138.47, 135.97, 132.09, 131.82, 129.13, 127.40, 125.67, 124.98, 124.81, 124.66, 112.08, 75.82, 34.13, 33.81, 33.65, 31.74, 31.38, 31.07, 23.41, 10.76, 4.06. Calcd. for C59H78O4Te: C, 72.40; H, 8.03. Found: C, 72.45; H, 8.08.
25-Hydroxy-26,28-dipropoxy-27-(3-phenyltelluropropoxy)-5,11,17,23-tetra-tert-butyl-calix[4]arene 12 (partial cone)
Compound 12 is synthesized as yellowish oil in 71% yield. FAB+-MS m/z 978.2 (M+). 1H NMR: 7.65 (d, 2H, J = 7.4 Hz, Te–Ph–H), 7.45 (s, 1H, OH), 7.30–7.22 (m, 3H, Te–Ph–H), 7.07 (s, 2H, Ar–H), 7.04 (s, 2H, Ar–H), 6.90 (s, 4H, Ar–H), 4.16 (d, 2H, J = 12.5 Hz, ArCH2Ar), 3.94–3.88 (m, 4H, OCH2), 3.83 (s, 4H, ArCH2Ar), 3.22 (d, 2H, J = 12.5 Hz, ArCH2Ar), 3.01 (t, 2H, J = 7.3 Hz, OCH2), 2.45 (t, 2H, J = 7.3 Hz, OCH2), 1.97–1.52 (m, 6H, CH2), 1.43 (s, 9H, t-Bu–H), 1.29 (s, 9H, t-Bu–H), 1.11 (s, 18H, t-Bu–H), 1.03–0.92 (m, 6H, CH3). 13C NMR: 154.37, 152.69, 150.10, 145.32, 143.90, 141.47, 138.49, 133.54, 133.28, 132.75, 129.16, 128.81, 128.38, 127.47, 125.93, 125.09, 124.59, 112.04, 72.55, 70.48, 38.75, 33.95, 31.73, 31.55, 29.78, 23.21, 19.20, 10.49, 3.54. Calcd. for C59H78O4Te: C, 72.40; H, 8.03. Found: C, 72.66; H, 8.18.
EMF Measurements. All EMF (electromotive force) measurements were made at 25 °C, using a pH/mV meter. The sample solution was magnetically stirred and kept in a thermostatted water bath. The EMF values were corrected by subtracting the liquid-junction potential between the external reference electrode and the sample solution in the higher Ag+ concentration.
Selectivity coefficients. The potentiometric selectivity coefficient, K, determined here is defined by the Nikolsky–Eisenman Equation (Equation 1).
where E represents the experimentally observed potential, R the gas constant, T the thermodynamic temperature in K, F the Faraday constant, aAg the Ag+ activity, aM the activity of the interfering cation, and ZM the charge of the interfering cation. The selectivity coefficients were determined by a mixed-solution method [65,66]. In this mixed-solution method, the concentration of silver ion is varied while that of the interfering ions such as Na+, K+, NH4+, Ca2+, Mg2+ are 0.1 M; Zn2+, Cu2+, Ni2+, Cd2+, Pb2+, Hg2+ are 0.01 M. According to this method, the potentiometric selectivity coefficients, K, can be evaluated from the potential measurements on solutions containing a fixed concentration of the interfering ions (Mn+) and varying the concentration of Ag+ ion using Equation 2.
The resulting logK values are summarized in Table 1.
Extraction experiments. Extraction experiments were carried out using a solution of metal picrate in water saturated with dichloromethane (1.00 × 10−4 mol·L−1) and solutions of the ligand in water saturated with dichloromethane (1.00 × 10−3 mol·L−1). Equal volumes (0.01 L) of the mutually saturated solvents containing the metal ion salt in the aqueous phase and the ligand in the organic phase were shaken for 30 min by 2D-2 oscillator and then left for 2 h at (25 ± 0.05) °C. For the determination of picrates in water phase, a cary-300 UV-visible spectrophotometer was used and absorbance readings were taken at 354 nm.
References
-
Gutsche, C. D. Calixarenes; The Royal Society of Chemistry: Cambridge, U.K., 1989.
