Abstract
Oligosaccharides are currently recognised as having functions that influence the entire spectrum of cell activities. However, a distinct disadvantage of naturally occurring oligosaccharides is their metabolic instability in biological systems. Therefore, much effort has been spent in the past two decades on the development of feasible routes to carbohydrate mimetics which can compete with their O-glycosidic counterparts in cell surface adhesion, inhibit carbohydrate processing enzymes, and interfere in the biosynthesis of specific cell surface carbohydrates. Such oligosaccharide mimetics are potential therapeutic agents against HIV and other infections, against cancer, diabetes and other metabolic diseases. An efficient strategy to access this type of compounds is the replacement of the glycosidic linkage by amide or pseudoamide functions such as thiourea, urea and guanidine. In this review we summarise the advances over the last decade in the synthesis of oligosaccharide mimetics that possess amide and pseudoamide linkages, as well as studies focussing on their supramolecular and recognition properties.
Graphical Abstract
Review
Among the major classes of biomolecules, carbohydrates are characterized by nearly unlimited structural diversity. Monosaccharide units can combine to produce oligosaccharides in a number of permutations that increases rapidly with the number of units present, more so than is the case with other biomolecules such as polypeptides or oligonucleotides. This is determined by the stereochemical identity of the monosaccharide units present (e.g. glucose, galactose, mannose), their glycosidic linkage positions (e.g. 1→4, 1→6), the nature at anomeric centres (α or β), the presence of additional substituents such as sulfate or acyl groups and the overall degree of branching. The molecular diversity of oligosaccharide offers a valuable tool for drug discovery in the areas of biologically important oligosaccharides, glycoconjugates and molecular scaffolds by investigating their structural and functional impact. Currently, oligosaccharides are known to have functions in a broad variety of cell–cell interactions related to invading bacteria, viruses and cancer cells [1-4] and to play central roles in post-translational modifications of proteins [5-7], cell–cell communication [8] and immune response to pathogens [7,9-11]. However, the application of oligosaccharides as potential therapeutic agents has its main drawback in the low instability of natural carbohydrates in biological systems. In addition, solid phase synthesis of oligosaccharides is not yet efficient enough for generating oligosaccharide-based libraries that may be useful in the future for the discovery of new therapeutic drugs. Taking into account the increasing importance of glycobiology and the difficulties associated to the synthesis of carbohydrate-based libraries, several approaches based on the assembly of sugar building blocks through amide and pseudoamide linkages have been developed by different research groups over the last few years. Sugar aminoacids (SAAs, Figure 1) have extensively been used in the development of a large variety of molecules. In the past 10 years, some reviews on SAAs have summarised the different synthetic methods used to obtain such molecules [12-14] as well as their applications to access to a diversity of linear, branched and cyclic pseudooligosaccharides and glycomimetics often referred to as carbopeptoids [13-17]. In addition, SAAs have been widely used in the field of material sciences as carbohydrate-derived monomers for the design of novel chiral polyamides [18]. Nevertheless, these review articles mainly focus on amide-linked sugars and do not pay too much attention to other types of pseudosugars. A notable exception is the contribution by Wessel and Dias Lucas, who recently published an interesting review where oligosaccharide mimetics which deviate from the natural linking pattern were discussed [19]. This review also included pseudoamide-linked sugars (Figure 1).
Figure 1: Schematic representation of sugar aminoacids (SAAs) and (pseudo)amide oligosaccharide mimetics.
Figure 1: Schematic representation of sugar aminoacids (SAAs) and (pseudo)amide oligosaccharide mimetics.
In this current review we have tried to highlight the recent advances in the synthesis of oligosaccharide mimetics. We have focussed on amide and pseudoamide-linked oligosaccharides, and their molecular recognition properties. We intend this survey to be wide-ranging and cover the most recent trends in the field.
Amide-linked sugars: carbopeptoids
The replacement of the glycosidic oxygen atom by an amide group leads to amide-linked sugars (Figure 1), which are synthesised by the sequential coupling of SAAs to form a class of compounds commonly referred to as carbopeptoids. The use of the amide functional group to connect sugar building blocks is inspired by the structure of peptides and the potential of carbohydrates to reproduce the structural features and biological properties of these polymers [12,13,17]. Poor bioavailability and metabolic stability of peptides have resulted in significant limitations as drug candidates. Another general problem is the loss of the original conformation in the isolated peptide fragments present in natural proteins, where the rest of molecule fixes a specific spatial disposition. Furthermore, short linear peptides cannot be restricted to adopt a particular conformation that enables effective interaction with a receptor [20]. As a consequence, a wide variety of methods to restrict the conformational freedom has been developed. One approach to get round this problem is the isosteric replacement of the amide bond in the peptide with a suitable mimetic to induce a specific secondary structure. Recent investigations have demonstrated that incorporation of SAAs in peptide structures can circumvent the adverse properties of bioactive natural systems. Thus, SAAs containing amino and carboxylic functional groups, and a rigid ring system (pyran, furan, oxetane) have emerged as versatile and conformationally constrained building blocks for the formation of peptide and oligosaccharide mimetics using standard peptide coupling techniques in solution or in the solid phase. Exploitation of the rigidity and diversity of the sugar backbone should permit subtle modifications and the rational design of oligomeric derivatives with tendency to adopt specific compact conformations (foldamers).
Linear naturally-occurring SAA homo-oligomers
SAAs occur extensively in nature as subunits of oligosaccharides in cell walls such as N-acetylneuraminic 1 and muramic acids 2, as well as in some nucleoside antibiotics (e.g., gougerotin 3 and aspiculamycin 4) (Figure 2).
Figure 2: Natural SAAs structures and natural nucleosidic antibiotics.
Figure 2: Natural SAAs structures and natural nucleosidic antibiotics.
N-Acetylneuraminic acid is a readily available naturally occurring δ amino acid. By applying solid phase peptide methods on Rink resin with Fmoc protecting group chemistry [21,22], Gervay and co-workers have reported a series of (1→5) amide-linked sialooligomers ranging from dimeric to octameric species (Scheme 1) [23,24]. All of these were soluble in water, DMSO and methanol, which allowed NH/ND exchange NMR experiments and circular dichroism (CD) studies to be carried out. Combined structural studies showed that a tetramer is required for an ordered secondary structure [23,25].
Scheme 1: Synthetic route to the target amide-linked sialooligomers. (a) Fmoc-Cl, NaHCO3, H2O, dioxane, 0 °C. (b) Ac2O, Pyridine, 0 °C. (c) BOP, DIPEA. (d) NH2NH2·H2O, MeOH.
Scheme 1: Synthetic route to the target amide-linked sialooligomers. (a) Fmoc-Cl, NaHCO3, H2O, dioxane, 0 °C....
Linear non-naturally-occurring SAA homo-oligomers
The first examples of amide-linked SAA-pyranose oligomers were reported as long ago as 1975 by Fuchs and Lehman [26,27]. In 2002, Ichikawa and co-workers developed a synthetic methodology to access oligomers of glycoamino acids, a family of non-natural SAAs with a carboxyl group at C-1 position and an amino group replacing one of the hydroxyl groups at either the C-2, 3, 4 or 6 positions (Figure 3).
Figure 3: The general structure of glycoamino acids and their corresponding oligomers.
Figure 3: The general structure of glycoamino acids and their corresponding oligomers.
Glucose-type building blocks were prepared and used to construct β(1→2), β(1→3), β(1→4) and β(1→6)-linked homo-oligomers [28-30] that were found to form rigid secondary structures predetermined by the linkage position as evidenced from CD and NMR measurements. Moreover, conformational analysis by molecular modelling calculation on the β(1→2)-linked decamer 9 supported a helical arrangement, stabilised by a intramolecular hydrogen bonding in the form of a 16-membered ring, characteristic for β-peptides (Figure 4) [29]. Analogously, NMR and IR data of a β(1→6)-linked unsaturated glycamino acid tetramer showed a turn-like structure in chloroform solution [31].
Figure 4: Conformational analysis of the β(1→2)-amide-linked glucooligomer 9.
Figure 4: Conformational analysis of the β(1→2)-amide-linked glucooligomer 9.
Glycoamino acid-based carbopeptoids are resistant to glycosidases and exhibit interesting biological activities. Thus, the O-sulfated β(1→2) and β(1→6)-linked homo-oligomers effectively inhibit L-selectin-mediated cell adhesion, HIV infection and heparanase acitivity in a linkage-specific manner [28].
SAAs can have a wide range of structural diversity as a consequence of differing on ring sizes, structural manipulation (such as chain branching, deoxygenation, hydroxyl protection, unsaturation), relative position between amine and carboxylic function, and, perhaps most importantly, the spatial arrangement of the amine and acid moieties. The limitations of stereo-control of glycosidic linkages can be circumvented by their replacement by an amide function. Furthermore, as a result of the presence of amine and acid moieties, SAAs are suitable for conventional combinatorial synthesis in both solid and solution phase. Many of these aspects have been extensively studied by Fleet’s group over the last 10 years. Efficient synthetic procedures for protected and unprotected homo-oligomeric derivatives of D-arabino- (Figure 5) [32-34] , D-talo- [35], L-allo- [35], D-galacto- [36], D-allo- [37], D-lyxo- [38], L-xylo- [39] and L-ribo-configured [39] tetrahydrofuran (THF) amino acids were optimised in order to study the influence of ring configuration and protecting groups on the secondary structure in these carbopeptoids.
Figure 5: Short oligomeric chains of C-glycosyl D-arabino THF amino acid oligomers.
Figure 5: Short oligomeric chains of C-glycosyl D-arabino THF amino acid oligomers.
Short oligomeric chains of C-glycosyl β-D-arabino THF amino acids 14 and 15 (where the C-2 and C-5 substituents of the THF ring are cis to each other) exhibit a well defined repeating turn secondary structure stabilised by inter-residual hydrogen bonds, whereas the epimeric α-D-arabinofuranose oligomer 12 (with the C-2 and C-5 substituents in trans relative disposition) do not show any secondary structure in solution [34]. NMR and IR studies on D-galacto- [36] and D-allo-configured [37] oligomeric carbopeptoids demonstrated that the inversion of a single stereocentre on the THF ring can have more pronounced effects for solution conformations than the presence or absence of different protecting groups. Thus, 2,4-cis-THF-L-ribonate oligomers adopt hydrogen bond stabilised conformations whereas 2,4-trans-THF-L-xylonate oligomers do not. Additionally, a number of structurally related THF aminoacid oligomers were examined by chiroptical spectroscopy to aid interpretation of their conformational preferences. The use of CD, in addition to NMR and solution IR, enabled the classification of the conformations adopted by carbopeptoids as hydrogen bonded regular, non-hydrogen bonded regular and non-hydrogen bonded irregular [40]. “Regular” is used to define a conformation that is either hydrogen bonded or non-hydrogen bonded and not in equilibrium with other multiple conformations. If conformational exchange operates, the term “irregular” is used. For example, these studies demonstrated that an octameric chain of C-glycosyl α-D-lyxo furanose amino acids 16 adopts a regular hydrogen bonded conformation similar to an π-helix (Figure 6) [38], whereas α-D-lyxo [38], D-talo and L-lyxo tetramers have regular non-hydrogen bonded conformations [40]. Also, based on these techniques, Andreini et al. [41] have recently demonstrated that homo-oligomers of β-SAA (β-N-mannofuranosyl-3-ulosonic acid) adopt eight-membered ring hydrogen bonded double-turn regular conformations in solution.
Figure 6: (A) Stereoview of the minimized structure of compound 16 (produced by a 500 ps simulation) that most satisfies the 27 NOE derived distance constraints. (B) View looking from C to N terminus. Hydrogen bonds are indicated by dotted lines.
Figure 6: (A) Stereoview of the minimized structure of compound 16 (produced by a 500 ps simulation) that mos...
Chakraborty’s group has also prepared protected and unprotected homo-oligomeric derivatives of D-manno- [42] and D-gluco-configured [43] tetrahydrofuran (THF) amino acids and studied their conformational properties by chiroptical and NMR techniques. Although both hydroxyl-protected linear oligomers displayed well-defined turn structures, their unprotected counterparts resulted in a less robust and regular conformation. However, in some cases the studies of the conformational preference in carbopeptoids is quite challenging and must be argued by computational methods [38,44] and infrared ion-dip spectroscopy [45] in order to interpret the data from CD, IR and NMR experiments reliably.
