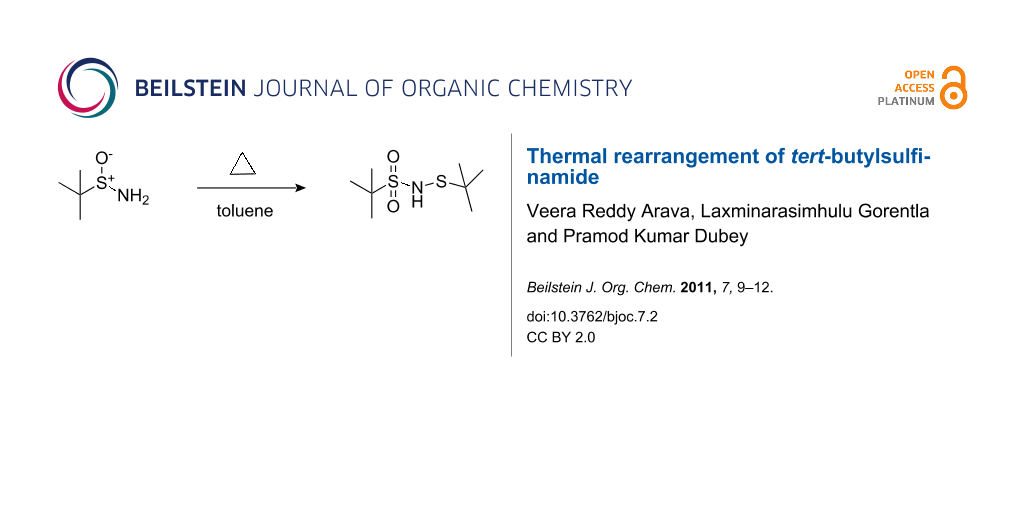
Abstract
tert-Butylsulfinamides are unstable above room temperature, and in chlorinated solvents they undergo rearrangement to form the more stable N-(tert-butylthio)-tert-butylsulfonamide.
Graphical Abstract
Introduction
Over the past decade, an ever increasing number of methods based upon the chiral amine reagent tert-butylsulfinamide (1) (Figure 1) has become one of the most extensively used synthetic approaches for both the production and discovery of drug candidates [1]. In particular, the tert-butylsulfinyl group showed high levels of asymmetric induction in many processes. The importance of these reagents (R and S) is evident by the number of manufacturers (>75) and by the number of publications (>400).
Results and Discussion
During our studies [2] on the industrial utilization of these two reagents (R and S), we found an interesting observation, i.e., when sulfinamide 1 was reacted with an organic acid in the presence of boric acid [3] to obtain an amide 2 (Scheme 1), no amide was obtained, which was confirmed by IR and NMR data, and the product was also devoid of an acid residue. The product appeared to be derived only from the reagent 1.
From the spectral data, structure 3 was assigned to the product shown in Figure 2. The structure of 3 was confirmed by chemical synthesis (Scheme 2) and finally by XRD [4] (Figure 3). Both tert-butylsulfanyl chloride 4 (Scheme 3) and tert-butylsulfonamide 5 (Scheme 4) were prepared by known procedures [5,6].
Figure 2: N-(tert-butylthio)-tert-butylsulfonamide.
Figure 2: N-(tert-butylthio)-tert-butylsulfonamide.
Scheme 3: Synthesis of tert-butylsulfanyl chloride.
Scheme 3: Synthesis of tert-butylsulfanyl chloride.
Scheme 4: Synthesis of tert-butylsulfonamide.
Scheme 4: Synthesis of tert-butylsulfonamide.
A possible mechanism for the proposed rearrangement is shown in Scheme 5.
Scheme 5: Proposed mechanism for rearrangement.
Scheme 5: Proposed mechanism for rearrangement.
First it is assumed that the product is formed only by the degradation of reagent 1. Further experiments, in the presence of acids (entries 7 to 13, Table 1) or the absence of acids (entries 1 to 6, Table 1), under sonication (entries 14 to 18) and with microwave irradiation (entry 26), confirmed the assumption that the reagent is thermally unstable. Also the rearrangement is likely not to proceed by a homolytic fission (radical) mechanism, because the rate of reaction is not affected either by benzoyl peroxide, by TEMPO a radical initiator (entries 22 and 23) or by a radical inhibitor 2,6-di-tert-butylphenol (entry 24).
Table 1: Screening of rearrangement conditions.
