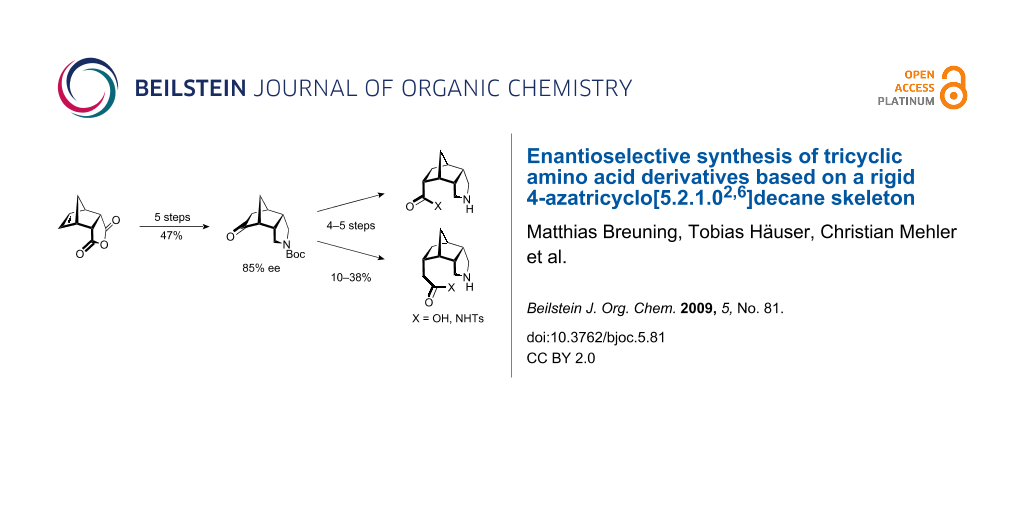
Abstract
An enantioselective route to four tricyclic amino acids and N-tosylamides, composed of a central norbornane framework with a 2-endo,3-endo-annelated pyrrolidine ring and a 5-endo-C1 or -C2 side chain, has been developed. A key intermediate was the chiral, N-Boc-protected ketone (1R,2S,6S,7R)-4-azatricyclo[5.2.1.02,6]decan-8-one, available from inexpensive endo-carbic anhydride in five steps and 47% yield. The rigid scaffold makes these amino acid derivatives promising candidates for β-turn-inducing building blocks in peptidomimetics and for chiral auxiliaries in asymmetric organocatalysis.
Graphical Abstract
Introduction
Unnatural amino acids with a rigid bowl-shaped backbone have received considerable interest in recent years. Incorporated in peptides or proteins, they may increase the metabolic stability and allow the introduction of novel structural motifs [1-4]. ß-Turns, for example, result if conformationally constrained spiro- or bicyclic amino acids such as 1 [5], 2 [6], and 3 [7,8] are embedded in peptidomimetics (Figure 1). Enantioselective organocatalysis [9-17] is another field of application for conformationally rigid amino acid derivatives. In this context, focus was also put on derivatives in which the activating acidic group is anchored at a more remote position of the molecule, but still in close spatial proximity to the amino function. Examples are β-proline (4) [18,19], the bispidinium salt 5 [20], and the binaphthyl-derived amino acid 6 [21-23], which provided excellent enantioselectivities in several aldol and Mannich reactions.
Figure 1: The conformationally rigid amino acid derivatives 1–3 (β-turn-inducing building blocks) and 4–6 (successful organocatalysts).
Figure 1: The conformationally rigid amino acid derivatives 1–3 (β-turn-inducing building blocks) and 4–6 (su...
Our studies targeted the chiral, tricyclic amino acid derivatives 7 and 8 (Figure 2), which possess a central norbornane framework equipped with a 2-endo,3-endo-annelated pyrrolidine ring. Due to the constrained, bowl-shaped backbone, these compounds may possess high potential as β-turn-inducing peptide building blocks and as bifunctional organocatalysts. In this paper we report on the first enantioselective synthesis of 7 and 8, which was achieved via the chiral ketone 9 as the key intermediate.
