Abstract
A bis-imine (prepared via a new FeCl3-based method) in combination with CoCl2 facilitated lipase-mediated acetylation of the (R)-isomer of a racemic benzylic secondary alcohol with 91% ees. The methodology was used for the preparation of the known drug rivastigmine.
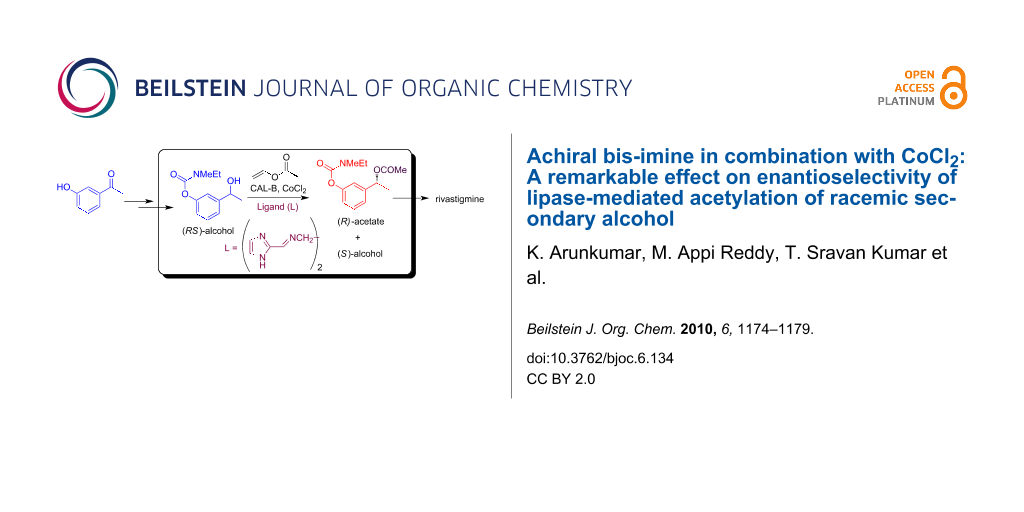
Graphical Abstract
Introduction
The development and use of newer synthetic methods for the stereoselective synthesis of chiral molecules have increased enormously in recent years especially in the chemical and pharmaceutical industry [1]. Biocatalysis, being an environmentally friendly process, has attracted particular attention for this purpose [2-6]. For example, high enantioselectivity was observed in lipase-mediated preparation of alcohols and amines [7-9]. These biocatalysts work under mild reaction conditions, and their immobilized forms, being stable in organic solvents, have allowed an easy separation of products and the potential recycling of the enzyme, thereby enhancing their economic viability [10,11]. Recently, we have observed that achiral bis-imines in combination with CoCl2 improved the enantioselectivity substantially in CAL-B (Candida antarctica lipase B) [12,13] mediated acetylation of a racemic secondary alcohol with vinyl acetate. Here we report our preliminary results on the synthesis and identification of a novel ligand for this process (Scheme 1) and its application in the preparation of the known drug rivastigmine [14]. While the uses of bis-imine/transition-metal complexes have been reported for the enantioselective synthesis of chiral compounds [15-19], their use as activators in an enzymatic reaction has not been previously explored.
Scheme 1: Lipase-catalyzed acetylation of racemic benzylic secondary alcohol [(RS)-4] and its application.
Scheme 1: Lipase-catalyzed acetylation of racemic benzylic secondary alcohol [(RS)-4] and its application.
