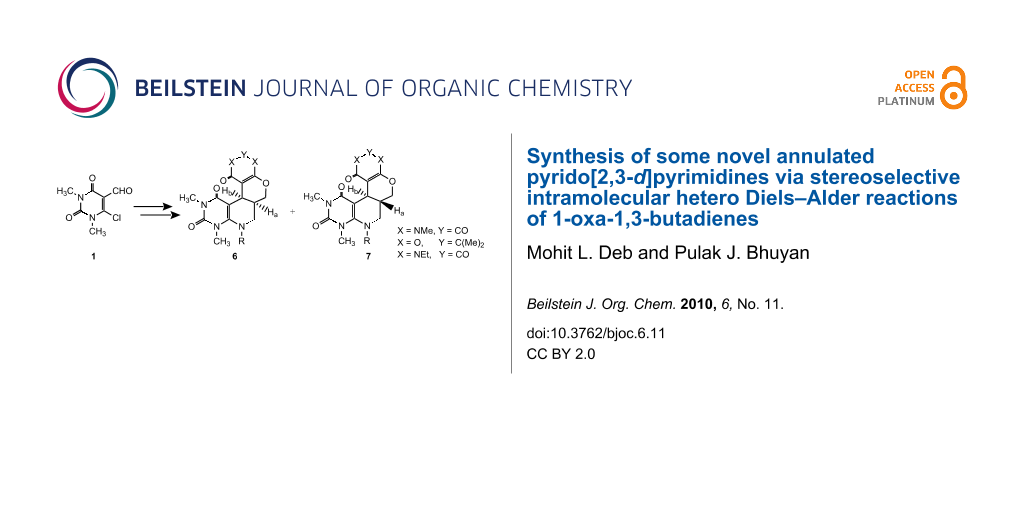
Abstract
Some novel annulated pyrido[2,3-d]pyrimidines 6 and 7 were synthesized stereoselectively by intramolecular hetero Diels–Alder reactions involving 1-oxa-1,3-butadienes.
Graphical Abstract
Introduction
The importance of uracil and its annulated derivatives is well recognized by synthetic as well as biological chemists [1-8]. Pyrido[2,3-d]pyrimidines represent a broad class of annelated uracils which have received considerable attention over the past years due to their wide range of biological activities such as antibacterial [9,10], antitumor [11,12], cardiotonic [13,14], hepatoprotective [13], antihypertensive [13], bronchiodilator [15] and vasodilator [16] properties. Additionally, some compounds of this type exhibit antialergic [17], antimalarial [18], analgesic [19,20] and antifungal [21] activity. Consequently, much effort has been directed towards the synthetic manipulation of uracil for the preparation of these complex molecules. However, there still remains many challenges in the synthesis of these naturally occurring complex molecules [22-31].
Hetero Diels–Alder reactions [32-35] are becoming a mainstay of heterocyclic and natural product synthesis. This powerful reaction method does not only allow the efficient synthesis of complex compounds starting from simple substrates but also permits the preparation of highly diversified molecules. One such reaction type, the oxabutadiene Diels–Alder reaction is a very useful method for the synthesis of dihydropyrans [36-38].
Heterocyclic β-halo aldehydes are very interesting compounds which can be transformed in a number of ways to fused heterocycles [39,40] by using the reactivity of halide for nucleophilic substitution in combination with a multitude of transformation possibilities from the aldehyde function.
Results and Discussion
As part of our continued interest in uracils [41-45] and the development of highly expedient methods for the synthesis of diverse heterocyclic compounds of biological importance [46-50], we now report the stereoselective synthesis of some new complex annulated pyrido[2,3-d]pyrimidines by intramolecular hetero Diels–Alder reactions involving 1-oxa-1,3-butadienes (Scheme 1).
Scheme 1: Stereoselective synthesis of some new complex annulated pyrido[2,3-d]pyrimidines by intramolecular hetero Diels–Alder reactions involving 1-oxa-1,3-butadienes.
Scheme 1: Stereoselective synthesis of some new complex annulated pyrido[2,3-d]pyrimidines by intramolecular ...
