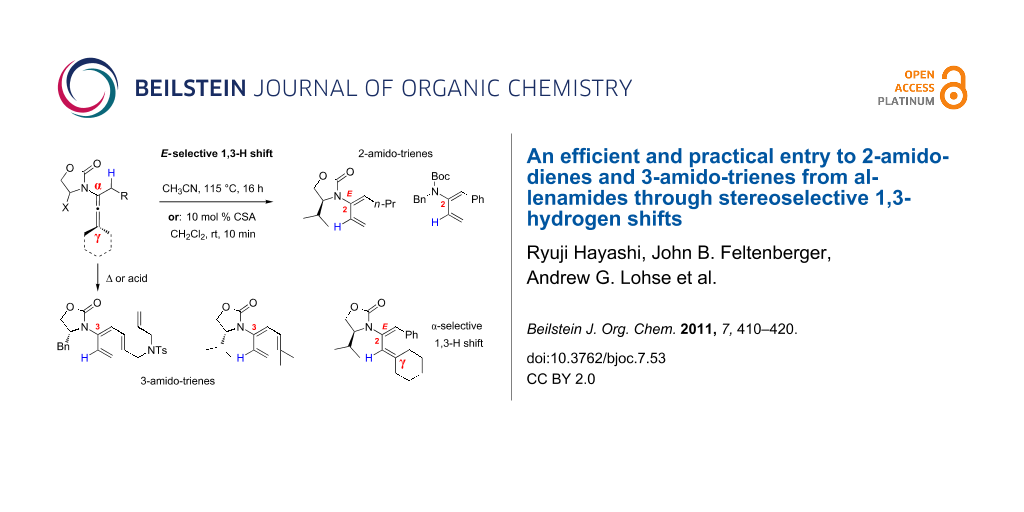
Abstract
Preparations of de novo acyclic 2-amido-dienes and 3-amido-trienes through 1,3-hydrogen shifts from allenamides are described. These 1,3-hydrogen shifts could be achieved thermally or they could be promoted by the use of Brønsted acids. Under either condition, these processes are highly regioselective in favour of the α-position, and highly stereoselective in favour of the E-configuration. In addition, 6π-electron electrocyclic ring-closure could be carried out with 3-amido-trienes to afford cyclic 2-amido-dienes, and such electrocyclic ring-closure could be rendered in tandem with the 1,3-hydrogen shift.
Graphical Abstract
Introduction
While allene isomerization to afford conjugated dienes is a well-known and thermodynamically favourable process, it is not trivial kinetically. A concerted allene isomerization leading to a diene involves a 1,3-hydrogen shift, which constitutes a four-electron (2π + 2σ) process that needs an antarafacial approach to fulfil the anti-Hückel (or Möbius) transition state based upon the Woodward–Hoffman rules [1]. Although there is no experimental precedent in an actual allylic system, it is relatively more feasible for an allenic system due to the presence of orthogonally oriented p-orbitals of the sp-hybridized central allenic carbon (Scheme 1), allowing a formal phase change required for an anti-Hückel transition state (in blue, for references on a possible radical pathway, see [2]), and a six-electron (2π + 2σ + 2π) process when considering the possible involvement of the second set of allenic π-electrons. Nevertheless, the calculated ∆Eact value remains high at 77.7 kcal·mol−1 [2]. Whether concerted or not, most thermal isomerizations of allenes require severe reaction conditions (for general reviews on allenes see [3], for some examples of thermal isomerization of exocyclic allenes to dienes via radical intermediates see [4-12]), whereby controlling E/Z ratios of the resulting diene remains a difficult problem. On the other hand, a stepwise isomerization of allenes via acid-, base-, or metal-mediated conditions seem to be more practical, but known examples have issues in controlling stereo- and regioselectivity [3] (for some examples see [13-20]). Therefore, solving these problems can be highly significant.
Scheme 1: 1,3-Hydrogen shifts of allenes.
Scheme 1: 1,3-Hydrogen shifts of allenes.
