Abstract
Several 2-(phenylethynyl)triphenylene derivatives bearing electron donor and acceptor substituents on the phenyl rings have been synthesized. The absorption and fluorescence emission properties of these molecules have been studied in solvents of different polarity. For a given derivative, solvent polarity had minimal effect on the absorption maxima. However, for a given solvent the absorption maxima red shifted with increasing conjugation of the substituent. The fluorescence emission of these derivatives was very sensitive to solvent polarity. In the presence of strongly electron withdrawing (–CN) and strongly electron donating (–NMe2) substituents large Stokes shifts (up to 130 nm, 7828 cm−1) were observed in DMSO. In the presence of carbonyl substituents (–COMe and –COPh), the largest Stokes shift (140 nm, 8163 cm−1) was observed in ethanol. Linear correlation was observed for the Stokes shifts in a Lippert–Mataga plot. Linear correlation of Stokes shift was also observed with ET(30) scale for protic and aprotic solvents but with different slopes. These results indicate that the fluorescence emission arises from excited state intramolecular charge transfer in these molecules where the triphenylene chromophore acts either as a donor or as an acceptor depending upon the nature of the substituent on the phenyl ring. HOMO–LUMO energy gaps have been estimated from the electrochemical and spectral data for these derivatives. The HOMO and LUMO surfaces were obtained from DFT calculations.
Graphical Abstract
Introduction
Fluorescent molecular probes that emit in the visible region and whose fluorescence emission is sensitive to environment and solvent polarity are of significant interest due to their versatile applications in chemistry, biology and environmental science [1-4]. Considerable effort has been expended into shifting the fluorescence emission of organic molecules into the visible region. The most commonly employed strategy for the bathochromic shifting of the emission wavelength is to extend the conjugation of the fluorophores with aryl, ethenyl and ethynyl groups. The addition of electron donating and withdrawing substituents to these conjugation enhancing groups also helps in shifting the emission wavelengths further into red region. For example, boron-dipyrrolomethenes (BODIPYs) are a class of molecules whose absorption and fluorescence emission have been fine tuned by suitable substituents [5-7]. Fluorophores emitting in the visible region are important especially in the in vivo study of biological samples. Otherwise the background blue emission of the biological samples interferes with the fluorescence sensing. The mechanism of fluorescence sensing often involves excited state intramolecular charge transfer (ICT) [8-11], photoinduced electron transfer [12-15] and metal ion induced enhancement or quenching of fluorescence [16-19]. Among these, fluorophores that exhibit excited state ICT are very popular. In this context fluorescent donor-π spacer-acceptor (d-π-a) type molecules are of considerable interest and importance. In this class of molecule, the excited state is generally highly polar compared to the ground state due to intramolecular charge transfer from the donor to the acceptor group. The intramolecular charge transfer results in a large dipole moment in the excited state compared to that of the ground state rendering its fluorescence emission sensitive to environment and solvent polarity [20-22].
Pyrene is a prototypical example of a fluorophore and its monomer emission occurs around 380 nm. It has been shifted to as high as 600 nm by multiple substitution by groups that extend the conjugation and also by substituting donor-acceptor groups along the conjugation [23-25]. In addition, pyrene also exhibits excimer emission at a longer wavelength compared to monomer emission which can be used in sensing applications [26-30]. The pyrene chromophore can act as a donor or as an acceptor depending upon the substituent. Pyrene- π spacer-donor and pyrene- π spacer-acceptor type molecules have been widely studied and they have been used in sensing, photo and electro-luminescence applications [31-36]. Unlike pyrene, the triphenylene chromophore has not been widely studied. In contrast to pyrene, triphenylene does not form an excimer in the excited state – emission from the excimer state is very rare for triphenylene chromophore [37-39]. The fluorescence of triphenylene occurs around 348 nm, which is more blue shifted than pyrene monomer emission. Only a few reports on the extension of conjugation of the triphenylene chromophore have appeared. 2,3,6,7,10,11-Hexakis(ethynyl)triphenylene derivatives have been used in photonics as organic light emitting diode (OLED) materials [40-42]. Therefore, it is worthwhile to tune the fluorescence emission of the triphenylene chromophore by the addition of suitable substituents.
Herein we report the synthesis of a series of 2-(phenylethynyl)triphenylene derivatives with donor and acceptor substituents on the phenyl ring (Scheme 1). The phenylethynyl group is used to extend the conjugation of the triphenylene chromophore and the substituents on the phenyl ring are used to enhance intramolecular charge transfer (ICT) in the excited state. We have studied the absorption and emission properties, in particular the ICT based solvatochromic fluorescence emission behavior and the correlation of the observed large Stokes shifts with orientation polarizibility (Δf) and solvent polarity (ET(30) scale). HOMO and LUMO surfaces of these derivatives, obtained from DFT calculations, help in identifying the triphenylene chromophore acting as an acceptor when substituted with electron donating groups and as a donor when substituted with electron withdrawing groups on the phenyl ring. HOMO–LUMO energy gaps have been estimated based on electrochemical studies and compared with those obtained from absorption spectroscopy.
Scheme 1: Structures of 2-phenylethynyltriphenylene derivatives.
Scheme 1: Structures of 2-phenylethynyltriphenylene derivatives.
Results and Discussion
Synthesis
2-Ethynyltriphenylene (4) was synthesized by coupling 2-iodotriphenylene (2) with 1,1-dimethylpropargyl alcohol followed by deprotection with KOH in refluxing toluene (Scheme 2). 2-Phenylethynyltriphenylene (1a) and those bearing electron withdrawing substituent (1b–e) were synthesized by the Sonogashira coupling of 2-iodotriphenylene (2) with the corresponding phenylacetylene derivatives. Although 1f–g were also synthesized by this procedure, their purification proved difficult due to an inseparable minor product formed in these reactions. Therefore compounds 1f–g were synthesized via the Sonogashira coupling of 2-ethynyltriphenylene (4) and the corresponding iodoarenes (Scheme 3). Compounds 1a–f were obtained as colorless solids in good yields. Derivative 1g was obtained as an orange solid in 58% yield. All compounds were purified by column chromatography and thoroughly characterized by various spectroscopic methods and analytical data.
Scheme 2: Synthesis of 2-ethynyltriphenylene (4).
Scheme 2: Synthesis of 2-ethynyltriphenylene (4).
Scheme 3: Synthesis of phenylethynyltriphenylene derivatives 1a–g.
Scheme 3: Synthesis of phenylethynyltriphenylene derivatives 1a–g.
Absorption and fluorescence emission studies
The UV–vis absorption spectra of 1a–g were recorded in various solvents ranging from non-polar cyclohexane to dipolar aprotic DMSO to polar protic ethanol and isopropanol. The absorption spectra of 1a–g in cyclohexane and acetonitrile are shown in Figure 1. The lowest energy absorption of triphenylene is symmetry forbidden and appears at 330–340 nm [23]. Compared to triphenylene, the absorption bands of derivatives 1a–g are consistently red shifted and more intense (symmetry allowed) due to extended conjugation with the phenylethynyl group and loss of symmetry, respectively. Compared to 1a and 1b, the lowest energy absorption bands of 1c–g are further red shifted in both cyclohexane and acetonitrile. Although there is no significant solvent effect on the absorption bands of 1a–g in these two solvents, the lowest energy absorption bands are red shifted by 5–10 nm in cyclohexane compared to acetonitrile. Moreover, the vibrational fine structure is more clearly seen in cyclohexane than in acetonitrile where the bands are relatively broadened, especially for 1g. These observations led to the conclusion that irrespective of the substituents on the phenyl ring, the ground state of these molecules is relatively non-polar and devoid of significant solvent and substituent effects. By contrast, the fluorescence emission bands of 1a–g showed significant substituent and solvent effects.