The key intermediate, the 2-chloro-3-formyl uracil derivative 1 (β-halo aldehyde), was prepared by the reaction of N,N-dimethyl barbituric acid with Vielsmeier reagent (DMF + POCl3) using excess phosphorous oxychloride as solvent following our published method [51]. The nucleophilic substitution of the chloro group of 1 by allyl amines 2 afforded the 6-N-allyl-1,3-dimethyl-5-formyl uracils 3. Compounds 3 were then reacted with cyclic β-diamides/β-diketones 4 in presence of a base catalyst (usually piperidine) to produce the 1-oxa-1,3-butadienes 5 which underwent intramolecular Diels–Alder reaction under reflux conditions in toluene (12 to 15 h) to give the cycloadducts. In most of cases the formation of two compounds was observed. The compounds were separated by column chromatography and their structures were determined from their spectroscopic data as the cis-(6) and trans-(7) isomers of the cycloadduct. The generality of the reaction was established by synthesizing a series of tetracyclic annulated uracil derivatives 6a–h and 7a–e. Our results are recorded in Table 1.
Table 1: Synthesis of some novel annulated pyrido[2,3-d]pyrimidines 6/7.a
| Entry | Allyl amines (2) | Compound (4) | Product yieldb | Reaction time (h) |
cis : trans
6 : 7 |
|---|---|---|---|---|---|
| 1 |
|
|
6a + 7a (64%) | 15 | 78 : 22 |
| 2 |
|
|
6b + 7b (55%) | 12 | 84 : 16 |
| 3 |
|
|
6c + 7c (62%) | 16 | 78 : 22 |
| 4 |
|
|
6d + 7d (50%) | 12 | 88 : 12 |
| 5 |
|
|
6e + 7e (60%) | 16 | 76 : 24 |
| 6 |
|
|
6f (62%) | 15 | 100 : 0 |
| 7 |
|
|
6g (60%) | 16 | 100 : 0 |
| 8 |
|
|
6h (57%) | 16 | 100 : 0 |
aAll the reactions were carried out under reflux conditions.
bIsolated yields.
In all of the reactions the cis-annelated products were formed either predominantly or exclusively. As the oxabutadiene is part of a cyclic compound and two carbon atoms to which the dienophile is attached are part of a ring system, the endo-transition state is energetically more favorable than the exo-transition state, and thus the cis-cycloadducts were formed predominantly (entries 1–5). In case of entries 6–8, the bulky ethyl group further favors the endo-transition and in these cases cis-annelated products were formed exclusively. In some of our previous efforts, we also obtained either cis-isomers or the mixtures of both the cis- and trans-isomers [52,53].
When N-methylallylamine (2b) or N-benzylallylamine (2c) was used for the reaction as shown in Scheme 1, the Knoevenagel condensation did not occur even after refluxing in the presence of piperidine. The reason might be the delocalization of the lone pair on the allyl nitrogen through the formyl group which makes it less electrophilic towards the active methylene compounds. However, the use of a stronger base, e.g. N,N-diisopropylethylamine (DIPEA) under refluxing conditions in ethanol for 3 h gave quite satisfactory results in all cases. In the case of N-phenylallylamine substituted uracils the condensation reactions proceeded normally in the presence of either of the bases.
Conclusion
In summary, we report the stereoselective preparation of some new complex tetracyclic annulated uracil derivatives by intramolecular hetero Diels–Alder reactions involving 1-oxa-1,3-butadiene. This reaction, which can also be investigated for the synthesis of many other heyterocyclic compounds of biological importance, is a valuable addition to the chemistry of uracils.
Experimental
All reagents and solvents were of reagent grade and were used without drying. The IR spectra were recorded on a Perkin Elmer system-2000 FTIR spectrometer. 1H NMR and 13C NMR spectra were recorded on Bruker Avance-DPX 300 MHz and 75 MHz FT NMR in CDCl3 using TMS as the internal standard. LR-MS were recorded on a Bruker Daltonics ESQUIRE 3000 LC ESI ion trap mass spectrometer and HRMS were obtained with a MALDI-TOF instrument. Elemental analyses were performed on a Perkin Elmer-2400 spectrometer. Analytical TLC and column chromatography were performed using E. Merck aluminum-backed silica gel plates coated with silica gel G and E. Merck silica gel (100–200 Mesh); melting points (uncorrected) were determined on a Büchi B-540 apparatus.
