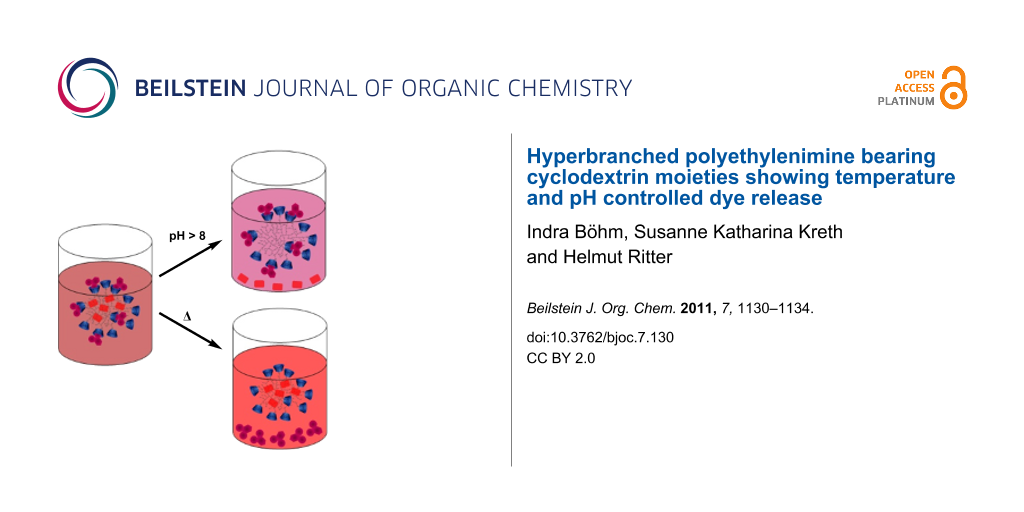
Abstract
The release of anthraquinone dyes from β-cyclodextrin modified, hyperbranched polyethylenimine (PEI-CD) was investigated. 5,8-Dichloro-1,4-dihydroxyanthraquinone (AQ-OH) was enclosed simply by ionic attraction between the phenolate groups of AQ-OH and the protonated amino groups of polyethylenimine (PEI). Additionally, the adamantyl moieties of 1,4-bis-N-adamantylaminoanthraquinone (AQ-Ada) were encapsulated by the cavity of CD modified PEI. Due to these different types of interaction, the dyes can be controllably released from the CD-cavity (AQ-Ada) by temperature variation or from salt encapsulation by pH variation (AQ-OH), as monitored by UV–vis spectroscopy.
Graphical Abstract
Introduction
The development of novel materials and their use as drug delivery systems has gained increased interest in the last few decades [1-3]. In this context, certain stimuli responsive behaviors are required [4,5]. Hence, the ability to release molecules in response to temperature or pH change is an important feature of new biopharmaceutical compounds [6-11].
Hyperbranched polyethylenimine (PEI) is a promising material for medical applications due to its high biocompatibility [12,13]. Active drugs bearing anionic groups can interact with the cationic hyperbranched structure of the polymeric matrix [14]. These components can be released by changing the pH value [15]. In this context we recently reported the synthesis of hyperbranched PEI bearing covalently attached β-cyclodextrin (CD) [16]. CD has been exploited to complex various hydrophobic guests such as proteins, dyes and anti tumor drugs [17-22]. Inclusion complexes with, e.g., adamantane derivates, are relatively stable due to their high binding constants [23,24]. These kinds of supramolecular complexes become less stable under heating [25]. For optical visualization of the attachment and release of suitable molecules, two anthraquinone dyes were chosen as model compounds in the present work [26].
Results and Discussion
5,8-Dichloro-1,4-dihydroxyanthraquinone (1) and 1,4-bis-N-adamantylaminoanthraquinone (2) were used as model compounds for a drug delivery system in combination with hyperbranched cationic polyethylenimine bearing covalently attached β-CDs 3.