To complement its research programme on foldamer design and because of the absence of structural investigations on linear homo-oligomers constrained by 4-membered rings, Fleet and co-workers undertook the synthesis of carbopeptoids based on the oxetane template (Figure 7) [46-48]. Conformational analysis was carried out for two oxetane β-SAA hexamers with the 6-deoxy-L-altro and D-arabino configurations by means of detailed NMR and IR studies in combination with molecular mechanics [49]. These studies identified a left-handed helical secondary structure for the 6-deoxy-L-altro-oxetane hexamer and a right-handed helical structure for the D-arabino-configured oxetane hexamer 21 (having the opposite absolute configuration at C-2 and C-3) stabilised in both instances by 10-membered ring hydrogen bond arrangement (Figure 8).
Figure 7: Structures of linear oxetane-β- and δ-SAA homo-oligomers 19–20.
Figure 7: Structures of linear oxetane-β- and δ-SAA homo-oligomers 19–20.
Figure 8: 10-Membered ring H-bonds in compound 21 consistent with NMR and modelling investigations.
Figure 8: 10-Membered ring H-bonds in compound 21 consistent with NMR and modelling investigations.
Similar conformational studies combined with CD spectroscopy have been carried out for homo-oligomers of silyl-protected δ-2,4-trans-oxetane-SAAs with different configurations. The results showed that 6-deoxy-L-altronate, D-fuconate and 6-deoxy-D-gulonate oligomers are characterised by irregular non-hydrogen bonded conformations whereas L-rhamnoate and D-lyxonate oligomers have regular conformations governed by sterical interactions [50]. In contrast to the δ-2,4-trans-oxetane-SAA oligomers, the δ-2,4-cis-oxetane-L-ribonate tetramer and hexamer adopted a repeating β-turn structure [51] dictated by internal 10-membered hydrogen bonded rings. Such conformations are similar to those reported for the δ-2,5-cis-oxetane-THF oligomers [37].
D’Onofrio et al. [52] have carried out solid phase synthesis of oligonucleotides conjugated at the 3′ terminus with (1→6)-amide-linked oligosaccharide mimics (Figure 9). The presence of the saccharide unit at the 3′-end of 18-mers significantly enhanced the stability of the oligonucleotides in bovine fetal serum, non-negatively interfering with their ability to form stable duplexes with complementary DNA strands, as evaluated by UV thermal denaturation studies.
Figure 9: General structure of carbopeptoid-oligonucleotide conjugates.
Figure 9: General structure of carbopeptoid-oligonucleotide conjugates.
Orthogonally protected sugar diamino acids (SDA) were first synthesised by Sicherl and Wittmann [53] and used to form linear and branched amide-linked mimetics (Figure 10).
Figure 10: Protected derivatives of 2,6-diamino-2,6-dideoxy-β-D-glucopyranosyl carboxylic acid 22 and 23.
Figure 10: Protected derivatives of 2,6-diamino-2,6-dideoxy-β-D-glucopyranosyl carboxylic acid 22 and 23.
Due to the various possibilities with which SDAs can be connected to each other, a high degree of diversity can be achieved by employing only a small set of different SDAs. Moreover, oligomeric SDAs with unprotected groups represent a novel type of aminoglycoside mimetics with potential recognition properties towards new RNA targets emerging in the post-genome era.
Cyclic SAA homo-oligomers
A cyclic array of desired ring size and defined secondary structure of alternating carbohydrate moieties and amide groups might conceivably lead to exquisite specificity of recognition and catalysis. One application of this concept is the mimicry of cyclodextrin inclusion complexes. Thus, Kessler and co-workers have reported the synthesis of cyclic oligomers containing glucopyranosyluronic acid by exploiting standard solid and solution phase coupling procedures [54]. These cyclic homo-oligomers of SAAs behave as host molecules that form inclusion complexes with p-nitrophenol and benzoic acid. Following the same objective, Xie’s group prepared orthogonally protected cyclic homo-oligomers with two to four SAA units that can be selectively or fully deprotected to afford macrocycles that can undergo further functionalisation (Figure 11) [55].
Figure 11: Cyclic homo-oligomers containing glucopyranoid-SAAs.
Figure 11: Cyclic homo-oligomers containing glucopyranoid-SAAs.
Conformational analysis by molecular modelling showed that the unprotected cyclic trimers and tetramers preferred a 4C1 chair conformation with the oxygen atoms of the sugar ring located in the interior of the cavity and the secondary hydroxyl groups outside. This conformation is in agreement with the capacity of these cyclic carbopeptoids to form inclusion complexes with aromatic guest molecules [54] and Cu(II) ion [56]. Additionally, van Well et al. have described a cyclization/cleavage strategy for solid phase synthesis of cyclic trimers and tetramers containing pyranoid δ-SAAs (Scheme 2) and reported their structural analysis from ROESY data in combination with molecular dynamics calculations [57]. The results showed that the furanoid and the pyranoid SAA trimers adopt well-defined structures, although the trimer composed of pyranoid SAAs is less flexible than its furanoid counterpart.
Scheme 2: Strategy for solid-phase synthesis of cyclic trimers and tetramers containing pyranoid δ-SAAs.
Scheme 2: Strategy for solid-phase synthesis of cyclic trimers and tetramers containing pyranoid δ-SAAs.
Chakraborty and co-workers have also synthesised cyclic homo-oligomers of mannose- and glucose-derived furanoid SAAs [58] in order to constrain their conformational degrees of freedom and to induce desirable structural elements essential for their biological activities, such as tubular structures for transporting ions or molecules across membranes. Detailed ROESY, temperature coefficient (Δδ/ΔT) measurements of amide protons and constrained MD simulations revealed that all the cyclic oligomers had symmetrical structures, although none of the unprotected cyclic oligomers displayed any ability to transport ions across model membranes according to ion flux studies.
Cyclic tetramers of L-rhamno- and D-gulo-configured oxetane-SAAs 24 and 25 (Figure 12) have been synthesised by Fleet et al. [59] as mimics of naturally occurring cyclic peptides and cyclodextrins, although no further conformational and recognition studies on these compounds have currently been published.
Figure 12: Cyclic tetramers of L-rhamno- and D-gulo-configured oxetane-SAAs.
Figure 12: Cyclic tetramers of L-rhamno- and D-gulo-configured oxetane-SAAs.
Urea, carbamate and thiourea-linked sugars
The replacement of amide groups by pseudoamide (NH–C = X)–Y; X, Y = O, N, S) has been widely used in peptide chemistry to induce well-defined secondary structures. In carbohydrate chemistry, the incorporation of pseudoamide-type intersaccharide linkages has an additional interest because these functional groups are isosteric with phosphodiesther groups occurring in oligoglycosylphosphates and nucleotides.
Some examples of natural compounds having saccharide units connected by pseudoamide linkage are known. For example, the family of glycocinnamoylspermidine antibiotics 26–28, whose structures were determined by Ellestad and co-workers [60] by NMR spectroscopy and X-ray diffraction, are characterised by the presence of a glycosylurea linkage (Figure 13). In 2005, Ichikawa and co-workers described the total synthesis of the glycocinnasperemicin D 29, a broad-spectrum antibiotic against Gram-negative organisms, which contains two highly functionalised aminosugars connected by an urea linkage [61]. While exploring the synthesis of this target molecule, this group established a new method for the stereoselective synthesis of novel β-urea-linked pseudooligosaccharides, which involves the reaction of amine-glycosides with Steyemark-type gluco- and galactopyranosyl oxazolidinones [62,63].
Figure 13: Aminoglycosidic antibiotics of the glycocinnamoylspermidine family.
Figure 13: Aminoglycosidic antibiotics of the glycocinnamoylspermidine family.
Another example of natural disaccharide analogue containing a pseudoamide linkage is trehazolin 33 [64], which possesses a cyclic isourea functionality between the α-D-glucose and aminocyclopentitol rings. Trehazolin is a potent trehalase inhibitor in which an aminocyclopentitol ring replaces the glucopyranosyl cation postulated as an intermediate in the enzymatic hydrolysis of α,α-trehalose. Although several synthetic methodologies had successfully been applied to prepare this inhibitor, Chiara and co-workers have described a novel complementary approach in which the oxazoline ring is generated by SN2 nucleophilic displacement reaction from the β-hydroxyurea 30 via the triflate intermediate 31 (Scheme 3) [65].
Scheme 3: Synthesis of (thio)trehazoline, via triflate, from β-hydroxy(thio)urea.
Scheme 3: Synthesis of (thio)trehazoline, via triflate, from β-hydroxy(thio)urea.
A similar methodology was used by the same authors to prepare the trehazolin analogue 37 with an isothiourea group instead of an isourea group, which is also a potent trehalase inhibitor [66,67]. D-Gluco trehazolin analogues have also been synthesised by means of novel methodology based on the coupling of O-unprotected β-D-glucopyranosyl isothiocyanate with different aminosugars followed by treatment with yellow mercury (II) oxide [68].
There are two general strategies to access pseudoamide-type oligosaccharide mimics: i) nucleophilic addition of sugar derivatives to carbohydrate isocyanates, isothiocyanates or isocyanides; and ii) conversion of sugar azides into glycosyl carbodiimides via a tandem Staudinger-aza-Wittig type reaction with triphenylphosphine and an isothiocyanate, followed by the addition of a nucleophile (H2O, H2S, NH3, amines) (Figure 14). Although this second methodology is more straightforward and versatile than the first, only a few examples of pseudooligosaccharides having cyanamide, urea and thiourea-linkages via the Staudinger reaction have been reported [69,70].
Figure 14: Approaches to access pseudoamide-type oligosaccharide mimics.
Figure 14: Approaches to access pseudoamide-type oligosaccharide mimics.
In our group, the carbodiimide approach was used to prepare calystegine B2 analogues with the urea-linked disaccharide structure (Figure 15). These compounds, however, did not show inhibitory activities, probably due to the presence of a hydrophilic sugar substituent at the nitrogen atom of the nor-tropane ring [71,72].
Figure 15: Calystegine B2 analogues 38 and 39 with urea-linked disaccharide structure.
Figure 15: Calystegine B2 analogues 38 and 39 with urea-linked disaccharide structure.
Several groups have shown that disaccharide ureas and carbamates with different bridging positions as α/β-anomers are readily accessible by the coupling reaction of aminosugars or sugars, respectively, and glycosyl-isocyanates [73,74] or isocyanides [75]. However, the experimental difficulties in handling isocyanates has led to the preferential use of sugar carbodiimides as key intermediates for the preparation of glycosylureido sugars [76-79].
From the large number of functional groups that can be employed as surrogates for the natural amide and phosphodiester linkages, the thiourea functionality ranks among the most popular. Firstly, it can generally be generated in high yield and are ideally suited for solid phase and combinatorial approaches. Secondly, thioureas can be easily transformed into carbodiimides and thus serve as key intermediate in the synthesis of other pseudoamide functionalities (urea, guanidine, etc.) [70-72,76-79]. Thirdly, thiourea bridges provide efficient anchoring points for bidentate hydrogen-bonding recognition, which can give rise to defined secondary structures or be exploited in molecular recognition. Conformational studies by temperature coefficient measurements and ROESY experiments showed that thioureidodisaccharides adopt the Z,E conformation at N–(C=S) bonds, which is stabilised by intramolecular hydrogen bonding with the formation of a 7-membered ring (e.g. 40) [80]. This conformation changes to the Z,Z-conformation in aqueous solution [70] or in the presence of carboxylate ligands due to the formation of a bidentate hydrogen bond (Figure 16) [80].
Figure 16: Rotameric equilibrium shift of 40 by formation of a bidentate hydrogen bond.
Figure 16: Rotameric equilibrium shift of 40 by formation of a bidentate hydrogen bond.