Results and Discussion
The report that bis-imine/Cu(I)-complexes were able to promote the direct and enantioselective addition of imines to alkylacetylenes [15] prompted us to evaluate a variety of bis-imines in combination with CoCl2 [16] in the lipase-mediated acetylation of a benzylic secondary alcohols. Accordingly, a number of achiral bis-imines were prepared and used to generate the desired complex. While a number of methods have been reported to prepare Schiff bases by reacting an amine with a carbonyl compound [20-24], in our hands the reaction of 1,2-amines with aromatic aldehydes under these reaction conditions provided a mixture of mono and bis-Schiff bases. We therefore developed a new and efficient method for the preparation of bis-imines 3 by reacting ethane-1,2-diamine (1) with a number of aryl and heteroaryl aldehydes 2 in the presence of anhydrous FeCl3 (Scheme 2, Table 1). Aldehydes containing electron donating (e.g., methoxy, hydroxy, fluoro and bromo) or withdrawing groups (e.g., nitro) were found to be equally effective in terms of product yields. The reactions were completed within 30 min in most cases.
Scheme 2: FeCl3-meditated synthesis of bis-imines.
Scheme 2: FeCl3-meditated synthesis of bis-imines.
Table 1: FeCl3-meditated synthesis of bis-imines (3) from (hetero)aryl aldehydes (2).
| Entry | Aldehyde (2) | Product (3) | Yielda (%) | Reaction Time |
|---|---|---|---|---|
| 1 |
2a |
3a |
90 | 0.5 |
| 2 |
2b |
3b |
90 | 0.15 |
| 3 |
2c |
3c |
88 | 0.5 |
| 4 |
2d |
3d |
80 | 0.5 |
| 5 |
2e |
3e |
80 | 0.5 |
| 6 |
2f |
3f |
88 | 0.5 |
| 7 |
2g |
3g |
80 | 0.5 |
| 8 |
2h |
3h |
85 | 0.5 |
| 9 |
2i |
3i |
90 | 0.5 |
| 10 |
2j |
3j |
90 | 0.5 |
aIsolated yield.
All bis-imines prepared were then screened in combination with CoCl2 for CAL-B mediated acetylation of a racemic secondary alcohol. Thus, 3-(1-hydroxyethyl)phenyl ethyl(methyl)carbamate [(RS)-4], prepared via the reaction of 3-hydroxyacetophenone (6) with N-ethyl-N-methylcarbamoyl chloride followed by reduction with NaBH4 (Scheme 3), was selected for our purpose. Subsequently, the enzymatic processes were carried out and the isolated reaction mixture was analysed by chiral HPLC.
Scheme 3: Preparation of racemic 3-(1-hydroxyethyl)phenyl ethyl(methyl)carbamate [(RS)-4]
Scheme 3: Preparation of racemic 3-(1-hydroxyethyl)phenyl ethyl(methyl)carbamate [(RS)-4]
Initially, the CAL-B mediated acetylation of (RS)-4 was carried out in the absence of any ligand and CoCl2. Vinyl acetate was used as a solvent as well as the acyl donor. No reaction was observed at room temperature even after 48 h. An increase in reaction temperature to 50–55 °C for 24 h facilitated the acetylation, however, the selectivity was not greater than 30%. In order to achieve better selectivity, we assessed the use of achiral bis-imines in combination with CoCl2 (Table 2). The reactions were complete within 10 h when diarylidene-ethane-1,2-diamines were used (entries 1–8, Table 2). While 35% enantiomeric excess was achieved in some of these cases (entries 2, 3 and 5, Table 2), the best results, however, were obtained with bis(heteroarylmethylene)ethane-1,2-diamines (entries 9 and 10, Table 2), especially 3i. The bis-imine 3i facilitated enantioselective acetylation of the (R)-isomer over the (S)-antipode with high enantiomeric excess (91% ees) and yield (80%). The reaction was complete within 12 h. The absolute configuration of the resolved chiral alcohol and its acetate was in accordance with Kazlauskas’ rule [25] (see Supporting Information File 1 for optical rotation values).