Preparation of 1,3-dimethyl-6-chloro-5-formyluracil (1): DMF (12 ml) in a 100 ml round bottomed flask was very slowly treated with phosphorous oxychloride (46 ml) with cooling after the addition of every 1 ml portion of POCl3. 1,3-Dimethylbarbituric acid (4 g) was added and the mixture heated under reflux for 1 h. Excess POCl3 was removed under reduced pressure. The viscous mixture was poured into ice-cold water and then extracted with dichloromethane (two to three times). After drying with sodium sulfate, the dichloromethane was removed under reduced pressure. The brown compound obtained contained some impurities and was used without further purification.
Reaction of 1,3-dimethyl-6-chloro-5-formyluracil (1) with N-allylanilines/N-allylamines (2) and preparation of 6-amino-5-formyluracils 3: Compound 1 (2 mmol, 404 mg) dissolved in dichloromethane, was treated with an equivalent amount of N-allylaniline (2a) (2 mmol, 266 mg) and triethylamine (2 mmol), and the mixture stirred at room temperature for 2 h. The solvent was evaporated and the residue was purified by column chromatography (silica gel, 100–200 Mesh) using dichloromethane to give 3a as a yellow solid 568 mg (95%). Similarly, 3b–c were prepared from the reaction of 1 with 2b–c.
Knoevenagel condensation of 3 with cyclic β-diamide/β-diketones 4 and synthesis of 5: Equimolar amounts of 3a (2 mmol, 598 mg) and 4a (2 mmol, 312 mg) were mixed thoroughly in a round-bottomed flask containing water (8 ml). Two drops of piperidine (in case of Meldrum’s acid (4b), piperidine acetate was used) were added and the mixture stirred for 4 h. The solid was removed by filtration and recrystallized from ethanol to afford a white solid (769 mg, 88%). The compound was assigned structure 5a from a consideration of its spectroscopic data. In case of N-methylallylamine 2b and N-benzylallylamine 2c, N,N-diisopropylethylamine (DIPEA) was used as catalyst for the Knoevenagel condensation and reactions were performed under reflux in ethanol for 3 h. The method gave satisfactory yields of up to 90%. Compounds 5b–h were synthesized similarly.
Intramolecular Diels–Alder reaction of compound 5: Compound 5a (1 mmol, 437 mg) was dissolved in toluene (6 ml) and heated under reflux for 15 h. After completion of the reaction (as monitored by TLC), the solvent was removed under reduced pressure. Two products (indicated by TLC) were separated by column chromatography using 65% ethyl acetate in petroleum ether. The structures were assigned from a combination of their spectral data and elemental analysis. From the value of coupling constant, it was established that the products 6a/7a are cis-/trans-isomers, respectively. Total yield = 64% (280 mg). The other hetero Diels–Alder products 6b–h and 7b–e were synthesized similarly.
cis-isomer 6a: mp 312–314 °C. IR (KBr); 3033, 2954, 1698, 1687, 1165, 744 cm−1. 1H NMR (300 MHz, CDCl3); δ, 2.96 (s, 3H), 2.98 (s, 3H), 3.21 (s, 3H), 3.47 (s, 3H), 3.59–3.68 (m, 3H), 4.23 (d, J = 5.28 Hz, 2H), 4.39 (d, J = 6.39 Hz, 1H), 7.0 (t, 2H, J = 7.41 Hz), 7.17–7.27 (m, 3H). 13C NMR (75 MHz, CDCl3); δ, 163.21, 156.35, 153.37, 149.54, 136.91, 129.95, 123.86, 118.69, 104.32, 89.61, 51.52, 37.05, 32.05, 29.0, 28.78, 28.31, 26.18. m/z 438.2 (M + H)+. Anal. Calcd for C22H23N5O5; C, 60.41; H, 5.26; N, 16.01; found C, 60.68; H, 5.33; N, 15.87.
trans-isomer 7a: mp 317–318 °C. IR (KBr); 3033, 2954, 1698, 1687, 1165, 744 cm−1. 1H NMR (300 MHz, CDCl3); δ, 2.96 (s, 3H), 2.98 (s, 3H), 3.21 (s, 3H), 3.45 (s, 3H), 3.53–3.67 (m, 3H), 4.24 (d, J = 5.8 Hz, 2H), 4.36 (d, J = 11.7 Hz, 1H), 7.08 (t, 2H, J = 7.36 Hz), 7.17–7.27 (m, 3H). 13C NMR (75 MHz, CDCl3); δ, 163.26, 156.35, 153.30, 149.47, 136.91, 129.63, 123.86, 118.65, 105.47, 89.61, 51.31, 37.43, 32.05, 29.11, 28.78, 28.31, 26.18. m/z 438.2 (M + H)+. Anal. Calcd for C22H23N5O5; C, 60.41; H, 5.26; N, 16.01; found C, 60.65; H, 5.37; N, 15.82.