As outlined in Scheme 1, each dye, 1 and 2, was mixed with 3 in water to form a salt 4 and a host–guest complex 5, respectively. Additionally, both dyes were mixed together with 3 to provide complex 6 (Scheme 1).
![[1860-5397-7-130-i1]](/bjoc/content/inline/1860-5397-7-130-i1.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 1: Preparation of salts and/or complexes 4, 5 and 6.
Scheme 1: Preparation of salts and/or complexes 4, 5 and 6.
The increase in hydrodynamic diameters (dH), measured by DLS, indicates the successful inclusion of both dyes 1 and 2 in polymer 3. The inclusion of slightly acidic 1 in the cationic PEI induces an increase of dH from 8 nm for compound 3 to 163 nm for the corresponding salt 4 (Supporting Information File 1, Figure S1) due to electrostatic expansion effects. In comparison, the dH of unmodified PEI also increases by the inclusion of 1 (Supporting Information File 1, Figure S2). The combination of unmodified PEI and dye 2 could not be measured since the dye is not water-soluble and cannot be enclosed by PEI.
In contrast, the two hydrophobic adamantyl moieties of 2 were enclosed in the CD cavity of PEI, yielding complex 5. An increased dH from 8 nm to 260 nm due to intermolecular effects was measured by DLS (Supporting Information File 1, Figure S3). This effect strongly indicates the formation of higher aggregates due to intermolecular interactions. DLS measurements of the complex of unmodified, randomly methylated, β-cyclodextrin (RAMEB-CD) and dye 2 were performed, giving a dH of 68 nm (Supporting Information File 1, Figure S6). The combination of RAMEB-CD and dye 1 could not be measured since the dye is not water-soluble and cannot be enclosed by CD.
Complex 6 exhibits enlarged networks with a dH of 480 nm, which verifies an inclusion of both dyes (1 and 2) through salt formation and host-guest complexation (Supporting Information File 1, Figure S4).
In water, a red solution of 4 was observed with λmax at 524 and 564 nm. The water insoluble dye 1 becomes water soluble since the deprotonated hydroxy groups form a salt with the cationic amino moieties of PEI. The system reaches an equilibrium state between the complexed and the released dye 1 at pH values below 9. In basic solution no interaction takes place due to deprotonation of the amino moieties. Regarding the UV–vis spectra, the absorption intensity at λmax (524 nm) for t = 0 corresponds to 100% of the dye being enclosed. Hence, 74% of the red dye was released after 24 h, a total amount of 88% after 48 h and a complete precipitate was observed after 72 h (Figure 1). This can be visualized even by the naked eye as the solution becomes light brownish (from the soluted polymer 3 which was also identified by DLS) and the red dye forms a residue.
Figure 1: UV–vis spectra of complex 4 in basic solution (pH 10, t = 0 (blue line), t = 24 h (red line), t = 48 h (green line), t > 72 h (orange line and violet line)), and corresponding photos and schematic illustrations of the solution.
Figure 1: UV–vis spectra of complex 4 in basic solution (pH 10, t = 0 (blue line), t = 24 h (red line), t = 4...
The water insoluble dye 2 becomes water soluble as the hydrophobic adamantyl moieties are encapsulated by CD. That leads to a purple aqueous solution with λmax at 552 and 668 nm. This complex 5 is not pH sensitive, which was verified by UV–vis spectroscopy (Supporting Information File 1, Figure S7).
After 30 min of heating at 60 °C the complexation equilibrium shifted towards the free purple colored dye 2. The complete release was monitored by eye and by UV–vis spectroscopy (Figure 2).
Figure 2: UV–vis spectra of complex 5 in solution before heating (solid line) and after 30 min of heating at 60 °C (dotted line), and corresponding photos and schematic illustrations of the solution.
Figure 2: UV–vis spectra of complex 5 in solution before heating (solid line) and after 30 min of heating at ...