![[1860-5397-6-112-1]](/bjoc/content/figures/1860-5397-6-112-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Absorption spectra of 1a–g in cyclohexane and acetonitrile (10−5 M).
Figure 1: Absorption spectra of 1a–g in cyclohexane and acetonitrile (10−5 M).
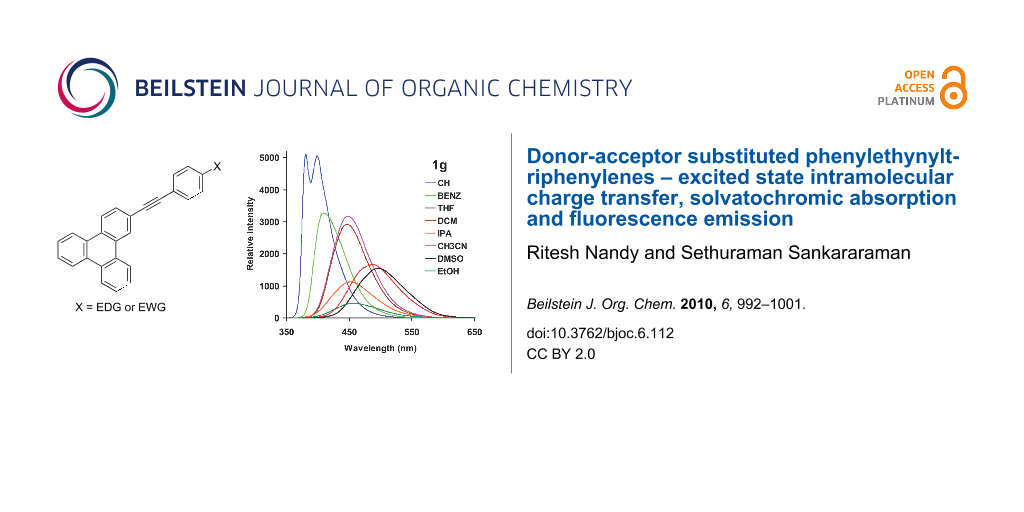
The fluorescence emission spectra of 1a–g in cyclohexane, and DMSO are shown in Figure 2. In cyclohexane, 1a–f showed emission maxima around 380 nm which is characteristic of the local excited state emission of the phenylethynyltriphenylene chromophore. The emission maximum was independent of the substituent, which implied that there was no significant ICT in the excited state in cyclohexane. In the case of 1g, two emission bands were observed at 381 and 400 nm. The 381 nm band was assigned to emission from the local excited state of the phenylethynyltriphenylene chromophore and the band at 400 nm to ICT from the dimethylamino group to triphenylene moiety [36]. In DMSO, the fluorescence emission of 1a–b appeared at around 380 nm, arising from the local excited state of phenylethynyltriphenylene chromophore. The emission maxima of all the other substrates (1c–g) were progressively red shifted and highly dependent on the substituent present. The maximum red shift (499 nm) was observed in case of 1g where excited state ICT is expected to be efficient due to the strong electron donating nature of the –NMe2 group. Compounds 1c–e bearing electron withdrawing substituents also showed progressive red shifts (1c, 412 nm; 1d, 430 nm and 1e, 448 nm) of the emission band due to ICT where charge transfer from triphenylene moiety to the phenyl group bearing the electron withdrawing substituent occurred. In case of 1e, two emission bands were observed at 394 and 448 nm due emission from local excitation of the triphenylene chromophore and the ICT transition, respectively. The fluorescence emission spectra of 1e and 1g measured in various solvents are shown in Figure 3. These two substrates, one with an electron withdrawing substituent (1e) and the other with an electron donating substituent (1g) are highlighted here because of the maximum Stokes shifts of the ICT band observed with these two compounds. With increasing solvent polarity the fluorescence maxima remained unchanged in the cases of 1a and 1b, whereas in all the other cases a progressive red shift of emission maxima was observed. The emission maxima of compounds 1a (with no substituent in the phenyl ring) and 1b (with a CF3 substituent ) were unaffected by a change of solvent polarity, whereas in derivatives 1c–g the substituent had a strong effect on the emission maxima with increasing solvent polarity.
![[1860-5397-6-112-2]](/bjoc/content/figures/1860-5397-6-112-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Fluorescence emission spectra of 1a–g in cyclohexane and DMSO (10−5 M), λex = 335 nm.
Figure 2: Fluorescence emission spectra of 1a–g in cyclohexane and DMSO (10−5 M), λex = 335 nm.
![[1860-5397-6-112-3]](/bjoc/content/figures/1860-5397-6-112-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Fluorescence emission spectra of 1e and 1g in various solvents (10−5 M). CH – cyclohexane (λex = 334 nm (1e) and 345 nm (1g)), BENZ – benzene (λex = 338 nm (1e) and 335 nm (1g)), DCM – dichloromethane (λex = 336 nm (1e) and 357 nm (1g)), IPA – isopropyl alcohol CH3CN (λex = 348 nm (1g)), DMSO (λex = 338 nm (1e) and 367 nm (1g). EtOH (λex = 335 nm (1e and 1g)), THF (λex = 356 nm (1g)).
Figure 3: Fluorescence emission spectra of 1e and 1g in various solvents (10−5 M). CH – cyclohexane (λex = 33...
In cases of 1c and 1f–g, the maximum Stokes shift was observed in DMSO where as in case of 1d–e the maximum Stokes shift was observed in ethanol. Compounds 1d–e are carbonyl derivatives and the maximum shift in hydroxylic solvents might be due to strong hydrogen bonding interactions between the solvent and the carbonyl group in the excited state. In order to probe further the effect of solvent polarity on the emission maximum (solvatochromic fluorescence emission), the fluorescence spectrum of 1g was recorded in binary solvent mixtures of cyclohexane and isopropyl alcohol. Initially in cyclohexane, two emission bands were observed. With the addition of isopropyl alcohol the emission maximum shifted to longer wavelengths and the two bands merged. In an approximately 10% isopropyl alcohol-cyclohexane mixture, the band that was assigned to emission from a local excited state vanished and only a band due to ICT was observed (Figure 4). With increasing solvent polarity the emission intensity decreased due to competing excited electron transfer quenching of fluorescence.
![[1860-5397-6-112-4]](/bjoc/content/figures/1860-5397-6-112-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Effect of binary solvent system (cyclohexane-isopropyl alcohol) on the fluorescence emission of 1g (10−5 M). CH – cyclohexane, IPA – isopropyl alcohol, λex = 335 nm.
Figure 4: Effect of binary solvent system (cyclohexane-isopropyl alcohol) on the fluorescence emission of 1g ...