Supporting Information
| Supporting Information File 1: Spectroscopic and elemental analyses data of the compounds 6b–h and 7b–e. | ||
| Format: PDF | Size: 91.0 KB | Download |
References
-
Marumoto, R.; Furukawa, Y. Chem. Pharm. Bull. 1977, 25, 2974.
Return to citation in text: [1] -
Griengl, H.; Wack, E.; Schwarz, W.; Streicher, W.; Rosenwirth, B.; De Clercq, E. J. Med. Chem. 1987, 30, 1199. doi:10.1021/jm00390a013
Return to citation in text: [1] -
De Clercq, E.; Bernaerts, R. J. Biol. Chem. 1987, 262, 14905.
Return to citation in text: [1] -
Jones, A. S.; Sayers, J. R.; Walker, R. T.; De Clercq, E. J. Med. Chem. 1988, 31, 268. doi:10.1021/jm00396a043
Return to citation in text: [1] -
Mitsuya, H.; Yarchoan, R.; Broder, S. Science 1990, 249, 1533. doi:10.1126/science.1699273
Return to citation in text: [1] -
Pontikis, R.; Monneret, C. Tetrahedron Lett. 1994, 35, 4351. doi:10.1016/S0040-4039(00)73352-1
Return to citation in text: [1] -
Wamhoff, H.; Winfried, S. J. Org. Chem. 1986, 51, 2787. doi:10.1021/jo00364a032
Return to citation in text: [1] -
Hirota, K.; Banno, K.; Yamada, Y.; Senda, S. J. Chem. Soc., Perkin Trans. 1 1985, 1137. doi:10.1039/P19850001137
Return to citation in text: [1] -
Gavrilov, M. Y.; Novoseleva, G. N.; Vakhrin, M. I.; Konshin, M. E. Khim.-Farm. Zh. 1996, 30, 39.
Return to citation in text: [1] -
Ghorab, M. M.; Hassan, A. Y. Phosphorus, Sulfur Silicon Relat. Elem. 1998, 141, 251. doi:10.1080/10426509808033737
Return to citation in text: [1] -
Broom, A. D.; Anderson, G. L.; Shim, J. L. J. Org. Chem. 1976, 41, 1095. doi:10.1021/jo00869a003
Return to citation in text: [1] -
Grivsky, E. M.; Lee, S.; Sigel, C. W.; Duch, D. S.; Nichol, C. A. J. Med. Chem. 1980, 23, 327. doi:10.1021/jm00177a025
Return to citation in text: [1] -
Furuya, S.; Ohtaki, T. Pyridopyrimidine derivatives, their production and use.. Eur. Pat. Appl., EP 0 608565 A1, Aug 3, 1994.
Chem. Abstr. 1994, 121, 205395.
Return to citation in text: [1] [2] [3] -
Heber, D.; Heers, C.; Ravens, U. Pharmazie 1993, 48, 537.
Return to citation in text: [1] -
Sakuma, Y.; Hasegawa, M.; Kataoka, K.; Hoshina, K.; Yamazaki, N.; Kadota, T.; Yamaguchi, H. 1,10-Phenanthroline Derivatives.. PCT Int. Appl. WO 91/05785, May 2, 1989.
Chem. Abstr. 1991, 115, 71646.
Return to citation in text: [1] -
Coates, W. Pyrimidopyrimidine Derivatives.. J. Eur. Pat. 0 351058 A1, Jan 17, 1990.
Chem. Abstr. 1990, 113, 40711.
