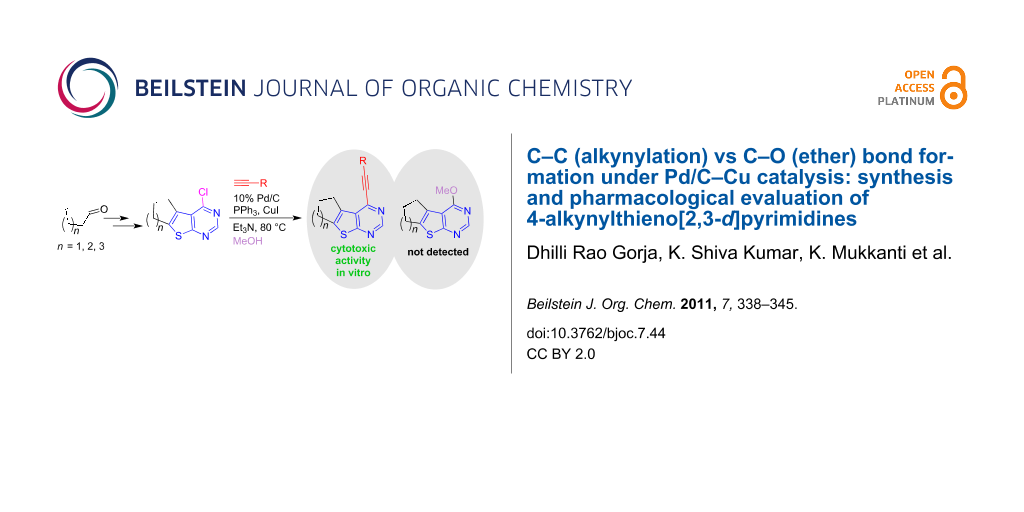
Abstract
The Pd/C–CuI–PPh3 catalytic system facilitated C–C bond formation between 4-chlorothieno[2,3-d]pyrimidines and terminal alkynes in methanol with high selectivity without generating any significant side products arising from C–O bond formation between the chloro compounds and methanol. A variety of novel 4-alkynylthieno[2,3- d]pyrimidines were prepared via alkynylation of 4-chlorothieno[2,3-d]pyrimidines in good to excellent yields. Some of the compounds synthesized were tested for cytotoxic activity in vitro.
Graphical Abstract
Introduction
Alkynyl substituted pyrimidines are of considerable pharmacological interest because of their notable biological activities [1], in particular, adenosine kinase inhibitory activity in the treatment of pain and inflammatory diseases [2] and thymidylate synthase inhibitory properties in cancer therapy [3]. On the other hand, the thiophene moiety is a common feature in many bioactive agents and drugs [4] and is considered as a bioisostere of the benzene ring [5]. Thus, one can anticipate that combining the pyrimidine ring of an alkynyl substituted pyrimidine moiety with a thiophene ring might afford compounds, i.e., alkynyl substituted thienopyrimidines of potential pharmacological interest. Notably, 6-ethynylthieno[3,2-d]- and 6-ethynylthieno[2,3-d]pyrimidin-4-aniline derivatives were found to be potent inhibitors of ErbB family receptor tyrosine kinases (EGFR, ErbB-2) and the proliferation of tumor cells that highly express these kinases [6]. In continuation of our research program into new drug discovery, we became interested in the generation of a small-molecule library A (Figure 1) based on thieno[2,3-d]pyrimidine for in-house pharmacological evaluation. Accordingly, we recently reported the synthesis of 4-(hetero)aryl substituted thieno[2,3-d]pyrimidines B [7]. As a further extension of this research and in view of possible pharmacological value of compounds containing alkyne, thiophene and pyrimidine moieties, we now wish to report the synthesis and in vitro cytotoxicity of novel 4-alkynylthieno[2,3-d]pyrimidines C (Figure 1). These derivatives are attractive due to the synthetic potential of C-4 alkynyl fragments for further use in library construction.
Figure 1: Diversity-based thieno[2,3-d]pyrimidine scaffold [7].