Examples of thiourea-connected saccharides have been typically limited to compounds where the tethering reaction involves only two moieties, with no possibility of chain elongation [68,70-72,81], except in cases where there are spacer substituents [82]. Interestingly, a thiourea-linked dinucleotide was elongated by solid-phase synthesis to incorporate positively charged isothiouronium inter-nucleoside linkages into otherwise negatively charged DNA (Figure 17) [83,84]. The binding of these artificial DNAs (DNmt or DNT) to its complementary DNA strand occurs as with the unmodified DNA·DNA duplex but with enhanced nuclease resistance.
Figure 17: Nucleotide analogues with thiourea and S-methylisothiouronium linkers.
Figure 17: Nucleotide analogues with thiourea and S-methylisothiouronium linkers.
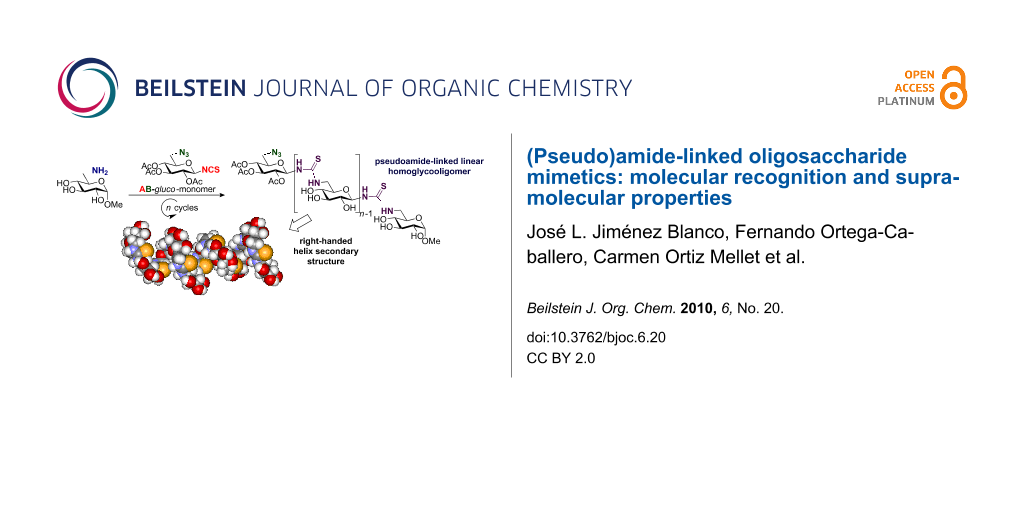
In order to design longer thiourea-linked glycooligomers with linear dendritic and branched architectures, our group has recently reported an efficient synthetic strategy based on the use of AB, AB2 and ABC-type monosaccharide building blocks containing isothiocyanate (A), azido (B) or carbamate groups (C) (Scheme 4) [85,87]. An iterative and efficient three-step reaction sequence was described for the assembly of monosaccharide units that involves: (i) a thiourea-forming reaction; (ii) deprotection of the hydroxy groups in the adducts and (iii) the generation of a new amino group in the growing chain. Branching points are incorporated by inserting building blocks bearing orthogonal amine functionalities at specific locations in the chain [86]. Conformational studies by dynamic NMR spectroscopy of β-(1→3)- and β-(1→6)-linked diglucosylthioureas (e.g. 41) detected the presence of Z,Z and Z,E conformers at the N–(C=S) bond (Figure 18).
Scheme 4: Retrosynthetic approach to synthesize thiourea-linked glycooligomers.
Scheme 4: Retrosynthetic approach to synthesize thiourea-linked glycooligomers.
Figure 18: Rotameric equilibria for β-(1→6)-thiourea-linked glucodimer 41.
Figure 18: Rotameric equilibria for β-(1→6)-thiourea-linked glucodimer 41.
β-(1→6)-Linked thioureido-di- and trisaccharides were used to obtain the corresponding ureido- and guanidine-linked oligomers. These compounds were evaluated as phosphate binders in water [76]. Association constants (Kas) for the binding of dimethyl and, especially, phenyl phosphate were obtained from NMR titration experiments for both series of glucooligomers. The binding affinity of the thiourea and guanidine oligomers was stronger than in the case of the urea analogues.
Stimulated by the interesting supramolecular properties and applications of cyclodextrins (CDs), a range of novel host molecules with differently shaped internal cavities has been obtained by replacement of the natural glycosidic bonds by urea and thiourea linkages. Thus, our group developed an efficient and modular strategy for the synthesis of cyclopseudooligosaccharide receptors relying on alternating α,α-trehalose motifs and semi-rigid thiourea segments (cyclotrehalans, CTs) [77-79,88,89]. Molecular diversity was introduced at the inter-saccharide connectors by exploiting the chemistry of macrocyclic carbodiimides [77-79,88,89] as well as by varying the size of the macrocycle (Figure 19).
Figure 19: Schematic representation of (a) cyclodextrin (CDs) and (b) cyclotrehalan (CTs) family members.
Figure 19: Schematic representation of (a) cyclodextrin (CDs) and (b) cyclotrehalan (CTs) family members.
Molecular modelling predicts the existence of a flexible, relatively hydrophobic cavity suited for molecular inclusion of non-polar guests [79,88]. NMR supports this fact and confirms the high symmetry of these hosts, where all glucopyranosyl units are magnetically equivalent as in the case of cyclodextrins. Determination of the inclusion capabilities towards a series of structurally diverse guests demonstrated that large-ring CTs are well suited for forming supramolecular complexes in water [77,79,88].
Guanidine-linked sugars
The use of the guanidine functional group to connect monosaccharide units in glycooligomers is particularly attractive with regard to molecular recognition processes. Like thioureas and ureas, guanidines can also form bidentate hydrogen bonds. In addition, because of their positively charged character, guanidines can exert strong electrostatic interactions with negatively charged functional groups such as phosphate or carboxylate groups in DNA, RNA and proteins.
Tóth and co-workers [90] synthesised a series of guanidine-linked sugars as a new class of oligosaccharide mimics to study their interactions with proteins. Thus, reaction of the carbodiimide-linked pseudooligosaccharide 42 with different secondary amines gave the corresponding guanidines 43–46 (Scheme 5) and their conformational behaviour and tautomerism was studied by NMR spectroscopy.
Scheme 5: Synthesis of guanidine-linked pseudodisaccharides via carbodiimide.
Scheme 5: Synthesis of guanidine-linked pseudodisaccharides via carbodiimide.
β(1→6)-Linked pseudodi- (47) and pseudotrisaccharides (48) incorporating guanidine intersaccharide bridges have been prepared and evaluated as phosphate binders in water (Figure 20) [76]. Association constants (Kas) for binding with dimethyl and phenyl phosphate were obtained from NMR titration experiments for both compounds. The results indicated that the binding strength depends strongly on the acidity of the NH protons and on solvating properties. The guanidine derivatives showed higher Kas values than the isosteric thiourea and urea analogues.
Figure 20: β(1→6)-Guanidine-linked pseudodi- and pseudotrisaccharides 47 and 48.
Figure 20: β(1→6)-Guanidine-linked pseudodi- and pseudotrisaccharides 47 and 48.
Ortiz Mellet’s group has reported the synthesis of N-benzylguanidine-linked dimeric cyclotrehalans (CT2s, 50) [78] via carbodiimide 49 by reaction with benzylamine hydrochloride and subsequent deacetylation (Scheme 6). Structural and conformational studies on 50 were carried out by NMR, which showed that the structure was stabilised by two anti-parallel seven-membered ring intramolecular hydrogen bonds, resulting in relatively high rotational barriers for the Z,E:E,Z/E,Z:Z,E equilibrium. The cavity collapses due to the presence of these intramolecular hydrogen bonds thus preventing the formation of inclusion complexes.
Scheme 6: Synthesis of N-benzylguanidine-linked CT2 50.
Scheme 6: Synthesis of N-benzylguanidine-linked CT2 50.
Most examples of linear pseudoamide-linked glycooligomers correspond to oligonucleotide analogues. The “antisense” strategy to regulate gene expression in living cells has required the development of modified oligonucleotides as potential therapeutic agents. A key goal in the design of such agents include increasing binding affinity while maintaining sequence specificity, resistance to degradation by nucleases and improved membrane permeability [83,84]. Numerous structural analogues of DNA/RNA designed to be effective antisense/antigene agents have been reported. An interesting approach involves replacing the negatively charged phosphodiester linkages of RNA/DNA by positively charged guanidinium linkages. The guanidinium linkage is resistant to nucleases [91,92] and the positive charge may give rise to cell membrane permeability through electrostatic attraction with the negatively charged groups of the proteoglycans at the cell surface. Using this strategy, Bruice and co-workers synthesised ribonucleic guanidine (RNG) and deoxyribonucleic guanidine (DNG) as analogues of RNA and DNA, respectively (Figure 21).
The methodology developed to generate internucleoside guanidinium linkages can be used in both solution phase and solid phase synthesis [91-94]. It involves the abstraction of the sulphur atom from a (Fmoc)-protected thiourea by Hg2+ to provide an activated carbodiimide which can react with a free amino group to give the protected guanidine (Figure 22).
Figure 22: Preparation of Fmoc-guanidinium derivatives.
Figure 22: Preparation of Fmoc-guanidinium derivatives.
Homo-oligomeric RNG sequences, containing trimeric and pentameric uridyl [95] and adenyl moieties (52-55) (Figure 23), and a mixed-base pentamer [96] (56, 5′-AUAUA-3′), were synthesised using this methodology (Figure 24).
Figure 23: Structures of the homo-oligomeric RNG derivatives 51–55.
Figure 23: Structures of the homo-oligomeric RNG derivatives 51–55.
Figure 24: Phosphoramidite building block 56.
Figure 24: Phosphoramidite building block 56.
Binding studies of uridyl ribonucleic guanidine 52 to DNA [97] and RNA [98] showed that it binds pentameric adenyl DNA and RNA with a 1:1 stoichiometry. The RNG 52 dimeric complex with DNA is thermodynamically favoured in comparison to RNA·DNA and DNA·DNA duplexes and was able to discriminate between complementary and non-complementary base pairs. These results suggest that 52 is a good candidate for an antisense agent.
A 21 base pair RNG/DNA chimera containing both anionic phosphodiester linkages of DNA and cationic guanidine linkages of RNG has been synthesised [99]. Phosphoramidite 56 (Figure 24) was used as a building block to introduce guanidinium linkages at desired positions in the chimeric oligonucleotides. The biological properties were evaluated using the bcr-abl oncogene (the cause of chronic myeloid leukaemia) as the target. The results showed that the binding of a 21-mer RNG/DNA chimera containing six guanidinium linkages is more than 104-fold stronger than the binding of its 21-mer DNA counterpart.
Deoxyribonucleic guanidine homo-oligomeric sequences, consisting of trimeric and octameric thymidyl [100], octameric cytidyl [101] and trimeric 7-deazaguanidyl moieties (57–60) [102] and a mixed-base tetramer [103] and hexamer [104] (61–63) were synthesised using the same strategy as noted above for RNG synthesis (Figure 25).
Binding studies of homo-oligomers 57 and 58 to complementary DNA oligomers showed a 1:2 DNG:DNA stoichiometry, similar to homo-polymeric thymidyl and adenyl DNA strands, which can form a triple helical structure. On the other hand, octameric cytidyl DNG 59 and mixed-based oligomers 61–63 form 1:1 complexes with complementary DNA oligomers. The trimeric 7-deazaguanidyl 60, in which guanine is replaced by 7-deazaguanine (c7G), forms a 1:1 complex with pentameric adenyl DNA. Deazaguanine nucleobase was chosen because of the unique glycoside bond stability and its ability to prevent G-quartet formation.
To increase the already strong binding of DNG to DNA, DNG was combined with a ligand capable of binding specifically to the minor groove of the DNA. A pentameric thymidyl DNG incorporating bis-benzimidazole (Hoechst 33258) ligand (64) was synthesised [105]. The stability of DNG-Hoechst conjugates 64 and 65 with a 30-mer double-strand DNA (dsDNA) and single-strand DNA (ssDNA) were evaluated. Fluorescent emission studies showed that hybridization of DNG-Hoechst conjugates 64 and 65 to dsDNA enhances the stability of the triple helix through simultaneous minor groove binding by the tethered Hoechst 33258 ligand. Furthermore, Hoechst 33258 is able to enhance the stability of the duplex DNG·DNA with a flexible minor groove. As in the case of RNG, a 20 base pair DNG/DNA chimera has been synthesised [106].