Return to citation in text: [1] -
Bennett, L. R.; Blankley, C. J.; Fleming, R. W.; Smith, R. D.; Tessonam, D. K. J. Med. Chem. 1981, 24, 382. doi:10.1021/jm00136a006
Return to citation in text: [1] -
Davoll, J.; Clarke, J.; Elslager, E. F. J. Med. Chem. 1972, 15, 837. doi:10.1021/jm00278a009
Return to citation in text: [1] -
Kretzschmer, E. Pharmazie 1980, 35, 253.
Return to citation in text: [1] -
Shigo, S.; Hiroshi, I. Yakugaku Zasshi 1969, 89, 266.
Return to citation in text: [1] -
Ahluwalia, V. K.; Bhatla, R.; Khurana, A.; Kumar, R. Indian J. Chem., Sect. B 1990, 29, 1141.
Return to citation in text: [1] -
Cheng, T.; Wang, Y.; Cai, M. Youji Huaxue 1988, 8, 250.
Return to citation in text: [1] -
Spada, M. R.; Klein, R. S.; Otter, B. A. J. Heterocycl. Chem. 1989, 26, 1851. doi:10.1002/jhet.5570260659
Return to citation in text: [1] -
Ahluwalia, V. K.; Kumar, R.; Khurana, K.; Bhatla, R. Tetrahedron 1990, 46, 3953. doi:10.1016/S0040-4020(01)90530-7
Return to citation in text: [1] -
Ahluwalia, V. K.; Bhatla, R.; Khurana, A.; Kumar, R. Indian J. Chem., Sect. B 1990, 29, 1141.
Return to citation in text: [1] -
Ahluwalia, V. K.; Sharma, H. R.; Tyagi, R. Tetrahedron 1986, 42, 4045. doi:10.1016/S0040-4020(01)87560-8
Return to citation in text: [1] -
Ahluwalia, V. K.; Aggarwal, R.; Alauddin, M.; Gill, G.; Khanduri, C. H. Heterocycles 1990, 31, 129. doi:10.3987/COM-89-5194
Return to citation in text: [1] -
Broom, A. D.; Shim, J. L.; Anderson, C. L. J. Org. Chem. 1976, 411, 1095. doi:10.1021/jo00869a003
Return to citation in text: [1] -
Wamhoff, H.; Muhr, J. Synthesis 1988, 11, 919. doi:10.1055/s-1988-27756
Return to citation in text: [1] -
Hirota, K.; Kuki, H.; Maki, Y. Heterocycles 1994, 37, 563. doi:10.3987/COM-93-S99
Return to citation in text: [1] -
Srivastava, P.; Saxena, A. S.; Ram, V. J. Synthesis 2000, 541. doi:10.1055/s-2000-6371
Return to citation in text: [1] -
Daly, J. W.; Spande, T. F. Amphibian Alkaloids: Chemistry, Pharmacology and Biology. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; Wiley: New York, 1986; Vol. 4, p 1.
Return to citation in text: [1] -
Foder, G. B.; Colasanti, B. The Pyridine and Piperidine Alkaloids: Chemistry and Pharmacology. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; Wiley: New York, 1985; Vol. 3, p 1.
Return to citation in text: [1] -
Boger, D.; Weinreb, S. M. Hetero Diels–Alder Methodology in Organic Synthesis; Academic Press: San Diego, 1987.
Chapter 2, p 34.