Figure 2: The targeted tricyclic amino acid derivatives 7 and 8, and the key intermediate 9.
Figure 2: The targeted tricyclic amino acid derivatives 7 and 8, and the key intermediate 9.
Results and Discussion
The key intermediate, the tricyclic amino ketone 9, was first prepared in racemic form starting from inexpensive endo-carbic anhydride (10, Scheme 1). Conversion of the succinyl anhydride moiety in 10 into the pyrrolidine ring in 11 was accomplished in three steps and 74% yield by imide formation, reduction [24], and N-protection. Hydroboration/oxidation of the alkene function of 11 delivered the exo-configured alcohol rac-12, which was oxidized with PCC furnishing rac-9 in 79% yield.
Scheme 1: Synthesis of the racemic ketone rac-9. i) NH4OAc, HOAc, Δ, 4 d, 100%; ii) LiAlH4, THF, Δ, 1 d, 87% [24]; iii) Boc2O, CH2Cl2, rt, 16 h, 85%; iv) NaBH4, Me2SO4, THF, rt, 6 h, then NaOH, H2O2, Δ, 90 min, 75%; v) PCC, Celite®, CH2Cl2, rt, 16 h, 79%.
Scheme 1: Synthesis of the racemic ketone rac-9. i) NH4OAc, HOAc, Δ, 4 d, 100%; ii) LiAlH4, THF, Δ, 1 d, 87% [24]...
The asymmetric synthesis of the ketone 9 was realized by enantioselective hydration of the meso-alkene 11 using Hayashi’s method (Scheme 2) [25-30]: Hydrosilylation with trichlorosilane in the presence of a catalytic amount of [Pd(C3H5)Cl]2 and (R)-MOP [(R)-2-diphenylphosphino-2′-methoxy-1,1′-binaphthyl], followed by SiCl3/OH exchange, delivered the exo-alcohol 12 in 81% yield and 85% ee, as determined from the (S)- and (R)-Mosher esters of 12. After oxidation (see Scheme 1), the chiral ketone 9 was thus available in overall five steps and 47% yield from 10. The X-ray crystal structure of 9 is shown in Figure 3.
Scheme 2: Enantioselective hydrosilylation/oxidation of 11. i) HSiCl3, [Pd(C3H5)Cl]2 (0.06 mol %), (R)-MOP (0.25 mol %), toluene, rt, 3 d, then evaporate, then KF, KHCO3, H2O2, THF/MeOH, rt, 1 d, 81%.
Scheme 2: Enantioselective hydrosilylation/oxidation of 11. i) HSiCl3, [Pd(C3H5)Cl]2 (0.06 mol %), (R)-MOP (0...
![[1860-5397-5-81-3]](/bjoc/content/figures/1860-5397-5-81-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: X-ray crystal structure of 9. X-ray data have been deposited with the Cambridge Crystallographic Data Centre (CCDC 743050).
Figure 3: X-ray crystal structure of 9. X-ray data have been deposited with the Cambridge Crystallographic Da...
Initial studies on the installation of the functionalized C1 side chain, as required for the amino acid derivatives 7, were done on racemic material and aimed at an oxidation of the alkene rac-13 (Scheme 3), which was available from the ketone rac-9 either by Wittig reaction or by a Tebbe-type olefination [31] using Mg, TiCl4, and CH2Cl2. Hydroboration/oxidation of rac-13 occurred highly diastereoselectively on the exo-side providing the desired endo-alcohol rac-14, as determined by NOE measurements. Further oxidation with PCC gave the aldehyde rac-15, albeit in low 13% overall yield from rac-9. As an alternative, the epoxidation of rac-13 with MCPBA was investigated, which delivered the spirocyclic exo-configured epoxide rac-16 in 46% overall yield from rac-9 as the sole diastereomer. Lewis acid-catalyzed rearrangement of rac-16 with BF3 etherate [32] furnished the desired aldehyde rac-15 in 26% yield (12% overall yield from rac-9) and the tetracyclic N,O-acetal rac-17 in 35% yield. The latter compound is presumably formed from rac-15 by a Lewis acid-catalyzed, intramolecular, and thus proximity-facilitated tandem hydride transfer/cyclization sequence [33].