Figure 1: Diversity-based thieno[2,3-d]pyrimidine scaffold [7].
A number of methods have been reported for the synthesis of alkynyl substituted pyrimidines and most of which involve the use of Sonogashira coupling of halopyrimidines with terminal alkynes [1-3] (for a review see [8]). While alkynylation of the thiophene ring of thienopyrimidines under Sonogashira conditions [9] has previously been reported [6], a similar coupling reaction on the pyrimidine ring of thieno[2,3-d]pyrimidines is uncommon in the literature [10]. The use of Pd/C–CuI–PPh3 as a less expensive catalyst system for efficient Sonogashira coupling has been explored earlier. Due to our continuing interest in Pd/C-mediated alkynylation of aryl and heteroaryl halides we decided to investigate the Pd/C-based methodology for the synthesis of our target compounds, i.e., 4-alkynylthieno[2,3-d]pyrimidines as shown in Scheme 1.
Scheme 1: Pd/C-mediated synthesis of 4-alkynyl-substituted thieno[2,3-d]pyrimidines.
Scheme 1: Pd/C-mediated synthesis of 4-alkynyl-substituted thieno[2,3-d]pyrimidines.
Results and Discussion
The key starting materials, i.e., 4-chlorothieno[2,3-d]pyrimidines 1a–c required for our synthesis were prepared according to a known method reported earlier [7]. The other chloro compound 4-chloro-6,7,8,9-tetrahydro-5H-cyclohepta[4,5]thieno[2,3-d]pyrimidine (1d) was prepared from cycloheptanone and ethyl cyanoacetate following a similar procedure as shown in Scheme 2.
Scheme 2: Preparation of 4-chloro-6,7,8,9-tetrahydro-5H-cyclohepta[4,5]thieno[2,3-d]pyrimidine 1d.
Scheme 2: Preparation of 4-chloro-6,7,8,9-tetrahydro-5H-cyclohepta[4,5]thieno[2,3-d]pyrimidine 1d.
Condensation of resulting amino ester, i.e., ethyl 2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxylate (4) with formamide gave 6,7,8,9-tetrahydro-5H-cyclohepta[4,5]thieno[2,3-d]pyrimidin-4-one (5) which on treatment with POCl3 under refluxing conditions provided the desired 4-chloro derivative 1d. All the terminal alkynes used were commercially available. Initially, we chose to examine the coupling reaction of 4-chloro-5,6,7,8-tetrahydrobenzothieno[2,3-d]pyrimidine (1a) with phenylacetylene (2a) in the presence of 10% Pd/C (0.023 mmol), PPh3 (0.17 mmol), CuI (0.04 mmol), and triethylamine (2.67 mmol) in various solvents. The results of this study are summarized in Table 1. The reaction was initially carried out in MeOH for 5 h and the desired product, i.e., 4-(phenylethynyl)-5,6,7,8-tetrahydrobenzothieno[2,3-d]pyrimidine (3a) was isolated in 67% yield (Table 1, entry 1). The yield of 3a however was significantly improved when the reaction time was increased to 10 h (Table 1, entry 2). Thus the reaction proceeded well in MeOH to give the expected product via a C–C bond forming reaction (Scheme 3, path a) and no side product as a result of C–O bond formation [11] due to participation of MeOH (Scheme 3, path b) was detected in the reaction mixture. The use of MeOH as a nucleophile in Pd-catalyzed reactions has been well documented in the literature, see for example [12]. Moreover, when the addition of alkyne was omitted without changing the other reaction conditions, compound 1a reacted with MeOH to give the corresponding ether, 4-methoxy-5,6,7,8-tetrahydrobenzothieno[2,3-d]pyrimidine, albeit in low yield. The use of other solvents such as THF, acetonitrile, 1,4-dioxane and DMF (Table 1, entries 3-6) also gave good yields of product but MeOH was found to be superior. Moreover, the reaction temperature (and duration in some cases) was higher, i.e., 80 °C in case of acetonitrile, 1,4-dioxane and DMF compared to 60–65 °C in case of MeOH. While triethylamine was used as a base in all these cases, the use of a secondary amine, e.g., pyrrolidine was also examined. A side product was observed in this case due to the C–N bond forming reaction between 1a and pyrrolidine and was identified as 4-(N-pyrrolidinyl)-5,6,7,8-tetrahydrobenzothieno[2,3-d]pyrimidine. The use of another palladium catalyst (PPh3)2PdCl2 was also examined and found to be effective (Table 1, entry 7). However, we preferred to use Pd/C because it is less expensive, easy to handle and separable from the product and has the potential for recyclability, for a review see [13]. To assess the recyclability of the recovered Pd/C-catalyst in the present coupling reaction, the reaction mixture of 1a and 2a (Table 1, entry 2) was allowed to cool to room temperature and filtered. The residue was washed with MeOH, acetone, and DCM. After drying under vacuum the recovered catalyst was used directly in the reaction of 1a with 2a in the presence of PPh3, CuI, and Et3N: A conversion of 85% was observed confirming the recyclability of the recovered Pd/C-catalyst.