Correlation of Stokes shifts with solvent polarity
Solvent induced spectral shifts are often interpreted in terms of the Lippert–Mataga [43-45] equation, which describes Stokes shifts in terms of the change in the dipole moment of the fluorophore and the dependence of the energy of the dipole on the dielectric constant and refractive index of the solvent. The Lippert–Mataga equation accounts for the general solvent effect and does not account for specific solvent–fluorophore interactions, for example, through hydrogen bonding etc. The Lippert–Mataga plot for 1c and 1g are shown in Figure 5 as representative examples. From the slope of these plots the change in the dipole moment (Δμ) of the fluorophore upon electronic excitation (μES − μGS) was estimated assuming the molecular radius as the cavity radius [11]. The molecules under consideration are non-spherical in nature. Hence, the above assumption of substituting molecular radius for cavity radius is only approximate. The molecular radii for 1c (8.8 Å), 1e (10.2 Å) and 1g (9.1 Å) were obtained from semi-empirical AM1 calculations [46]. The change in the dipole moments (Δμ) were, 25.7 D for 1c, 43.0 D for 1e and 40.0 D for 1g, respectively (Table 1). The change in dipole moment for 1g is higher than that of the corresponding pyrene derivative (30 D) [36]. Whenever there is an excited state charge transfer process, Reichardt–Dimroth’s ET(30) [47,48] scale is more useful to correlate the solvent induced Stokes shift. Correlation using ET(30) scale often follows two distinct lines, one for the non-protic solvents and other for the protic solvents. The data points corresponding to ethanol and isopropyl alcohol are indicated in Figure 6. In protic solvents specific solvent–fluorophore interaction such as hydrogen bonding is possible and the extent of this interaction would depend upon the functional groups present in the fluorophore. In the case of 1e all the data points of the correlation between Stokes shift and ET(30) lie on the straight line with a correlation coefficient of 0.98. A similar trend is observed in the case of 1d. However, in cases of 1c and 1g, the data points corresponding to the protic solvents do not lie on the straight line since the observed Stokes shifts are much lower than expected for these solvents. Derivative 1e is a carbonyl compound and stabilization due to hydrogen bonding interaction with protic solvents is expected both in the ground and excited state. Such a specific solvent-fluorophore interaction might be weak in the case of other derivatives. Thus, the correlation of Stokes shift with ET(30) helps in identifying specific solvent–fluorophore interactions.
![[1860-5397-6-112-5]](/bjoc/content/figures/1860-5397-6-112-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Lippert–Mataga plot showing Stokes shift as a function of solvent orientation polarizibility (Δf).
Figure 5: Lippert–Mataga plot showing Stokes shift as a function of solvent orientation polarizibility (Δf).
![[1860-5397-6-112-6]](/bjoc/content/figures/1860-5397-6-112-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Correlation of Stokes shift with ET(30) scale.
Figure 6: Correlation of Stokes shift with ET(30) scale.
Quantum yield of fluorescence
From Figure 3 and Figure 4 it is clear that with increasing solvent polarity, the intensity of emission decreases along with the bathochromic shift of the wavelength of emission. The fluorescence intensity of molecular systems that undergo efficient ICT upon photoexcitation decreases due to competing electron transfer from the donor to the acceptor site that quenches the fluorescence [49-51]. For compounds 1c, 1f and 1g, the fluorescence quantum yield decreased upon changing the solvent from cyclohexane to DMSO (Table 1). For compounds 1a and 1b, which did not show strong solvatochromic emission, the reverse trend was observed. The quantum yield of fluorescence increased in DMSO compared to cyclohexane. This might be due to the increase in the viscosity of the medium which quenches the non-radiative pathways. In the cases of the carbonyl derivatives 1d and 1e, the quantum yield of fluorescence was measured in ethanol. The fluorescence quantum yields for these derivatives in ethanol were low, presumably due to facile electron transfer and hydrogen bonding interaction with the solvent which enhances the non-radiative processes. In polar solvents electron transfer from the aromatic moiety to the benzophenone has been previously shown by time resolved spectroscopy to result in the formation of a radical ion pair [52-54].
Table 1: Representative absorption, emission and fluorescence quantum yield data.
| Substrate | Solventa |
Absorption
λmax (nm) |
Emission
λmax (nm) |
Φfb | Δμ (D)c |
|---|---|---|---|---|---|
| 1a |
CH
DMSO |
334
336 |
370
371 |
0.20
0.28 |
|
| 1b |
CH
DMSO |
334
337 |
368
373 |
0.17
0.35 |
|
| 1c |
CH
DMSO |
349
353 |
362
412 |
0.60
0.54 |
25.7 |
| 1d |
CH
EtOH |
351
353 |
383
434 |
—
0.32 |
29.5 |
| 1e |
CH
EtOH |
354
350 |
381
490 |
—
0.11 |
43 |
| 1f |
CH
DMSO |
343
348 |
365
421 |
0.58
0.43 |
28 |
| 1g |
CH
DMSO |
367
367 |
380
499 |
0.99
0.36 |
40 |
aCH = cyclohexane, bfluorescence quantum yield relative to quinine sulfate standard, cchange in dipole moment (μES − μGS) due to excited state ICT calculated from Lippert–Mataga plot.
HOMO and LUMO surfaces and energy gaps
Cyclic voltammograms of 1a–e and 1g were recorded in acetonitrile in order to obtain the redox potentials, as well as to estimate the HOMO–LUMO gap of these derivatives [31-35]. All the compounds showed a single irreversible oxidation peak and multiple reduction peaks. The HOMO–LUMO gap was estimated from the CV data as the difference between the oxidation peak potential and the reduction peak potential (Table 2) and compared with the HOMO–LUMO gap estimated from the onset of optical absorption from the UV–vis spectra in acetonitrile. The HOMO–LUMO gaps obtained by these two methods are comparable considering the approximate nature of these methods of estimation. The optimized structures, HOMO and LUMO surfaces of 1c and 1g were obtained by DFT calculations [46]. In case of 1c, the molecule is planar whereas 1g is twisted. The dihedral angle between the plane of the triphenylene ring and the plane of the phenyl ring is 96° for 1g. These observations are comparable with the geometry of the corresponding pyrene derivatives reported earlier [31,32]. The HOMO and LUMO surfaces are shown in Figure 7. In case of 1g, the HOMO density is mainly located on the dimethylaminophenylethynyl moiety and the triphenylene moiety is devoid of any HOMO density. The LUMO of 1g is mainly located on the triphenylene moiety indicating that the dimethylaminophenylethynyl group is the donor and the triphenylene group the acceptor. In case of 1c the situation is reversed, the HOMO density is located mainly on the triphenylene moiety and the LUMO density is on the cyanophenylethynyl group. This indicates that there is role reversal of the triphenylene moiety either as a donor or as an acceptor depending upon the nature of the functional group attached to the phenylethynyl unit. These findings are consistent with the earlier reports on the pyrene derivatives [31].
Table 2: HOMO-LUMO energy gap and change in dipole moment due to ICT.
| Substrate | ΔE (eV)a | Ep(ox) (V)b | Ep(red) (V)b | ΔE (eV)c |
|---|---|---|---|---|
| 1a | 3.42 | +1.60 | −2.14 | 3.74 |
| 1b | 3.41 | +1.65 | −2.02 | 3.67 |
| 1c | 3.30 | +1.68 | −1.85 | 3.53 |
| 1d | 3.27 | +1.66 | −1.70 | 3.36 |
| 1e | 3.20 | +1.63 | −1.63 | 3.26 |
| 1f | 3.19 | — | — | — |
| 1g | 2.97 | +0.76 | −2.27 | 3.03 |
aHOMO–LUMO energy gap estimated on the basis of absorption data, bat 0.1 Vs−1 scan rate, cHOMO–LUMO energy gap estimated on the basis of electrochemical data.
![[1860-5397-6-112-7]](/bjoc/content/figures/1860-5397-6-112-7.png?scale=2.4&max-width=1024&background=FFFFFF)
Figure 7: HOMO and LUMO surfaces of 1c and 1g according to DFT calculations.