Return to citation in text: [1] [2] -
Ikeda, A.; Shinkai, S. Chem. Rev. 1997, 97, 1713–1734. doi:10.1021/cr960385x
Return to citation in text: [1] [2] -
Böhmer, V. Angew. Chem., Int. Ed. Engl. 1995, 34, 713–745. doi:10.1002/anie.199507131
Return to citation in text: [1] [2] -
Kim, J. S.; Quang, D. T. Chem. Rev. 2007, 107, 3780–3799. doi:10.1021/cr068046j
Return to citation in text: [1] [2] -
Bühlmann, P.; Pretsch, E.; Bakker, E. Chem. Rev. 1998, 98, 1593–1687. doi:10.1021/cr970113+
Return to citation in text: [1] -
Bakker, E.; Bühlmann, P.; Pretsch, E. Chem. Rev. 1997, 97, 3083–3132. doi:10.1021/cr940394a
Return to citation in text: [1] -
Ganjali, M. R.; Norouzi, P.; Rezapour, M.; Faridbod, F.; Pourjavid, M. R. Sensors 2006, 6, 1018–1086. doi:10.3390/s6081018
Return to citation in text: [1] -
Lockhart, J. C. In Comprehensive Supramolecular Chemistry; Gokel, G. W., Ed.; Pergamon: Oxford, 1996; Vol. 1, pp 605–634.
Return to citation in text: [1] -
Odashima, K.; Koga, K. In Comprehensive Supramolecular Chemistry; Vögtle, F., Ed.; Pergamon: Oxford, 1996; Vol. 2, pp 143–194.
Return to citation in text: [1] -
Brazózka, Z. In Comprehensive Supramolecular Chemistry; Reinhoudt, D. N., Ed.; Pergamon: Oxford, 1996; Vol. 10, p 187.
Return to citation in text: [1] -
Bakker, E. Anal. Chem. 1997, 69, 1061–1069. doi:10.1021/ac960891m
Return to citation in text: [1] -
Tsujimura, Y.; Yokoyama, M.; Kimura, K. Anal. Chem. 1995, 67, 2401–2404. doi:10.1021/ac00110a012
Return to citation in text: [1] -
Shortreed, M.; Bakker, E.; Kopelman, R. Anal. Chem. 1996, 68, 2656–2662. doi:10.1021/ac960035a
Return to citation in text: [1] -
O’Connor, K. M.; Cherry, M.; Svehla, G.; Harris, S. J.; McKervey, M. A. Talanta 1994, 41, 1207–1211. doi:10.1016/0039-9140(94)80093-6
Return to citation in text: [1] -
Grady, T.; Cadogan, A.; McKittrick, T.; Harris, S. J.; Diamond, D.; McKervey, M. A. Anal. Chim. Acta 1996, 336, 1–12. doi:10.1016/S0003-2670(96)00380-7
Return to citation in text: [1] -
Brunink, J. A. J.; Lugtenberg, R. J. W.; Brzózka, Z.; Engbersen, J. F. J.; Reinhoudt, D. N. J. Electroanal. Chem. 1994, 378, 185–200. doi:10.1016/0022-0728(94)87071-3
Return to citation in text: [1] -
Shibutani, Y.; Yoshinaga, H.; Yakabe, K.; Shono, T.; Tanaka, M. J. Inclusion Phenom. Mol. Recognit. Chem. 1994, 19, 333–342. doi:10.1007/BF00708991
Return to citation in text: [1] -
Careri, M.; Casnati, A.; Guarinoni, A.; Mangia, A.; Mori, G.; Pochini, A.; Ungaro, R. Anal. Chem. 1993, 65, 3156–3160. doi:10.1021/ac00069a034
Return to citation in text: [1] -
Cadogan, A.; Gao, Z.; Lewenstam, A.; Ivaska, A.; Diamond, D. Anal. Chem. 1992, 64, 2496–2501. doi:10.1021/ac00045a007
Return to citation in text: [1] -
Telting-Diaz, M.; Diamond, D.; Smyth, M. R. Anal. Chim. Acta 1991, 251, 149–155. doi:10.1016/0003-2670(91)87128-T
Return to citation in text: [1] -
Casnati, A.; Pochini, A.; Ungaro, R.; Bocchi, C.; Ugozzoli, F.; Egberink, R. J. M.; Struijk, H.; Lugtenberg, R.; de Jong, F.; Reinhoudt, D. N. Chem.–Eur. J. 1996, 2, 436–439. doi:10.1002/chem.19960020413
Return to citation in text: [1] -
Brzozka, Z.; Lammerink, B.; Reinhoudt, D. N.; Ghidini, E.; Ungaro, R. J. Chem. Soc., Perkin Trans. 2 1993, 1037–1040. doi:10.1039/p29930001037
Return to citation in text: [1] -
Cadogan, A.; Diamond, D.; Cremin, S.; McKervey, M. A.; Harris, S. J. Anal. Proc. 1991, 28, 13–14. doi:10.1039/AP9912800008
Return to citation in text: [1] -
Zeng, X.; Weng, L.; Chen, L.; Ju, H.; Leng, X.; He, X.; Zhang, Z. Z. Chin. J. Chem. 2001, 19 (5), 493–499.