Table 1: Effect of solvent on the coupling reaction of 4-chloro-5,6,7,8-tetrahydrobenzothieno[2,3-d]pyrimidine (1a) with phenylacetylene (2a).a
|
|
|||
| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| 1 | MeOH | 5 | 67 |
| 2 | MeOH | 10 | 90 |
| 3 | THF | 14 | 80 |
| 4 | MeCN | 12 | 57 (80c) |
| 5 | 1,4-dioxane | 10 | 59 (80c) |
| 6 | DMF | 10 | 49 (75c) |
| 7 | MeOH | 10 | 83d |
aAll reactions were carried out by using 1 (0.89 mmol), 2a (1.33 mmol), 10% Pd/C (0.023 mmol), PPh3 (0.17 mmol), CuI (0.04 mmol), and Et3N (2.67 mmol) at 60–65 °C under a nitrogen atmosphere. bIsolated yields. cThe reaction was carried out at 80 °C. d(PPh3)2PdCl2–CuI was used instead of 10% Pd/C–PPh3–CuI.
Scheme 3: Reactivity of 4-chlorothieno[2,3-d]pyrimidines 1 towards terminal alkynes and MeOH under Pd/C–Cu catalysis.
Scheme 3: Reactivity of 4-chlorothieno[2,3-d]pyrimidines 1 towards terminal alkynes and MeOH under Pd/C–Cu ca...
Having established the optimum reaction conditions for the preparation of 3a we decided to test the generality and scope of this protocol further. Thus, a variety of commercially available terminal alkynes was employed under the reaction conditions indicated in entry 2 of Table 1 and the results are summarized in Table 2.
Table 2: Pd/C-mediated synthesis of 4-alkynylthieno[2,3-d]pyrimidine in methanol.a
| Entry | 4-Chlorothieno[2,3-d]pyrimidine | R–≡ (R=) | Productsb | Yieldc (%) |
|---|---|---|---|---|
| 1 |
1a |
–C6H5 (2a) |
3a |
90 |
| 2 | 1a | –CH2OH (2b) |
3b |
88 |
| 3 | 1a | –CH2CH2OH (2c) |
3c |
85 |
| 4 | 1a | –(CH2)2CH3 (2d) |
3d |
75 |
| 5 | 1a | –C(CH3)2OH (2e) |
3e |
77 |
| 6 |
1b |
–C(CH3)2OH (2e) |
3f |
80 |
| 7 |
1c |
–C6H5 (2a) |
3g |
90 |
| 8 | 1c | –CH2OH (2b) |
3h |
85 |
| 9 | 1c | –(CH2)2CH3 (2d) |
3i |
79 |
| 10 |
1d |
–CH2OH (2b) |
3j |
87 |
| 11 | 1d | –CH2CH2OH (2c) |
3k |
88 |
| 12 | 1d | –C(CH3)3 (2f) |
3l |
85 |
| 13 | 1d | –C(CH3)2OH (2e) |
3m |
85 |
| 14 | 1d | –C(CH2)3CN (2g) |
3n |
80 |
aAll the reactions were carried out with 1 (0.89 mmol), 2 (1.33 mmol), 10% Pd/C (0.023 mmol), PPh3 (0.17 mmol), CuI (0.04 mmol), and Et3N (2.67 mmol) in MeOH (5.0 mL) at 60–65 °C for 10–12 h. bIdentified by 1H NMR, IR, and MS. cIsolated yields.
