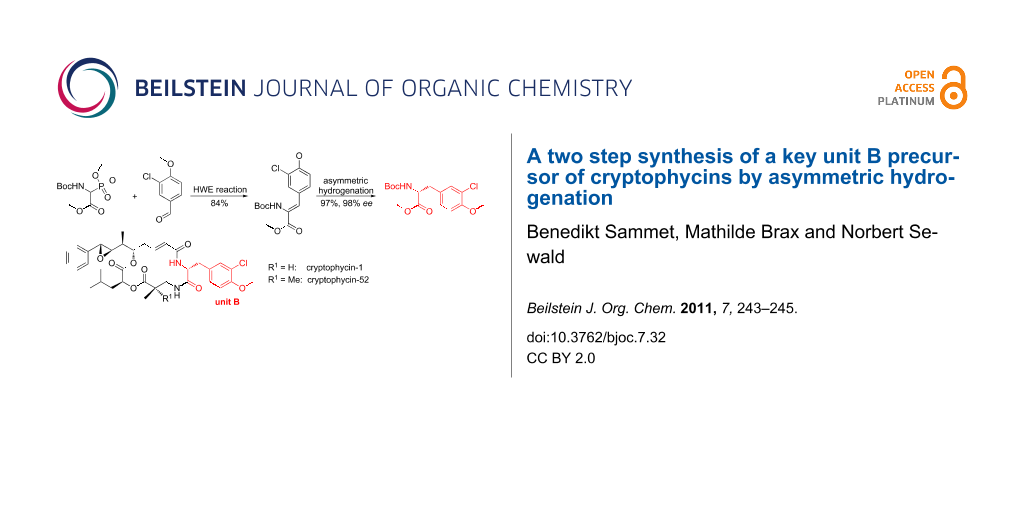
Abstract
A novel highly enantioselective two step access to a unit B precursor of cryptophycins in good yields from commercially available starting materials has been developed. The key step is an asymmetric hydrogenation using the commercially available [(COD)Rh-(R,R)-Et-DuPhos]BF4 catalyst. The synthetic route provides the advantage of less synthetic steps, proceeds with high yields and enantioselectivity, and avoids hazardous reaction conditions.
Graphical Abstract
Introduction
Cryptophycins are macrocyclic depsipeptides, which show very high cytotoxicity even against multidrug-resistant cell lines. They inhibit mitosis of eukaryotic cells by interacting with the β-subunit of α/β-tubulin heterodimers. Numerous natural and artificial analogs have been analysed in structure–activity relationship (SAR) studies. The unit B of cryptophycins contains a considerably modified D-tyrosine derivative (Figure 1). Substituent variations at unit B are not well tolerated. Both the methoxy and the chloro substituent are required for full biological activity [1-4].
Figure 1: The four building blocks (units) A–D of cryptophycin-1 (1) and cryptophycin-52 (2).
Figure 1: The four building blocks (units) A–D of cryptophycin-1 (1) and cryptophycin-52 (2).
The previously published synthetic route to unit B precursor 4 involves a three-step modification of D-tyrosine by chlorination, protecting group introduction and double methylation followed by a final saponification reaction to give carboxylic acid 5 (Scheme 1). A number of experimental procedures for this route have been published [5-7]. The selective monochlorination of D-tyrosine is quite cumbersome since the formation of the dichlorinated product must be minimized and the presence of unreacted D-tyrosine after the reaction must be completely avoided. The dichlorinated by-product has to be separated by column chromatography when purifying the desired methyl ester 4 [7,8]. In addition, another major drawback of this synthetic route is the use of highly toxic and carcinogenic dimethyl sulfate.
Scheme 1: Synthesis of the unit B precursor from D-tyrosine (3). Reagents and conditions [7]: a) SO2Cl2, AcOH, rt, 90 min, (75%); b) Boc2O, NaOH, t-BuOH/H2O, rt, 16 h (quant.) c) Me2SO4, K2CO3, acetone, reflux, 4 h (99%); d) LiOH, H2O/THF/MeOH, rt, 1 h (93%).