Figure 7: HOMO and LUMO surfaces of 1c and 1g according to DFT calculations.
Conclusion
Several 2-phenylethynyltriphenylene derivatives bearing electron donating and electron releasing groups on the phenyl ring were synthesized. Their absorption and fluorescence emission were studied in several solvents. The absorption maximum of these derivatives was not siginificantly altered by solvent polarity. However, the fluorescence emission maxima showed strong solvent polarity dependence and large Stokes shifts were observed. These observations are explained on the basis of an excited state intramolecular charge transfer (ICT) process. Derivative 1g, with dimethylamino substituent, showed the maximum solvent effect with a Stokes shift of nearly 130 nm (7828 cm−1) in DMSO in comparison to that observed in cyclohexane. Derivatives bearing carbonyl substituents (1d–e) showed large Stokes shift in polar protic solvents such as ethanol and isopropyl alcohol, presumeably due to the hydrogen bonding stabilization of the excited state by these solvents. The Stokes shifts were correlated with solvent orientation polarizibility by the Lippert–Mataga equation and Reichardt’s ET(30) solvent polarity scale. HOMO–LUMO gaps were calculated from both optical and electrochemical data. HOMO and LUMO surfaces based on DFT calculations show that the triphenylene chromophore can act either as an electron donor or as an electron acceptor in the ICT process, depending upon the nature of substituent on the phenyl ring. Derivatives 1e and 1g are potential candidates for use as solvent polarity probes. However, their performance is only comparable to those of the corresponding pyrene derivatives.
Experimental
Synthesis of 2-methyl-4-(triphenylen-2-yl)but-3-yn-2-ol (3). A Schlenk flask was charged with a mixture of 2-iodotriphenylene and triphenylene (2.0 g, 45:55 by 1H NMR) (see Supporting Information File 1), Pd(PPh3)2Cl2 (0.2 g, 0.3 mmol), PPh3 (0.15 g, 0.6 mmol), CuI (0.105 g, 0.6 mmol), degassed THF (30 mL) and diisopropylamine (30 mL). The mixture was stirred at room temperature for 15 min and 2-methyl-3-butyn-2-ol (0.32 g, 3.8 mmol) was added. Stirring was continued for 2 h at 60 °C after which time the solvent was removed and the residue dissolved in dichloromethane (100 mL). The solution was washed successively with 5% aq HCl (2 × 60 mL) and water (60 mL). The organic layer was dried over anhydrous sodium sulfate and solvent removed under reduced pressure. The crude product was purified by column chromatography on silica gel. Elution with hexane to remove unreacted triphenylene followed by elution with a mixture of hexane and ethyl acetate (9:1, v/v) gave 3 as a colorless solid (0.95 g, 78%), mp 153–155 °C; IR (neat) 3331, 2978, 2212 cm−1; 1H NMR (CDCl3, 400 MHz) δH = 8.71 (d, J = 1.2 Hz, 1H), 8.53–8.63 (m, 5H), 7.63–7.67 (m, 5H), 2.24 (s, 1H), 1.72 (s, 6H) ppm. 13C NMR (CDCl3, 100 MHz) δc = 130.0, 129.9, 129.6, 129.5, 129.2, 129.0, 127.6, 127.5, 1273, 126.9, 123.5, 123.4, 123.38, 123.31, 123.27, 121.3, 94.5, 82.5, 65.8, 31.6 ppm. ESI Q-TOF MS m/z 333 [M + Na]+, 293 [M − OH]+; HRMS calcd for C23H18ONa [M − Na]+ 333.1255; found, 333.1257.
Synthesis of 2-ethynyltriphenylene (4). To a degassed solution of 3 (0.79 g, 2.5 mmol) in toluene (100 mL), KOH (0.57 g, 10.2 mmol) was added and the reaction mixtureheated under reflux for 2.5 h. Upon completion of the reaction, the hot reaction mixture was filtered and the residue washed with toluene (10 mL). The combined filtrate and washings were washed with water (2 × 60 mL). The organic layer was separated and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and the crude product purified by column chromatography on silica gel with hexane and dichloromethane (95:5, v/v) as eluant to give 4 as a colorless solid (0.508 g, 79%); mp 149–151 °C; IR (neat) 3280, 2194 cm−1; 1H NMR (CDCl3, 400 MHz) δH = 8.80 (d, J = 1.2 Hz, 1H), 8.57–8.65 (m, 5H), 7.74 (dd, J = 1.2, 8.8 Hz, 1H), 7.65–7.68 (m, 4H), 3.23 (s, 1H) ppm; 13C NMR (CDCl3, 100 MHz) δc 130.2, 130.1, 129.9, 129.1, 128.9, 127.7, 127.6, 127.5, 127.4, 127.3, 123.5, 123.4, 123.3, 120.6, 84.1, 77.9 ppm; MALDI-TOF MS m/z (%) 252 (72) [M+], 253 (100) [M+ + 1], 254 (22) [M+ + 2]; Anal. calcd. for C20H12 C, 95.23; H, 4.75. Found C, 95.04, H 4.60.
General procedure for the synthesis of 1a–e. 1a–e were synthesized by coupling 2-iodotriphenylene (2 mmol) with the corresponding arylethyne (1.9 mmol) (see Scheme 3) according to the procedure described above for the synthesis of 3.
2-(Phenylethynyl)triphenylene (1a). The crude product was purified by column chromatography with hexane as eluant to afford 1a as a colorless solid (0.495 g, 80%), mp 180–182 °C; IR (neat) 3069, 2217 cm−1; 1H NMR (CDCl3, 500 MHz) δH = 8.80 (d, J = 1.5 Hz, 1H), 8.55–8.64 (m, 5H), 7.75 (dd, J = 1.5, 8.5 Hz, 1H), 7.61–7.66 (m, 6H), 7.36-7.40 (m, 3H) ppm; 13C NMR (CDCl3, 125 MHz) δc 131.7, 130.1, 130.0, 129.9, 129.7, 129.6, 129.3, 129.1, 128.5, 128.4, 127.59, 127.58, 127.4, 127.3, 126.8, 123.5, 123.43, 123.41, 123.36, 123.31, 121.9, 90.2, 89.9 ppm; The mass spectrum was recorded as the silver ion adduct of 1a by adding silver triflate to a solution of 1a in acetonitrile prior to measurement. ESI Q-TOF MS m/z 435 [M + Ag]+ along with the isotope peaks in the expected intensity ratios; HRMS calcd for C26H16Ag [M + Ag]+ 435.0303; found, 435.0298.
2-(3-Trifluoromethylphenylethynyl)triphenylene (1b). The crude product was purified three times by column chromatography with hexane as eluant to yield 1b as a colorless solid (0.654 g, 87%), mp 137–139 °C; IR (neat) 3069, 2213 cm−1; 1H NMR (CDCl3, 500 MHz) δH = 8.80 (d, J = 1.5 Hz, 1H), 8.55–8.68 (m, 5H), 7.8 (m, 1H), 7.73–7.77 (m, 2H), 7.59–7.67 (m, 5H), 7.48-7.51 (m, 1H) ppm; 13C NMR (CDCl3, 125 MHz) δc 134.7, 131 (q, 2JC-F = 32.5 Hz), 130.1, 130.0, 129.9, 129.8, 129.7, 129.2, 129.0, 128.9, 128.5 (q, 3JC-F = 3.75 Hz), 127.7, 127.6, 127.41, 127.39, 127.0, 124.87 (q, 3JC-F = 3.75 Hz), 124.3, 123.5, 123.4, 123.3, 122.7, 121.1, 91.4, 88.6 ppm; MALDI-TOF MS C27H15F3 m/z (%) 396 (100) [M+], 397 (84) [M+ + 1], 398 (22) [M+ + 2].