Table 2: Screening of bis-imines as achiral ligands in CAL-B mediated acetylation of (RS)-4 (step 1, Scheme 1)a.
| Entry | Ligand 3 | Time (h) | ees | eep | Conversionb (%) | Ec |
|---|---|---|---|---|---|---|
| 1 | 3a | 10 | 0 | 0 | 0 | 0 |
| 2 | 3b | 5 | 35 | >99 | ~26 | >200 |
| 3 | 3c | 5 | 35 | >99 | ~26 | >200 |
| 4 | 3d | 10 | 0 | 0 | 0 | 0 |
| 5 | 3e | 5 | 35 | >99 | ~26 | >200 |
| 6 | 3f | 5 | 0 | 0 | 0 | 0 |
| 7 | 3g | 5 | 0 | 0 | 0 | 0 |
| 8 | 3h | 5 | 0 | 0 | 0 | 0 |
| 9 | 3i | 12 | 91 | >99 | ~48 | >500 |
| 10 | 3j | 10 | 83 | >99 | ~46 | >300 |
aAll the reactions were carried out at 1.0 g scale of (RS)-4 with vinyl acetate (20 mL) as acyl donor, in the presence of CAL-B (150 mg), bis-imine (3, 0.3 mmol) and CoCl2 (0.3 mmol); ees = enantiomeric excess of substrate (the ees is mentioned as the enzyme is active with only one enantiomer) and eep = enantiomeric excess of product. Both ees and eep were determined by HPLC [column: chiralpak IC (250 x 4.6 mm, 5.0 μm), mobile phase A: 0.05% TFA in water, mobile phase B: n-hexane: IPA (80:20), concentration: 0.5 mg/mL, diluent: ethanol, run time: 30.0 min, temperature: 25 °C, flow: 1.0 mL/min, UV: 220 nm]. bConversion = ees/(ees + eep). cE = {ln[eep(1-ees)]/(eep+ees)}/{ln[eep(1 + ees)]/(eep + ees)}.
Mechanistically [12], the special H-bonding rearrangement of the “catalytic triad” (i.e., serine, histidine, and aspartate) at the active site of CAL-B increases the nucleophilicity of the serine residue. This then interacts with the carbonyl group of the vinyl acetate to form the “acyl-enzyme intermediate” T-1 (Scheme 4) which finally transfers the acyl group to the substrate alcohol 4 via T-2, affording the desired product 5. The CoCl2 in combination with 3i perhaps forms a tight complex with T-1 as well as 4 which facilitates the acyl transfer process (Scheme 4). However, the reason for selective acylation was not clearly understood. It was speculated that the orientation of the hydroxy group of the (R)-isomer was possibly in the proximal position of the acyl-transfer site and the imidazole moiety for proton abstraction.
Scheme 4: Proposed reaction mechanism of lipase-catalyzed acetylation of racemic alcohol 4.
Scheme 4: Proposed reaction mechanism of lipase-catalyzed acetylation of racemic alcohol 4.
Finally, application of this methodology was demonstrated in preparing the well-known drug rivastigmine which has been used to treat mild to moderate dementia associated with Alzheimer’s and Parkinson’s disease. Thus the enantiopure acetate (R)-5 was treated with excess of dimethylamine in toluene to afford the desired (S)-8 [(S)-rivastigmine] in 60% yield (final step, Scheme 5). Notably, the earlier method for the synthesis of (S)-8 involved asymmetric reduction of the ketone 6 to give the alcohol with the required chirality followed by mesylation and subsequent treatment with dimethylamine [26,27].
Scheme 5: Complete synthesis of rivastigmine.
Scheme 5: Complete synthesis of rivastigmine.
Conclusion
We have developed a novel lipase-based method for acetylation of a benzylic secondary alcohol with high enantioselectivity and yield. The methodology involves the use of CoCl2 in combination with a bis-imine (prepared via a new FeCl3-based method) and its application has been demonstrated in preparing rivastigmine.