Because of the popularity of dienes as one of the most utilized organic building blocks, a number of stereoselective preparations are known. The major question here is how viable is it to access conjugated dienes from structurally more challenging allenes through a kinetically difficult and stereochemically undistinguished isomerization. It might not seem like a logical approach; however, our justification is that since there are few well established routes for preparing amido-dienes, our allenamide isomerization strategy (for reviews on allenamide chemistry see [21-23], for reports in 2009, 2010 and 2011 see [24-43], for earlier studies on allenamides see [44-46]) can open the door to construct synthetically useful amido-dienes (for a review on the synthesis of enamides see [47], for reviews on the chemistry of dienamides see [48-50], for reviews on the chemistry of 2-amino or 2-amido-dienes see [51,52]). Problems with the two primary approaches to access amido-dienes [47] are that acid-mediated condensations suffer from functional group tolerances, and metal-mediated coupling methods (for reviews on Cu-mediated C–N and C–O bond formations see [53-55], for some examples see [56-58]) suffer from limited access as well as the instability of halo-dienes (Scheme 2).
Scheme 2: Synthesizing amido-dienes from allenamides.
Scheme 2: Synthesizing amido-dienes from allenamides.
In contrast, multi-substituted allenamides can be concisely prepared through α-alkylations of a parent allenamide [47,59] (for the synthesis of parent allenamides see [60]) or amidative cross-couplings of allenyl halides [61,62]. Therefore, our allenamide isomerization strategy has a much greater synthetic potential in constructing amido-dienes. While the chemistry of 1-amido-dienes has been explored in some detail (see Scheme 3 for success in preparing 1-amido-dienes via allenamide isomerizations) [63,64] (for examples see [65-72]), herein, we report details of an efficient entry to synthetically rare 2-amido-dienes [73-77] via a regio- and stereoselective 1,3-hydrogen shift of allenamides.
Scheme 3: Synthesis of 1-amido-dienes from allenamides.
Scheme 3: Synthesis of 1-amido-dienes from allenamides.
Results and Discussion
As part of our initial screening efforts, both the thermal and acidic conditions were investigated as shown in Table 1. Allenamide 1 smoothly underwent isomerization via a 1,3-hydrogen shift when heated at 115 °C in CH3CN (sealed tube) to give the desired 2-amido-diene product 2 in 78% isolated yield with a 16:1 E/Z selectivity (Table 1, entry 1). There appears to be some solvent effect on the E/Z selectivity with more polar solvents providing the best ratio (Table 1, entries 2–4). In addition to thermal conditions, we screened several Brønsted acids at room temperature in order to investigate a milder condition. While PTSA resulted in poor E/Z ratio (Table 1, entry 5), a range of Brønsted acids were quite effective in affording the desired 2-amido-diene 2 (Supporting Information File 1 and Supporting Information File 2) with excellent E/Z selectivity [Table 1, entries 6–9].
Table 1: 1,3-Hydrogen shift of allenamides.
|
|
||||||
| entry | solvent | acid [10 mol %] | temp [°C] | time [h] | yield [%]a,b | E:Zc |
|---|---|---|---|---|---|---|
| 1 | CH3CN | — | 115 | 16 | 91 (78) | 16:1 |
| 2 | THF | — | 115 | 16 | 51 | 9:1 |
| 3 | ClCH2CH2Cl | — | 115 | 16 | 79 | 7:1 |
| 4 | toluene | — | 150 | 16 | 55 | 4:1 |
| 5 | CH2Cl2 | p-toluenesulfonic acid (PTSA) | 25 | 1 | 66 | 2:1 |
| 6 | CH2Cl2 | p-NO2-ArCO2H | 25 | 16 | 81 | 15:1 |
| 7 | CH2Cl2 | PhCO2H | 25 | 16 | 85 (55) | 18:1 |
| 8 | CH2Cl2 | pyridinium p-toluenesulfonate (PPTS) | 25 | 16 | 77 | 15:1 |
| 9 | CH2Cl2 | camphorsulfonic acid (CSA) | 25 | 10 min | 95 (74) | 18:1 |
aNMR yields. bIsolated yields are shown in brackets. cRatios were determined by 1H NMR.