Return to citation in text: [1] -
Chen, L.; Ju, H.; Zeng, X.; He, X.; Zhang, Z. Z. Anal. Chim. Acta 2001, 447, 41–46. doi:10.1016/S0003-2670(01)01300-9
Return to citation in text: [1] -
Bocchi, C.; Careri, M.; Casnati, A.; Mori, G. Anal. Chem. 1995, 67, 4234–4238. doi:10.1021/ac00119a005
Return to citation in text: [1] -
Cadogan, A.; Diamond, D.; Smyth, M. R.; Svehla, G.; McKervey, M. A.; Seward, E. M.; Harris, S. J. Analyst 1990, 115, 1207–1210. doi:10.1039/an9901501207
Return to citation in text: [1] -
Lugtenberg, R. J. W.; Brzózka, Z.; Casnati, A.; Ungaro, R.; Engbersen, J. F. J.; Reinhoudt, D. N. Anal. Chim. Acta 1995, 310, 263–267. doi:10.1016/0003-2670(95)00139-Q
Return to citation in text: [1] -
Péres-Jiménez, C.; Escriche, L.; Casabó, J. Anal. Chim. Acta 1998, 371, 155–162. doi:10.1016/S0003-2670(98)00325-0
Return to citation in text: [1] -
Kim, J. S.; Ohki, A.; Ueki, R.; Ishizuka, T.; Shimotashiro, T.; Maeda, S. Talanta 1999, 48, 705–710. doi:10.1016/S0039-9140(98)00291-4
Return to citation in text: [1] [2] -
Kimura, K.; Tatsumi, K.; Yokoyama, M.; Ouchi, M.; Mocerino, M. Anal. Commun. 1999, 36, 229–230. doi:10.1039/a902803j
Return to citation in text: [1] -
Cadogan, F.; Kane, P.; McKervey, M. A.; Diamond, D. Anal. Chem. 1999, 71, 5544–5550. doi:10.1021/ac990303f
Return to citation in text: [1] -
Malinowska, E.; Brzozka, Z.; Kasiura, K.; Egberink, R. J. M.; Reinhoudt, D. N. Anal. Chim. Acta 1994, 298, 253–258. doi:10.1016/0003-2670(94)00266-5
Return to citation in text: [1] -
Odashima, K.; Yagi, K.; Tohda, K.; Umezawa, Y. Anal. Chem. 1993, 65, 1074–1083. doi:10.1021/ac00056a022
Return to citation in text: [1] -
Chan, W. H.; Shiu, K. K.; Gu, X. H. Analyst 1993, 118, 863–867. doi:10.1039/an9931800863
Return to citation in text: [1] -
Shvedene, N. V.; Nemilova, M. Yu.; Kovalev, V. V.; Shokova, E. A.; Rozov, A. K.; Pletnev, I. V. Sens. Actuators, B 1995, 27, 372–376. doi:10.1016/0925-4005(94)01620-W
Return to citation in text: [1] -
Cobben, P. L. H. M.; Egberink, R. J. M.; Bomer, J. G.; Bergveld, P.; Verboom, W.; Reinhoudt, D. N. J. Am. Chem. Soc. 1992, 114, 10573–10582. doi:10.1021/ja00052a063
Return to citation in text: [1] -
Cooper, S. R. Acc. Chem. Res. 1988, 21, 141–146. doi:10.1021/ar00148a002
Return to citation in text: [1] -
Murray, S. G.; Hartley, F. R. Chem. Rev. 1981, 81, 365–414. doi:10.1021/cr00044a003
Return to citation in text: [1] -
Beard, C. D.; Carr, L.; Davis, M. F.; Evans, J.; Levason, W.; Norman, L. D.; Reid, G.; Webster, M. Eur. J. Inorg. Chem. 2006, 4399–4406. doi:10.1002/ejic.200600573
Return to citation in text: [1] -
Küppers, H.-J.; Wieghardt, K.; Nuber, B.; Weiss, J.; Bill, E.; Trautwein, A. X. Inorg. Chem. 1987, 26, 3762–3769. doi:10.1021/ic00269a028
Return to citation in text: [1] -
Edema, J. J. H.; Buter, J.; Schoonbeek, F. S.; Kellogg, R. M.; Van Bolhuis, F.; Spek, A. L. Inorg. Chem. 1994, 33, 2448–2452. doi:10.1021/ic00089a022
Return to citation in text: [1] -
Lucas, C. R.; Liang, W.; Miller, D. O.; Bridson, J. N. Inorg. Chem. 1997, 36, 4508–4513. doi:10.1021/ic961536h