Phosphoramidite 66 (Figure 26) was used as the starting material for the introduction of guanidinium linkages at desired positions in the chimeric oligonucleotides. The biological properties were evaluated using the juvenile esterase gene as the target. The results showed that binding of a 20-mer DNG/DNA hexameric (ATATAT) chimera containing guanidinium linkages is more than 105.7 times stronger than the binding of the corresponding 20-mer of DNA. The hexameric DNG binds to DNA in a 1:1 ratio and is able to discriminate between complementary and non-complementary base pairs. In addition, the DNG·DNA complex is more stable than the DNA·DNA duplex.
Figure 26: Structure of the phosphoramidite building block 66.
Figure 26: Structure of the phosphoramidite building block 66.
Cancer cell immortality is due to relatively high concentrations of telomerase enzyme which maintains the telomer sequence during cell division. One approach to prevent telomer replacement in cancer cells is by inhibiting the telomerase enzyme by blocking the telomeric RNA 11-base template (5′-CUAACCCAAC-3′). For this purpose, Bruice and co-workers [107] prepared complementary DNG (5′-GATTGGGATTG-3′) to telomeric RNA complex. Binding studies demonstrated that the DNG/telomeric RNA complex is favoured over the telomer substrate, and could be of use as an anticancer agent.
Conclusion
Over the last 10 years considerable efforts have been expended in the synthesis of novel linear and cyclic pseudoamide-linked oligosaccharide mimics. In particular, important advances in synthetic methodologies for thioureido and ureido glycooligomers have made thus enabling the preparation of virtually any oligomeric structure (linear, branched, dendritic and cyclic). The secondary structures adopted by these pseudooligosaccharides have been extensively studied by current NMR and molecular modelling techniques. The results have shown that, in many cases, the three-dimensional structure of naturally occurring biomolecules can be mimicked by carbopeptoids and pseudoamide-linked oligomeric surrogates. Nevertheless, further investigations are required to clarify the structure-activity relationships in order to design novel biologically active analogues of potential therapeutic value.
References
-
Mareel, M.; Leroy, A. Physiol. Rev. 2003, 83, 337–376.
Return to citation in text: [1] -
Burleigh, B. A.; Woolsey, A. M. Cell. Microbiol. 2002, 4, 701–711. doi:10.1046/j.1462-5822.2002.00226.x
Return to citation in text: [1] -
Shi, X.; Friess, H.; Kleeff, J.; Ozawa, F.; Büchler, M. W. Pancreatology 2001, 1, 517–524. doi:10.1159/000055854
Return to citation in text: [1] -
Muramatsu, T. Glycoconjugate J. 2000, 17, 577–595. doi:10.1023/A:1011078627247
Return to citation in text: [1] -
Dwek, R. A. Chem. Rev. 1996, 96, 683–720. doi:10.1021/cr940283b
Return to citation in text: [1] -
Dwek, R. A.; Butters, T. D.; Platt, F. M.; Zitzmann, N. Nat. Rev. Drug Discovery 2002, 1, 65–75. doi:10.1038/nrd708
Return to citation in text: [1] -
Wilson, I. B. H. Curr. Opin. Struct. Biol. 2002, 12, 569–577. doi:10.1016/S0959-440X(02)00367-6
Return to citation in text: [1] [2] -
Crocker, P. R.; Feizi, T. Curr. Opin. Struct. Biol. 1996, 6, 679–691. doi:10.1016/S0959-440X(96)80036-4
Return to citation in text: [1] -
Crocker, P. R. Curr. Opin. Struct. Biol. 2002, 12, 609–615. doi:10.1016/S0959-440X(02)00375-5
Return to citation in text: [1] -
Harnett, W.; Harnett, M. M.; Byron, O. Curr. Protein Pept. Sci. 2003, 4, 59–71. doi:10.2174/1389203033380368
Return to citation in text: [1] -
Pendse, S.; Sayegh, M. H.; Frank, M. H. Curr. Drug Targets 2003, 4, 469–476. doi:10.2174/1389450033490894
Return to citation in text: [1] -
Risseeuw, M. D. P.; Overhand, M.; Fleet, G. W. J.; Simona, M. I. Tetrahedron: Asymmetry 2007, 18, 2001–2010. doi:10.1016/j.tetasy.2007.08.004
Return to citation in text: [1] [2] -
Gruner, S. A. W.; Locardi, E.; Lohof, E.; Kessler, H. Chem. Rev. 2002, 102, 491–514. doi:10.1021/cr0004409
Return to citation in text: [1] [2] [3] -
Franz, A. H.; Gross, P. H.; Samoshin, V. V. ARKIVOC 2008, i, 271–308.
Return to citation in text: [1] [2] -
Smith, M. D.; Fleet, G. W. J. J. Pept. Sci. 1999, 5, 425–441. doi:10.1002/(SICI)1099-1387(199910)5:10<425::AID-PSC222>3.0.CO;2-O
Return to citation in text: [1] -
Chakraborty, T. K.; Srinivasu, P.; Tapadar, S.; Mohan, B. K. Glycoconjugate J. 2005, 22, 83–93. doi:10.1007/s10719-005-0844-x
Return to citation in text: [1] -
Schweizer, F. Angew. Chem., Int. Ed. 2002, 41, 230–253. doi:10.1002/1521-3773(20020118)41:2<230::AID-ANIE230>3.0.CO;2-L
Return to citation in text: [1] [2] -
Varela, O.; Orgueira, H. A. Adv. Carbohydr. Chem. Biochem. 2000, 55, 137–174. doi:10.1016/S0065-2318(00)55005-7
Return to citation in text: [1] -
Wessel, H. P.; Lucas Dias, S. In Glycoscience; Fraser-Reid, B.; Tatsuta, K.; Thiem, J., Eds.; Springer-Verlag: Berlin Heidelberg, Germany, 2008; pp 2079–2112. doi:10.1007/978-3-540-30429-6_52
Return to citation in text: [1] -
Chakraborty, T. K.; Ghosh, S.; Jayaprakash, S. Curr. Med. Chem. 2002, 9, 421–435.
Return to citation in text: [1] -
Müller, C.; Kitas, E.; Wessel, H. P. J. Chem. Soc., Chem. Commun. 1995, 2425–2426. doi:10.1039/C39950002425
Return to citation in text: [1] -
Wessel, H. P.; Mitchell, C. M.; Lobato, C. M.; Schmid, G. Angew. Chem., Int. Ed. Engl. 1996, 34, 2712–2713. doi:10.1002/anie.199527121
Return to citation in text: [1] -
Szabo, L.; Smith, B. L.; McReynolds, D.; Parrill, A. L.; Morris, E. R.; Gervay, J. J. Org. Chem. 1998, 63, 1074–1078. doi:10.1021/jo971415e
Return to citation in text: [1] [2] -
Gregar, T. Q.; Gervay-Hague, J. J. Org. Chem. 2004, 69, 1001–1009. doi:10.1021/jo035312+
Return to citation in text: [1] -
McReynolds, K. D.; Gervay-Hague, J. Tetrahedron: Asymmetry 2000, 11, 337–362. doi:10.1016/S0957-4166(99)00560-1
Return to citation in text: [1] -
Fuchs, E.-F.; Lehmann, J. Chem. Ber. 1975, 108, 2254–2260. doi:10.1002/cber.19751080712
Return to citation in text: [1] -
Fuchs, E.-F.; Lehmann, J. Carbohydr. Res. 1976, 49, 267–273. doi:10.1016/S0008-6215(00)83144-4
Return to citation in text: [1] -
Suhara, Y.; Yamaguchi, Y.; Collins, B.; Schnaar, R. L.; Yanagishita, M.; Hildreth, J. E. K.; Shimada, I.; Ichikawa, Y. Bioorg. Med. Chem. 2002, 10, 1999–2013. doi:10.1016/S0968-0896(02)00020-2
Return to citation in text: [1] [2] -
Suhara, Y.; Kurihara, M.; Kittaka, A.; Ichikawa, Y. Tetrahedron 2006, 62, 8207–8217. doi:10.1016/j.tet.2006.05.080
Return to citation in text: [1] [2] -
Durrat, F.; Xie, J.; Valéry, J.-M. Tetrahedron Lett. 2004, 45, 1477–1479. doi:10.1016/j.tetlet.2003.12.032
Return to citation in text: [1] -
Chung, Y.-K.; Claridge, T. D. W.; Fleet, G. W. J.; Johnson, S. W.; Jones, J. H.; Lumbard, K. W.; Stalchulski, A. V. J. Pept. Sci. 2004, 10, 1–7. doi:10.1002/psc.488
Return to citation in text: [1] -
Smith, M. D.; Long, D. D.; Martin, A.; Marquess, D. G.; Claridge, T. D. W.; Fleet, G. W. J. Tetrahedron Lett. 1999, 40, 2191–2194. doi:10.1016/S0040-4039(99)00109-4
Return to citation in text: [1] -
Claridge, T. D. W.; Long, D. D.; Hungerford, N. L.; Aplin, R. T.; Smith, M. D.; Marquess, D. G.; Fleet, G. W. J. Tetrahedron Lett. 1999, 40, 2199–2202. doi:10.1016/S0040-4039(99)00111-2
Return to citation in text: [1] -
Smith, M. D.; Claridge, T. D. W.; Sansom, M. S. P.; Fleet, G. W. J. Org. Biomol. Chem. 2003, 1, 3647–3655. doi:10.1039/b307707c
Return to citation in text: [1] [2] -
Long, D. D.; Hungerford, N. L.; Smith, M. D.; Brittain, D. E. A.; Marquess, D. G.; Claridge, T. D. W.; Fleet, G. W. J. Tetrahedron Lett. 1999, 40, 2195–2198. doi:10.1016/S0040-4039(99)00110-0
Return to citation in text: [1] [2] -
Brittain, D. E. A.; Watterson, M. P.; Claridge, T. D. W.; Smith, M. D.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 2000, 3655–3665. doi:10.1039/b002995p
Return to citation in text: [1] [2] -
Hungerford, N. L.; Claridge, T. D. W.; Watterson, M. P.; Aplin, R. T.; Moreno, A.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 2000, 3666–3679. doi:10.1039/b002996n
Return to citation in text: [1] [2] [3] -
Claridge, T. D. W.; Long, D. D.; Baker, C. M.; Odell, B.; Grant, G. H.; Edwards, A. A.; Tranter, G. E.; Fleet, G. W. J.; Smith, M. D. J. Org. Chem. 2005, 70, 2082–2090. doi:10.1021/jo0480040
Return to citation in text: [1] [2] [3] [4] -
Edwards, A. A.; Sanjayan, G. J.; Hachisu, S.; Tranter, G. E.; Fleet, G. W. J. Tetrahedron 2006, 62, 7718–7725. doi:10.1016/j.tet.2006.05.067
Return to citation in text: [1] [2] -
Edwards, A. A.; Fleet, G. W. J.; Tranter, G. E. Chirality 2006, 18, 265–272. doi:10.1002/chir.20248
Return to citation in text: [1] [2] -