Scheme 3: Initial route to the aldehyde rac-15. i) MePPh3+Br−, t-BuOK, toluene, Δ, 7 h, 77% or Mg, TiCl4, CH2Cl2, 0 °C → rt, 2 h, 55%; ii) NaBH4, Me2SO4, THF, 0 °C → rt, 18 h, then NaOH, H2O2, rt, 3 h, 34%; iii) PCC, CH2Cl2, rt, 6 h, 51%; iv) MCPBA, CH2Cl2, rt, 3 h, 60%; v) BF3•OEt2, toluene, 0 °C, 5 min, 35% (rac-17) and 26% (rac-15).
Scheme 3: Initial route to the aldehyde rac-15. i) MePPh3+Br−, t-BuOK, toluene, Δ, 7 h, 77% or Mg, TiCl4, CH2...
Since the yields of rac-15 from the alkene rac-13 were low, we turned our attention to an alternative approach via the enol ether 18, which was available from 9 as a 1:1 mixture of E/Z-isomers by Wittig reaction with MeOCH=PPh3 (Scheme 4). The selective hydrolysis of the enol ether moiety in 18 in the presence of the N-Boc-protective group was achieved by using trichloroacetic acid. The desired endo-configured aldehyde 15 was thus available in only two steps in good 64% overall yield from 9. After oxidation of 15 to the acid 19, the target amino acid 7a•HCl was obtained by N-deprotection with aqueous HCl in overall four steps and 38% yield from 9. The N-tosylamide 7b•HCl was accessed from 19 by condensation with TsNH2 under Steglich conditions followed by N-deprotection with ethereal HCl (overall five steps and 12% yield from 9).
Scheme 4: Assembly of the amino acid 7a•HCl and the N-tosylamide 7b•HCl. i) MeOCH2PPh3+Cl−, t-BuOK, toluene/THF, rt, 1 d, 84%; ii) Cl3CCO2H, H2O, CH2Cl2, rt, 1.5 h, 76%; iii) NaClO2, H2O2, KH2PO4, H2O/MeCN, rt, 6 h, 75%; iv) HCl, H2O, Δ, 1 d, 79%; v) TsNH2, DCC, DMAP, CH2Cl2, rt, 1 d, 64%; vi) HCl, Et2O, MeOH, rt, 3 h, 38%.
Scheme 4: Assembly of the amino acid 7a•HCl and the N-tosylamide 7b•HCl. i) MeOCH2PPh3+Cl−, t-BuOK, toluene/T...
The preparation of the amino acid 8a•HCl and the N-tosylamide 8b•HCl required the attachment of an endo-oriented acetic acid substituent at the position of the keto group in 9 (Scheme 5). Initial attempts to introduce such a side chain by Wittig or Horner-Wadsworth-Emmons reactions, for example with MeO2CCH=PPh3 or MeO2CCH2P(O)(OEt)2/n-BuLi, failed. By contrast, Peterson-type olefination using TMSCH2CO2Et/LDA cleanly afforded the α,β-unsaturated ester 20 as a 77:23 mixture of the E/Z-isomers in 50% yield. The reduction of the conjugated double bond with Mg in methanol furnished, after saponification, the endo-configured acid 21 as a single diastereomer. The further conversion of 21 into the target molecules was carried out in analogy to the preparation of 7a/b•HCl from 19 (see Scheme 4), giving 8a•HCl in overall 24% yield from 9 (four steps) and 8b•HCl in overall 10% yield (five steps). The required endo-orientation of the acetic acid moiety in 8a•HCl was confirmed by the X-ray structure of the corresponding free base 8a•MeOH (Figure 4).