As evident from Table 2, the reaction proceeded well with both aliphatic (Table 2, entries 2–6 and 8–14) and aromatic alkynes (Table 2, entry 1 and 7). The chloro compounds containing a five (1b), a six (1a) or a seven membered cycloalkane ring (1d) or without a ring (1c) were found to be equally effective under the reaction conditions employed. All the reactions were generally complete within 10 h irrespective of the nature of substituents present in the terminal alkynes 2 to afford the desired products 3a–n in good to excellent yields.
A plausible mechanism for the Pd/C–Cu mediated alkynylation of 4-chlorothieno[2,3-d]pyrimidines 1 is shown in Scheme 4. The alkynylation proceeds via generation of an active Pd(0) species in situ that undergoes oxidative addition with 1 to give the organo-Pd(II) species E. The active Pd(0) species is generated from the minor portion of the bound palladium (Pd/C) via a Pd leaching process in the solution [13]. The leached Pd then becomes an active species by interacting with phosphine ligands. Thus, a soluble Pd(0)–PPh3 complex is the active species that actually catalyzes the C–C bond forming reaction in solution. The catalytic cycle therefore works in solution rather than on the surface, and at the end of the reaction, re-precipitation of Pd occurs on the surface of the charcoal. Once generated, the organo-Pd(II) species E then facilitates the stepwise formation of C–C bond via (i) trans organometallation with copper acetylide generated in situ from CuI and the terminal alkyne followed by (ii) reductive elimination of Pd(0) to afford 4-alkynylthieno[2,3-d]pyrimidine (3). A C–O bond forming reaction between 1 and MeOH (Scheme 2, path b) was not observed perhaps due to the higher reactivity of copper acetylide over MeOH (even although present in excess) towards E.
Scheme 4: Plausible mechanism of Pd/C-mediated alkynylation of 4-chlorothieno[2,3-d]pyrimidines 1.
Scheme 4: Plausible mechanism of Pd/C-mediated alkynylation of 4-chlorothieno[2,3-d]pyrimidines 1.
We have shown that an alkynyl moiety can be introduced efficiently at the C-4 position of a thieno[2,3-d]pyrimidine ring via C–C coupling under Pd/C–Cu catalysis. To the best of our knowledge, only two examples of similar coupling have been reported using the system (PPh3)2PdCl2/CuI as catalyst in the presence of Et3N [10]. The alkynyl substituent of compound 3 could be utilized for further structural elaboration leading to the functionalized derivatives of thieno[2,3-d]pyrimidine preparation which may be difficult to access by other methods. Some of the 4-alkynyl-derivatives synthesized were screened for their cytotoxic activity against chronic myelogenous leukemia (CML) cell line in vitro. The percentage of cell death measured for each compound at two different concentrations, i.e., 10 and 20 µM is shown in Table 3. As evident from Table 3, most of the compounds showed moderate to low activity against CML under the in vitro condition employed. The compounds 3f and 3g however, showed reasonable activity when tested at 10 µM which doubled at 20 µM. Comparing the structural features of compounds 3f and 3g with the other compounds tested, it may be noted that the presence of CMe2OH moiety played an important role in the cytotoxic activities of these compounds. It is known that alkynyl substituted pyrimidine derivatives exhibit their anticancer properties by inhibiting a key enzyme, i.e., thymidylate synthase (TS) which is essential for cellular growth [14]. A possible explanation for observed cytotoxic activities of present series of compounds 3 therefore could be their potential inhibition of TS [15]. The CMe2OH group next to the alkynyl moiety may facilitate the binding of the TS enzyme through its sulfhydryl (–SH) moiety with compounds 3f and 3g thereby generating the corresponding drug–enzyme allene intermediates (Figure 2). Nevertheless, the present study indicated that 4-alkynylthieno[2,3-d]pyrimidine can be used as a template for the identification of novel and potential anticancer agents.