Return to citation in text: [1] -
Buomora, P.; Olsen, J. C.; Oh, T. Tetrahedron 2001, 57, 6099. doi:10.1016/S0040-4020(01)00438-0
Return to citation in text: [1] -
Tietze, L. F.; Rackelmann, N. Pure Appl. Chem. 2004, 76, 1967. doi:10.1351/pac200476111967
Return to citation in text: [1] -
Khoshkholgh, M. J.; Lotfi, M.; Balalaie, S.; Rominger, F. Tetrahedron 2009, 65, 4228. doi:10.1016/j.tet.2009.03.032
Return to citation in text: [1] -
Lee, Y. R.; Kim, Y. M.; Kim, S. H. Tetrahedron 2009, 65, 101. doi:10.1016/j.tet.2008.10.101
Return to citation in text: [1] -
Brahma, S.; Ray, J. K. Tetrahedron 2008, 64, 2883. doi:10.1016/j.tet.2007.11.112
Return to citation in text: [1] -
Ceulemans, E.; Voets, M.; Emmers, S.; Uytterhoeven, K.; Meervelt, L. V.; Dehaen, W. Tetrahedron 2002, 58, 531. doi:10.1016/S0040-4020(01)01153-X
Return to citation in text: [1] -
Devi, I.; Borah, H. N.; Bhuyan, P. J. Tetrahedron Lett. 2004, 45, 2405. doi:10.1016/j.tetlet.2004.01.094
Return to citation in text: [1] -
Devi, I.; Bhuyan, P. J. Tetrahedron Lett. 2004, 45, 8625. doi:10.1016/j.tetlet.2004.09.158
Return to citation in text: [1] -
Devi, I.; Bhuyan, P. J. Synlett 2004, 2, 283. doi:10.1055/s-2003-43367
Return to citation in text: [1] -
Devi, I.; Bhuyan, P. J. Tetrahedron Lett. 2005, 46, 5727. doi:10.1016/j.tetlet.2005.06.075
Return to citation in text: [1] -
Baruah, B.; Bhuyan, P. J. Tetrahedron Lett. 2009, 50, 243. doi:10.1016/j.tetlet.2008.10.125
Return to citation in text: [1] -
Baruah, B.; Deb, M. L.; Bhuyan, P. J. Synlett 2007, 12, 1873. doi:10.1055/s-2007-982568
Return to citation in text: [1] -
Deb, M. L.; Bhuyan, P. J. Synlett 2008, 3, 325. doi:10.1055/s-2008-1032052
Return to citation in text: [1] -
Deb, M. L.; Bhuyan, P. J. Synthesis 2008, 18, 2891. doi:10.1055/s-2008-1067217
Return to citation in text: [1] -
Deb, M. L.; Baruah, B.; Bhuyan, P. J. Synthesis 2007, 2, 286. doi:10.1055/s-2008-1000849
Return to citation in text: [1] -
Deb, M. L.; Bhuyan, P. J. Tetrahedron Lett. 2005, 46, 6453. doi:10.1016/j.tetlet.2005.07.111
Return to citation in text: [1] -
Prajapati, D.; Bhuyan, P. J.; Sandhu, J. S. J. Chem. Soc., Perkin Trans. 1 1988, 607. doi:10.1039/P19880000607
Return to citation in text: [1] -
Devi, I.; Bhuyan, P. J. Tetrahedron Lett. 2004, 45, 7727. doi:10.1016/j.tetlet.2004.08.072
Return to citation in text: [1] -
Borah, H. N.; Deb, M. L.; Boruah, R. C.; Bhuyan, P. J. Tetrahedron Lett. 2005, 46, 3391. doi:10.1016/j.tetlet.2005.03.091
Return to citation in text: [1]
| 51. | Prajapati, D.; Bhuyan, P. J.; Sandhu, J. S. J. Chem. Soc., Perkin Trans. 1 1988, 607. doi:10.1039/P19880000607 |
| 41. | Devi, I.; Borah, H. N.; Bhuyan, P. J. Tetrahedron Lett. 2004, 45, 2405. doi:10.1016/j.tetlet.2004.01.094 |
| 42. | Devi, I.; Bhuyan, P. J. Tetrahedron Lett. 2004, 45, 8625. doi:10.1016/j.tetlet.2004.09.158 |
| 43. | Devi, I.; Bhuyan, P. J. Synlett 2004, 2, 283. doi:10.1055/s-2003-43367 |