The absorption plot of complex 6 (a combination of the red and the purple dyes) exhibits maxima at 524, 564 and 656 nm. To show the separate release of dye 2, complex 6 was heated for 30 min at 60 °C. At pH 1–8 the absorption undergoes a hypsochromic shift and a decrease of the maximum of the purple dye, resulting in a red solution (Figure 3).
Figure 3: UV–vis spectra of complex 6 in solution (pH 6) before heating (solid line) and after 30 min heating at 60 °C (dotted line), and corresponding photos and schematic illustrations of the solution.
Figure 3: UV–vis spectra of complex 6 in solution (pH 6) before heating (solid line) and after 30 min heating...
In contrast, the separate release of dye 1 was forced by pH adjustment. At pH 9–14 the characteristic maximum of the red dye 1 (524 nm) decreases and a bathochromic shift is observed (Figure 4).
Figure 4: UV–vis spectra of complex 6 in basic solution (pH 10) before heating (solid line), after 24 h without heating (dashed line) and after 30 min of heating at 60 °C (dotted line), and corresponding photos and schematic illustrations of the solution.
Figure 4: UV–vis spectra of complex 6 in basic solution (pH 10) before heating (solid line), after 24 h witho...
Furthermore, a complete release (~1 mg of each dye from 5 mg of complex 6) of both dyes was achieved by heating the solution of complex 6 at pH 9–14 (Figure 4). Hence, two different components can be enclosed in one polymer and released separately or simultaneous in response to different external stimuli.
Conclusion
The controlled release of different dyes from a drug delivery system based on hyperbranched polyethylenimine was investigated. The ionic interactions between the PEI scaffold and the hydroxy moieties of 5,8-dichloro-1,4-dihydroxyanthraquinone (AQ-OH) can be dissolved by pH adjustment. In contrast, the adamantyl modified compound 1,4-di-N-adamantylaminoanthraquinone (AQ-Ada) forms a host–guest complex with the PEI attached CDs and can be released by heating. These dyes serve as model compounds to illustrate the effectiveness of PEI-CD as drug delivery system in which two different components can be enclosed and released, either separately or simultaneously, in response to different external stimuli.
Experimental
Measurements: Dynamic Light Scattering (DLS) experiments were carried out with a Malvern Nano ZS ZEN 3600 in a temperature range from 6 to 50 °C. The particle size distribution was derived by deconvoluting the measured intensity autocorrelation function of the sample with the NNLS general purpose mode algorithm included in the DTS software. UV–vis spectra were recorded in water using the UV 540 system of the company Unicam.
Materials: All reagents used were commercially available and were used without further purification.
Synthesis of the host-molecule hyperbranched polyethylenimine covalently attached with β-cyclodextrin (3): Polymer 3 was synthesized according to [16] (Supporting Information File 1, Scheme S1). Briefly, compound 3 can be obtained through polymer-analogous amidation with 5-hexynoic acid and subsequent reaction with mono-(6-azido-6-desoxy)-β-cyclodextrin by click reaction under microwave assisted conditions.
Synthesis of 1,4-di-N-adamantylaminoanthraquinone (2): 2 was synthesized according to literature procedure [27] (Supporting Information File 1, Scheme S2). Briefly, 1,4-dichloroanthraquinone, adamantylamine and disodium hydrogen phosphate were suspended in N-methylpyrrolidone and heated for several hours. The reaction mixture was poured into water, filtered off and dried.
Synthesis of host–guest complexes 4, 5 and 6: 4, 5: In 20 mL water, 0.15 g of 3 and 0.05 g of the anthraquinone derivative (either 1 or 2) were added and stirred for 24 h; 6: In 20 mL water, 0.15 g of 3 and 0.05 g of both anthraquinone derivatives 1 and 2 were added and stirred for 24 h.