Scheme 5: Preparation of the amino acid 8a•HCl and the N-tosylamide 8b•HCl. i) TMSCH2CO2Et, LDA, THF, −78 °C → rt, 19 h, 50%; ii) Mg, MeOH, rt, 16 h, 76%; iii) KOH, EtOH/H2O, Δ, 1 d, 90%; iv) HCl, H2O, Δ, 1 d, 71%; v) TsNH2, DCC, DMAP, CH2Cl2, rt, 4 d, 72%; vi) HCl, Et2O, rt, 20 h, 42%.
Scheme 5: Preparation of the amino acid 8a•HCl and the N-tosylamide 8b•HCl. i) TMSCH2CO2Et, LDA, THF, −78 °C ...
![[1860-5397-5-81-4]](/bjoc/content/figures/1860-5397-5-81-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: X-ray crystal structure of 8a•MeOH. X-ray data have been deposited with the Cambridge Crystallographic Data Centre (CCDC 742656).
Figure 4: X-ray crystal structure of 8a•MeOH. X-ray data have been deposited with the Cambridge Crystallograp...
A first evaluation of the bowl-shaped amino acid derivatives 7 and 8 in standard organocatalytic aldol and Mannich reactions showed that these compounds are capable of promoting these reactions, albeit with low yields and enantioselectivities. Further investigations on this issue and on the use of 7 and 8 as β-turns are in progress.
Conclusion
The enantioselective syntheses of the bowl-shaped, tricyclic amino acids and N-tosylamides 7 and 8 were successfully accomplished in 9–10 steps starting with inexpensive endo-carbic anhydride (10). The key stereochemical step was the desymmetrization of the meso-alkene 11 using Hayashi’s hydrosilylation/oxidation procedure, which provided the endo-alcohol 12 in 85% ee. The target molecules are promising candidates as ß-turn-inducing building blocks in peptidomimetics and as chiral auxiliaries in organocatalysis.
Supporting Information
| Supporting Information File 1: Full experimental details and characterization data for all new compounds. | ||
| Format: PDF | Size: 208.6 KB | Download |
| Supporting Information File 2: NMR spectra of all new compounds. | ||
| Format: PDF | Size: 520.5 KB | Download |
| Supporting Information File 3: Crystallographic data of the compounds 8a•MeOH and 9. | ||
| Format: PDF | Size: 191.8 KB | Download |
References
-
Hill, D. J.; Mio, M. J.; Prince, R. B.; Hughes, T. S.; Moore, J. S. Chem. Rev. 2001, 101, 3893–4011. doi:10.1021/cr990120t
Return to citation in text: [1] -
Komarov, I. V.; Grigorenko, A. O.; Turov, A. V.; Khilya, V. P. Russ. Chem. Rev. 2004, 73, 785–810. doi:10.1070/RC2004v073n08ABEH000912
Return to citation in text: [1] -
Gardiner, J.; Abell, A. D. Org. Biomol. Chem. 2004, 2, 2365–2370. doi:10.1039/b406450j
Return to citation in text: [1] -
Cativiela, C.; Díaz-de-Villegas, M. D. Tetrahedron: Asymmetry 2000, 11, 645–732. doi:10.1016/S0957-4166(99)00565-0
Return to citation in text: [1] -
Lesma, G.; Landoni, N.; Pilati, T.; Sacchetti, A.; Silvani, A. J. Org. Chem. 2009, 74, 8098–8105. doi:10.1021/jo901480d
Return to citation in text: [1] -
Danieli, E.; Trabocchi, A.; Menchi, G.; Guarna, A. Eur. J. Org. Chem. 2007, 1659–1668. doi:10.1002/ejoc.200600650
Return to citation in text: [1] -
Dietrich, E.; Lubell, W. D. J. Org. Chem. 2003, 68, 6988–6996. doi:10.1021/jo034739d
Return to citation in text: [1] -
Ramana Rao, M. H. V.; Pinyol, E.; Lubell, W. D. J. Org. Chem. 2007, 72, 736–743. doi:10.1021/jo0616761
Return to citation in text: [1] -
Melchiorre, P.; Marigo, M.; Carlone, A.; Bartoli, G. Angew. Chem. 2008, 120, 6232–6265. doi:10.1002/ange.200705523
Angew. Chem., Int. Ed. 2008, 47, 6138–6171. doi:10.1002/anie.200705523
Return to citation in text: [1] -
Mukherjee, S.; Yang, J. W.; Hoffmann, S.; List, B. Chem. Rev. 2007, 107, 5471–5569. doi:10.1021/cr0684016
Return to citation in text: [1] -
Tanaka, F.; Barbas, C. F., III. Aldol and Mannich-Type Reactions. In Enantioselective Organocatalysis; Dalko, P. I., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp 19–55.