4-(2-Triphenylenylethynyl)benzonitrile (1c). The crude product was purified by column chromatography with a mixture of hexane and dichloromethane (85:15, v/v) as eluant to yield 1c as a colorless solid (0.529 g, 79%), mp 192–194 °C; IR (neat) 3059, 2221 cm−1; 1H NMR (CDCl3, 400 MHz) δH = 8.82 (d, J = 1.2 Hz, 1H), 8.60–8.66 (m, 5H), 7.77 (dd, J = 1.2, 8.8 Hz, 1H), 7.66–7.69 (m, 8H) ppm;13C NMR (CDCl3, 100 MHz) δc 132.1, 130.2, 130.0, 129.8, 129.1, 128.9, 128.2, 127.9, 127.8, 127.4, 127.2, 123.6, 123.5, 123.4, 123.37, 123.3, 120.8, 119.6, 111.5, 94.3, 88.8 ppm; ESI Q-TOF MS C27H15N m/z 376 [M +Na]+, 354 [M + H]+; HRMS calcd for C27H16N [M + H]+ 354.1283; found, 354.1287.
4-(2-Triphenylenylethynyl)acetophenone (1d). The crude product was purified by column chromatography with a mixture of hexane and dichloromethane (4:1, v/v) as eluant to yield 1d as a colorless solid (0.506 g, 72%), mp 181–183 °C; IR (neat) 3067, 2218, 1669 cm−1; 1H NMR (CDCl3, 400 MHz) δH = 8.86 (d, J = 1.6 Hz, 1H), 8.62–8.68 (m, 5H), 7.97–8.0 (m, 2H), 7.80 (dd, J = 1.6, 8.8 Hz, 1H), 7.66–7.71 (m, 6H), 2.64 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz) δc 191.3, 136.6, 131.8, 130.2, 130.0, 129.9, 129.8, 129.2, 129.0, 128.4, 128.2, 127.8, 127.7, 127.4, 127.1, 123.6, 123.4, 121.3, 93.2, 89.4, 26.6 ppm; ESI Q-TOF MS m/z 371 [M + H]+; HRMS calcd for C28H19O [M + H]+ 371.1436; found, 371.1433.
4-(2-Triphenylenylethynyl)benzophenone (1e). The crude product was purified by column chromatography with a mixture of hexane and dichloromethane (80:20, v/v) as eluant to yield 1e as a colorless solid (0.517 g, 63%), mp 172–174 °C; IR (neat) 3080, 2211, 1658 cm−1; 1H NMR (CDCl3, 400 MHz) δH = 8.85 (d, J = 1.2 Hz, 1H), 8.61–8.66 (m, 5H), 7.79–7.86 (m, 5H), 7.59–7.73 (m, 7H), 7.49–7.53 (m, 2H) ppm; 13C NMR (CDCl3, 100 MHz) δc 196.0, 136.9, 132.6, 131.5, 130.20, 130.16, 130.06, 130.03, 129.9, 129.8, 128.4, 127.8, 127.7, 127.5, 127.1, 123.6, 123.5, 123.4, 121.4, 93.0, 89.5 ppm; ESI Q-TOF MS m/z 433 [M + H]+; HRMS calcd for C33H21O [M + H]+ 433.1592; found, 433.1593.
General procedure for the synthesis of 1f–g. A Schlenk flask was charged with the corresponding aryl iodide (0.6 mmol), Pd(PPh3)2Cl2 (0.011 g, 0.015 mmol), PPh3 (0.008 g, 0.03 mmol), CuI (0.006 g, 0.03 mmol), degassed THF (30 mL) and diisopropylamine (10 mL). The reaction mixture was stirred at room temperature for 15 min and a solution of 2-ethynyltriphenylene (4) (0.5 mmol) in THF (3mL) added dropwise. Stirring was continued for 2.5 h. Removal of solvent and other volatile materials under reduced pressure gave the crude product which was purified by column chromatography on silica gel.
2-(3,4-Bis(decyloxy)phenylethynyl)triphenylene (1f). The crude product was purified by column chromatography with a mixture of hexane and dichloromethane (85:15, v/v) as eluant to yield 1f as a colorless solid (0.201 g, 62%), mp 118–120 °C; IR (neat) 3084, 2959, 2918, 2850 cm−1; 1H NMR (CDCl3, 400 MHz) δH = 8.82 (d, J = 1.2 Hz, 1H), 8.58–8.65 (m, 5H), 7.78 (dd, J = 1.6, 8.4 Hz, 1H), 7.64–7.68 (m, 4H), 7.20 (dd, J = 1.2 Hz, 8.8 Hz, 1H), 7.15 (d, J = 1.6 Hz, 1H), 6.87 (d, J = 8.4 Hz, 1H), 4.05 (qt , J = 7.2, 8.4 Hz, 4H), 1.83–1.88 (m, 4H), 1.47–1.52 (m, 4H), 1.27–1.29 (m, 24H), 0.88-0.92 (m, 6H) ppm; 13C NMR (CDCl3, 100 MHz) δc 149.9, 148.9, 130.0, 129.9, 129.8, 129.4, 129.3, 229.2, 127.5, 127.3, 126.6, 125.1, 123.5, 123.4, 123.35, 123.3, 122.3, 116, 115.4, 113.5, 113.5, 90.6, 88.2, 69.4, 69.2, 31.9, 29.7, 29.6, 29.4, 29.36, 29.30, 29.26, 26.1, 22.7, 14.1 ppm; MALDI-TOF MS: m/z (%) 640 (100) [M+], 641 (49) [M+ + 1], 642 (9) [M+ + 2].
N,N-Dimethyl-4-(2-triphenylenylethynyl)aniline (1g). The crude product was purified by column chromatography with a mixture of hexane and dichloromethane (9:1, v/v) as eluant to yield 1g as an orange solid (0.108 g, 58%), mp 212–214 °C (decomposed during melting); IR (neat) 3064, 2798, 2190 cm−1; 1H NMR (CDCl3, 400 MHz) δH = 8.80 (d, J = 1.2 Hz, 1H), 8.61–8.66 (m, 4H), 8.59 (d, J = 8.4 Hz, 1H), 7.77 (dd, J = 1.6, 8.4 Hz, 1H), 7.65–7.69 (m, 4H), 7.51 (d, J = 9.2 Hz, 2H), 6.71 (d, J = 9.2 Hz, 2H), 3.02 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz) δc 150.2, 132.8, 130.0, 129.9, 129.8, 129.7, 129.3, 128.9, 127.5, 127.4, 127.3, 126.3, 123.5, 123.4, 123.3, 123.2, 122.9, 111.9, 110.0, 91.6, 87.9, 40.2 ppm; ESI Q-TOF MS m/z 372 [M + H]+; HRMS calcd for C28H22N [M + H]+ 372.1752; found, 372.1759.
Supporting Information
Supporting Information includes the NMR spectra of all compounds (1a–g, 2–4), UV–vis data in various solvents, electrochemical data for 1a–g and calculated atomic coordinates for 1c and 1g.
| Supporting Information File 1: Experimental data for compounds 1a–g and 2–4. | ||
| Format: PDF | Size: 1.2 MB | Download |
References
-
Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kulwer Academic: New York, 1999.