| Entry | Reaction conditions | Yield (%)a | ||||
|---|---|---|---|---|---|---|
|
Starting
material |
Reagent / condition
type |
Solvent | T (°C) | Time (h) | ||
| 1 | (R)-isomer | - | - | 110 | 3 | 27 |
| 2 | (R)-isomer | - | toluene | 110 | 48 | 70 |
| 3 | (R)-isomer | - | o-xylene | 140 | 48 | 64 |
| 4 | (R)-isomer | - | ethylene dichloride | 80 | 72 | 40 + 20c |
| 5 | (R)-isomer | - | CHCl3 | 65 | 72 | 25 |
| 6 | (R)-isomer | - | toluene | 110 | 48 | 70 |
| 7 | (R)-isomer | boric acid (1.0 equiv) | toluene (u/N2) | 110 | 24 | 65 |
| 8 | (R)-isomer | boric acid (1.0 equiv) | toluene | 110 | 24 | 65 |
| 9 | (R)-isomer | boric acid (0.5 equiv) | toluene | 110 | 48 | 60 |
| 10 | (R)-isomer | MeSO3H | toluene | 110 | 1 | 27 |
| 11 | (R)-isomer | tartaric acid | toluene | 110 | 24 | 38 |
| 12 | (R)-isomer | citric acid | toluene | 110 | 24 | 16 |
| 13 | (R)-isomer | p-TSA | toluene | 110 | 24 | 38 |
| 14 | (R)-isomer | sonication | CHCl3 | RT | 2 | 34b |
| 15 | (R)-isomer | sonication | DMF | RT | 1 | 2b |
| 16 | (R)-isomer | sonication | ethyl acetate | RT | 1 | 5b |
| 17 | (R)-isomer | sonication + boric acid | CHCl3 | RT | 1 | 16b |
| 18 | (R)-isomer | sonication + p-TSA | CHCl3 | RT | 0.5 | 23b |
| 19 | (R)-isomer | - | toluene | RT | 144 | 3 |
| 20 | (R)-isomer | - | CHCl3 | RT | 144 | 21b |
| 21 | (R)-isomer | - | MeOH | RT | 144 | 3b |
| 22 | (R)-isomer | benzoyl peroxide | toluene | 110 | 48 | 60 |
| 23 | (R)-isomer | TEMPO | toluene | RT - 110 | 40 | 9b |
| 24 | (R)-isomer | 2,6-di-tert-butylphenol | toluene | 110 | 24 | 75 |
| 25 | (S)-isomer | - | toluene | 110 | 48 | 70 |
| 26 | (R)-isomer | MW 150 Watt | DMF | 150 | 0.5 | 70 |
aIsolated yield, b% conversion in HPLC, crecovered starting material with SOR +5°
When the reagent 1 alone was subjected to thermal rearrangement (entry 1), complete consumption of starting material was observed. Only 27% product was isolated and 73% of the material was lost by vaporitation. When the reaction was carried out in the presence of solvents such as toluene (entries 2, 6 and 24), o-xylene (entry 3), or solvents with reagents such as boric acid (entries 7, 8 and 9), methanesulphonic acid (entry 10), p-TSA (entry 13), benzoyl peroxide (entry 22), 2,6-di-tert-butylphenol (entry 23) or with microwave irradiation (entry 25), complete consumption of starting material was observed. In other cases (entries 4, 5, 11 and 12), 10 to 30% of the starting material was recovered without racemization.
Conclusion
We found that both (R and S) tert-butanesulfinamides are unstable above room temperature and in chlorinated solvents.
Supporting Information
| Supporting Information File 1: Full experimental data. | ||
| Format: PDF | Size: 34.4 KB | Download |
References
-
Robak, M. T.; Herbage, M. A.; Ellman, J. A. Chem. Rev. 2010, 110, 3600–3740. doi:10.1021/cr900382t
Return to citation in text: [1] -
Reddy, A. V.; Rao, S. U. B.; Narasimha, G. L.; Dubey, P. K. Synth. Commun. 2009, 39, 1451–1456. doi:10.1080/00397910802519216
Return to citation in text: [1] -
Mylavarapu, R. K.; Kondaiah, G. C. M.; Kolla, N.; Veeramalla, R.; Koilkonda, P.; Bhattacharya, A.; Bandichhor, R. Org. Process Res. Dev. 2007, 11, 1065–1068. doi:10.1021/op700098w
Return to citation in text: [1] -
Deposited as CCDC 773082 (2010).
Return to citation in text: [1] -
Himel, C. M.; Park, M. Production of tertiary alkyl thio sulfenyl chlorides. U.S. Patent 2,934,563, April 26, 1960.
Return to citation in text: [1] -
Sharpless, K. B.; Gontcharov, A. V.; Liu, H. Aminohydroxylation of olefins with tert-alkyl sulfonamides. U.S. Patent 6,008,376, Aug 21, 1999.
Return to citation in text: [1]
| 1. | Robak, M. T.; Herbage, M. A.; Ellman, J. A. Chem. Rev. 2010, 110, 3600–3740. doi:10.1021/cr900382t |
| 5. | Himel, C. M.; Park, M. Production of tertiary alkyl thio sulfenyl chlorides. U.S. Patent 2,934,563, April 26, 1960. |
| 6. | Sharpless, K. B.; Gontcharov, A. V.; Liu, H. Aminohydroxylation of olefins with tert-alkyl sulfonamides. U.S. Patent 6,008,376, Aug 21, 1999. |
| 3. | Mylavarapu, R. K.; Kondaiah, G. C. M.; Kolla, N.; Veeramalla, R.; Koilkonda, P.; Bhattacharya, A.; Bandichhor, R. Org. Process Res. Dev. 2007, 11, 1065–1068. doi:10.1021/op700098w |
| 2. | Reddy, A. V.; Rao, S. U. B.; Narasimha, G. L.; Dubey, P. K. Synth. Commun. 2009, 39, 1451–1456. doi:10.1080/00397910802519216 |
© 2011 Arava et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)




![[1860-5397-7-2-3]](/bjoc/content/figures/1860-5397-7-2-3.png?scale=2.0&max-width=1024&background=FFFFFF)