Return to citation in text: [1] -
Kulatilleke, C. P.; Goldie, S. N.; Ochrymowycz, L. A.; Rorabacher, D. B. Inorg. Chem. 1999, 38, 5906–5909. doi:10.1021/ic9907213
Return to citation in text: [1] -
Rijn, J. V.; Driessen, W. L.; Reedijk, J.; Lehn, J.-M. Inorg. Chem. 1984, 23, 3584–3588. doi:10.1021/ic00190a030
Return to citation in text: [1] -
Panda, S.; Singh, H. B.; Butcher, R. J. Chem. Commun. 2004, 322–323. doi:10.1039/b312358h
Return to citation in text: [1] -
Patel, U.; Singh, H. B.; Butcher, R. J. Eur. J. Inorg. Chem. 2006, 5089–5097. doi:10.1002/ejic.200600495
Return to citation in text: [1] -
Vasilescu, I. M.; Bray, D. J.; Clegg, J. K.; Lindoy, L. F.; Meehan, G. V.; Wei, G. Dalton Trans. 2006, 5115–5117. doi:10.1039/b613636m
Return to citation in text: [1] -
Panda, S.; Singh, H. B.; Butcher, R. J. Inorg. Chem. 2004, 43, 8532–8537. doi:10.1021/ic0351949
Return to citation in text: [1] -
Zeng, X.; Weng, L.; Chen, L.; Leng, X.; Zhang, Z. Z.; He, X. Tetrahedron Lett. 2000, 41, 4917–4921. doi:10.1016/S0040-4039(00)00746-2
Return to citation in text: [1] [2] -
Zeng, X.; Weng, L.; Chen, L.; Leng, X.; Ju, H.; He, X.; Zhang, Z. Z. J. Chem. Soc., Perkin Trans. 2 2001, 545–549. doi:10.1039/b008114k
Return to citation in text: [1] [2] -
Zeng, X.; Leng, X.; Chen, L.; Sun, H.; Xu, F.; Li, Q.; He, X.; Zhang, Z.-Z. J. Chem. Soc., Perkin Trans. 2 2002, 796–801. doi:10.1039/b109238c
Return to citation in text: [1] [2] -
Zeng, X.; Weng, L.; Chen, L.; Xu, F.; Li, Q.; Leng, X.; He, X.; Zhang, Z.-Z. Tetrahedron 2002, 58, 2647–2658. doi:10.1016/S0040-4020(02)00124-2
Return to citation in text: [1] [2] -
Zeng, X.; Sun, H.; Chen, L.; Leng, X.; Xu, F.; Li, Q.; He, X.; Zhang, W.; Zhang, Z.-Z. Org. Biomol. Chem. 2003, 1, 1073–1079. doi:10.1039/b211381c
Return to citation in text: [1] [2] [3] [4] [5] -
Li, Z. T.; Ji, G. Z.; Zhao, C. X.; Yuan, S. D.; Ding, H.; Huang, C.; Du, A. L.; Wei, M. J. Org. Chem. 1999, 64, 3572–3584. doi:10.1021/jo9824100
Return to citation in text: [1] [2] [3] -
van Loon, J.-D.; Groenen, L. C.; Wijmenga, S. S.; Verboom, W.; Reinhoudt, D. N. J. Am. Chem. Soc. 1991, 113, 2378–2384. doi:10.1021/ja00007a005
Return to citation in text: [1] -
Bodenant, B.; Weil, T.; Businelli-Pourcel, M.; Fages, F.; Barbe, B.; Pianet, I.; Laguerre, M. J. Org. Chem. 1999, 64, 7034–7039. doi:10.1021/jo9904720
Return to citation in text: [1] -
Morf, W. E. Principles of Ion-Selective Electrodes and Membrane Transport; Elesevier: New York, 1981.
Return to citation in text: [1] -
Bricker, J.; Daunert, S.; Bachas, L. G.; Valiente, M. Anal. Chem. 1991, 63, 1585–1589. doi:10.1021/ac00015a016
Return to citation in text: [1] -
Kimura, K.; Yajima, S.; Tatsumi, K.; Yokoyama, M.; Oue, M. Anal. Chem. 2000, 72, 5290–5294. doi:10.1021/ac000490d
Return to citation in text: [1] -
Ma, J. C.; Dougherty, D. A. Chem. Rev. 1997, 97, 1303–1324. doi:10.1021/cr9603744
Return to citation in text: [1] -
Xu, F.; Weng, L.; Sun, L.; Zhang, Z. Z.; Zhou, Z. Organometallics 2000, 19, 2658–2660. doi:10.1021/om9909237
Our work about Ag+-p interaction in the solid state.