Return to citation in text: [1] -
Goldys, E. M., Ed. Fluorescence Applications in Biotechnology and the Life Sciences; Wiley Blackwell: New Jersey, 2009.
Return to citation in text: [1] -
Giepmans, B. N. G.; Adams, S. R.; Ellisman, M. H.; Tsien, R. Y. Science 2006, 312, 217–224.
Return to citation in text: [1] -
Baker, A. Environ. Sci. Technol. 2001, 35, 948–953. doi:10.1021/es000177t
Return to citation in text: [1] -
Loudet, A.; Burgess, K. Chem. Rev. 2007, 107, 4891–4932.
Return to citation in text: [1] -
Ulrich, G.; Ziessel, R.; Harriman, A. Angew. Chem., Int. Ed. 2008, 47, 1184–1201. doi:10.1002/anie.200702070
Return to citation in text: [1] -
Kowada, T.; Yamaguchi, S.; Ohe, K. Org. Lett. 2010, 12, 296–299. doi:10.1021/ol902631d
Return to citation in text: [1] -
Thiagarajan, V.; Ramamurthy, P.; Thirumalai, D.; Ramakrishnan, V. T. Org. Lett. 2005, 7, 657–660. doi:10.1021/ol047463k
Return to citation in text: [1] -
Badugu, R.; Lakowicz, J. R.; Geddes, C. D. J. Am. Chem. Soc. 2005, 127, 3635–3641. doi:10.1021/ja044421i
Return to citation in text: [1] -
Srikun, D.; Miller, E. W.; Domaille, D. W.; Chang, C. J. J. Am. Chem. Soc. 2008, 130, 4596–4597. doi:10.1021/ja711480f
Return to citation in text: [1] -
Zhang, L.; Clark, R. J.; Zhu, L. Chem.–Eur. J. 2008, 14, 2894–2903. doi:10.1002/chem.200701419
Return to citation in text: [1] [2] -
Andrew, T. L.; Swager, T. M. J. Am. Chem. Soc. 2007, 129, 7254–7255. doi:10.1021/ja071911c
Return to citation in text: [1] -
Dale, T. J.; Rebek, J., Jr. J. Am. Chem. Soc. 2006, 128, 4500–4501. doi:10.1021/ja057449i
Return to citation in text: [1] -
Fan, L.-J.; Jones, W. E., Jr. J. Phys. Chem. B 2006, 110, 7777–7782. doi:10.1021/jp056381q
Return to citation in text: [1] -
Kim, S. K.; Yoon, J. Chem. Commun. 2002, 770–771. doi:10.1039/b110139k
Return to citation in text: [1] -
Kim, S. H.; Kim, J. S.; Park, S. M.; Chang, S.-K. Org. Lett. 2006, 8, 371–374. doi:10.1021/ol052282j
Return to citation in text: [1] -
Jung, H. S.; Park, M.; Han, D. Y.; Kim, E.; Lee, C.; Ham, S.; Kim, J. S. Org. Lett. 2009, 11, 3378–3381. doi:10.1021/ol901221q
Return to citation in text: [1] -
Lee, S. H.; Kim, S. H.; Kim, S. K.; Jung, J. H.; Kim, J. S. J. Org. Chem. 2005, 70, 9288–9295. doi:10.1021/jo051302s
Return to citation in text: [1] -
Park, S. Y.; Yoon, J. H.; Hong, C. S.; Souane, R.; Kim, J. S.; Matthews, S. E.; Vicens, J. J. Org. Chem. 2008, 73, 8212–8218. doi:10.1021/jo8012918
Return to citation in text: [1] -
Beinhoff, M.; Weigel, W.; Jurczok, M.; Rettig, W.; Modrakowski, C.; Brüdgam, I.; Hartl, H.; Schlüter, A. D. Eur. J. Org. Chem. 2001, 3819–3829. doi:10.1002/1099-0690(200110)2001:20<3819::AID-EJOC3819>3.0.CO;2-W
Return to citation in text: [1] -
Beinhoff, M.; Weigel, W.; Rettig, W.; Bruedgam, I.; Hartl, H.; Schlüter, A. D. J. Org. Chem. 2005, 70, 6583–6591. doi:10.1021/jo050302p
Return to citation in text: [1] -
Techert, S.; Wiessner, A.; Schmatz, S.; Staerk, H. J. Phys. Chem. B 2001, 105, 7579–7587. doi:10.1021/jp004370l
and references 2-3 cited therein.
Return to citation in text: [1] -
Berlman, I. B. Handbook of Fluorescence Spectra of Aromatic Molecules; Academic Press: New York, 1965; p 173.
Return to citation in text: [1] [2] -
Venkataramana, G.; Sankararaman, S. Eur. J. Org. Chem. 2005, 4162–4166. doi:10.1002/ejoc.200500222
Return to citation in text: [1] -
Venkataramana, G.; Sankararaman, S. Org. Lett. 2006, 8, 2739–2742. doi:10.1021/ol060767h
Return to citation in text: [1] -
Yuasa, H.; Miyagawa, N.; Izumi, T.; Nakatani, M.; Izumi, M.; Hashimoto, H. Org. Lett. 2004, 6, 1489–1492. doi:10.1021/ol049628v
Return to citation in text: [1] -
Kim, S. K.; Bok, J. H.; Bartsch, R. A.; Lee, J. Y.; Kim, J. S. Org. Lett. 2005, 7, 4839–4842. doi:10.1021/ol051609d
Return to citation in text: [1] -
Kim, J. S.; Quang, D. T. Chem. Rev. 2007, 107, 3780–3799.
Return to citation in text: [1] -
Strauss, J.; Daub, J. Org. Lett. 2002, 4, 683–686. doi:10.1021/ol0170354
Return to citation in text: [1] -
Cho, H. K.; Lee, D. H.; Hong, J.-I. Chem. Commun. 2005, 1690–1692. doi:10.1039/b417845a
Return to citation in text: [1] -
Yang, S.-W.; Elangovan, A.; Hwang, K.-C.; Ho, T.-I. J. Phys. Chem. B 2005, 109, 16628–16635. doi:10.1021/jp052086u
Return to citation in text: [1] [2] [3] [4] -
Kim, H. M.; Lee, Y. O.; Lim, C. S.; Kim, J. S.; Cho, B. R. J. Org. Chem. 2008, 73, 5127–5130. doi:10.1021/jo800363v
Return to citation in text: [1] [2] [3] -
Oh, J.-W.; Lee, Y. O.; Kim, T. H.; Ko, K. C.; Lee, J. Y.; Kim, H.; Kim, J. S. Angew. Chem., Int. Ed. 2009, 48, 2522–2524. doi:10.1002/anie.200804669
Return to citation in text: [1] [2] -
Dobkowski, J.; Retting, W.; Waluk, J. Phys. Chem. Chem. Phys. 2002, 4, 4334–4339. doi:10.1039/b204749g
Return to citation in text: [1] [2] -
Ji, S.; Yang, J.; Yang, Q.; Liu, S.; Chen, M.; Zhao, J. J. Org. Chem. 2009, 74, 4855–4865. doi:10.1021/jo900588e
Return to citation in text: [1] [2] -
Subuddhi, U.; Haldar, S.; Sankararaman, S.; Mishra, A. K. Photochem. Photobiol. Sci. 2006, 5, 459–466. doi:10.1039/b600009f
Return to citation in text: [1] [2] [3] -
Birks, J. B.; Christophorou, L. G. Proc. R. Soc. London, Ser. A 1964, 277, 571–582. doi:10.1098/rspa.1964.0041
Return to citation in text: [1] -
Ikeda, M.; Takeuchi, M.; Shinkai, S. Chem. Commun. 2003, 1354–1355. doi:10.1039/b302415f
Return to citation in text: [1] -
Nandy, R.; Sankararaman, S. Org. Biomol. Chem. 2010, 8, 2260–2266. doi:10.1039/b923441a
Return to citation in text: [1] -
Praefcke, K.; Kohne, B.; Singer, D. Angew. Chem., Int. Ed. Engl. 1990, 29, 177–179. doi:10.1002/anie.199001771
Return to citation in text: [1] -
Matsumoto, H.; Kyushin, S.; Negishi, K.; Ono, Y.; Uchida, M.; Oomori, H. Triphenylene compounds, method of manufacturing the same and organic electroluminescent devices employing the same. US 2008/0265750 A1, Oct 30, 2008.