Figure 2: Possible interactions of compounds 3f and 3g with TS enzyme.
Figure 2: Possible interactions of compounds 3f and 3g with TS enzyme.
Conclusion
In conclusion, the present study demonstrates the first efficient synthesis 4-alkynylthieno[2,3-d]pyrimidines in good to excellent yields. The combination of Pd/C–CuI–PPh3 proved to be an efficient and selective catalytic system for the C–C bond formation between 4-chlorothieno[2,3-d]pyrimidine and terminal alkynes in methanol providing a general and practical method for the preparation of these novel compounds. The reaction proceeds well with both hydrophobic and hydrophilic terminal alkynes without generating any significant side products arising from C–O bond formation or dimerization of the terminal alkynes. The reaction does not involve the use of expensive catalysts or reagents and is easy to perform. Some of the compounds synthesized were tested for cytotoxic activities in vitro. The methodology is amenable to the diversity-oriented synthesis of thieno[2,3-d]pyrimidine derivatives of potential pharmacological significance and therefore may find use in organic and medicinal chemistry.
Supporting Information
Supporting Information features details on experimental procedures and spectral data as well as NMR spectra of compounds 3a–n.
| Supporting Information File 1: Experimental procedures and spectral data. | ||
| Format: PDF | Size: 267.9 KB | Download |
| Supporting Information File 2: NMR spectra of compounds 3a–n. | ||
| Format: PDF | Size: 2.7 MB | Download |
References
-
Rai, D.; Johar, M.; Manning, T.; Agrawal, B.; Kunimoto, D. Y.; Kumar, R. J. Med. Chem. 2005, 48, 7012–7017. doi:10.1021/jm058167w
Return to citation in text: [1] [2] -
Gomtsyan, A.; Didomenico, S.; Lee, C.-H.; Matulenko, M. A.; Kim, K.; Kowaluk, E. A.; Wismer, C. T.; Mikusa, J.; Yu, H.; Kohlhaas, K.; Jarvis, M. F.; Bhagwat, S. S. J. Med. Chem. 2002, 45, 3639–3648. doi:10.1021/jm020049a
Return to citation in text: [1] [2] -
Spector, T.; Porter, D. J. T.; Nelson, D. J.; Baccanari, D. P.; Davis, S. T.; Almond, M. R.; Khor, S. P.; Amyx, H.; Cao, S.; Rustum, Y. M. Drugs Future 1994, 19, 565–571.
Return to citation in text: [1] [2] -
Campaigne, E. In Comprehensive Heterocyclic Chemistry; Katritzky, A. R., Ed.; Pergamon Press: New York, 1984; Vol. 4, pp 911–913.
Return to citation in text: [1] -
Sanfilippo, P. J.; McNally, J. J.; Press, J. B.; Fitzpatrick, L.; Urbanski, M. J.; Katz, L. B.; Giardino, E.; Falotico, R.; Salata, J.; Moore, J. B., Jr.; Miller, W. J. Med. Chem. 1992, 35, 4425–4433. doi:10.1021/jm00101a020
Return to citation in text: [1] -
Wood, E. R.; Shewchuk, L. M.; Ellis, B.; Brignola, P.; Brashear, R. L.; Caferro, T. R.; Dickerson, S. H.; Dickson, H. D.; Donaldson, K. H.; Gaul, M.; Griffin, R. J.; Hassell, A. M.; Keith, B.; Mullin, R.; Petrov, K. G.; Reno, M. J.; Rusnak, D. W.; Tadepalli, S. M.; Ulrich, J. C.; Wagner, C. D.; Vanderwall, D. E.; Waterson, A. G.; Williams, J. D.; White, W. L.; Uehling, D. E. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 2773–2778. doi:10.1073/pnas.0708281105
Return to citation in text: [1] [2] -
Kumar, K. S.; Chamakuri, S.; Vishweshwar, P.; Iqbal, J.; Pal, M. Tetrahedron Lett. 2010, 51, 3269–3273. doi:10.1016/j.tetlet.2010.04.057
Return to citation in text: [1] [2] [3] -
Schröter, S.; Stock, C.; Bach, T. Tetrahedron 2005, 61, 2245–2267. doi:10.1016/j.tet.2004.11.074
Return to citation in text: [1] -
Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron Lett. 1975, 16, 4467–4470. doi:10.1016/S0040-4039(00)91094-3
Return to citation in text: [1] -
Konno, S.; Watanabe, R.; Yamanaka, H. Yakugaku Zasshi 1989, 109, 642–649.