Return to citation in text: [1] -
Marigo, M.; Jørgensen, K. A. Chem. Commun. 2006, 2001–2011. doi:10.1039/b517090g
Return to citation in text: [1] -
List, B. Chem. Commun. 2006, 819–824. doi:10.1039/b514296m
Return to citation in text: [1] -
Notz, W.; Tanaka, F.; Barbas, C. F., III. Acc. Chem. Res. 2004, 37, 580–591. doi:10.1021/ar0300468
Return to citation in text: [1] -
List, B. Tetrahedron 2002, 58, 5573–5590. doi:10.1016/S0040-4020(02)00516-1
Return to citation in text: [1] -
Jarvo, E. R.; Miller, S. J. Tetrahedron 2002, 58, 2481–2495. doi:10.1016/S0040-4020(02)00122-9
Return to citation in text: [1] -
List, B. Synlett 2001, 1675–1686. doi:10.1055/s-2001-18074
Return to citation in text: [1] -
Zhang, H.; Mifsud, M.; Tanaka, F.; Barbas, C. F., III. J. Am. Chem. Soc. 2006, 128, 9630–9631. doi:10.1021/ja062950b
Return to citation in text: [1] -
Zhang, H.; Mitsumori, S.; Utsumi, N.; Imai, M.; Garcia-Delgado, N.; Mifsud, M.; Albertshofer, K.; Cheong, P. H.-Y.; Houk, K. N.; Tanaka, F.; Barbas, C. F., III. J. Am. Chem. Soc. 2008, 130, 875–886. doi:10.1021/ja074907+
Return to citation in text: [1] -
Liu, J.; Yang, Z.; Wang, Z.; Wang, F.; Chen, X.; Liu, X.; Feng, X.; Su, Z.; Hu, C. J. Am. Chem. Soc. 2008, 130, 5654–5655. doi:10.1021/ja800839w
Return to citation in text: [1] -
Kano, T.; Takai, J.; Tokuda, O.; Maruoka, K. Angew. Chem. 2005, 117, 3115–3117. doi:10.1002/ange.200500408
Angew. Chem., Int. Ed. 2005, 44, 3055–3057. doi:10.1002/anie.200500408
Return to citation in text: [1] -
Kano, T.; Tokuda, O.; Takai, J.; Maruoka, K. Chem.–Asian J. 2006, 1, 210–215. doi:10.1002/asia.200600077
Return to citation in text: [1] -
Kano, T.; Maruoka, K. Chem. Commun. 2008, 5465–5473. doi:10.1039/b809301f
Return to citation in text: [1] -
Michaelis, S.; Blechert, S. Chem.–Eur. J. 2007, 13, 2358–2368. doi:10.1002/chem.200601183
Return to citation in text: [1] [2] -
Hayashi, T. Acc. Chem. Res. 2000, 33, 354–362. doi:10.1021/ar990080f
Return to citation in text: [1] -
Hayashi, T. Catal. Today 2000, 62, 3–15. doi:10.1016/S0920-5861(00)00404-1
Return to citation in text: [1] -
Hayashi, T. Catal. Surv. Jpn. 1999, 3, 127–135. doi:10.1023/A:1019075719151
Return to citation in text: [1] -
Hayashi, T. Acta Chem. Scand. 1996, 50, 259–266. doi:10.3891/acta.chem.scand.50-0259
Return to citation in text: [1] -
Hayashi, T.; Uozumi, Y. Pure Appl. Chem. 1992, 64, 1911–1916. doi:10.1351/pac199264121911