After having established the 1,3-hydrogen shift under thermal and protic conditions, a diverse array of 2-amido-dienes was prepared as summarized in Table 2. Some notable features are: (1) a variety of novel chiral 2-amido-dienes 8–10 were obtained from chiral allenamides 5–7 in synthetically useful yields and with high E/Z ratios (≥95:5) under both thermal or acidic conditions (Table 2, entries 2–12); (2) unsubstituted 2-amido-dienes 8d and 9c could also be prepared in good yields (see R = H in Table 2, entries 7 and 10); (3) even allenamide containing an acyclic carbamate such as 11 underwent an efficient 1,3-hydrogen shift; and (4) the X-ray structure (Supporting Information File 3) of a single crystal of 2-amido-diene 10b was successfully obtained to assign unambiguously the E-configuration (Figure 1).
Table 2: Synthesis of 2-amido-dienes.
| entry | allenamides | conditions (time)a | amido-dienes | yield [%]b,c | ||
|---|---|---|---|---|---|---|
| 1 |
|
3 | 115 °C (16 h) |
|
4 | 71 |
| 2 |
|
5a: R = n-Pr | 115 °C (6 h) |
|
8a | 77 |
| 3 | 5a: R = n-Pr | CSA (4 h)d | 8a | 87 | ||
| 4 | 5b: R = Ph | 115 °C (16 h) | 8b | 74 | ||
| 5 | 5b: R = Ph | CSA (2 h) | 8b | 83 | ||
| 6 | 5a: R = 2Nape | 115 °C (16 h)f | 8c | 73 | ||
| 7 | 5a: R = H | 115 °C (16 h) | 8d | 69 | ||
| 8 |
|
6a: R = n-Pr | CSA (10 min) |
|
9a | 82 |
| 9 | 6b: R = Ph | CSA (10 min) | 9b | 76 | ||
| 10 | 6c: R = H | 115 °C (16 h) | 9c | 69 | ||
| 11 |
|
7a: R = n-Pr | 115 °C (16 h) |
|
10a | 62 |
| 12 | 7b: R = Ph | 115 °C (16 h) | 10b | 82 | ||
| 13 |
|
11 | 135 °C (16 h) |
|
12 | 45 |
| 14 | 11 | CSAg (2 h) | 12 | 61 | ||
aUnless otherwise indicated, CH3CN was the solvent for thermal conditions and CH2Cl2 was the solvent when using 10 mol % of CSA at rt. For all reactions, concn = 0.10 M. bAll are isolated yields. cAll 1,3-H shifts were highly E-selective [≥95:5] except for entry 1 in which the E:Z ratio is 6:1 for 4. Ratios were determined by 1H NMR. dTemp started at –78 °C. eThe group 2Nap stands for 2-naphthyl. fClCH2CH2Cl was used. g4Å MS was used.
Encouraged by this highly stereoselective isomerization, we turned our attention to the possibility of constructing synthetically much more challenging 3-amido-trienes from allenamides through 1,3-hydrogen shifts. As shown in Table 3, to our satisfaction, a wide variety of 3-amido-trienes could be readily accessed from corresponding α-allylated allenamides. When using a catalytic amount of CSA, both achiral and chiral 3-amido-trienes were obtained in high yields with exclusive E-selectivity, including structurally intriguing examples such as 24–28 (Table 3, entries 9–15). Moreover, a protected alcohol or amine in the allenamide did not impede the isomerization process (Table 3, entries 10–14), leading to more functionalized trienes.