| 44. | Devi, I.; Bhuyan, P. J. Tetrahedron Lett. 2005, 46, 5727. doi:10.1016/j.tetlet.2005.06.075 |
| 45. | Baruah, B.; Bhuyan, P. J. Tetrahedron Lett. 2009, 50, 243. doi:10.1016/j.tetlet.2008.10.125 |
| 46. | Baruah, B.; Deb, M. L.; Bhuyan, P. J. Synlett 2007, 12, 1873. doi:10.1055/s-2007-982568 |
| 47. | Deb, M. L.; Bhuyan, P. J. Synlett 2008, 3, 325. doi:10.1055/s-2008-1032052 |
| 48. | Deb, M. L.; Bhuyan, P. J. Synthesis 2008, 18, 2891. doi:10.1055/s-2008-1067217 |
| 49. | Deb, M. L.; Baruah, B.; Bhuyan, P. J. Synthesis 2007, 2, 286. doi:10.1055/s-2008-1000849 |
| 50. | Deb, M. L.; Bhuyan, P. J. Tetrahedron Lett. 2005, 46, 6453. doi:10.1016/j.tetlet.2005.07.111 |
| 1. | Marumoto, R.; Furukawa, Y. Chem. Pharm. Bull. 1977, 25, 2974. |
| 2. | Griengl, H.; Wack, E.; Schwarz, W.; Streicher, W.; Rosenwirth, B.; De Clercq, E. J. Med. Chem. 1987, 30, 1199. doi:10.1021/jm00390a013 |
| 3. | De Clercq, E.; Bernaerts, R. J. Biol. Chem. 1987, 262, 14905. |
| 4. | Jones, A. S.; Sayers, J. R.; Walker, R. T.; De Clercq, E. J. Med. Chem. 1988, 31, 268. doi:10.1021/jm00396a043 |
| 5. | Mitsuya, H.; Yarchoan, R.; Broder, S. Science 1990, 249, 1533. doi:10.1126/science.1699273 |
| 6. | Pontikis, R.; Monneret, C. Tetrahedron Lett. 1994, 35, 4351. doi:10.1016/S0040-4039(00)73352-1 |
| 7. | Wamhoff, H.; Winfried, S. J. Org. Chem. 1986, 51, 2787. doi:10.1021/jo00364a032 |
| 8. | Hirota, K.; Banno, K.; Yamada, Y.; Senda, S. J. Chem. Soc., Perkin Trans. 1 1985, 1137. doi:10.1039/P19850001137 |
| 13. |
Furuya, S.; Ohtaki, T. Pyridopyrimidine derivatives, their production and use.. Eur. Pat. Appl., EP 0 608565 A1, Aug 3, 1994.
Chem. Abstr. 1994, 121, 205395. |
| 36. | Tietze, L. F.; Rackelmann, N. Pure Appl. Chem. 2004, 76, 1967. doi:10.1351/pac200476111967 |
| 37. | Khoshkholgh, M. J.; Lotfi, M.; Balalaie, S.; Rominger, F. Tetrahedron 2009, 65, 4228. doi:10.1016/j.tet.2009.03.032 |
| 38. | Lee, Y. R.; Kim, Y. M.; Kim, S. H. Tetrahedron 2009, 65, 101. doi:10.1016/j.tet.2008.10.101 |
| 13. |
Furuya, S.; Ohtaki, T. Pyridopyrimidine derivatives, their production and use.. Eur. Pat. Appl., EP 0 608565 A1, Aug 3, 1994.
Chem. Abstr. 1994, 121, 205395. |
| 14. | Heber, D.; Heers, C.; Ravens, U. Pharmazie 1993, 48, 537. |
| 39. | Brahma, S.; Ray, J. K. Tetrahedron 2008, 64, 2883. doi:10.1016/j.tet.2007.11.112 |
| 40. | Ceulemans, E.; Voets, M.; Emmers, S.; Uytterhoeven, K.; Meervelt, L. V.; Dehaen, W. Tetrahedron 2002, 58, 531. doi:10.1016/S0040-4020(01)01153-X |
| 11. | Broom, A. D.; Anderson, G. L.; Shim, J. L. J. Org. Chem. 1976, 41, 1095. doi:10.1021/jo00869a003 |
| 12. | Grivsky, E. M.; Lee, S.; Sigel, C. W.; Duch, D. S.; Nichol, C. A. J. Med. Chem. 1980, 23, 327. doi:10.1021/jm00177a025 |
| 22. | Cheng, T.; Wang, Y.; Cai, M. Youji Huaxue 1988, 8, 250. |
| 23. | Spada, M. R.; Klein, R. S.; Otter, B. A. J. Heterocycl. Chem. 1989, 26, 1851. doi:10.1002/jhet.5570260659 |