Andreini, M.; Taillefumier, C.; Chrétien, F.; Thery, V.; Chapleur, Y. J. Org. Chem. 2009, 74, 7651–7659. doi:10.1021/jo900966b
Return to citation in text: [1] -
Chakraborty, T. K.; Jayaprakash, S.; Srinivasu, P.; Govardhana Chary, M.; Diwan, P. V.; Nagaraj, R.; Ravi Sankar, A.; Kunwar, A. C. Tetrahedron Lett. 2000, 41, 8167–8171. doi:10.1016/S0040-4039(00)01425-8
Return to citation in text: [1] -
Chakraborty, T. K.; Srinivasu, P.; Sakunthala Madhavendra, S.; Kiran Kumar, S.; Kunwar, A. C. Tetrahedron Lett. 2004, 45, 3573–3577. doi:10.1016/j.tetlet.2004.03.048
Return to citation in text: [1] -
Edwards, A. A.; Alexander, B. D.; Fleet, G. W. J.; Tranter, G. E. Chirality 2008, 20, 969–972. doi:10.1002/chir.20538
Return to citation in text: [1] -
Jockusch, R. A.; Talbot, F. O.; Rogers, P. S.; Simone, M. I.; Fleet, G. W. J.; Simons, J. P. J. Am. Chem. Soc. 2006, 128, 16771–16777. doi:10.1021/ja0607133
Return to citation in text: [1] -
Barker, S. F.; Angus, D.; Taillefumier, C.; Probert, M. R.; Watkin, D. J.; Watterson, M. P.; Claridge, T. D. W.; Hungerford, N. L.; Fleet, G. W. J. Tetrahedron Lett. 2001, 42, 4247–4250. doi:10.1016/S0040-4039(01)00660-8
Return to citation in text: [1] -
Johnson, S. W.; Jenkinson, S. F.; Angus, D.; Pérez-Victoria, I.; Claridge, T. D. W.; Fleet, G. W. J.; Jones, J. H. J. Pept. Sci. 2005, 11, 303–318. doi:10.1002/psc.622
Return to citation in text: [1] -
Lopez-Ortega, B.; Jenkinson, S. F.; Claridge, T. D. W.; Fleet George, W. J. Tetrahedron: Asymmetry 2008, 19, 976–983. doi:10.1016/j.tetasy.2008.03.030
Return to citation in text: [1] -
Claridge, T. D. W.; Goodman, J. M.; Moreno, A.; Angus, D.; Barker, S. F.; Taillefumier, C.; Watterson, M. P.; Fleet, G. W. J. Tetrahedron Lett. 2001, 42, 4251–4255. doi:10.1016/S0040-4039(01)00661-X
Return to citation in text: [1] -
Johnson, S. W.; Jenkinson, S. F.; Pérez-Victoria, I.; Edwards, A. A.; Claridge, T. D. W.; Tranter, G. E.; Fleet, G. W. J. J. Pept. Sci. 2005, 11, 517–524. doi:10.1002/psc.658
Return to citation in text: [1] -
Claridge, T. D. W.; Lopez-Ortega, B.; Jenkinson, S. F.; Fleet, G. W. J. Tetrahedron: Asymmetry 2008, 19, 984–988. doi:10.1016/j.tetasy.2008.03.029
Return to citation in text: [1] -
D’Onofrio, J.; De Champdoré, M.; De Napoli, L.; Montesarchio, D.; Di Fabio, G. Bioconjugate Chem. 2005, 16, 1299–1309. doi:10.1021/bc0501606
Return to citation in text: [1] -
Sicherl, F.; Wittmann, V. Angew. Chem., Int. Ed. 2005, 44, 2096–2099. doi:10.1002/anie.200462595
Return to citation in text: [1] -
Locardi, E.; Stöckle, M.; Gruner, S.; Kessler, H. J. Am. Chem. Soc. 2001, 123, 8189–8196. doi:10.1021/ja010181k
Return to citation in text: [1] [2] -
Ménand, M.; Blais, J.-C.; Hamon, L.; Valéry, J.-M.; Xie, J. J. Org. Chem. 2005, 70, 4423–4430. doi:10.1021/jo0502899
Return to citation in text: [1] -
Fournier, F.; Afonso, C.; Ménand, M.; Hamon, L.; Xie, J.; Tabeta, J.-C. Eur. J. Mass Spectrom. 2008, 14, 61–69. doi:10.1255/ejms.912
Return to citation in text: [1] -
van Well, R. M.; Marinelli, L.; Erkelens, K.; van der Marel, G. A.; Lavecchia, A.; Overkleeft, H. S.; van Boom, J. H.; Kessler, H.; Overhand, M. Eur. J. Org. Chem. 2003, 2303–2313. doi:10.1002/ejoc.200300031
Return to citation in text: [1] -
Charkraborty, T. K.; Srinivasu, P.; Bikshapathy, E.; Nagaraj, R.; Vairamani, M.; Kiran Kumar, S.; Kunwar, A. C. J. Org. Chem. 2003, 68, 6257–6263. doi:10.1021/jo034586u
Return to citation in text: [1] -
Fleet, G. W. J.; Johnson, S. W.; Jones, J. H. J. Pept. Sci. 2006, 12, 559–561. doi:10.1002/psc.759
Return to citation in text: [1] -
Ellestad, G. A.; Cosulich, D. B.; Broschard, R. W.; Martin, J. H.; Kuntsmann, M. P.; Morton, G. O.; Lancaster, J. E.; Fulmor, W.; Lovell, F. M. J. Am. Chem. Soc. 1978, 100, 2515–2524. doi:10.1021/ja00476a041
Return to citation in text: [1] -
Nishiyama, T.; Isobe, M.; Ichikawa, Y. Angew. Chem., Int. Ed. 2005, 44, 4372–4375. doi:10.1002/anie.200500892
Return to citation in text: [1] -
Ichikawa, Y.; Matsukawa, Y.; Tamura, M.; Ohara, F.; Isobe, M.; Kotsuki, H. Chem.– Asian J. 2006, 1, 717–723. doi:10.1002/asia.200600190
Return to citation in text: [1] -
Ichikawa, Y.; Matsukawa, Y.; Isobe, M. J. Am. Chem. Soc. 2006, 128, 3934–3938. doi:10.1021/ja056253f
Return to citation in text: [1] -
Ando, O.; Satake, H.; Itoi, K.; Sato, A.; Nakajima, M.; Takahashi, S.; Haruyama, H.; Ohkuma, Y.; Kinishita, T.; Enokita, R. J. Antibiot. 1991, 44, 1165–1168.
Return to citation in text: [1] -
Storch de Gracia, I.; Bobo, S.; Martín-Ortega, M. D.; Chiara, J. L. Org. Lett. 1999, 1, 1705–1708. doi:10.1021/ol990904t
Return to citation in text: [1] -
Chiara, J. L.; Storch de Gracia, I.; García, A.; Batida, A.; Bobo, M.; Martín-Ortega, M. D. ChemBioChem 2005, 6, 186–191. doi:10.1002/cbic.200400231
Return to citation in text: [1] -
Gibson, R. P.; Gloster, T. M.; Roberts, S.; Warren, R. A. J.; Storch de Gracia, I.; García, A.; Chiara, J. L.; Davies, G. J. Angew. Chem., Int. Ed. 2007, 46, 4115–4119. doi:10.1002/anie.200604825
Return to citation in text: [1] -
López, O.; Maya, I.; Fuentes, J.; Fernández-Bolaños, J. G. Tetrahedron 2004, 60, 61–72. doi:10.1016/j.tet.2003.10.092
Return to citation in text: [1] [2] -
Kovács, L.; Osz, E.; Györdeák, Z. Carbohydr. Res. 2002, 337, 1171–1178. doi:10.1016/S0008-6215(02)00108-8
Return to citation in text: [1] -
Díaz Pérez, V. M.; Ortiz Mellet, C.; Fuentes, J.; García Fernández, J. M. Carbohydr. Res. 2000, 326, 161–175. doi:10.1016/S0008-6215(00)00040-9
Return to citation in text: [1] [2] [3] [4] -
García-Moreno, M. I.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2001, 66, 7604–7614. doi:10.1021/jo015639f
Return to citation in text: [1] [2] [3] -
García-Moreno, M. I.; Ortiz Mellet, C.; García Fernández, J. M. Eur. J. Org. Chem. 2004, 1803–1819. doi:10.1002/ejoc.200300731
Return to citation in text: [1] [2] [3] -
Prosperi, D.; Ronchi, S.; Lay, L.; Rencurosi, A.; Russo, G. Eur. J. Org. Chem. 2004, 395–405. doi:10.1002/ejoc.200300483
Return to citation in text: [1] -
López, O.; Maza, S.; Maya, I.; Fuentes, J.; Fernández-Bolaños, J. G. Tetrahedron 2005, 61, 9058–9069. doi:10.1016/j.tet.2005.07.041
Return to citation in text: [1] -
Prosperi, D.; Ronchi, S.; Lay, L.; Rencurosi, A.; Russo, G. Synlett 2004, 1529–1532. doi:10.1055/s-2004-829074
Return to citation in text: [1] -
Jiménez Blanco, J. L.; Bootello, P.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2006, 71, 5136–5143. doi:10.1021/jo060360q
Return to citation in text: [1] [2] [3] [4] -
Benito, J. M.; Rodríguez-Lucena, D.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. J. Inclusion Phenom. Macrocyclic Chem. 2007, 57, 147–150. doi:10.1007/s10847-006-9203-1
Return to citation in text: [1] [2] [3] [4] [5] -
Rodríguez-Lucena, D.; Benito, J. M.; Álvarez, E.; Jaime, C.; Perez-Miron, J.; García Fernández, J. M. J. Org. Chem. 2008, 73, 2967–2979. doi:10.1021/jo800048s
Return to citation in text: [1] [2] [3] [4] [5] -
Rodríguez-Lucena, D.; Ortiz Mellet, C.; Jaime, C.; Burusco, K. K.; García Fernández, J. M.; Benito, J. M. J. Org. Chem. 2009, 74, 2997–3008. doi:10.1021/jo802796p
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Jiménez Blanco, J. L.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. Org. Lett. 1999, 1, 1217–1220. doi:10.1021/ol990889s
Return to citation in text: [1] [2] -
Benito, J. M.; Ortiz Mellet, C.; Sadalapure, K.; Lindhorst, T. K.; Defaye, J.; García Fernández, J. M. Carbohydr. Res. 1999, 320, 37–48. doi:10.1016/S0008-6215(99)00141-X
Return to citation in text: [1] -
Patel, A.; Lindhorst, T. K. J. Org. Chem. 2001, 66, 2674–2680. doi:10.1021/jo005671u
Return to citation in text: [1] -
Arya, D. P.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2000, 10, 691–693. doi:10.1016/S0960-894X(00)00085-8
Return to citation in text: [1] [2] -
Challa, H.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2001, 11, 2423–2427. doi:10.1016/S0960-894X(01)00455-3
Return to citation in text: [1] [2] -
Jiménez Blanco, J. L.; Bootello, P.; Ortiz Mellet, C.; Gutiérrez Gallego, R.; García Fernández, J. M. Chem. Commun. 2004, 92–93. doi:10.1039/b312743p
Return to citation in text: [1] -
Jiménez Blanco, J. L.; Bootello, P.; Ortiz Mellet, C.; García Fernández, J. M. Eur. J. Org. Chem. 2006, 183–196. doi:10.1002/ejoc.200500472
Return to citation in text: [1] -
Jiménez Blanco, J. L.; Bootello, P.; Gutiérrez Gallego, R.; Ortiz Mellet, C.; García Fernández, J. M. Synthesis 2007, 2545–2558. doi:10.1055/s-2007-983794
Return to citation in text: [1] -
Benito, J. M.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. Angew. Chem., Int. Ed. 2002, 41, 3674–3676. doi:10.1002/1521-3773(20021004)41:19<3674::AID-ANIE3674>3.0.CO;2-F
Return to citation in text: [1] [2] [3] [4] -