Scheme 1: Synthesis of the unit B precursor from D-tyrosine (3). Reagents and conditions [7]: a) SO2Cl2, AcOH, r...
A completely different route to unit B precursor 8 (Scheme 2) is based on a phase transfer catalyst (PTC) mediated asymmetric alkylation. However, the required cinchonine derived chiral catalyst is not commercially available [9].
Scheme 2: Unit B synthesis by a chiral PTC approach. Reagents and conditions [9]: a) N-(Diphenylmethylene)glycine tert-butyl ester, 50% KOH, toluene/CHCl3, chiral phase transfer catalyst (0.01 equiv), 0 °C, 20 h (87%; 96% ee); b) 15% citric acid, THF, rt, 16 h; c) FmocCl, Na2CO3, THF, rt, 14 h, (72% over two steps).
Scheme 2: Unit B synthesis by a chiral PTC approach. Reagents and conditions [9]: a) N-(Diphenylmethylene)glycin...
Results and Discussion
We envisaged a two step synthesis for the unit B precursor 4 (Scheme 1) from commercially available non-toxic starting materials based on an asymmetric hydrogenation approach to make the unit B precursor synthesis shorter and safer. In general, there is also a whole variety of possible stereoselective synthetic methods available to synthesize modified α-amino acids, such as the classical Schöllkopf-method [10] or catalytic approaches [11,12]. The unit B precursor of cryptophycin is a phenylalanine derivative. An asymmetric hydrogenation approach for the synthesis of such α-amino acids is well-established [12]. In the first step of the developed synthesis 3-chloro-4-methoxybenzaldehyde is reacted with rac-Boc-α-phosphonoglycine trimethyl ester (9) [13,14] to yield olefin 10 in a completely Z-selective Horner–Wadsworth–Emmons (HWE) reaction (Scheme 3). Asymmetric hydrogenation using the commercially available [(COD)Rh-(R,R)-Et-DuPhos]BF4 catalyst [14,15] gave the anticipated methyl ester 4 (Scheme 1) in 97% yield with an ee exceeding 98% (determined by chiral HPLC). Hydrogenation of 10 with 10% Pd/C was envisaged to obtain rac-4 as a reference for the determination of the ee. Interestingly, due to this more reactive catalyst a complete reductive dehalogenation was observed to give rac-Boc-Tyr(Me)-OMe (rac-11) as reported for a similar case [16]. Therefore, ent-4 was synthesized analogously also using the commercially available enantiomeric catalyst ([(COD)Rh-(S,S)-Et-DuPhos]BF4).
Scheme 3: Unit B precursor 4 synthesis by asymmetric hydrogenation. Reagents and conditions: a) 3-Chloro-4-methoxybenzaldehyde, 1,1,3,3-tetramethylguanidine, CH2Cl2, 0 °C to rt, 16 h (84%); b) [(COD)Rh-(R,R)-Et-DuPhos]BF4 (1.9 mol %), H2, dry and degassed MeOH, 3–6 bar, 21.5 h (97%; 98% ee); c) 10% Pd/C, H2, MeOH, 16 h, (76%).
Scheme 3: Unit B precursor 4 synthesis by asymmetric hydrogenation. Reagents and conditions: a) 3-Chloro-4-me...
Conclusion
A novel two step synthesis of the important cryptophycin unit B precursor 4 is disclosed based on a HWE reaction followed by a highly enantioselective [(COD)Rh-(R,R)-Et-DuPhos]BF4 mediated asymmetric hydrogenation. This high-yielding access is more convenient and avoids hazardous chemicals in contrast to the established method.