Table 3: Synthesis of 3-amido-trienes.a
| entry | α-allylated allenamides | 3-amido-trienes | yield [%]b | ||
|---|---|---|---|---|---|
| 1 |
|
13 |
|
14 | 86 |
| 2 |
|
15a |
|
22 | 79 |
| 3 |
|
16a: R = Bn |
|
23a | 89 |
| 4 | 16b: R = Ph | 23b | 89 | ||
| 5 | 16c: R = iPr | 23c | 91 | ||
| 6 |
|
16d: R1 = (R)-Me, R2 = H |
|
23d | 74 |
| 7 | 16e: R1 = (S)-Ph, R2 = H | 23e | 89 | ||
| 8 | 16f: R1 = (R)-Ph, R2 = Ph | 23f | 86 | ||
| 9 |
|
17 |
|
24 | 95 |
| 10 |
|
18a: R = TBDPS |
|
25a | 84 |
| 11 | 18b: R = allyl | 25b | 75 | ||
| 12 | 18c: R = cinnamyl | 25c | 54 | ||
| 13 |
|
19 |
|
26 | 62 |
| 14 |
|
20 |
|
27 | 72 |
| 15 |
|
21 |
|
28 | 72 |
aAll reactions were run in CH2Cl2 [concn = 0.10 M] with 10 mol % of CSA for 10 min at rt. bAll were isolated yields.
To continue elevating the level of complexity, we examined allenamides with both α- and γ-substitutions and hoped to observe regioselectivity during the 1,3-hydrogen shift. Consequently, as shown in Table 4, isomerizations of tetra-substituted allenamides were examined. When heating α- and γ-substituted allenamides 29a and 30 in CH3CN at 115 °C in a sealed tube, 1,3-hydrogen shift took place exclusively from the α-position affording highly substituted (E)-2-amido-dienes 33a and 34 in 71% and 79% yields, respectively (Table 4, entries 1 and 3). The E-geometry in 33a and 34 was assigned by NOE (Supporting Information File 2).
Table 4: A regioselective 1,3-hydrogen shift.a
| entry | allenamides | conditions (time) | amido-alkenes | yield [%]b,c | ||
|---|---|---|---|---|---|---|
| 1 |
|
29a: R = Ph | 115 °C (16 h) |
|
33a | 71 |
| 2 | 29b: R = H | —d | 33b | 90 | ||
| 3 |
|
30 | 115 °C (16 h) |
|
34 | 79 |
| 4 |
|
31a: R = H | CSA (10 min) |
|
35a | 68 |
| 5 | 31b: R = Me | CSA (10 min) | 35b | 80 | ||
| 6 |
|
32 | CSA (10 min) |
|
36 | 84 |
aUnless otherwise noted, CH3CN was the solvent for thermal conditions and CH2Cl2 was the solvent when using 10 mol % of CSA at rt. For all reactions, concn = 0.10 M. bAll were isolated yields. cAll amido-di- and trienes were exclusively E-selective [≥95:5]. dSee text for this isomerization.
Intriguingly, allenamide 29b underwent a 1,3-hydrogen shift at room temperature when simply in contact with silica gel during the purification stage; but again, only the 1,3-hydrogen shift was favoured proceeding from the α-position to give (E)-2-amido-diene 33b (Table 4, entry 2). In addition, highly substituted 3-amido-trienes 35a, 35b, and 36 were regioselectively synthesized in overall high yields using the CSA-catalyzed conditions (Table 4, entries 4–6). Not only are the products from this regioselective isomerization structurally unique, but also mechanistically intriguing.
One of the probable explanations for the significantly lowered thermal activation barrier of 1,3-hydrogen-shifts of allenamides is that the nitrogen atom can serve to stabilize the biradical intermediate [2,4-12] (for another leading reference on related radical intermediates see [78]) which are presumed to be electron deficient. Based on the model in Figure 2 (left side), stabilization of the biradical intermediate is direct when isomerizations proceed from the α-position, whereas the isomerization from the γ-position is “vinylogous”, or remotely stabilized through the olefin. Therefore, thermal isomerizations at the α-position should be faster than at the γ-position.
While under thermal conditions, a biradical intermediate is at play [2,4,24], under acidic conditions, the isomerization clearly proceeds through an N-acyl iminium intermediate via protonation of the allenamide (Figure 2, center). Consequently, a similar argument could be used to rationalize the regioselective 1,3-hydrogen shift when acid was used. It is noteworthy that this charged transition state could also be adopted for the thermal isomerization. While still being a neutral transition state, the nitrogen atom could facilitate a polarized transition state through increasing negative charge density at the β-carbon. This action would lead to an N-acyl iminium ion-like character with the migrating hydrogen being proton-like with the α-position being favoured. This polarized transition state should also have a lower thermal activation barrier for the 1,3-hydrogen shift than the neutral one.