Return to citation in text: [1] -
Huang, C.-C.; Lin, Y.-C.; Lin, P.-Y.; Chen, Y.-J. Eur. J. Org. Chem. 2006, 4510–4518. doi:10.1002/ejoc.200600380
Return to citation in text: [1] -
Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kulwer Academic: New York, 1999; pp 187–194.
(See for Lippert equation and its applications.)
Return to citation in text: [1] -
Lippert, E. V. Z. Elektrochem. 1957, 61, 962–975.
Return to citation in text: [1] -
Mataga, K. N.; Kaifu, Y.; Koizumi, M. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. doi:10.1246/bcsj.29.465
Return to citation in text: [1] -
PC Spartan Pro, Version 1.1; Wavefunction, Inc: Irvine, CA, 1999.
Return to citation in text: [1] [2] -
Reichardt, C. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH: Weinheim, 2003; p 329.
Return to citation in text: [1] -
Reichardt, C. Chem. Rev. 1994, 94, 2319–2358. doi:10.1021/cr00032a005
Return to citation in text: [1] -
Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kulwer Academic: New York, 1999; p 551.
Return to citation in text: [1] -
Beeson, J. C.; Huston, M. E.; Pollard, D. A.; Venkatachalam, T. K.; Czarnik, A. W. J. Fluoresc. 1993, 3, 65–68. doi:10.1007/BF00865319
Return to citation in text: [1] -
Duke, R. M.; Gunnlaugsson, T. Tetrahedron Lett. 2007, 48, 8043–8047. doi:10.1016/j.tetlet.2007.09.026
Return to citation in text: [1] -
Schaefer, C. G.; Peters, K. S. J. Am. Chem. Soc. 1980, 102, 7566–7567. doi:10.1021/ja00545a030
Return to citation in text: [1] -
Peters, K. S.; Lee, J. J. Phys. Chem. 1993, 97, 3761–3764. doi:10.1021/j100117a022
Return to citation in text: [1] -
Manring, L. E.; Peters, K. S. J. Am. Chem. Soc. 1985, 107, 6452–6458. doi:10.1021/ja00309a004
Return to citation in text: [1]
| 49. | Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kulwer Academic: New York, 1999; p 551. |
| 50. | Beeson, J. C.; Huston, M. E.; Pollard, D. A.; Venkatachalam, T. K.; Czarnik, A. W. J. Fluoresc. 1993, 3, 65–68. doi:10.1007/BF00865319 |
| 51. | Duke, R. M.; Gunnlaugsson, T. Tetrahedron Lett. 2007, 48, 8043–8047. doi:10.1016/j.tetlet.2007.09.026 |
| 36. | Subuddhi, U.; Haldar, S.; Sankararaman, S.; Mishra, A. K. Photochem. Photobiol. Sci. 2006, 5, 459–466. doi:10.1039/b600009f |
| 47. | Reichardt, C. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH: Weinheim, 2003; p 329. |
| 48. | Reichardt, C. Chem. Rev. 1994, 94, 2319–2358. doi:10.1021/cr00032a005 |
| 1. | Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kulwer Academic: New York, 1999. |
| 2. | Goldys, E. M., Ed. Fluorescence Applications in Biotechnology and the Life Sciences; Wiley Blackwell: New Jersey, 2009. |
| 3. | Giepmans, B. N. G.; Adams, S. R.; Ellisman, M. H.; Tsien, R. Y. Science 2006, 312, 217–224. |
| 4. | Baker, A. Environ. Sci. Technol. 2001, 35, 948–953. doi:10.1021/es000177t |
| 16. | Kim, S. H.; Kim, J. S.; Park, S. M.; Chang, S.-K. Org. Lett. 2006, 8, 371–374. doi:10.1021/ol052282j |
| 17. | Jung, H. S.; Park, M.; Han, D. Y.; Kim, E.; Lee, C.; Ham, S.; Kim, J. S. Org. Lett. 2009, 11, 3378–3381. doi:10.1021/ol901221q |
| 18. | Lee, S. H.; Kim, S. H.; Kim, S. K.; Jung, J. H.; Kim, J. S. J. Org. Chem. 2005, 70, 9288–9295. doi:10.1021/jo051302s |
| 19. | Park, S. Y.; Yoon, J. H.; Hong, C. S.; Souane, R.; Kim, J. S.; Matthews, S. E.; Vicens, J. J. Org. Chem. 2008, 73, 8212–8218. doi:10.1021/jo8012918 |
| 11. | Zhang, L.; Clark, R. J.; Zhu, L. Chem.–Eur. J. 2008, 14, 2894–2903. doi:10.1002/chem.200701419 |
| 12. | Andrew, T. L.; Swager, T. M. J. Am. Chem. Soc. 2007, 129, 7254–7255. doi:10.1021/ja071911c |
| 13. | Dale, T. J.; Rebek, J., Jr. J. Am. Chem. Soc. 2006, 128, 4500–4501. doi:10.1021/ja057449i |
| 14. | Fan, L.-J.; Jones, W. E., Jr. J. Phys. Chem. B 2006, 110, 7777–7782. doi:10.1021/jp056381q |
| 15. | Kim, S. K.; Yoon, J. Chem. Commun. 2002, 770–771. doi:10.1039/b110139k |
| 8. | Thiagarajan, V.; Ramamurthy, P.; Thirumalai, D.; Ramakrishnan, V. T. Org. Lett. 2005, 7, 657–660. doi:10.1021/ol047463k |
| 9. | Badugu, R.; Lakowicz, J. R.; Geddes, C. D. J. Am. Chem. Soc. 2005, 127, 3635–3641. doi:10.1021/ja044421i |
| 10. | Srikun, D.; Miller, E. W.; Domaille, D. W.; Chang, C. J. J. Am. Chem. Soc. 2008, 130, 4596–4597. doi:10.1021/ja711480f |
| 11. | Zhang, L.; Clark, R. J.; Zhu, L. Chem.–Eur. J. 2008, 14, 2894–2903. doi:10.1002/chem.200701419 |
| 36. | Subuddhi, U.; Haldar, S.; Sankararaman, S.; Mishra, A. K. Photochem. Photobiol. Sci. 2006, 5, 459–466. doi:10.1039/b600009f |
| 31. | Yang, S.-W.; Elangovan, A.; Hwang, K.-C.; Ho, T.-I. J. Phys. Chem. B 2005, 109, 16628–16635. doi:10.1021/jp052086u |
| 5. | Loudet, A.; Burgess, K. Chem. Rev. 2007, 107, 4891–4932. |
| 6. | Ulrich, G.; Ziessel, R.; Harriman, A. Angew. Chem., Int. Ed. 2008, 47, 1184–1201. doi:10.1002/anie.200702070 |
| 7. | Kowada, T.; Yamaguchi, S.; Ohe, K. Org. Lett. 2010, 12, 296–299. doi:10.1021/ol902631d |
| 43. |
Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kulwer Academic: New York, 1999; pp 187–194.