Return to citation in text: [1] -
Uozumi, Y.; Lee, S.-Y.; Hayashi, T. Tetrahedron Lett. 1992, 33, 7185–7188. doi:10.1016/S0040-4039(00)60868-7
Return to citation in text: [1] -
Yan, T.-H.; Tsai, C.-C.; Chien, C.-T.; Cho, C.-C.; Huang, P.-C. Org. Lett. 2004, 6, 4961–4963. doi:10.1021/ol0478887
Return to citation in text: [1] -
Basabe, P.; Delgado, S.; Marcos, I. S.; Diez, D.; Diego, A.; De Román, M.; Urones, J. G. J. Org. Chem. 2005, 70, 9480–9485. doi:10.1021/jo0515529
Return to citation in text: [1] -
Pastine, S. J.; Sames, D. Org. Lett. 2005, 7, 5429–5431. doi:10.1021/ol0522283
Return to citation in text: [1]
| 1. | Hill, D. J.; Mio, M. J.; Prince, R. B.; Hughes, T. S.; Moore, J. S. Chem. Rev. 2001, 101, 3893–4011. doi:10.1021/cr990120t |
| 2. | Komarov, I. V.; Grigorenko, A. O.; Turov, A. V.; Khilya, V. P. Russ. Chem. Rev. 2004, 73, 785–810. doi:10.1070/RC2004v073n08ABEH000912 |
| 3. | Gardiner, J.; Abell, A. D. Org. Biomol. Chem. 2004, 2, 2365–2370. doi:10.1039/b406450j |
| 4. | Cativiela, C.; Díaz-de-Villegas, M. D. Tetrahedron: Asymmetry 2000, 11, 645–732. doi:10.1016/S0957-4166(99)00565-0 |
| 9. |
Melchiorre, P.; Marigo, M.; Carlone, A.; Bartoli, G. Angew. Chem. 2008, 120, 6232–6265. doi:10.1002/ange.200705523
Angew. Chem., Int. Ed. 2008, 47, 6138–6171. doi:10.1002/anie.200705523 |
| 10. | Mukherjee, S.; Yang, J. W.; Hoffmann, S.; List, B. Chem. Rev. 2007, 107, 5471–5569. doi:10.1021/cr0684016 |
| 11. | Tanaka, F.; Barbas, C. F., III. Aldol and Mannich-Type Reactions. In Enantioselective Organocatalysis; Dalko, P. I., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp 19–55. |
| 12. | Marigo, M.; Jørgensen, K. A. Chem. Commun. 2006, 2001–2011. doi:10.1039/b517090g |
| 13. | List, B. Chem. Commun. 2006, 819–824. doi:10.1039/b514296m |
| 14. | Notz, W.; Tanaka, F.; Barbas, C. F., III. Acc. Chem. Res. 2004, 37, 580–591. doi:10.1021/ar0300468 |
| 15. | List, B. Tetrahedron 2002, 58, 5573–5590. doi:10.1016/S0040-4020(02)00516-1 |
| 16. | Jarvo, E. R.; Miller, S. J. Tetrahedron 2002, 58, 2481–2495. doi:10.1016/S0040-4020(02)00122-9 |
| 17. | List, B. Synlett 2001, 1675–1686. doi:10.1055/s-2001-18074 |
| 7. | Dietrich, E.; Lubell, W. D. J. Org. Chem. 2003, 68, 6988–6996. doi:10.1021/jo034739d |
| 8. | Ramana Rao, M. H. V.; Pinyol, E.; Lubell, W. D. J. Org. Chem. 2007, 72, 736–743. doi:10.1021/jo0616761 |