Return to citation in text: [1] [2] -
Snégaroff, K.; Lassagne, F.; Bentabed-Ababsa, G.; Nassar, E.; Ely, S. C. S.; Hesse, S.; Perspicace, E.; Derdour, A.; Mongin, F. Org. Biomol. Chem. 2009, 7, 4782–4788. doi:10.1039/b915274a
Return to citation in text: [1] -
van der Deen, H.; van Oeveren, A.; Kellogg, R. M.; Feringa, B. L. Tetrahedron Lett. 1999, 40, 1755–1758. doi:10.1016/S0040-4039(98)02683-5
Return to citation in text: [1] -
Pal, M. Synlett 2009, 2896–2912. doi:10.1055/s-0029-1218021
Return to citation in text: [1] [2] -
Kundu, N. G.; Das, B.; Spears, C. P.; Majumdar, A.; Kang, S. I. J. Med. Chem. 1990, 33, 1975–1979. doi:10.1021/jm00169a026
Return to citation in text: [1] -
Rao, K. N.; Bhattacharya, R. K.; Venkatachalam, S. R. Cancer Lett. 1998, 128, 183–188. doi:10.1016/S0304-3835(98)00061-5
Return to citation in text: [1]
| 1. | Rai, D.; Johar, M.; Manning, T.; Agrawal, B.; Kunimoto, D. Y.; Kumar, R. J. Med. Chem. 2005, 48, 7012–7017. doi:10.1021/jm058167w |
| 5. | Sanfilippo, P. J.; McNally, J. J.; Press, J. B.; Fitzpatrick, L.; Urbanski, M. J.; Katz, L. B.; Giardino, E.; Falotico, R.; Salata, J.; Moore, J. B., Jr.; Miller, W. J. Med. Chem. 1992, 35, 4425–4433. doi:10.1021/jm00101a020 |
| 11. | Snégaroff, K.; Lassagne, F.; Bentabed-Ababsa, G.; Nassar, E.; Ely, S. C. S.; Hesse, S.; Perspicace, E.; Derdour, A.; Mongin, F. Org. Biomol. Chem. 2009, 7, 4782–4788. doi:10.1039/b915274a |
| 4. | Campaigne, E. In Comprehensive Heterocyclic Chemistry; Katritzky, A. R., Ed.; Pergamon Press: New York, 1984; Vol. 4, pp 911–913. |
| 12. | van der Deen, H.; van Oeveren, A.; Kellogg, R. M.; Feringa, B. L. Tetrahedron Lett. 1999, 40, 1755–1758. doi:10.1016/S0040-4039(98)02683-5 |
| 3. | Spector, T.; Porter, D. J. T.; Nelson, D. J.; Baccanari, D. P.; Davis, S. T.; Almond, M. R.; Khor, S. P.; Amyx, H.; Cao, S.; Rustum, Y. M. Drugs Future 1994, 19, 565–571. |
| 2. | Gomtsyan, A.; Didomenico, S.; Lee, C.-H.; Matulenko, M. A.; Kim, K.; Kowaluk, E. A.; Wismer, C. T.; Mikusa, J.; Yu, H.; Kohlhaas, K.; Jarvis, M. F.; Bhagwat, S. S. J. Med. Chem. 2002, 45, 3639–3648. doi:10.1021/jm020049a |
| 7. | Kumar, K. S.; Chamakuri, S.; Vishweshwar, P.; Iqbal, J.; Pal, M. Tetrahedron Lett. 2010, 51, 3269–3273. doi:10.1016/j.tetlet.2010.04.057 |