Return to citation in text: [1] -
Ikeda, A.; Shinkai, S. J. Am. Chem. Soc. 1994, 116, 3102–3110. doi:10.1021/ja00086a045
Return to citation in text: [1] -
Inokuchi, F.; Miyahara, Y.; Inazu, T.; Shinkai, S. Angew. Chem., Int. Ed. Engl. 1995, 34, 1364–1366. doi:10.1002/anie.199513641
Return to citation in text: [1] -
Kimura, K.; Shono, T. Complexation of cationic species by crown ethers. In Cation Binding by Macrocycles; Inoue, Y.; Gokel, G. W., Eds.; Marcel Dekker: New York, 1990; pp 429–463.
Return to citation in text: [1] -
Umezawa, Y.; Umezawa, K.; Sato, H. Pure Appl. Chem. 1995, 67, 507–518. doi:10.1351/pac199567030507
Return to citation in text: [1]
| 60. | Kimura, K.; Yajima, S.; Tatsumi, K.; Yokoyama, M.; Oue, M. Anal. Chem. 2000, 72, 5290–5294. doi:10.1021/ac000490d |
| 61. | Ma, J. C.; Dougherty, D. A. Chem. Rev. 1997, 97, 1303–1324. doi:10.1021/cr9603744 |
| 62. |
Xu, F.; Weng, L.; Sun, L.; Zhang, Z. Z.; Zhou, Z. Organometallics 2000, 19, 2658–2660. doi:10.1021/om9909237
Our work about Ag+-p interaction in the solid state. |
| 63. | Ikeda, A.; Shinkai, S. J. Am. Chem. Soc. 1994, 116, 3102–3110. doi:10.1021/ja00086a045 |
| 64. | Inokuchi, F.; Miyahara, Y.; Inazu, T.; Shinkai, S. Angew. Chem., Int. Ed. Engl. 1995, 34, 1364–1366. doi:10.1002/anie.199513641 |
| 54. | Zeng, X.; Sun, H.; Chen, L.; Leng, X.; Xu, F.; Li, Q.; He, X.; Zhang, W.; Zhang, Z.-Z. Org. Biomol. Chem. 2003, 1, 1073–1079. doi:10.1039/b211381c |
| 58. | Morf, W. E. Principles of Ion-Selective Electrodes and Membrane Transport; Elesevier: New York, 1981. |
| 59. | Bricker, J.; Daunert, S.; Bachas, L. G.; Valiente, M. Anal. Chem. 1991, 63, 1585–1589. doi:10.1021/ac00015a016 |
| 1. | Gutsche, C. D. Calixarenes; The Royal Society of Chemistry: Cambridge, U.K., 1989. |
| 2. | Ikeda, A.; Shinkai, S. Chem. Rev. 1997, 97, 1713–1734. doi:10.1021/cr960385x |
| 3. | Böhmer, V. Angew. Chem., Int. Ed. Engl. 1995, 34, 713–745. doi:10.1002/anie.199507131 |
| 4. | Kim, J. S.; Quang, D. T. Chem. Rev. 2007, 107, 3780–3799. doi:10.1021/cr068046j |
| 21. | Casnati, A.; Pochini, A.; Ungaro, R.; Bocchi, C.; Ugozzoli, F.; Egberink, R. J. M.; Struijk, H.; Lugtenberg, R.; de Jong, F.; Reinhoudt, D. N. Chem.–Eur. J. 1996, 2, 436–439. doi:10.1002/chem.19960020413 |
| 22. | Brzozka, Z.; Lammerink, B.; Reinhoudt, D. N.; Ghidini, E.; Ungaro, R. J. Chem. Soc., Perkin Trans. 2 1993, 1037–1040. doi:10.1039/p29930001037 |
| 23. | Cadogan, A.; Diamond, D.; Cremin, S.; McKervey, M. A.; Harris, S. J. Anal. Proc. 1991, 28, 13–14. doi:10.1039/AP9912800008 |
| 24. | Zeng, X.; Weng, L.; Chen, L.; Ju, H.; Leng, X.; He, X.; Zhang, Z. Z. Chin. J. Chem. 2001, 19 (5), 493–499. |
| 25. | Chen, L.; Ju, H.; Zeng, X.; He, X.; Zhang, Z. Z. Anal. Chim. Acta 2001, 447, 41–46. doi:10.1016/S0003-2670(01)01300-9 |
| 54. | Zeng, X.; Sun, H.; Chen, L.; Leng, X.; Xu, F.; Li, Q.; He, X.; Zhang, W.; Zhang, Z.-Z. Org. Biomol. Chem. 2003, 1, 1073–1079. doi:10.1039/b211381c |