| 6. | Danieli, E.; Trabocchi, A.; Menchi, G.; Guarna, A. Eur. J. Org. Chem. 2007, 1659–1668. doi:10.1002/ejoc.200600650 |
| 32. | Basabe, P.; Delgado, S.; Marcos, I. S.; Diez, D.; Diego, A.; De Román, M.; Urones, J. G. J. Org. Chem. 2005, 70, 9480–9485. doi:10.1021/jo0515529 |
| 5. | Lesma, G.; Landoni, N.; Pilati, T.; Sacchetti, A.; Silvani, A. J. Org. Chem. 2009, 74, 8098–8105. doi:10.1021/jo901480d |
| 33. | Pastine, S. J.; Sames, D. Org. Lett. 2005, 7, 5429–5431. doi:10.1021/ol0522283 |
| 24. | Michaelis, S.; Blechert, S. Chem.–Eur. J. 2007, 13, 2358–2368. doi:10.1002/chem.200601183 |
| 25. | Hayashi, T. Acc. Chem. Res. 2000, 33, 354–362. doi:10.1021/ar990080f |
| 26. | Hayashi, T. Catal. Today 2000, 62, 3–15. doi:10.1016/S0920-5861(00)00404-1 |
| 27. | Hayashi, T. Catal. Surv. Jpn. 1999, 3, 127–135. doi:10.1023/A:1019075719151 |
| 28. | Hayashi, T. Acta Chem. Scand. 1996, 50, 259–266. doi:10.3891/acta.chem.scand.50-0259 |
| 29. | Hayashi, T.; Uozumi, Y. Pure Appl. Chem. 1992, 64, 1911–1916. doi:10.1351/pac199264121911 |
| 30. | Uozumi, Y.; Lee, S.-Y.; Hayashi, T. Tetrahedron Lett. 1992, 33, 7185–7188. doi:10.1016/S0040-4039(00)60868-7 |
| 21. |
Kano, T.; Takai, J.; Tokuda, O.; Maruoka, K. Angew. Chem. 2005, 117, 3115–3117. doi:10.1002/ange.200500408
Angew. Chem., Int. Ed. 2005, 44, 3055–3057. doi:10.1002/anie.200500408 |
| 22. | Kano, T.; Tokuda, O.; Takai, J.; Maruoka, K. Chem.–Asian J. 2006, 1, 210–215. doi:10.1002/asia.200600077 |
| 23. | Kano, T.; Maruoka, K. Chem. Commun. 2008, 5465–5473. doi:10.1039/b809301f |
| 31. | Yan, T.-H.; Tsai, C.-C.; Chien, C.-T.; Cho, C.-C.; Huang, P.-C. Org. Lett. 2004, 6, 4961–4963. doi:10.1021/ol0478887 |
| 20. | Liu, J.; Yang, Z.; Wang, Z.; Wang, F.; Chen, X.; Liu, X.; Feng, X.; Su, Z.; Hu, C. J. Am. Chem. Soc. 2008, 130, 5654–5655. doi:10.1021/ja800839w |
| 18. | Zhang, H.; Mifsud, M.; Tanaka, F.; Barbas, C. F., III. J. Am. Chem. Soc. 2006, 128, 9630–9631. doi:10.1021/ja062950b |
| 19. | Zhang, H.; Mitsumori, S.; Utsumi, N.; Imai, M.; Garcia-Delgado, N.; Mifsud, M.; Albertshofer, K.; Cheong, P. H.-Y.; Houk, K. N.; Tanaka, F.; Barbas, C. F., III. J. Am. Chem. Soc. 2008, 130, 875–886. doi:10.1021/ja074907+ |
| 24. | Michaelis, S.; Blechert, S. Chem.–Eur. J. 2007, 13, 2358–2368. doi:10.1002/chem.200601183 |
© 2009 Breuning et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)