Lastly, a non-radical proton-transfer like mechanism could also be at play under conditions using protic solvents or owing to the presence of trace of amount of water (Figure 2, right). These last two models also reveal some insight into the E-selectivity given the pro-E configured transition state (TS) (see the R1 group). Along the same line, if the reaction proceeds through a radical pathway, the observed E-selectivity in the thermal 1,3-hydrogen shift should be favoured because the pro-Z transition state experiences a greater allylic strain compared to the pro-E transition state (Scheme 4). A thermodynamically driven equilibration from (Z)- to (E)-enamide post-isomerization is a real possibility that cannot be ruled out, and the observed solvent effect on the (E/Z)-selectivity would particularly support this possible notion.
An interesting discovery was made during this work. As shown in Scheme 5, when subjected to CSA catalyzed isomerization for protected allyl alcohol-substituted allenamides, the reactions with 37a and 37b did not stop at the intermediate 38, but an unexpected 1,7-H-shift (for some examples of an antarafacial 1,7-H shift see [79-82]) took place at room temperature to afford 5-amido-trienes 39a and 39b stereoselectively in good yields. Furthermore, when heating the protected homo-allyl alcohol-substituted allenamide 40, after the 1,3-hydrogen shift an unprecedented double 1,7-H-shift through intermediate 41 and 42 took place to afford the 6-amido-triene 43 in 45% yield. It is noteworthy that amido-triene 41 could be isolated in 65% yield when using 10 mol % CSA.
Scheme 5: Unexpected competing 1,7-hydrogen shifts.
Scheme 5: Unexpected competing 1,7-hydrogen shifts.
The synthesis of 3-amido-trienes from α-isomerization of allenamides allowed us to explore an important pericyclic process for yet another amido-diene synthesis. As shown in Scheme 6, isomerizations of α-allylated allenamides 15a and 13 under acidic conditions can afford 3-amido-trienes 22 and 14 in excellent yields. Given the E-selectivity of this isomerization, these 3-amido-trienes are perfectly suited for thermal 6π-electron electrocyclic ring-closure (for reviews on pericyclic ring-closures see [83,84], for reviews on ring-closure in natural product synthesis see [85,86], for recent examples of 6π-electron electrocyclic ring-closure see [87-93], for examples on accelerated ring-closures of 1,3,5-hexatrienes see [94-99]) to access cyclic 2-amido-dienes that are quite rare (for examples see [100-102]). Chiral amido-triene 22 underwent electrocyclization efficiently to give chiral cyclic 2-amido-diene 44a in 84% yield. Although obtained in only 35% yield, the achiral cyclic 2-amido-diene 45 could also be prepared.
Scheme 6: Applications in pericyclic ring-closure.
Scheme 6: Applications in pericyclic ring-closure.
Finally, this overall process was rendered in tandem under thermal conditions to directly prepare cyclic 2-amido-dienes 44a–c from allenamides 15a–c, respectively, in good yields (Scheme 7). Notably these 6π-electron pericyclic ring-closures took place at relatively low temperature (135 °C), thereby implying an accelerated process of electrocyclization. This feature is consistently observed in related ring-closures of 1,3,5-hexatrienes with an electron-donating substituent at the C3 position of the triene [94-99] (for theoretical studies on substituent effects on electrocyclic ring-closures of 1,3,5-hexatrienes see [97,103-105]. It is also noteworthy that while acyclic 2-amido-dienes and 3-amido-trienes are synthetically challenging to make, cyclic amido-dienes are almost inaccessible synthetically [100-102].
Scheme 7: Cyclic 2-amido-diene synthesis.
Scheme 7: Cyclic 2-amido-diene synthesis.
Conclusion
Herein, we have accomplished the preparation of de novo acyclic 2-amido-dienes and 3-amido-trienes through 1,3-hydrogen shifts from allenamides. These 1,3-hydrogen shifts could be achieved under thermal conditions or they could be promoted with Brønsted acids. Under either condition, these processes are highly regioselective in favour of the α-position, and highly stereoselective in favour of the E-configuration. Additionally, 6π-electron electrocyclic ring-closure could be carried out from 3-amido-trienes to afford cyclic 2-amido-dienes, and such electrocyclic ring-closure could be rendered in tandem with the 1,3-hydrogen shift, thereby constituting a facile construction of synthetically rare cyclic 2-amido-dienes.