García Fernández, J. M.; Ortiz Mellet, C.; Defaye, J. J. Inclusion Phenom. Macrocyclic Chem. 2006, 56, 149–159. doi:10.1007/s10847-006-9076-3
Return to citation in text: [1] [2] -
Tóth, G.; Gáti, T.; Pintér, I.; Kovács, J.; Haessner, R. Magn. Reson. Chem. 2001, 39, 283–287. doi:10.1002/mrc.835
Return to citation in text: [1] -
Barawkar, D.; Bruice, T. C. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 11047–11052. doi:10.1073/pnas.95.19.11047
Return to citation in text: [1] [2] -
Barawkar, D.; Linkletter, B. A.; Bruice, T. C. Bioorg. Med. Chem. Lett. 1998, 8, 1517–1520. doi:10.1016/S0960-894X(98)00251-0
Return to citation in text: [1] [2] -
Linkletter, B. A.; Szabo, I. E.; Bruice, T. C. J. Am. Chem. Soc. 1999, 121, 3888–3896. doi:10.1021/ja984212w
Return to citation in text: [1] -
Linkletter, B. A.; Bruice, T. C. Bioorg. Med. Chem. 2000, 8, 1893–1901. doi:10.1016/S0968-0896(00)00146-2
Return to citation in text: [1] -
Kojima, N.; Bruice, T. C. Org. Lett. 2000, 2, 81–84. doi:10.1021/ol9912478
Return to citation in text: [1] -
Kojima, N.; Szabo, I. E.; Bruice, T. C. Tetrahedron 2002, 58, 867–879. doi:10.1016/S0040-4020(01)01185-1
Return to citation in text: [1] -
Park, M.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2005, 15, 3247–3251. doi:10.1016/j.bmcl.2005.04.073
Return to citation in text: [1] -
Park, M.; Toporowski, J. W.; Bruice, T. C. Bioorg. Med. Chem. 2006, 14, 1743–1749. doi:10.1016/j.bmc.2005.10.028
Return to citation in text: [1] -
Park, M.; Canzio, D.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2008, 18, 2377–2384. doi:10.1016/j.bmcl.2008.02.063
Return to citation in text: [1] -
Linkletter, B. A.; Szabo, I. E.; Bruice, T. C. Nucleic Acids Res. 2001, 29, 2370–2376. doi:10.1093/nar/29.11.2370
Return to citation in text: [1] -
Szabo, I. E.; Bruice, T. C. Bioorg. Med. Chem. 2004, 12, 4233–4244. doi:10.1016/j.bmc.2004.05.010
Return to citation in text: [1] -
Jain, M. L.; Bruice, T. C. Bioorg. Med. Chem. 2006, 14, 7333–7346. doi:10.1016/j.bmc.2006.05.074
Return to citation in text: [1] -
Challa, H.; Bruice, T. C. Bioorg. Med. Chem. 2004, 12, 1475–1481. doi:10.1016/j.bmc.2003.12.043
Return to citation in text: [1] -
Reddy, P. M.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2003, 13, 1281–1285. doi:10.1016/S0960-894X(03)00119-7
Return to citation in text: [1] -
Reddy, P. M.; Bruice, T. C. J. Am. Chem. Soc. 2004, 126, 3736–3747. doi:10.1021/ja031557s
Return to citation in text: [1] -
Park, M.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2008, 18, 3488–3491. doi:10.1016/j.bmcl.2008.05.030
Return to citation in text: [1] -
Zhang, X.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2008, 18, 665–669. doi:10.1016/j.bmcl.2007.11.061
Return to citation in text: [1]
| 86. | Jiménez Blanco, J. L.; Bootello, P.; Ortiz Mellet, C.; García Fernández, J. M. Eur. J. Org. Chem. 2006, 183–196. doi:10.1002/ejoc.200500472 |
| 76. | Jiménez Blanco, J. L.; Bootello, P.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2006, 71, 5136–5143. doi:10.1021/jo060360q |
| 83. | Arya, D. P.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2000, 10, 691–693. doi:10.1016/S0960-894X(00)00085-8 |
| 84. | Challa, H.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2001, 11, 2423–2427. doi:10.1016/S0960-894X(01)00455-3 |
| 85. | Jiménez Blanco, J. L.; Bootello, P.; Ortiz Mellet, C.; Gutiérrez Gallego, R.; García Fernández, J. M. Chem. Commun. 2004, 92–93. doi:10.1039/b312743p |
| 87. | Jiménez Blanco, J. L.; Bootello, P.; Gutiérrez Gallego, R.; Ortiz Mellet, C.; García Fernández, J. M. Synthesis 2007, 2545–2558. doi:10.1055/s-2007-983794 |
| 82. | Patel, A.; Lindhorst, T. K. J. Org. Chem. 2001, 66, 2674–2680. doi:10.1021/jo005671u |
| 90. | Tóth, G.; Gáti, T.; Pintér, I.; Kovács, J.; Haessner, R. Magn. Reson. Chem. 2001, 39, 283–287. doi:10.1002/mrc.835 |
| 79. | Rodríguez-Lucena, D.; Ortiz Mellet, C.; Jaime, C.; Burusco, K. K.; García Fernández, J. M.; Benito, J. M. J. Org. Chem. 2009, 74, 2997–3008. doi:10.1021/jo802796p |
| 88. | Benito, J. M.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. Angew. Chem., Int. Ed. 2002, 41, 3674–3676. doi:10.1002/1521-3773(20021004)41:19<3674::AID-ANIE3674>3.0.CO;2-F |
| 77. | Benito, J. M.; Rodríguez-Lucena, D.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. J. Inclusion Phenom. Macrocyclic Chem. 2007, 57, 147–150. doi:10.1007/s10847-006-9203-1 |
| 79. | Rodríguez-Lucena, D.; Ortiz Mellet, C.; Jaime, C.; Burusco, K. K.; García Fernández, J. M.; Benito, J. M. J. Org. Chem. 2009, 74, 2997–3008. doi:10.1021/jo802796p |
| 88. | Benito, J. M.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. Angew. Chem., Int. Ed. 2002, 41, 3674–3676. doi:10.1002/1521-3773(20021004)41:19<3674::AID-ANIE3674>3.0.CO;2-F |
| 77. | Benito, J. M.; Rodríguez-Lucena, D.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. J. Inclusion Phenom. Macrocyclic Chem. 2007, 57, 147–150. doi:10.1007/s10847-006-9203-1 |
| 78. | Rodríguez-Lucena, D.; Benito, J. M.; Álvarez, E.; Jaime, C.; Perez-Miron, J.; García Fernández, J. M. J. Org. Chem. 2008, 73, 2967–2979. doi:10.1021/jo800048s |
| 79. | Rodríguez-Lucena, D.; Ortiz Mellet, C.; Jaime, C.; Burusco, K. K.; García Fernández, J. M.; Benito, J. M. J. Org. Chem. 2009, 74, 2997–3008. doi:10.1021/jo802796p |
| 88. | Benito, J. M.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. Angew. Chem., Int. Ed. 2002, 41, 3674–3676. doi:10.1002/1521-3773(20021004)41:19<3674::AID-ANIE3674>3.0.CO;2-F |
| 89. | García Fernández, J. M.; Ortiz Mellet, C.; Defaye, J. J. Inclusion Phenom. Macrocyclic Chem. 2006, 56, 149–159. doi:10.1007/s10847-006-9076-3 |
| 77. | Benito, J. M.; Rodríguez-Lucena, D.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. J. Inclusion Phenom. Macrocyclic Chem. 2007, 57, 147–150. doi:10.1007/s10847-006-9203-1 |
| 78. | Rodríguez-Lucena, D.; Benito, J. M.; Álvarez, E.; Jaime, C.; Perez-Miron, J.; García Fernández, J. M. J. Org. Chem. 2008, 73, 2967–2979. doi:10.1021/jo800048s |
| 79. | Rodríguez-Lucena, D.; Ortiz Mellet, C.; Jaime, C.; Burusco, K. K.; García Fernández, J. M.; Benito, J. M. J. Org. Chem. 2009, 74, 2997–3008. doi:10.1021/jo802796p |
| 88. | Benito, J. M.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. Angew. Chem., Int. Ed. 2002, 41, 3674–3676. doi:10.1002/1521-3773(20021004)41:19<3674::AID-ANIE3674>3.0.CO;2-F |
| 89. | García Fernández, J. M.; Ortiz Mellet, C.; Defaye, J. J. Inclusion Phenom. Macrocyclic Chem. 2006, 56, 149–159. doi:10.1007/s10847-006-9076-3 |
| 91. | Barawkar, D.; Bruice, T. C. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 11047–11052. doi:10.1073/pnas.95.19.11047 |
| 92. | Barawkar, D.; Linkletter, B. A.; Bruice, T. C. Bioorg. Med. Chem. Lett. 1998, 8, 1517–1520. doi:10.1016/S0960-894X(98)00251-0 |
| 93. | Linkletter, B. A.; Szabo, I. E.; Bruice, T. C. J. Am. Chem. Soc. 1999, 121, 3888–3896. doi:10.1021/ja984212w |
| 94. | Linkletter, B. A.; Bruice, T. C. Bioorg. Med. Chem. 2000, 8, 1893–1901. doi:10.1016/S0968-0896(00)00146-2 |
| 83. | Arya, D. P.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2000, 10, 691–693. doi:10.1016/S0960-894X(00)00085-8 |
| 84. | Challa, H.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2001, 11, 2423–2427. doi:10.1016/S0960-894X(01)00455-3 |
| 91. | Barawkar, D.; Bruice, T. C. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 11047–11052. doi:10.1073/pnas.95.19.11047 |
| 92. | Barawkar, D.; Linkletter, B. A.; Bruice, T. C. Bioorg. Med. Chem. Lett. 1998, 8, 1517–1520. doi:10.1016/S0960-894X(98)00251-0 |
| 76. | Jiménez Blanco, J. L.; Bootello, P.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2006, 71, 5136–5143. doi:10.1021/jo060360q |
| 78. | Rodríguez-Lucena, D.; Benito, J. M.; Álvarez, E.; Jaime, C.; Perez-Miron, J.; García Fernández, J. M. J. Org. Chem. 2008, 73, 2967–2979. doi:10.1021/jo800048s |
| 98. | Park, M.; Toporowski, J. W.; Bruice, T. C. Bioorg. Med. Chem. 2006, 14, 1743–1749. doi:10.1016/j.bmc.2005.10.028 |
| 99. | Park, M.; Canzio, D.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2008, 18, 2377–2384. doi:10.1016/j.bmcl.2008.02.063 |
| 96. | Kojima, N.; Szabo, I. E.; Bruice, T. C. Tetrahedron 2002, 58, 867–879. doi:10.1016/S0040-4020(01)01185-1 |
| 97. | Park, M.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2005, 15, 3247–3251. doi:10.1016/j.bmcl.2005.04.073 |
| 58. | Charkraborty, T. K.; Srinivasu, P.; Bikshapathy, E.; Nagaraj, R.; Vairamani, M.; Kiran Kumar, S.; Kunwar, A. C. J. Org. Chem. 2003, 68, 6257–6263. doi:10.1021/jo034586u |
| 59. | Fleet, G. W. J.; Johnson, S. W.; Jones, J. H. J. Pept. Sci. 2006, 12, 559–561. doi:10.1002/psc.759 |