| 55. | Li, Z. T.; Ji, G. Z.; Zhao, C. X.; Yuan, S. D.; Ding, H.; Huang, C.; Du, A. L.; Wei, M. J. Org. Chem. 1999, 64, 3572–3584. doi:10.1021/jo9824100 |
| 11. | Bakker, E. Anal. Chem. 1997, 69, 1061–1069. doi:10.1021/ac960891m |
| 12. | Tsujimura, Y.; Yokoyama, M.; Kimura, K. Anal. Chem. 1995, 67, 2401–2404. doi:10.1021/ac00110a012 |
| 13. | Shortreed, M.; Bakker, E.; Kopelman, R. Anal. Chem. 1996, 68, 2656–2662. doi:10.1021/ac960035a |
| 14. | O’Connor, K. M.; Cherry, M.; Svehla, G.; Harris, S. J.; McKervey, M. A. Talanta 1994, 41, 1207–1211. doi:10.1016/0039-9140(94)80093-6 |
| 15. | Grady, T.; Cadogan, A.; McKittrick, T.; Harris, S. J.; Diamond, D.; McKervey, M. A. Anal. Chim. Acta 1996, 336, 1–12. doi:10.1016/S0003-2670(96)00380-7 |
| 16. | Brunink, J. A. J.; Lugtenberg, R. J. W.; Brzózka, Z.; Engbersen, J. F. J.; Reinhoudt, D. N. J. Electroanal. Chem. 1994, 378, 185–200. doi:10.1016/0022-0728(94)87071-3 |
| 17. | Shibutani, Y.; Yoshinaga, H.; Yakabe, K.; Shono, T.; Tanaka, M. J. Inclusion Phenom. Mol. Recognit. Chem. 1994, 19, 333–342. doi:10.1007/BF00708991 |
| 18. | Careri, M.; Casnati, A.; Guarinoni, A.; Mangia, A.; Mori, G.; Pochini, A.; Ungaro, R. Anal. Chem. 1993, 65, 3156–3160. doi:10.1021/ac00069a034 |
| 19. | Cadogan, A.; Gao, Z.; Lewenstam, A.; Ivaska, A.; Diamond, D. Anal. Chem. 1992, 64, 2496–2501. doi:10.1021/ac00045a007 |
| 20. | Telting-Diaz, M.; Diamond, D.; Smyth, M. R. Anal. Chim. Acta 1991, 251, 149–155. doi:10.1016/0003-2670(91)87128-T |
| 54. | Zeng, X.; Sun, H.; Chen, L.; Leng, X.; Xu, F.; Li, Q.; He, X.; Zhang, W.; Zhang, Z.-Z. Org. Biomol. Chem. 2003, 1, 1073–1079. doi:10.1039/b211381c |
| 1. | Gutsche, C. D. Calixarenes; The Royal Society of Chemistry: Cambridge, U.K., 1989. |
| 2. | Ikeda, A.; Shinkai, S. Chem. Rev. 1997, 97, 1713–1734. doi:10.1021/cr960385x |
| 3. | Böhmer, V. Angew. Chem., Int. Ed. Engl. 1995, 34, 713–745. doi:10.1002/anie.199507131 |
| 4. | Kim, J. S.; Quang, D. T. Chem. Rev. 2007, 107, 3780–3799. doi:10.1021/cr068046j |
| 54. | Zeng, X.; Sun, H.; Chen, L.; Leng, X.; Xu, F.; Li, Q.; He, X.; Zhang, W.; Zhang, Z.-Z. Org. Biomol. Chem. 2003, 1, 1073–1079. doi:10.1039/b211381c |
| 55. | Li, Z. T.; Ji, G. Z.; Zhao, C. X.; Yuan, S. D.; Ding, H.; Huang, C.; Du, A. L.; Wei, M. J. Org. Chem. 1999, 64, 3572–3584. doi:10.1021/jo9824100 |
| 5. | Bühlmann, P.; Pretsch, E.; Bakker, E. Chem. Rev. 1998, 98, 1593–1687. doi:10.1021/cr970113+ |
| 6. | Bakker, E.; Bühlmann, P.; Pretsch, E. Chem. Rev. 1997, 97, 3083–3132. doi:10.1021/cr940394a |
| 7. | Ganjali, M. R.; Norouzi, P.; Rezapour, M.; Faridbod, F.; Pourjavid, M. R. Sensors 2006, 6, 1018–1086. doi:10.3390/s6081018 |
| 8. | Lockhart, J. C. In Comprehensive Supramolecular Chemistry; Gokel, G. W., Ed.; Pergamon: Oxford, 1996; Vol. 1, pp 605–634. |