Supporting Information
Supporting Information features detailed information on synthesis, purification and characterization data of all substances given in this article, proton and selected carbon NMR spectra, and X-ray data of compound 10b.
| Supporting Information File 1: Experimental section. | ||
| Format: PDF | Size: 412.2 KB | Download |
| Supporting Information File 2: Proton and Carbon NMR spectra, and NOE data. | ||
| Format: PDF | Size: 3.1 MB | Download |
| Supporting Information File 3: X-Ray structural analysis and information for compound 10b. | ||
| Format: RES | Size: 5.2 KB | Download |
References
-
Woodward, R. B.; Hoffmann, R. Angew. Chem., Int. Ed. Engl. 1969, 8, 781. doi:10.1002/anie.196907811
Return to citation in text: [1] -
Jensen, F. J. Am. Chem. Soc. 1995, 117, 7487. doi:10.1021/ja00133a021
Return to citation in text: [1] [2] [3] [4] -
Krause, N.; Hashmi, A. S. K., Eds. Modern allene chemistry; Wiley-VCH: Weinheim, Germany, 2004.
Return to citation in text: [1] [2] -
Crandall, J. K.; Paulson, D. R. J. Am. Chem. Soc. 1966, 88, 4302. doi:10.1021/ja00970a063
Return to citation in text: [1] [2] [3] -
Paulson, D. R.; Crandall, J. K.; Bunnell, C. A. J. Org. Chem. 1970, 35, 3708. doi:10.1021/jo00836a027
Return to citation in text: [1] [2] -
Bloch, R.; Perchec, P. L.; Conia, J.-M. Angew. Chem., Int. Ed. Engl. 1970, 9, 798. doi:10.1002/anie.197007981
Return to citation in text: [1] [2] -
Jones, M.; Hendrick, M. E.; Hardie, J. A. J. Org. Chem. 1971, 36, 3061. doi:10.1021/jo00819a042
Return to citation in text: [1] [2] -
Patrick, T. B.; Haynie, E. C.; Probst, W. J. Tetrahedron Lett. 1971, 27, 423. doi:10.1016/S0040-4039(01)96457-3
Return to citation in text: [1] [2] -
Lenk, W.; Hopf, H. Tetrahedron Lett. 1982, 23, 4073. doi:10.1016/S0040-4039(00)88350-1
Return to citation in text: [1] [2] -
Hopf, H.; Gottschild, D.; Lenk, W. Isr. J. Chem. 1985, 26, 79.
Return to citation in text: [1] [2] -
Lehrich, F.; Hopf, H. Tetrahedron Lett. 1987, 28, 2697. doi:10.1016/S0040-4039(00)96184-7
Return to citation in text: [1] [2] -
Meier, H.; Schmitt, M. Tetrahedron Lett. 1989, 30, 5873. doi:10.1016/S0040-4039(01)93493-8
Return to citation in text: [1] [2] -
Tsuboi, S.; Masuda, T.; Takeda, A. J. Org. Chem. 1982, 47, 4478. doi:10.1021/jo00144a015
Return to citation in text: [1] -
Peng, W.; Zhu, S. Tetrahedron 2003, 59, 4641. doi:10.1016/S0040-4020(03)00663-X
Return to citation in text: [1] -
Al-Masum, M.; Yamamoto, Y. J. Am. Chem. Soc. 1998, 120, 3809. doi:10.1021/ja974223+
Return to citation in text: [1] -
Bibas, H.; Koch, R.; Wentrup, C. J. Org. Chem. 1998, 63, 2619. doi:10.1021/jo972137m
Return to citation in text: [1] -
Jacobs, T. L.; Johnson, R. N. J. Am. Chem. Soc. 1960, 82, 6397. doi:10.1021/ja01509a050
Return to citation in text: [1] -
Buzas, A. K.; Istrate, F. M.; Gagosz, F. Org. Lett. 2007, 9, 985. doi:10.1021/ol063031t
Return to citation in text: [1] -
Trost, B. M.; Kazmaier, U. J. Am. Chem. Soc. 1992, 114, 7933. doi:10.1021/ja00046a062
Return to citation in text: [1] -
Guo, C.; Lu, X. J. Chem. Soc., Perkin Trans. 1 1993, 1921. doi:10.1039/P19930001921
Return to citation in text: [1] -
Wei, L.-L.; Xiong, H.; Hsung, R. P. Acc. Chem. Res. 2003, 36, 773. doi:10.1021/ar030029i
Return to citation in text: [1] -
Standen, P. E.; Kimber, M. C. Curr. Opin. Drug Discovery Dev. 2010, 13, 645.