Supporting Information
| Supporting Information File 1: Experimental procedures and spectral data. | ||
| Format: PDF | Size: 171.3 KB | Download |
References
-
Carey, J. S.; Laffan, D.; Thomson, C.; Williams, M. T. Org. Biomol. Chem. 2006, 4, 2337–2347. doi:10.1039/b602413k
Return to citation in text: [1] -
Straathof, J. J.; Panke, S.; Schmid, A. Curr. Opin. Biotechnol. 2002, 13, 548–556. doi:10.1016/S0958-1669(02)00360-9
Return to citation in text: [1] -
Panke, S.; Held, M.; Wubbolts, M. Curr. Opin. Biotechnol. 2004, 15, 272–279. doi:10.1016/j.copbio.2004.06.011
Return to citation in text: [1] -
Gotor-Fernandez, V.; Gotor, V. Use of lipases in organic synthesis. In Industrial enzymes: Structure, function and applications; Polaina, J.; MacCabe, A. P., Eds.; Chapter 18; Springer: Dordrecht, The Netherlands, 2007; pp 301–315.
Return to citation in text: [1] -
Ran, N.; Zhao, L.; Chen, Z.; Tao, J. Green Chem. 2008, 10, 361–372. doi:10.1039/b716045c
Return to citation in text: [1] -
Woodley, J. M. Trends Biotechnol. 2008, 26, 321–327. doi:10.1016/j.tibtech.2008.03.004
Return to citation in text: [1] -
Gotor-Fernandez, V.; Gotor, V.; Rebolledo, F. Preparation of Chiral Pharmaceuticals through Enzymatic Acylation of Alcohols and Amines. In Biocatalysis in the Pharmaceutical and Biotechnology Industries; Patel, R. N., Ed.; Chapter 7; CRC Press: Boca Raton, 2007; pp 203–248.
Return to citation in text: [1] -
Gotor-Fernandez, V.; Brieva, R.; Gotor, V. J. Mol. Catal. B: Enzym. 2006, 40, 111–120. doi:10.1016/j.molcatb.2006.02.010
Return to citation in text: [1] -
Patel, R. N. Coord. Chem. Rev. 2008, 252, 659–701. doi:10.1016/j.ccr.2007.10.031
Return to citation in text: [1] -
Ghanem, A.; Aboul-Enein, H. Y. Tetrahedron: Asymmetry 2004, 15, 3331–3351. doi:10.1016/j.tetasy.2004.09.019
Return to citation in text: [1] -
Ghanem, A. Tetrahedron 2007, 63, 1721–1754. doi:10.1016/j.tet.2006.09.110
Return to citation in text: [1] -
Pàmies, O.; Bäckvall, J.-E. Chem. Rev. 2003, 103, 3247–3262. doi:10.1021/cr020029g
Return to citation in text: [1] [2] -
García-Urdiales, E.; Rebolledo, F.; Gotor, V. Tetrahedron: Asymmetry 2001, 12, 3047–3052. doi:10.1016/S0957-4166(01)00532-8
Return to citation in text: [1] -
Jann, M. W. Pharmacotherapy 2000, 20, 1–12. doi:10.1592/phco.20.1.1.34664
Return to citation in text: [1] -
Colombo, F.; Benaglia, M.; Orlandi, S.; Usuelli, F.; Celentano, G. J. Org. Chem. 2006, 71, 2064–2070. doi:10.1021/jo052481g
Return to citation in text: [1] [2] -
Iqbal, J.; Mukhopadhyay, M.; Mandal, A. K. Synlett 1997, 876–886. doi:10.1055/s-1997-924
Return to citation in text: [1] [2] -
Saito, T.; Takekawa, K.; Nishimura, J.-I.; Kawamura, M. J. Chem. Soc., Perkin Trans. 1 1997, 2957–2960. doi:10.1039/a703590j
Return to citation in text: [1] -
Evans, D. A.; Lectka, T.; Miller, S. J. Tetrahedron Lett. 1993, 34, 7027–7030. doi:10.1016/S0040-4039(00)61588-5
Return to citation in text: [1] -
Li, Z.; Conser, K. R.; Jacobsen, E. N. J. Am. Chem. Soc. 1993, 115, 5326–5327. doi:10.1021/ja00065a067
Return to citation in text: [1] -
Vazzana, I.; Terranova, E.; Mattioli, F.; Sparatore, F. ARKIVOC 2004, v, 364–374.