| 9. | Odashima, K.; Koga, K. In Comprehensive Supramolecular Chemistry; Vögtle, F., Ed.; Pergamon: Oxford, 1996; Vol. 2, pp 143–194. |
| 10. | Brazózka, Z. In Comprehensive Supramolecular Chemistry; Reinhoudt, D. N., Ed.; Pergamon: Oxford, 1996; Vol. 10, p 187. |
| 56. | van Loon, J.-D.; Groenen, L. C.; Wijmenga, S. S.; Verboom, W.; Reinhoudt, D. N. J. Am. Chem. Soc. 1991, 113, 2378–2384. doi:10.1021/ja00007a005 |
| 57. | Bodenant, B.; Weil, T.; Businelli-Pourcel, M.; Fages, F.; Barbe, B.; Pianet, I.; Laguerre, M. J. Org. Chem. 1999, 64, 7034–7039. doi:10.1021/jo9904720 |
| 34. | Odashima, K.; Yagi, K.; Tohda, K.; Umezawa, Y. Anal. Chem. 1993, 65, 1074–1083. doi:10.1021/ac00056a022 |
| 35. | Chan, W. H.; Shiu, K. K.; Gu, X. H. Analyst 1993, 118, 863–867. doi:10.1039/an9931800863 |
| 36. | Shvedene, N. V.; Nemilova, M. Yu.; Kovalev, V. V.; Shokova, E. A.; Rozov, A. K.; Pletnev, I. V. Sens. Actuators, B 1995, 27, 372–376. doi:10.1016/0925-4005(94)01620-W |
| 38. | Cooper, S. R. Acc. Chem. Res. 1988, 21, 141–146. doi:10.1021/ar00148a002 |
| 39. | Murray, S. G.; Hartley, F. R. Chem. Rev. 1981, 81, 365–414. doi:10.1021/cr00044a003 |
| 40. | Beard, C. D.; Carr, L.; Davis, M. F.; Evans, J.; Levason, W.; Norman, L. D.; Reid, G.; Webster, M. Eur. J. Inorg. Chem. 2006, 4399–4406. doi:10.1002/ejic.200600573 |
| 41. | Küppers, H.-J.; Wieghardt, K.; Nuber, B.; Weiss, J.; Bill, E.; Trautwein, A. X. Inorg. Chem. 1987, 26, 3762–3769. doi:10.1021/ic00269a028 |
| 42. | Edema, J. J. H.; Buter, J.; Schoonbeek, F. S.; Kellogg, R. M.; Van Bolhuis, F.; Spek, A. L. Inorg. Chem. 1994, 33, 2448–2452. doi:10.1021/ic00089a022 |
| 43. | Lucas, C. R.; Liang, W.; Miller, D. O.; Bridson, J. N. Inorg. Chem. 1997, 36, 4508–4513. doi:10.1021/ic961536h |
| 44. | Kulatilleke, C. P.; Goldie, S. N.; Ochrymowycz, L. A.; Rorabacher, D. B. Inorg. Chem. 1999, 38, 5906–5909. doi:10.1021/ic9907213 |
| 45. | Rijn, J. V.; Driessen, W. L.; Reedijk, J.; Lehn, J.-M. Inorg. Chem. 1984, 23, 3584–3588. doi:10.1021/ic00190a030 |
| 46. | Panda, S.; Singh, H. B.; Butcher, R. J. Chem. Commun. 2004, 322–323. doi:10.1039/b312358h |
| 47. | Patel, U.; Singh, H. B.; Butcher, R. J. Eur. J. Inorg. Chem. 2006, 5089–5097. doi:10.1002/ejic.200600495 |
| 48. | Vasilescu, I. M.; Bray, D. J.; Clegg, J. K.; Lindoy, L. F.; Meehan, G. V.; Wei, G. Dalton Trans. 2006, 5115–5117. doi:10.1039/b613636m |
| 49. | Panda, S.; Singh, H. B.; Butcher, R. J. Inorg. Chem. 2004, 43, 8532–8537. doi:10.1021/ic0351949 |
| 65. | Kimura, K.; Shono, T. Complexation of cationic species by crown ethers. In Cation Binding by Macrocycles; Inoue, Y.; Gokel, G. W., Eds.; Marcel Dekker: New York, 1990; pp 429–463. |
| 66. | Umezawa, Y.; Umezawa, K.; Sato, H. Pure Appl. Chem. 1995, 67, 507–518. doi:10.1351/pac199567030507 |
| 32. | Cadogan, F.; Kane, P.; McKervey, M. A.; Diamond, D. Anal. Chem. 1999, 71, 5544–5550. doi:10.1021/ac990303f |