| 24. | Ahluwalia, V. K.; Kumar, R.; Khurana, K.; Bhatla, R. Tetrahedron 1990, 46, 3953. doi:10.1016/S0040-4020(01)90530-7 |
| 25. | Ahluwalia, V. K.; Bhatla, R.; Khurana, A.; Kumar, R. Indian J. Chem., Sect. B 1990, 29, 1141. |
| 26. | Ahluwalia, V. K.; Sharma, H. R.; Tyagi, R. Tetrahedron 1986, 42, 4045. doi:10.1016/S0040-4020(01)87560-8 |
| 27. | Ahluwalia, V. K.; Aggarwal, R.; Alauddin, M.; Gill, G.; Khanduri, C. H. Heterocycles 1990, 31, 129. doi:10.3987/COM-89-5194 |
| 28. | Broom, A. D.; Shim, J. L.; Anderson, C. L. J. Org. Chem. 1976, 411, 1095. doi:10.1021/jo00869a003 |
| 29. | Wamhoff, H.; Muhr, J. Synthesis 1988, 11, 919. doi:10.1055/s-1988-27756 |
| 30. | Hirota, K.; Kuki, H.; Maki, Y. Heterocycles 1994, 37, 563. doi:10.3987/COM-93-S99 |
| 31. | Srivastava, P.; Saxena, A. S.; Ram, V. J. Synthesis 2000, 541. doi:10.1055/s-2000-6371 |
| 9. | Gavrilov, M. Y.; Novoseleva, G. N.; Vakhrin, M. I.; Konshin, M. E. Khim.-Farm. Zh. 1996, 30, 39. |
| 10. | Ghorab, M. M.; Hassan, A. Y. Phosphorus, Sulfur Silicon Relat. Elem. 1998, 141, 251. doi:10.1080/10426509808033737 |
| 32. | Daly, J. W.; Spande, T. F. Amphibian Alkaloids: Chemistry, Pharmacology and Biology. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; Wiley: New York, 1986; Vol. 4, p 1. |
| 33. | Foder, G. B.; Colasanti, B. The Pyridine and Piperidine Alkaloids: Chemistry and Pharmacology. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; Wiley: New York, 1985; Vol. 3, p 1. |
| 34. |
Boger, D.; Weinreb, S. M. Hetero Diels–Alder Methodology in Organic Synthesis; Academic Press: San Diego, 1987.
Chapter 2, p 34. |
| 35. | Buomora, P.; Olsen, J. C.; Oh, T. Tetrahedron 2001, 57, 6099. doi:10.1016/S0040-4020(01)00438-0 |
| 17. | Bennett, L. R.; Blankley, C. J.; Fleming, R. W.; Smith, R. D.; Tessonam, D. K. J. Med. Chem. 1981, 24, 382. doi:10.1021/jm00136a006 |
| 19. | Kretzschmer, E. Pharmazie 1980, 35, 253. |
| 20. | Shigo, S.; Hiroshi, I. Yakugaku Zasshi 1969, 89, 266. |
| 16. |
Coates, W. Pyrimidopyrimidine Derivatives.. J. Eur. Pat. 0 351058 A1, Jan 17, 1990.
Chem. Abstr. 1990, 113, 40711. |
| 21. | Ahluwalia, V. K.; Bhatla, R.; Khurana, A.; Kumar, R. Indian J. Chem., Sect. B 1990, 29, 1141. |
| 15. |
Sakuma, Y.; Hasegawa, M.; Kataoka, K.; Hoshina, K.; Yamazaki, N.; Kadota, T.; Yamaguchi, H. 1,10-Phenanthroline Derivatives.. PCT Int. Appl. WO 91/05785, May 2, 1989.
Chem. Abstr. 1991, 115, 71646. |
| 52. | Devi, I.; Bhuyan, P. J. Tetrahedron Lett. 2004, 45, 7727. doi:10.1016/j.tetlet.2004.08.072 |
| 53. | Borah, H. N.; Deb, M. L.; Boruah, R. C.; Bhuyan, P. J. Tetrahedron Lett. 2005, 46, 3391. doi:10.1016/j.tetlet.2005.03.091 |
| 13. |
Furuya, S.; Ohtaki, T. Pyridopyrimidine derivatives, their production and use.. Eur. Pat. Appl., EP 0 608565 A1, Aug 3, 1994.
Chem. Abstr. 1994, 121, 205395. |
| 18. | Davoll, J.; Clarke, J.; Elslager, E. F. J. Med. Chem. 1972, 15, 837. doi:10.1021/jm00278a009 |
© 2010 Deb and Bhuyan; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)