Preparation for UV–vis spectroscopy: 0.5 mL of the solutions 4, 5 or 6 was added to 3 mL of different aqueous solutions (of sodium hydroxide or hydrochloric acid) with pH values from 1 to 14.
Supporting Information
Supporting Information features detailed data on syntheses, and DLS and UV–vis measurements.
| Supporting Information File 1: Experimental details. | ||
| Format: PDF | Size: 450.1 KB | Download |
References
-
Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Angew. Chem., Int. Ed. 2003, 42, 4640–4643. doi:10.1002/anie.200250653
Return to citation in text: [1] -
Zhang, J.-T.; Xue, Y.-N.; Gao, F.-Z.; Huang, S.-W.; Zhuo, R.-X. J. Appl. Polym. Sci. 2008, 108, 3031–3037. doi:10.1002/app.27931
Return to citation in text: [1] -
Tang, G. P.; Guo, H. Y.; Alexis, F.; Wang, X.; Zeng, S.; Lim, T. M.; Ding, J.; Yang, Y. Y.; Wang, S. J. Gene Med. 2006, 8, 736–744. doi:10.1002/jgm.874
Return to citation in text: [1] -
Goldin, D. S.; Dahl, C. A.; Olsen, K. L.; Ostrach, L. H.; Klausner, R. D. Science 2001, 292, 443–444. doi:10.1126/science.1059744
Return to citation in text: [1] -
Medeiros, S. F.; Santos, A. M.; Fessi, H.; Elaissari, A. Int. J. Pharm. 2011, 403, 139–161. doi:10.1016/j.ijpharm.2010.10.011
Return to citation in text: [1] -
Koutroumanis, K. P.; Avgoustakis, K.; Bikiaris, D. Carbohydr. Polym. 2010, 82, 181–188. doi:10.1016/j.carbpol.2010.04.044
Return to citation in text: [1] -
Connors, K. A. Chem. Rev. 1997, 97, 1325–1358. doi:10.1021/cr960371r
Return to citation in text: [1] -
Ihre, H. R.; Padilla de Jesús, O. L.; Szoka, F. C., Jr.; Fréchet, M. J. Bioconjugate Chem. 2002, 13, 443–452. doi:10.1021/bc010102u
Return to citation in text: [1] -
Krämer, M.; Stumbé, J. F.; Türk, H.; Krause, S.; Komp, A.; Delineau, L.; Prokhovova, S.; Kautz, H.; Haag, R. Angew. Chem. 2002, 114, 4426–4431. doi:10.1002/1521-3757(20021115)114:22<4426::AID-ANGE4426>3.0.CO;2-1
Return to citation in text: [1] -
Discher, D. E.; Eisenberg, A. Science 2002, 297, 967–973. doi:10.1126/science.1074972
Return to citation in text: [1] -
Langer, R. Science 2001, 293, 58–59. doi:10.1126/science.1063273
Return to citation in text: [1] -
Kabanov, A. V.; Felgner, P. L.; Seymour, L. W., Eds. Self-Assembling Complexes for Gene Delivery: From Laboratory to Clinical Trial; John Wiley and Sons: New York, 1998.