Return to citation in text: [1] -
Sridhar, S. K.; Saravanan, M.; Ramesh, A. Eur. J. Med. Chem. 2001, 36, 615–625. doi:10.1016/S0223-5234(01)01255-7
Return to citation in text: [1] -
Karupaiyan, K.; Puranik, V. G.; Deshmukh, A. R. A. S.; Bhawal, B. M. Tetrahedron 2000, 56, 8555–8560. doi:10.1016/S0040-4020(00)00800-0
Return to citation in text: [1] -
Kunz, H.; Pfrengle, W.; Rück, K.; Sager, W. Synthesis 1991, 1039–1042. doi:10.1055/s-1991-26641
Return to citation in text: [1] -
Kunz, H.; Sager, W. Angew. Chem., Int. Ed. Engl. 1987, 26, 557–559. doi:10.1002/anie.198705571
Return to citation in text: [1] -
Kazlauskas, R. J.; Weissfloch, A. N. E.; Rappaport, A. T.; Cuccia, L. A. J. Org. Chem. 1991, 56, 2656–2665. doi:10.1021/jo00008a016
Return to citation in text: [1] -
Gaitonde, A.; Mangle, M.; Pawar, S. R. Novel processes for the preparation of aminoalkyl phenylcarbamates. World Pat. Appl. WO2005/061446, July 7, 2005.
Return to citation in text: [1] -
Fieldhouse, R. Process for the preparation of tertiary amines attached to a secondary carbon centre. World Pat. Appl. WO2005/058804, June 30, 2005.
Return to citation in text: [1]
| 1. | Carey, J. S.; Laffan, D.; Thomson, C.; Williams, M. T. Org. Biomol. Chem. 2006, 4, 2337–2347. doi:10.1039/b602413k |
| 12. | Pàmies, O.; Bäckvall, J.-E. Chem. Rev. 2003, 103, 3247–3262. doi:10.1021/cr020029g |
| 13. | García-Urdiales, E.; Rebolledo, F.; Gotor, V. Tetrahedron: Asymmetry 2001, 12, 3047–3052. doi:10.1016/S0957-4166(01)00532-8 |
| 10. | Ghanem, A.; Aboul-Enein, H. Y. Tetrahedron: Asymmetry 2004, 15, 3331–3351. doi:10.1016/j.tetasy.2004.09.019 |
| 11. | Ghanem, A. Tetrahedron 2007, 63, 1721–1754. doi:10.1016/j.tet.2006.09.110 |
| 7. | Gotor-Fernandez, V.; Gotor, V.; Rebolledo, F. Preparation of Chiral Pharmaceuticals through Enzymatic Acylation of Alcohols and Amines. In Biocatalysis in the Pharmaceutical and Biotechnology Industries; Patel, R. N., Ed.; Chapter 7; CRC Press: Boca Raton, 2007; pp 203–248. |
| 8. | Gotor-Fernandez, V.; Brieva, R.; Gotor, V. J. Mol. Catal. B: Enzym. 2006, 40, 111–120. doi:10.1016/j.molcatb.2006.02.010 |
| 9. | Patel, R. N. Coord. Chem. Rev. 2008, 252, 659–701. doi:10.1016/j.ccr.2007.10.031 |
| 26. | Gaitonde, A.; Mangle, M.; Pawar, S. R. Novel processes for the preparation of aminoalkyl phenylcarbamates. World Pat. Appl. WO2005/061446, July 7, 2005. |
| 27. | Fieldhouse, R. Process for the preparation of tertiary amines attached to a secondary carbon centre. World Pat. Appl. WO2005/058804, June 30, 2005. |