(See for Lippert equation and its applications.) |
| 44. | Lippert, E. V. Z. Elektrochem. 1957, 61, 962–975. |
| 45. | Mataga, K. N.; Kaifu, Y.; Koizumi, M. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. doi:10.1246/bcsj.29.465 |
| 31. | Yang, S.-W.; Elangovan, A.; Hwang, K.-C.; Ho, T.-I. J. Phys. Chem. B 2005, 109, 16628–16635. doi:10.1021/jp052086u |
| 32. | Kim, H. M.; Lee, Y. O.; Lim, C. S.; Kim, J. S.; Cho, B. R. J. Org. Chem. 2008, 73, 5127–5130. doi:10.1021/jo800363v |
| 33. | Oh, J.-W.; Lee, Y. O.; Kim, T. H.; Ko, K. C.; Lee, J. Y.; Kim, H.; Kim, J. S. Angew. Chem., Int. Ed. 2009, 48, 2522–2524. doi:10.1002/anie.200804669 |
| 34. | Dobkowski, J.; Retting, W.; Waluk, J. Phys. Chem. Chem. Phys. 2002, 4, 4334–4339. doi:10.1039/b204749g |
| 35. | Ji, S.; Yang, J.; Yang, Q.; Liu, S.; Chen, M.; Zhao, J. J. Org. Chem. 2009, 74, 4855–4865. doi:10.1021/jo900588e |
| 36. | Subuddhi, U.; Haldar, S.; Sankararaman, S.; Mishra, A. K. Photochem. Photobiol. Sci. 2006, 5, 459–466. doi:10.1039/b600009f |
| 40. | Praefcke, K.; Kohne, B.; Singer, D. Angew. Chem., Int. Ed. Engl. 1990, 29, 177–179. doi:10.1002/anie.199001771 |
| 41. | Matsumoto, H.; Kyushin, S.; Negishi, K.; Ono, Y.; Uchida, M.; Oomori, H. Triphenylene compounds, method of manufacturing the same and organic electroluminescent devices employing the same. US 2008/0265750 A1, Oct 30, 2008. |
| 42. | Huang, C.-C.; Lin, Y.-C.; Lin, P.-Y.; Chen, Y.-J. Eur. J. Org. Chem. 2006, 4510–4518. doi:10.1002/ejoc.200600380 |
| 26. | Yuasa, H.; Miyagawa, N.; Izumi, T.; Nakatani, M.; Izumi, M.; Hashimoto, H. Org. Lett. 2004, 6, 1489–1492. doi:10.1021/ol049628v |
| 27. | Kim, S. K.; Bok, J. H.; Bartsch, R. A.; Lee, J. Y.; Kim, J. S. Org. Lett. 2005, 7, 4839–4842. doi:10.1021/ol051609d |
| 28. | Kim, J. S.; Quang, D. T. Chem. Rev. 2007, 107, 3780–3799. |
| 29. | Strauss, J.; Daub, J. Org. Lett. 2002, 4, 683–686. doi:10.1021/ol0170354 |
| 30. | Cho, H. K.; Lee, D. H.; Hong, J.-I. Chem. Commun. 2005, 1690–1692. doi:10.1039/b417845a |
| 23. | Berlman, I. B. Handbook of Fluorescence Spectra of Aromatic Molecules; Academic Press: New York, 1965; p 173. |
| 31. | Yang, S.-W.; Elangovan, A.; Hwang, K.-C.; Ho, T.-I. J. Phys. Chem. B 2005, 109, 16628–16635. doi:10.1021/jp052086u |
| 32. | Kim, H. M.; Lee, Y. O.; Lim, C. S.; Kim, J. S.; Cho, B. R. J. Org. Chem. 2008, 73, 5127–5130. doi:10.1021/jo800363v |
| 23. | Berlman, I. B. Handbook of Fluorescence Spectra of Aromatic Molecules; Academic Press: New York, 1965; p 173. |
| 24. | Venkataramana, G.; Sankararaman, S. Eur. J. Org. Chem. 2005, 4162–4166. doi:10.1002/ejoc.200500222 |
| 25. | Venkataramana, G.; Sankararaman, S. Org. Lett. 2006, 8, 2739–2742. doi:10.1021/ol060767h |
| 52. | Schaefer, C. G.; Peters, K. S. J. Am. Chem. Soc. 1980, 102, 7566–7567. doi:10.1021/ja00545a030 |
| 53. | Peters, K. S.; Lee, J. J. Phys. Chem. 1993, 97, 3761–3764. doi:10.1021/j100117a022 |
| 54. | Manring, L. E.; Peters, K. S. J. Am. Chem. Soc. 1985, 107, 6452–6458. doi:10.1021/ja00309a004 |
| 20. | Beinhoff, M.; Weigel, W.; Jurczok, M.; Rettig, W.; Modrakowski, C.; Brüdgam, I.; Hartl, H.; Schlüter, A. D. Eur. J. Org. Chem. 2001, 3819–3829. doi:10.1002/1099-0690(200110)2001:20<3819::AID-EJOC3819>3.0.CO;2-W |
| 21. | Beinhoff, M.; Weigel, W.; Rettig, W.; Bruedgam, I.; Hartl, H.; Schlüter, A. D. J. Org. Chem. 2005, 70, 6583–6591. doi:10.1021/jo050302p |
| 22. |
Techert, S.; Wiessner, A.; Schmatz, S.; Staerk, H. J. Phys. Chem. B 2001, 105, 7579–7587. doi:10.1021/jp004370l
and references 2-3 cited therein. |
| 37. | Birks, J. B.; Christophorou, L. G. Proc. R. Soc. London, Ser. A 1964, 277, 571–582. doi:10.1098/rspa.1964.0041 |
| 38. | Ikeda, M.; Takeuchi, M.; Shinkai, S. Chem. Commun. 2003, 1354–1355. doi:10.1039/b302415f |
| 39. | Nandy, R.; Sankararaman, S. Org. Biomol. Chem. 2010, 8, 2260–2266. doi:10.1039/b923441a |
| 31. | Yang, S.-W.; Elangovan, A.; Hwang, K.-C.; Ho, T.-I. J. Phys. Chem. B 2005, 109, 16628–16635. doi:10.1021/jp052086u |
| 32. | Kim, H. M.; Lee, Y. O.; Lim, C. S.; Kim, J. S.; Cho, B. R. J. Org. Chem. 2008, 73, 5127–5130. doi:10.1021/jo800363v |
| 33. | Oh, J.-W.; Lee, Y. O.; Kim, T. H.; Ko, K. C.; Lee, J. Y.; Kim, H.; Kim, J. S. Angew. Chem., Int. Ed. 2009, 48, 2522–2524. doi:10.1002/anie.200804669 |
| 34. | Dobkowski, J.; Retting, W.; Waluk, J. Phys. Chem. Chem. Phys. 2002, 4, 4334–4339. doi:10.1039/b204749g |
| 35. | Ji, S.; Yang, J.; Yang, Q.; Liu, S.; Chen, M.; Zhao, J. J. Org. Chem. 2009, 74, 4855–4865. doi:10.1021/jo900588e |
© 2010 Nandy and Sankararaman; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)