Return to citation in text: [1] -
Peng, Q.; Zhong, Z.; Zhuo, R. Bioconjugate Chem. 2008, 19, 499–506. doi:10.1021/bc7003236
Return to citation in text: [1] -
Pastor-Pérez, L.; Chen, Y.; Shen, Z.; Lahoz, A.; Stiriba, S.-E. Macromol. Rapid Commun. 2007, 28, 1404–1409. doi:10.1002/marc.200700190
Return to citation in text: [1] -
Haag, R. Angew. Chem., Int. Ed. 2004, 43, 278–282. doi:10.1002/anie.200301694
Return to citation in text: [1] -
Boehm, I.; Ritter, H. Macromol. Chem. Phys. 2011, 212, 1080–1085. doi:10.1002/macp.201100006
Return to citation in text: [1] [2] -
Forrest, M. L.; Gabrielson, N.; Pack, D. W. Biotechnol. Bioeng. 2005, 89, 416–423. doi:10.1002/bit.20356
Return to citation in text: [1] -
Kretschmann, O.; Choi, S. W.; Miyauchi, M.; Tomatsu, I.; Harada, A.; Ritter, H. Angew. Chem., Int. Ed. 2006, 45, 4361–4365. doi:10.1002/anie.200504539
Return to citation in text: [1] -
Kretschmann, O.; Ritter, H. Macromol. Chem. Phys. 2006, 207, 987–992. doi:10.1002/macp.200600120
Return to citation in text: [1] -
Antonietti, L.; Aymonier, C.; Schlotterbeck, U.; Garamus, V. M.; Maksimova, T.; Richtering, W.; Mecking, S. Macromolecules 2005, 38, 5914–5920. doi:10.1021/ma047458w
Return to citation in text: [1] -
Maciollek, A.; Munteanu, M.; Ritter, H. Macromol. Chem. Phys. 2010, 211, 245–249. doi:10.1002/macp.200900436
Return to citation in text: [1] -
Zhou, J.; Ritter, H. Polym. Chem. 2010, 1, 1552–1559. doi:10.1039/c0py00219d
Return to citation in text: [1] -
Rekharsky, M. V.; Inoue, Y. Chem. Rev. 1998, 98, 1875–1918. doi:10.1021/cr970015o
Return to citation in text: [1] -
Koopmans, C.; Ritter, H. Macromolecules 2008, 41, 7418–7422. doi:10.1021/ma801202f
Return to citation in text: [1] -
Feil, H.; Bae, Y. H.; Feijen, J.; Kim, S. W. Macromolecules 1993, 26, 2496–2500. doi:10.1021/ma00062a016
Return to citation in text: [1] -
Dang, X.-J.; Tong, J.; Li, H.-L. J. Inclusion Phenom. Mol. Recognit. Chem. 1996, 24, 275–286. doi:10.1007/BF01041113
Return to citation in text: [1] -
Böhm, I.; Kreth, S. K.; Ritter, H.; Branscheid, R.; Kolb, U. Macromol. Chem. Phys. 2011, in press. doi:10.1002/macp.201100259
Return to citation in text: [1]
| 1. | Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Angew. Chem., Int. Ed. 2003, 42, 4640–4643. doi:10.1002/anie.200250653 |
| 2. | Zhang, J.-T.; Xue, Y.-N.; Gao, F.-Z.; Huang, S.-W.; Zhuo, R.-X. J. Appl. Polym. Sci. 2008, 108, 3031–3037. doi:10.1002/app.27931 |
| 3. | Tang, G. P.; Guo, H. Y.; Alexis, F.; Wang, X.; Zeng, S.; Lim, T. M.; Ding, J.; Yang, Y. Y.; Wang, S. J. Gene Med. 2006, 8, 736–744. doi:10.1002/jgm.874 |
| 14. | Pastor-Pérez, L.; Chen, Y.; Shen, Z.; Lahoz, A.; Stiriba, S.-E. Macromol. Rapid Commun. 2007, 28, 1404–1409. doi:10.1002/marc.200700190 |
| 12. | Kabanov, A. V.; Felgner, P. L.; Seymour, L. W., Eds. Self-Assembling Complexes for Gene Delivery: From Laboratory to Clinical Trial; John Wiley and Sons: New York, 1998. |