Supporting Information
| Supporting Information File 1: Full experimental procedures and detailed analytical data for the synthesis of 10 and 4 including chiral HPLC spectra. | ||
| Format: PDF | Size: 712.1 KB | Download |
References
-
Trimurtulu, G.; Ohtani, I.; Patterson, G. M. L.; Moore, R. E.; Corbett, T. H.; Valeriote, F. A.; Demchik, L. J. Am. Chem. Soc. 1994, 116, 4729–4737. doi:10.1021/ja00090a020
Return to citation in text: [1] -
Eißler, S.; Stoncius, A.; Nahrwold, M.; Sewald, N. Synthesis 2006, 3747–3789. doi:10.1055/s-2006-950332
Return to citation in text: [1] -
Nahrwold, M.; Bogner, T.; Eissler, S.; Verma, S.; Sewald, N. Org. Lett. 2010, 12, 1064–1067. doi:10.1021/ol1000473
Return to citation in text: [1] -
Sammet, B.; Bogner, T.; Nahrwold, M.; Weiss, C.; Sewald, N. J. Org. Chem. 2010, 75, 6953–6960. doi:10.1021/jo101563s
Return to citation in text: [1] -
Barrow, R. A.; Hemscheidt, T.; Liang, J.; Paik, S.; Moore, R. E.; Tius, M. A. J. Am. Chem. Soc. 1995, 117, 2479–2490. doi:10.1021/ja00114a011
Return to citation in text: [1] -
McCubbin, J. A.; Maddess, M. L.; Lautens, M. Org. Lett. 2006, 8, 2993–2996. doi:10.1021/ol0609356
Return to citation in text: [1] -
Nahrwold, M. β2-Aminosäuren als Bausteine funktionalisierter Cryptophycin-Analoga. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, 2009.
http://bieson.ub.uni-bielefeld.de/volltexte/2010/1673/
Return to citation in text: [1] [2] [3] -
Eißler, S. Synthese von Cryptophycinen für SAR-Studien. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, 2008.
http://bieson.ub.uni-bielefeld.de/volltexte/2008/1301/
Return to citation in text: [1] -
Danner, P.; Bauer, M.; Phukan, P.; Maier, M. E. Eur. J. Org. Chem. 2005, 317–325. doi:10.1002/ejoc.200400558
Return to citation in text: [1] [2] -
Lim, H. J.; Gallucci, J. C.; RajanBabu, T. V. Org. Lett. 2010, 12, 2162–2165. doi:10.1021/ol100663y
Return to citation in text: [1] -
Zuend, S. J.; Coughlin, M. P.; Lalonde, M. P.; Jacobsen, E. N. Nature 2009, 461, 968–970. doi:10.1038/nature08484
Return to citation in text: [1] -
Nájera, C.; Sansano, J. M. Chem. Rev. 2007, 107, 4584–4671. doi:10.1021/cr050580o
Return to citation in text: [1] [2] -
Schmidt, U.; Griesser, H.; Leitenberger, V.; Lieberknecht, A.; Mangold, R.; Meyer, R.; Riedl, B. Synthesis 1992, 487–490. doi:10.1055/s-1992-26143
Return to citation in text: [1] -
Bower, J. F.; Szeto, P.; Gallagher, T. Chem. Commun. 2005, 5793–5795. doi:10.1039/b510761j
Return to citation in text: [1] [2] -
Burk, M. J.; Feaster, J. E.; Nugent, W. A.; Harlow, R. L. J. Am. Chem. Soc. 1993, 115, 10125–10138. doi:10.1021/ja00075a031
Return to citation in text: [1] -
Melillo, D. G.; Larsen, R. D.; Mathre, D. J.; Shukis, W. F.; Wood, A. W.; Colleluori, J. R. J. Org. Chem. 1987, 52, 5143–5150. doi:10.1021/jo00232a016
Return to citation in text: [1]
| 1. | Trimurtulu, G.; Ohtani, I.; Patterson, G. M. L.; Moore, R. E.; Corbett, T. H.; Valeriote, F. A.; Demchik, L. J. Am. Chem. Soc. 1994, 116, 4729–4737. doi:10.1021/ja00090a020 |
| 2. | Eißler, S.; Stoncius, A.; Nahrwold, M.; Sewald, N. Synthesis 2006, 3747–3789. doi:10.1055/s-2006-950332 |
| 3. | Nahrwold, M.; Bogner, T.; Eissler, S.; Verma, S.; Sewald, N. Org. Lett. 2010, 12, 1064–1067. doi:10.1021/ol1000473 |
| 4. | Sammet, B.; Bogner, T.; Nahrwold, M.; Weiss, C.; Sewald, N. J. Org. Chem. 2010, 75, 6953–6960. doi:10.1021/jo101563s |
| 9. | Danner, P.; Bauer, M.; Phukan, P.; Maier, M. E. Eur. J. Org. Chem. 2005, 317–325. doi:10.1002/ejoc.200400558 |
| 7. |
Nahrwold, M. β2-Aminosäuren als Bausteine funktionalisierter Cryptophycin-Analoga. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, 2009.