| 2. | Straathof, J. J.; Panke, S.; Schmid, A. Curr. Opin. Biotechnol. 2002, 13, 548–556. doi:10.1016/S0958-1669(02)00360-9 |
| 3. | Panke, S.; Held, M.; Wubbolts, M. Curr. Opin. Biotechnol. 2004, 15, 272–279. doi:10.1016/j.copbio.2004.06.011 |
| 4. | Gotor-Fernandez, V.; Gotor, V. Use of lipases in organic synthesis. In Industrial enzymes: Structure, function and applications; Polaina, J.; MacCabe, A. P., Eds.; Chapter 18; Springer: Dordrecht, The Netherlands, 2007; pp 301–315. |
| 5. | Ran, N.; Zhao, L.; Chen, Z.; Tao, J. Green Chem. 2008, 10, 361–372. doi:10.1039/b716045c |
| 6. | Woodley, J. M. Trends Biotechnol. 2008, 26, 321–327. doi:10.1016/j.tibtech.2008.03.004 |
| 16. | Iqbal, J.; Mukhopadhyay, M.; Mandal, A. K. Synlett 1997, 876–886. doi:10.1055/s-1997-924 |
| 25. | Kazlauskas, R. J.; Weissfloch, A. N. E.; Rappaport, A. T.; Cuccia, L. A. J. Org. Chem. 1991, 56, 2656–2665. doi:10.1021/jo00008a016 |
| 15. | Colombo, F.; Benaglia, M.; Orlandi, S.; Usuelli, F.; Celentano, G. J. Org. Chem. 2006, 71, 2064–2070. doi:10.1021/jo052481g |
| 12. | Pàmies, O.; Bäckvall, J.-E. Chem. Rev. 2003, 103, 3247–3262. doi:10.1021/cr020029g |
| 15. | Colombo, F.; Benaglia, M.; Orlandi, S.; Usuelli, F.; Celentano, G. J. Org. Chem. 2006, 71, 2064–2070. doi:10.1021/jo052481g |
| 16. | Iqbal, J.; Mukhopadhyay, M.; Mandal, A. K. Synlett 1997, 876–886. doi:10.1055/s-1997-924 |
| 17. | Saito, T.; Takekawa, K.; Nishimura, J.-I.; Kawamura, M. J. Chem. Soc., Perkin Trans. 1 1997, 2957–2960. doi:10.1039/a703590j |
| 18. | Evans, D. A.; Lectka, T.; Miller, S. J. Tetrahedron Lett. 1993, 34, 7027–7030. doi:10.1016/S0040-4039(00)61588-5 |
| 19. | Li, Z.; Conser, K. R.; Jacobsen, E. N. J. Am. Chem. Soc. 1993, 115, 5326–5327. doi:10.1021/ja00065a067 |
| 20. | Vazzana, I.; Terranova, E.; Mattioli, F.; Sparatore, F. ARKIVOC 2004, v, 364–374. |
| 21. | Sridhar, S. K.; Saravanan, M.; Ramesh, A. Eur. J. Med. Chem. 2001, 36, 615–625. doi:10.1016/S0223-5234(01)01255-7 |
| 22. | Karupaiyan, K.; Puranik, V. G.; Deshmukh, A. R. A. S.; Bhawal, B. M. Tetrahedron 2000, 56, 8555–8560. doi:10.1016/S0040-4020(00)00800-0 |
| 23. | Kunz, H.; Pfrengle, W.; Rück, K.; Sager, W. Synthesis 1991, 1039–1042. doi:10.1055/s-1991-26641 |
| 24. | Kunz, H.; Sager, W. Angew. Chem., Int. Ed. Engl. 1987, 26, 557–559. doi:10.1002/anie.198705571 |
© 2010 Arunkumar et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)