| 33. | Malinowska, E.; Brzozka, Z.; Kasiura, K.; Egberink, R. J. M.; Reinhoudt, D. N. Anal. Chim. Acta 1994, 298, 253–258. doi:10.1016/0003-2670(94)00266-5 |
| 50. | Zeng, X.; Weng, L.; Chen, L.; Leng, X.; Zhang, Z. Z.; He, X. Tetrahedron Lett. 2000, 41, 4917–4921. doi:10.1016/S0040-4039(00)00746-2 |
| 51. | Zeng, X.; Weng, L.; Chen, L.; Leng, X.; Ju, H.; He, X.; Zhang, Z. Z. J. Chem. Soc., Perkin Trans. 2 2001, 545–549. doi:10.1039/b008114k |
| 52. | Zeng, X.; Leng, X.; Chen, L.; Sun, H.; Xu, F.; Li, Q.; He, X.; Zhang, Z.-Z. J. Chem. Soc., Perkin Trans. 2 2002, 796–801. doi:10.1039/b109238c |
| 53. | Zeng, X.; Weng, L.; Chen, L.; Xu, F.; Li, Q.; Leng, X.; He, X.; Zhang, Z.-Z. Tetrahedron 2002, 58, 2647–2658. doi:10.1016/S0040-4020(02)00124-2 |
| 31. | Kimura, K.; Tatsumi, K.; Yokoyama, M.; Ouchi, M.; Mocerino, M. Anal. Commun. 1999, 36, 229–230. doi:10.1039/a902803j |
| 54. | Zeng, X.; Sun, H.; Chen, L.; Leng, X.; Xu, F.; Li, Q.; He, X.; Zhang, W.; Zhang, Z.-Z. Org. Biomol. Chem. 2003, 1, 1073–1079. doi:10.1039/b211381c |
| 55. | Li, Z. T.; Ji, G. Z.; Zhao, C. X.; Yuan, S. D.; Ding, H.; Huang, C.; Du, A. L.; Wei, M. J. Org. Chem. 1999, 64, 3572–3584. doi:10.1021/jo9824100 |
| 26. | Bocchi, C.; Careri, M.; Casnati, A.; Mori, G. Anal. Chem. 1995, 67, 4234–4238. doi:10.1021/ac00119a005 |
| 27. | Cadogan, A.; Diamond, D.; Smyth, M. R.; Svehla, G.; McKervey, M. A.; Seward, E. M.; Harris, S. J. Analyst 1990, 115, 1207–1210. doi:10.1039/an9901501207 |
| 28. | Lugtenberg, R. J. W.; Brzózka, Z.; Casnati, A.; Ungaro, R.; Engbersen, J. F. J.; Reinhoudt, D. N. Anal. Chim. Acta 1995, 310, 263–267. doi:10.1016/0003-2670(95)00139-Q |
| 29. | Péres-Jiménez, C.; Escriche, L.; Casabó, J. Anal. Chim. Acta 1998, 371, 155–162. doi:10.1016/S0003-2670(98)00325-0 |
| 30. | Kim, J. S.; Ohki, A.; Ueki, R.; Ishizuka, T.; Shimotashiro, T.; Maeda, S. Talanta 1999, 48, 705–710. doi:10.1016/S0039-9140(98)00291-4 |
| 30. | Kim, J. S.; Ohki, A.; Ueki, R.; Ishizuka, T.; Shimotashiro, T.; Maeda, S. Talanta 1999, 48, 705–710. doi:10.1016/S0039-9140(98)00291-4 |
| 37. | Cobben, P. L. H. M.; Egberink, R. J. M.; Bomer, J. G.; Bergveld, P.; Verboom, W.; Reinhoudt, D. N. J. Am. Chem. Soc. 1992, 114, 10573–10582. doi:10.1021/ja00052a063 |
| 50. | Zeng, X.; Weng, L.; Chen, L.; Leng, X.; Zhang, Z. Z.; He, X. Tetrahedron Lett. 2000, 41, 4917–4921. doi:10.1016/S0040-4039(00)00746-2 |
| 51. | Zeng, X.; Weng, L.; Chen, L.; Leng, X.; Ju, H.; He, X.; Zhang, Z. Z. J. Chem. Soc., Perkin Trans. 2 2001, 545–549. doi:10.1039/b008114k |
| 52. | Zeng, X.; Leng, X.; Chen, L.; Sun, H.; Xu, F.; Li, Q.; He, X.; Zhang, Z.-Z. J. Chem. Soc., Perkin Trans. 2 2002, 796–801. doi:10.1039/b109238c |
| 53. | Zeng, X.; Weng, L.; Chen, L.; Xu, F.; Li, Q.; Leng, X.; He, X.; Zhang, Z.-Z. Tetrahedron 2002, 58, 2647–2658. doi:10.1016/S0040-4020(02)00124-2 |
© 2009 Lu et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)