http://bieson.ub.uni-bielefeld.de/volltexte/2010/1673/ |
| 7. |
Nahrwold, M. β2-Aminosäuren als Bausteine funktionalisierter Cryptophycin-Analoga. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, 2009.
http://bieson.ub.uni-bielefeld.de/volltexte/2010/1673/ |
| 8. |
Eißler, S. Synthese von Cryptophycinen für SAR-Studien. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, 2008.
http://bieson.ub.uni-bielefeld.de/volltexte/2008/1301/ |
| 5. | Barrow, R. A.; Hemscheidt, T.; Liang, J.; Paik, S.; Moore, R. E.; Tius, M. A. J. Am. Chem. Soc. 1995, 117, 2479–2490. doi:10.1021/ja00114a011 |
| 6. | McCubbin, J. A.; Maddess, M. L.; Lautens, M. Org. Lett. 2006, 8, 2993–2996. doi:10.1021/ol0609356 |
| 7. |
Nahrwold, M. β2-Aminosäuren als Bausteine funktionalisierter Cryptophycin-Analoga. Ph.D. Thesis, Bielefeld University, Bielefeld, Germany, 2009.
http://bieson.ub.uni-bielefeld.de/volltexte/2010/1673/ |
| 12. | Nájera, C.; Sansano, J. M. Chem. Rev. 2007, 107, 4584–4671. doi:10.1021/cr050580o |
| 14. | Bower, J. F.; Szeto, P.; Gallagher, T. Chem. Commun. 2005, 5793–5795. doi:10.1039/b510761j |
| 15. | Burk, M. J.; Feaster, J. E.; Nugent, W. A.; Harlow, R. L. J. Am. Chem. Soc. 1993, 115, 10125–10138. doi:10.1021/ja00075a031 |
| 11. | Zuend, S. J.; Coughlin, M. P.; Lalonde, M. P.; Jacobsen, E. N. Nature 2009, 461, 968–970. doi:10.1038/nature08484 |
| 12. | Nájera, C.; Sansano, J. M. Chem. Rev. 2007, 107, 4584–4671. doi:10.1021/cr050580o |
| 16. | Melillo, D. G.; Larsen, R. D.; Mathre, D. J.; Shukis, W. F.; Wood, A. W.; Colleluori, J. R. J. Org. Chem. 1987, 52, 5143–5150. doi:10.1021/jo00232a016 |
| 10. | Lim, H. J.; Gallucci, J. C.; RajanBabu, T. V. Org. Lett. 2010, 12, 2162–2165. doi:10.1021/ol100663y |
| 9. | Danner, P.; Bauer, M.; Phukan, P.; Maier, M. E. Eur. J. Org. Chem. 2005, 317–325. doi:10.1002/ejoc.200400558 |
| 13. | Schmidt, U.; Griesser, H.; Leitenberger, V.; Lieberknecht, A.; Mangold, R.; Meyer, R.; Riedl, B. Synthesis 1992, 487–490. doi:10.1055/s-1992-26143 |
| 14. | Bower, J. F.; Szeto, P.; Gallagher, T. Chem. Commun. 2005, 5793–5795. doi:10.1039/b510761j |
© 2011 Sammet et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)