| 57. | van Well, R. M.; Marinelli, L.; Erkelens, K.; van der Marel, G. A.; Lavecchia, A.; Overkleeft, H. S.; van Boom, J. H.; Kessler, H.; Overhand, M. Eur. J. Org. Chem. 2003, 2303–2313. doi:10.1002/ejoc.200300031 |
| 1. | Mareel, M.; Leroy, A. Physiol. Rev. 2003, 83, 337–376. |
| 2. | Burleigh, B. A.; Woolsey, A. M. Cell. Microbiol. 2002, 4, 701–711. doi:10.1046/j.1462-5822.2002.00226.x |
| 3. | Shi, X.; Friess, H.; Kleeff, J.; Ozawa, F.; Büchler, M. W. Pancreatology 2001, 1, 517–524. doi:10.1159/000055854 |
| 4. | Muramatsu, T. Glycoconjugate J. 2000, 17, 577–595. doi:10.1023/A:1011078627247 |
| 12. | Risseeuw, M. D. P.; Overhand, M.; Fleet, G. W. J.; Simona, M. I. Tetrahedron: Asymmetry 2007, 18, 2001–2010. doi:10.1016/j.tetasy.2007.08.004 |
| 13. | Gruner, S. A. W.; Locardi, E.; Lohof, E.; Kessler, H. Chem. Rev. 2002, 102, 491–514. doi:10.1021/cr0004409 |
| 14. | Franz, A. H.; Gross, P. H.; Samoshin, V. V. ARKIVOC 2008, i, 271–308. |
| 68. | López, O.; Maya, I.; Fuentes, J.; Fernández-Bolaños, J. G. Tetrahedron 2004, 60, 61–72. doi:10.1016/j.tet.2003.10.092 |
| 7. | Wilson, I. B. H. Curr. Opin. Struct. Biol. 2002, 12, 569–577. doi:10.1016/S0959-440X(02)00367-6 |
| 9. | Crocker, P. R. Curr. Opin. Struct. Biol. 2002, 12, 609–615. doi:10.1016/S0959-440X(02)00375-5 |
| 10. | Harnett, W.; Harnett, M. M.; Byron, O. Curr. Protein Pept. Sci. 2003, 4, 59–71. doi:10.2174/1389203033380368 |
| 11. | Pendse, S.; Sayegh, M. H.; Frank, M. H. Curr. Drug Targets 2003, 4, 469–476. doi:10.2174/1389450033490894 |
| 8. | Crocker, P. R.; Feizi, T. Curr. Opin. Struct. Biol. 1996, 6, 679–691. doi:10.1016/S0959-440X(96)80036-4 |
| 65. | Storch de Gracia, I.; Bobo, S.; Martín-Ortega, M. D.; Chiara, J. L. Org. Lett. 1999, 1, 1705–1708. doi:10.1021/ol990904t |
| 5. | Dwek, R. A. Chem. Rev. 1996, 96, 683–720. doi:10.1021/cr940283b |
| 6. | Dwek, R. A.; Butters, T. D.; Platt, F. M.; Zitzmann, N. Nat. Rev. Drug Discovery 2002, 1, 65–75. doi:10.1038/nrd708 |
| 7. | Wilson, I. B. H. Curr. Opin. Struct. Biol. 2002, 12, 569–577. doi:10.1016/S0959-440X(02)00367-6 |
| 66. | Chiara, J. L.; Storch de Gracia, I.; García, A.; Batida, A.; Bobo, M.; Martín-Ortega, M. D. ChemBioChem 2005, 6, 186–191. doi:10.1002/cbic.200400231 |
| 67. | Gibson, R. P.; Gloster, T. M.; Roberts, S.; Warren, R. A. J.; Storch de Gracia, I.; García, A.; Chiara, J. L.; Davies, G. J. Angew. Chem., Int. Ed. 2007, 46, 4115–4119. doi:10.1002/anie.200604825 |
| 12. | Risseeuw, M. D. P.; Overhand, M.; Fleet, G. W. J.; Simona, M. I. Tetrahedron: Asymmetry 2007, 18, 2001–2010. doi:10.1016/j.tetasy.2007.08.004 |
| 13. | Gruner, S. A. W.; Locardi, E.; Lohof, E.; Kessler, H. Chem. Rev. 2002, 102, 491–514. doi:10.1021/cr0004409 |
| 17. | Schweizer, F. Angew. Chem., Int. Ed. 2002, 41, 230–253. doi:10.1002/1521-3773(20020118)41:2<230::AID-ANIE230>3.0.CO;2-L |
| 62. | Ichikawa, Y.; Matsukawa, Y.; Tamura, M.; Ohara, F.; Isobe, M.; Kotsuki, H. Chem.– Asian J. 2006, 1, 717–723. doi:10.1002/asia.200600190 |
| 63. | Ichikawa, Y.; Matsukawa, Y.; Isobe, M. J. Am. Chem. Soc. 2006, 128, 3934–3938. doi:10.1021/ja056253f |
| 19. | Wessel, H. P.; Lucas Dias, S. In Glycoscience; Fraser-Reid, B.; Tatsuta, K.; Thiem, J., Eds.; Springer-Verlag: Berlin Heidelberg, Germany, 2008; pp 2079–2112. doi:10.1007/978-3-540-30429-6_52 |
| 64. | Ando, O.; Satake, H.; Itoi, K.; Sato, A.; Nakajima, M.; Takahashi, S.; Haruyama, H.; Ohkuma, Y.; Kinishita, T.; Enokita, R. J. Antibiot. 1991, 44, 1165–1168. |
| 18. | Varela, O.; Orgueira, H. A. Adv. Carbohydr. Chem. Biochem. 2000, 55, 137–174. doi:10.1016/S0065-2318(00)55005-7 |
| 60. | Ellestad, G. A.; Cosulich, D. B.; Broschard, R. W.; Martin, J. H.; Kuntsmann, M. P.; Morton, G. O.; Lancaster, J. E.; Fulmor, W.; Lovell, F. M. J. Am. Chem. Soc. 1978, 100, 2515–2524. doi:10.1021/ja00476a041 |
| 13. | Gruner, S. A. W.; Locardi, E.; Lohof, E.; Kessler, H. Chem. Rev. 2002, 102, 491–514. doi:10.1021/cr0004409 |
| 14. | Franz, A. H.; Gross, P. H.; Samoshin, V. V. ARKIVOC 2008, i, 271–308. |
| 15. | Smith, M. D.; Fleet, G. W. J. J. Pept. Sci. 1999, 5, 425–441. doi:10.1002/(SICI)1099-1387(199910)5:10<425::AID-PSC222>3.0.CO;2-O |
| 16. | Chakraborty, T. K.; Srinivasu, P.; Tapadar, S.; Mohan, B. K. Glycoconjugate J. 2005, 22, 83–93. doi:10.1007/s10719-005-0844-x |
| 17. | Schweizer, F. Angew. Chem., Int. Ed. 2002, 41, 230–253. doi:10.1002/1521-3773(20020118)41:2<230::AID-ANIE230>3.0.CO;2-L |
| 61. | Nishiyama, T.; Isobe, M.; Ichikawa, Y. Angew. Chem., Int. Ed. 2005, 44, 4372–4375. doi:10.1002/anie.200500892 |
| 73. | Prosperi, D.; Ronchi, S.; Lay, L.; Rencurosi, A.; Russo, G. Eur. J. Org. Chem. 2004, 395–405. doi:10.1002/ejoc.200300483 |
| 74. | López, O.; Maza, S.; Maya, I.; Fuentes, J.; Fernández-Bolaños, J. G. Tetrahedron 2005, 61, 9058–9069. doi:10.1016/j.tet.2005.07.041 |
| 75. | Prosperi, D.; Ronchi, S.; Lay, L.; Rencurosi, A.; Russo, G. Synlett 2004, 1529–1532. doi:10.1055/s-2004-829074 |
| 69. | Kovács, L.; Osz, E.; Györdeák, Z. Carbohydr. Res. 2002, 337, 1171–1178. doi:10.1016/S0008-6215(02)00108-8 |
| 70. | Díaz Pérez, V. M.; Ortiz Mellet, C.; Fuentes, J.; García Fernández, J. M. Carbohydr. Res. 2000, 326, 161–175. doi:10.1016/S0008-6215(00)00040-9 |
| 71. | García-Moreno, M. I.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2001, 66, 7604–7614. doi:10.1021/jo015639f |
| 72. | García-Moreno, M. I.; Ortiz Mellet, C.; García Fernández, J. M. Eur. J. Org. Chem. 2004, 1803–1819. doi:10.1002/ejoc.200300731 |
| 80. | Jiménez Blanco, J. L.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. Org. Lett. 1999, 1, 1217–1220. doi:10.1021/ol990889s |
| 68. | López, O.; Maya, I.; Fuentes, J.; Fernández-Bolaños, J. G. Tetrahedron 2004, 60, 61–72. doi:10.1016/j.tet.2003.10.092 |
| 70. | Díaz Pérez, V. M.; Ortiz Mellet, C.; Fuentes, J.; García Fernández, J. M. Carbohydr. Res. 2000, 326, 161–175. doi:10.1016/S0008-6215(00)00040-9 |
| 71. | García-Moreno, M. I.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2001, 66, 7604–7614. doi:10.1021/jo015639f |
| 72. | García-Moreno, M. I.; Ortiz Mellet, C.; García Fernández, J. M. Eur. J. Org. Chem. 2004, 1803–1819. doi:10.1002/ejoc.200300731 |
| 81. | Benito, J. M.; Ortiz Mellet, C.; Sadalapure, K.; Lindhorst, T. K.; Defaye, J.; García Fernández, J. M. Carbohydr. Res. 1999, 320, 37–48. doi:10.1016/S0008-6215(99)00141-X |
| 80. | Jiménez Blanco, J. L.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. Org. Lett. 1999, 1, 1217–1220. doi:10.1021/ol990889s |
| 70. | Díaz Pérez, V. M.; Ortiz Mellet, C.; Fuentes, J.; García Fernández, J. M. Carbohydr. Res. 2000, 326, 161–175. doi:10.1016/S0008-6215(00)00040-9 |
| 76. | Jiménez Blanco, J. L.; Bootello, P.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2006, 71, 5136–5143. doi:10.1021/jo060360q |
| 77. | Benito, J. M.; Rodríguez-Lucena, D.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. J. Inclusion Phenom. Macrocyclic Chem. 2007, 57, 147–150. doi:10.1007/s10847-006-9203-1 |
| 78. | Rodríguez-Lucena, D.; Benito, J. M.; Álvarez, E.; Jaime, C.; Perez-Miron, J.; García Fernández, J. M. J. Org. Chem. 2008, 73, 2967–2979. doi:10.1021/jo800048s |
| 79. | Rodríguez-Lucena, D.; Ortiz Mellet, C.; Jaime, C.; Burusco, K. K.; García Fernández, J. M.; Benito, J. M. J. Org. Chem. 2009, 74, 2997–3008. doi:10.1021/jo802796p |
| 70. | Díaz Pérez, V. M.; Ortiz Mellet, C.; Fuentes, J.; García Fernández, J. M. Carbohydr. Res. 2000, 326, 161–175. doi:10.1016/S0008-6215(00)00040-9 |
| 71. | García-Moreno, M. I.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2001, 66, 7604–7614. doi:10.1021/jo015639f |
| 72. | García-Moreno, M. I.; Ortiz Mellet, C.; García Fernández, J. M. Eur. J. Org. Chem. 2004, 1803–1819. doi:10.1002/ejoc.200300731 |
| 76. | Jiménez Blanco, J. L.; Bootello, P.; Benito, J. M.; Ortiz Mellet, C.; García Fernández, J. M. J. Org. Chem. 2006, 71, 5136–5143. doi:10.1021/jo060360q |
| 77. | Benito, J. M.; Rodríguez-Lucena, D.; Jiménez Blanco, J. L.; Ortiz Mellet, C.; García Fernández, J. M. J. Inclusion Phenom. Macrocyclic Chem. 2007, 57, 147–150. doi:10.1007/s10847-006-9203-1 |
| 78. | Rodríguez-Lucena, D.; Benito, J. M.; Álvarez, E.; Jaime, C.; Perez-Miron, J.; García Fernández, J. M. J. Org. Chem. 2008, 73, 2967–2979. doi:10.1021/jo800048s |
| 79. | Rodríguez-Lucena, D.; Ortiz Mellet, C.; Jaime, C.; Burusco, K. K.; García Fernández, J. M.; Benito, J. M. J. Org. Chem. 2009, 74, 2997–3008. doi:10.1021/jo802796p |
| 36. | Brittain, D. E. A.; Watterson, M. P.; Claridge, T. D. W.; Smith, M. D.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 2000, 3655–3665. doi:10.1039/b002995p |
| 37. | Hungerford, N. L.; Claridge, T. D. W.; Watterson, M. P.; Aplin, R. T.; Moreno, A.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 2000, 3666–3679. doi:10.1039/b002996n |