| 13. | Peng, Q.; Zhong, Z.; Zhuo, R. Bioconjugate Chem. 2008, 19, 499–506. doi:10.1021/bc7003236 |
| 6. | Koutroumanis, K. P.; Avgoustakis, K.; Bikiaris, D. Carbohydr. Polym. 2010, 82, 181–188. doi:10.1016/j.carbpol.2010.04.044 |
| 7. | Connors, K. A. Chem. Rev. 1997, 97, 1325–1358. doi:10.1021/cr960371r |
| 8. | Ihre, H. R.; Padilla de Jesús, O. L.; Szoka, F. C., Jr.; Fréchet, M. J. Bioconjugate Chem. 2002, 13, 443–452. doi:10.1021/bc010102u |
| 9. | Krämer, M.; Stumbé, J. F.; Türk, H.; Krause, S.; Komp, A.; Delineau, L.; Prokhovova, S.; Kautz, H.; Haag, R. Angew. Chem. 2002, 114, 4426–4431. doi:10.1002/1521-3757(20021115)114:22<4426::AID-ANGE4426>3.0.CO;2-1 |
| 10. | Discher, D. E.; Eisenberg, A. Science 2002, 297, 967–973. doi:10.1126/science.1074972 |
| 11. | Langer, R. Science 2001, 293, 58–59. doi:10.1126/science.1063273 |
| 27. | Böhm, I.; Kreth, S. K.; Ritter, H.; Branscheid, R.; Kolb, U. Macromol. Chem. Phys. 2011, in press. doi:10.1002/macp.201100259 |
| 4. | Goldin, D. S.; Dahl, C. A.; Olsen, K. L.; Ostrach, L. H.; Klausner, R. D. Science 2001, 292, 443–444. doi:10.1126/science.1059744 |
| 5. | Medeiros, S. F.; Santos, A. M.; Fessi, H.; Elaissari, A. Int. J. Pharm. 2011, 403, 139–161. doi:10.1016/j.ijpharm.2010.10.011 |
| 23. | Rekharsky, M. V.; Inoue, Y. Chem. Rev. 1998, 98, 1875–1918. doi:10.1021/cr970015o |
| 24. | Koopmans, C.; Ritter, H. Macromolecules 2008, 41, 7418–7422. doi:10.1021/ma801202f |
| 26. | Dang, X.-J.; Tong, J.; Li, H.-L. J. Inclusion Phenom. Mol. Recognit. Chem. 1996, 24, 275–286. doi:10.1007/BF01041113 |
| 17. | Forrest, M. L.; Gabrielson, N.; Pack, D. W. Biotechnol. Bioeng. 2005, 89, 416–423. doi:10.1002/bit.20356 |
| 18. | Kretschmann, O.; Choi, S. W.; Miyauchi, M.; Tomatsu, I.; Harada, A.; Ritter, H. Angew. Chem., Int. Ed. 2006, 45, 4361–4365. doi:10.1002/anie.200504539 |
| 19. | Kretschmann, O.; Ritter, H. Macromol. Chem. Phys. 2006, 207, 987–992. doi:10.1002/macp.200600120 |
| 20. | Antonietti, L.; Aymonier, C.; Schlotterbeck, U.; Garamus, V. M.; Maksimova, T.; Richtering, W.; Mecking, S. Macromolecules 2005, 38, 5914–5920. doi:10.1021/ma047458w |
| 21. | Maciollek, A.; Munteanu, M.; Ritter, H. Macromol. Chem. Phys. 2010, 211, 245–249. doi:10.1002/macp.200900436 |
| 22. | Zhou, J.; Ritter, H. Polym. Chem. 2010, 1, 1552–1559. doi:10.1039/c0py00219d |
| 16. | Boehm, I.; Ritter, H. Macromol. Chem. Phys. 2011, 212, 1080–1085. doi:10.1002/macp.201100006 |
| 16. | Boehm, I.; Ritter, H. Macromol. Chem. Phys. 2011, 212, 1080–1085. doi:10.1002/macp.201100006 |
| 15. | Haag, R. Angew. Chem., Int. Ed. 2004, 43, 278–282. doi:10.1002/anie.200301694 |
| 25. | Feil, H.; Bae, Y. H.; Feijen, J.; Kim, S. W. Macromolecules 1993, 26, 2496–2500. doi:10.1021/ma00062a016 |
© 2011 Böhm et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)