| 1. | Rai, D.; Johar, M.; Manning, T.; Agrawal, B.; Kunimoto, D. Y.; Kumar, R. J. Med. Chem. 2005, 48, 7012–7017. doi:10.1021/jm058167w |
| 2. | Gomtsyan, A.; Didomenico, S.; Lee, C.-H.; Matulenko, M. A.; Kim, K.; Kowaluk, E. A.; Wismer, C. T.; Mikusa, J.; Yu, H.; Kohlhaas, K.; Jarvis, M. F.; Bhagwat, S. S. J. Med. Chem. 2002, 45, 3639–3648. doi:10.1021/jm020049a |
| 3. | Spector, T.; Porter, D. J. T.; Nelson, D. J.; Baccanari, D. P.; Davis, S. T.; Almond, M. R.; Khor, S. P.; Amyx, H.; Cao, S.; Rustum, Y. M. Drugs Future 1994, 19, 565–571. |
| 9. | Sonogashira, K.; Tohda, Y.; Hagihara, N. Tetrahedron Lett. 1975, 16, 4467–4470. doi:10.1016/S0040-4039(00)91094-3 |
| 7. | Kumar, K. S.; Chamakuri, S.; Vishweshwar, P.; Iqbal, J.; Pal, M. Tetrahedron Lett. 2010, 51, 3269–3273. doi:10.1016/j.tetlet.2010.04.057 |
| 6. | Wood, E. R.; Shewchuk, L. M.; Ellis, B.; Brignola, P.; Brashear, R. L.; Caferro, T. R.; Dickerson, S. H.; Dickson, H. D.; Donaldson, K. H.; Gaul, M.; Griffin, R. J.; Hassell, A. M.; Keith, B.; Mullin, R.; Petrov, K. G.; Reno, M. J.; Rusnak, D. W.; Tadepalli, S. M.; Ulrich, J. C.; Wagner, C. D.; Vanderwall, D. E.; Waterson, A. G.; Williams, J. D.; White, W. L.; Uehling, D. E. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 2773–2778. doi:10.1073/pnas.0708281105 |
| 7. | Kumar, K. S.; Chamakuri, S.; Vishweshwar, P.; Iqbal, J.; Pal, M. Tetrahedron Lett. 2010, 51, 3269–3273. doi:10.1016/j.tetlet.2010.04.057 |
| 14. | Kundu, N. G.; Das, B.; Spears, C. P.; Majumdar, A.; Kang, S. I. J. Med. Chem. 1990, 33, 1975–1979. doi:10.1021/jm00169a026 |
| 6. | Wood, E. R.; Shewchuk, L. M.; Ellis, B.; Brignola, P.; Brashear, R. L.; Caferro, T. R.; Dickerson, S. H.; Dickson, H. D.; Donaldson, K. H.; Gaul, M.; Griffin, R. J.; Hassell, A. M.; Keith, B.; Mullin, R.; Petrov, K. G.; Reno, M. J.; Rusnak, D. W.; Tadepalli, S. M.; Ulrich, J. C.; Wagner, C. D.; Vanderwall, D. E.; Waterson, A. G.; Williams, J. D.; White, W. L.; Uehling, D. E. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 2773–2778. doi:10.1073/pnas.0708281105 |
| 8. | Schröter, S.; Stock, C.; Bach, T. Tetrahedron 2005, 61, 2245–2267. doi:10.1016/j.tet.2004.11.074 |
| 15. | Rao, K. N.; Bhattacharya, R. K.; Venkatachalam, S. R. Cancer Lett. 1998, 128, 183–188. doi:10.1016/S0304-3835(98)00061-5 |
© 2011 Gorja et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)