| 40. | Edwards, A. A.; Fleet, G. W. J.; Tranter, G. E. Chirality 2006, 18, 265–272. doi:10.1002/chir.20248 |
| 38. | Claridge, T. D. W.; Long, D. D.; Baker, C. M.; Odell, B.; Grant, G. H.; Edwards, A. A.; Tranter, G. E.; Fleet, G. W. J.; Smith, M. D. J. Org. Chem. 2005, 70, 2082–2090. doi:10.1021/jo0480040 |
| 44. | Edwards, A. A.; Alexander, B. D.; Fleet, G. W. J.; Tranter, G. E. Chirality 2008, 20, 969–972. doi:10.1002/chir.20538 |
| 45. | Jockusch, R. A.; Talbot, F. O.; Rogers, P. S.; Simone, M. I.; Fleet, G. W. J.; Simons, J. P. J. Am. Chem. Soc. 2006, 128, 16771–16777. doi:10.1021/ja0607133 |
| 42. | Chakraborty, T. K.; Jayaprakash, S.; Srinivasu, P.; Govardhana Chary, M.; Diwan, P. V.; Nagaraj, R.; Ravi Sankar, A.; Kunwar, A. C. Tetrahedron Lett. 2000, 41, 8167–8171. doi:10.1016/S0040-4039(00)01425-8 |
| 43. | Chakraborty, T. K.; Srinivasu, P.; Sakunthala Madhavendra, S.; Kiran Kumar, S.; Kunwar, A. C. Tetrahedron Lett. 2004, 45, 3573–3577. doi:10.1016/j.tetlet.2004.03.048 |
| 40. | Edwards, A. A.; Fleet, G. W. J.; Tranter, G. E. Chirality 2006, 18, 265–272. doi:10.1002/chir.20248 |
| 41. | Andreini, M.; Taillefumier, C.; Chrétien, F.; Thery, V.; Chapleur, Y. J. Org. Chem. 2009, 74, 7651–7659. doi:10.1021/jo900966b |
| 38. | Claridge, T. D. W.; Long, D. D.; Baker, C. M.; Odell, B.; Grant, G. H.; Edwards, A. A.; Tranter, G. E.; Fleet, G. W. J.; Smith, M. D. J. Org. Chem. 2005, 70, 2082–2090. doi:10.1021/jo0480040 |
| 38. | Claridge, T. D. W.; Long, D. D.; Baker, C. M.; Odell, B.; Grant, G. H.; Edwards, A. A.; Tranter, G. E.; Fleet, G. W. J.; Smith, M. D. J. Org. Chem. 2005, 70, 2082–2090. doi:10.1021/jo0480040 |
| 46. | Barker, S. F.; Angus, D.; Taillefumier, C.; Probert, M. R.; Watkin, D. J.; Watterson, M. P.; Claridge, T. D. W.; Hungerford, N. L.; Fleet, G. W. J. Tetrahedron Lett. 2001, 42, 4247–4250. doi:10.1016/S0040-4039(01)00660-8 |
| 47. | Johnson, S. W.; Jenkinson, S. F.; Angus, D.; Pérez-Victoria, I.; Claridge, T. D. W.; Fleet, G. W. J.; Jones, J. H. J. Pept. Sci. 2005, 11, 303–318. doi:10.1002/psc.622 |
| 48. | Lopez-Ortega, B.; Jenkinson, S. F.; Claridge, T. D. W.; Fleet George, W. J. Tetrahedron: Asymmetry 2008, 19, 976–983. doi:10.1016/j.tetasy.2008.03.030 |
| 49. | Claridge, T. D. W.; Goodman, J. M.; Moreno, A.; Angus, D.; Barker, S. F.; Taillefumier, C.; Watterson, M. P.; Fleet, G. W. J. Tetrahedron Lett. 2001, 42, 4251–4255. doi:10.1016/S0040-4039(01)00661-X |
| 50. | Johnson, S. W.; Jenkinson, S. F.; Pérez-Victoria, I.; Edwards, A. A.; Claridge, T. D. W.; Tranter, G. E.; Fleet, G. W. J. J. Pept. Sci. 2005, 11, 517–524. doi:10.1002/psc.658 |
| 54. | Locardi, E.; Stöckle, M.; Gruner, S.; Kessler, H. J. Am. Chem. Soc. 2001, 123, 8189–8196. doi:10.1021/ja010181k |
| 56. | Fournier, F.; Afonso, C.; Ménand, M.; Hamon, L.; Xie, J.; Tabeta, J.-C. Eur. J. Mass Spectrom. 2008, 14, 61–69. doi:10.1255/ejms.912 |
| 54. | Locardi, E.; Stöckle, M.; Gruner, S.; Kessler, H. J. Am. Chem. Soc. 2001, 123, 8189–8196. doi:10.1021/ja010181k |
| 55. | Ménand, M.; Blais, J.-C.; Hamon, L.; Valéry, J.-M.; Xie, J. J. Org. Chem. 2005, 70, 4423–4430. doi:10.1021/jo0502899 |
| 52. | D’Onofrio, J.; De Champdoré, M.; De Napoli, L.; Montesarchio, D.; Di Fabio, G. Bioconjugate Chem. 2005, 16, 1299–1309. doi:10.1021/bc0501606 |
| 53. | Sicherl, F.; Wittmann, V. Angew. Chem., Int. Ed. 2005, 44, 2096–2099. doi:10.1002/anie.200462595 |
| 51. | Claridge, T. D. W.; Lopez-Ortega, B.; Jenkinson, S. F.; Fleet, G. W. J. Tetrahedron: Asymmetry 2008, 19, 984–988. doi:10.1016/j.tetasy.2008.03.029 |
| 37. | Hungerford, N. L.; Claridge, T. D. W.; Watterson, M. P.; Aplin, R. T.; Moreno, A.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 2000, 3666–3679. doi:10.1039/b002996n |
| 105. | Reddy, P. M.; Bruice, T. C. J. Am. Chem. Soc. 2004, 126, 3736–3747. doi:10.1021/ja031557s |
| 106. | Park, M.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2008, 18, 3488–3491. doi:10.1016/j.bmcl.2008.05.030 |
| 103. | Challa, H.; Bruice, T. C. Bioorg. Med. Chem. 2004, 12, 1475–1481. doi:10.1016/j.bmc.2003.12.043 |
| 104. | Reddy, P. M.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2003, 13, 1281–1285. doi:10.1016/S0960-894X(03)00119-7 |
| 101. | Szabo, I. E.; Bruice, T. C. Bioorg. Med. Chem. 2004, 12, 4233–4244. doi:10.1016/j.bmc.2004.05.010 |
| 102. | Jain, M. L.; Bruice, T. C. Bioorg. Med. Chem. 2006, 14, 7333–7346. doi:10.1016/j.bmc.2006.05.074 |
| 100. | Linkletter, B. A.; Szabo, I. E.; Bruice, T. C. Nucleic Acids Res. 2001, 29, 2370–2376. doi:10.1093/nar/29.11.2370 |
| 28. | Suhara, Y.; Yamaguchi, Y.; Collins, B.; Schnaar, R. L.; Yanagishita, M.; Hildreth, J. E. K.; Shimada, I.; Ichikawa, Y. Bioorg. Med. Chem. 2002, 10, 1999–2013. doi:10.1016/S0968-0896(02)00020-2 |
| 29. | Suhara, Y.; Kurihara, M.; Kittaka, A.; Ichikawa, Y. Tetrahedron 2006, 62, 8207–8217. doi:10.1016/j.tet.2006.05.080 |
| 30. | Durrat, F.; Xie, J.; Valéry, J.-M. Tetrahedron Lett. 2004, 45, 1477–1479. doi:10.1016/j.tetlet.2003.12.032 |
| 29. | Suhara, Y.; Kurihara, M.; Kittaka, A.; Ichikawa, Y. Tetrahedron 2006, 62, 8207–8217. doi:10.1016/j.tet.2006.05.080 |
| 23. | Szabo, L.; Smith, B. L.; McReynolds, D.; Parrill, A. L.; Morris, E. R.; Gervay, J. J. Org. Chem. 1998, 63, 1074–1078. doi:10.1021/jo971415e |
| 25. | McReynolds, K. D.; Gervay-Hague, J. Tetrahedron: Asymmetry 2000, 11, 337–362. doi:10.1016/S0957-4166(99)00560-1 |
| 26. | Fuchs, E.-F.; Lehmann, J. Chem. Ber. 1975, 108, 2254–2260. doi:10.1002/cber.19751080712 |
| 27. | Fuchs, E.-F.; Lehmann, J. Carbohydr. Res. 1976, 49, 267–273. doi:10.1016/S0008-6215(00)83144-4 |
| 21. | Müller, C.; Kitas, E.; Wessel, H. P. J. Chem. Soc., Chem. Commun. 1995, 2425–2426. doi:10.1039/C39950002425 |
| 22. | Wessel, H. P.; Mitchell, C. M.; Lobato, C. M.; Schmid, G. Angew. Chem., Int. Ed. Engl. 1996, 34, 2712–2713. doi:10.1002/anie.199527121 |
| 23. | Szabo, L.; Smith, B. L.; McReynolds, D.; Parrill, A. L.; Morris, E. R.; Gervay, J. J. Org. Chem. 1998, 63, 1074–1078. doi:10.1021/jo971415e |
| 24. | Gregar, T. Q.; Gervay-Hague, J. J. Org. Chem. 2004, 69, 1001–1009. doi:10.1021/jo035312+ |
| 107. | Zhang, X.; Bruice, T. C. Bioorg. Med. Chem. Lett. 2008, 18, 665–669. doi:10.1016/j.bmcl.2007.11.061 |
| 20. | Chakraborty, T. K.; Ghosh, S.; Jayaprakash, S. Curr. Med. Chem. 2002, 9, 421–435. |
| 32. | Smith, M. D.; Long, D. D.; Martin, A.; Marquess, D. G.; Claridge, T. D. W.; Fleet, G. W. J. Tetrahedron Lett. 1999, 40, 2191–2194. doi:10.1016/S0040-4039(99)00109-4 |
| 33. | Claridge, T. D. W.; Long, D. D.; Hungerford, N. L.; Aplin, R. T.; Smith, M. D.; Marquess, D. G.; Fleet, G. W. J. Tetrahedron Lett. 1999, 40, 2199–2202. doi:10.1016/S0040-4039(99)00111-2 |
| 34. | Smith, M. D.; Claridge, T. D. W.; Sansom, M. S. P.; Fleet, G. W. J. Org. Biomol. Chem. 2003, 1, 3647–3655. doi:10.1039/b307707c |
| 31. | Chung, Y.-K.; Claridge, T. D. W.; Fleet, G. W. J.; Johnson, S. W.; Jones, J. H.; Lumbard, K. W.; Stalchulski, A. V. J. Pept. Sci. 2004, 10, 1–7. doi:10.1002/psc.488 |
| 28. | Suhara, Y.; Yamaguchi, Y.; Collins, B.; Schnaar, R. L.; Yanagishita, M.; Hildreth, J. E. K.; Shimada, I.; Ichikawa, Y. Bioorg. Med. Chem. 2002, 10, 1999–2013. doi:10.1016/S0968-0896(02)00020-2 |
| 39. | Edwards, A. A.; Sanjayan, G. J.; Hachisu, S.; Tranter, G. E.; Fleet, G. W. J. Tetrahedron 2006, 62, 7718–7725. doi:10.1016/j.tet.2006.05.067 |
| 34. | Smith, M. D.; Claridge, T. D. W.; Sansom, M. S. P.; Fleet, G. W. J. Org. Biomol. Chem. 2003, 1, 3647–3655. doi:10.1039/b307707c |
| 38. | Claridge, T. D. W.; Long, D. D.; Baker, C. M.; Odell, B.; Grant, G. H.; Edwards, A. A.; Tranter, G. E.; Fleet, G. W. J.; Smith, M. D. J. Org. Chem. 2005, 70, 2082–2090. doi:10.1021/jo0480040 |
| 39. | Edwards, A. A.; Sanjayan, G. J.; Hachisu, S.; Tranter, G. E.; Fleet, G. W. J. Tetrahedron 2006, 62, 7718–7725. doi:10.1016/j.tet.2006.05.067 |
| 36. | Brittain, D. E. A.; Watterson, M. P.; Claridge, T. D. W.; Smith, M. D.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 2000, 3655–3665. doi:10.1039/b002995p |
| 37. | Hungerford, N. L.; Claridge, T. D. W.; Watterson, M. P.; Aplin, R. T.; Moreno, A.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 2000, 3666–3679. doi:10.1039/b002996n |
| 35. | Long, D. D.; Hungerford, N. L.; Smith, M. D.; Brittain, D. E. A.; Marquess, D. G.; Claridge, T. D. W.; Fleet, G. W. J. Tetrahedron Lett. 1999, 40, 2195–2198. doi:10.1016/S0040-4039(99)00110-0 |
| 35. | Long, D. D.; Hungerford, N. L.; Smith, M. D.; Brittain, D. E. A.; Marquess, D. G.; Claridge, T. D. W.; Fleet, G. W. J. Tetrahedron Lett. 1999, 40, 2195–2198. doi:10.1016/S0040-4039(99)00110-0 |
© 2010 Jiménez Blanco et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)