Return to citation in text: [1] -
Deagostino, A.; Prandi, C.; Tabasso, S.; Venturello, P. Molecules 2010, 15, 2667. doi:10.3390/molecules15042667
Return to citation in text: [1] -
Zhu, Y.; Yin, G.; Hong, D.; Lu, P.; Wang, Y. Org. Lett. 2011, 13, 1024. doi:10.1021/ol103074d
Return to citation in text: [1] [2] -
Yin, G.; Zhu, Y.; Zhang, L.; Lu, P.; Wang, Y. Org. Lett. 2011, 13, 940. doi:10.1021/ol102992n
Return to citation in text: [1] -
Hayashi, R.; Walton, M. C.; Hsung, R. P.; Schwab, J. H.; Yu, X. Org. Lett. 2010, 12, 5768. doi:10.1021/ol102693e
Return to citation in text: [1] -
Lohse, A. G.; Krenske, E. H.; Antoline, J. E.; Houk, K. N.; Hsung, R. P. Org. Lett. 2010, 12, 5506. doi:10.1021/ol1023745
Return to citation in text: [1] -
Beccalli, E. M.; Bernasconi, A.; Borsini, E.; Broggini, G.; Rigamonti, M.; Zecchi, G. J. Org. Chem. 2010, 75, 6923. doi:10.1021/jo101501u
Return to citation in text: [1] -
Hill, A. W.; Elsegood, M. R. J.; Kimber, M. C. J. Org. Chem. 2010, 75, 5406. doi:10.1021/jo101035n
Return to citation in text: [1] -
Persson, A. K. Å.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2010, 49, 4624. doi:10.1002/anie.201000726
Return to citation in text: [1] -
Krenske, E. H.; Houk, K. N.; Lohse, A. G.; Antoline, J. E.; Hsung, R. P. Chem. Sci. 2010, 1, 387. doi:10.1039/c0sc00280a
Return to citation in text: [1] -
Danowitz, A. M.; Taylor, C. E.; Shrikian, T. M.; Mapp, A. K. Org. Lett. 2010, 12, 2574. doi:10.1021/ol1007845
Return to citation in text: [1] -
Zbieg, J. R.; McInturff, E. L.; Krische, M. J. Org. Lett. 2010, 12, 2514. doi:10.1021/ol1007235
Return to citation in text: [1] -
Cordier, P.; Aubert, C.; Malacria, M.; Gandon, V.; Lacôte, E. Chem.–Eur. J. 2010, 16, 9973. doi:10.1002/chem.201000914
Return to citation in text: [1] -
Kimber, M. C. Org. Lett. 2010, 12, 1128. doi:10.1021/ol1001494
Return to citation in text: [1] -
Hashimoto, K.; Horino, Y.; Kuroda, S. Heterocycles 2010, 80, 187. doi:10.3987/COM-09-S(S)54
Return to citation in text: [1] -
Persson, A. K. Å.; Johnston, E. V.; Bäckvall, J.-E. Org. Lett. 2009, 11, 3814. doi:10.1021/ol901294j
Return to citation in text: [1] -
Skucas, E.; Zbieg, J. R.; Krische, M. J. J. Am. Chem. Soc. 2009, 131, 5054. doi:10.1021/ja900827p
Return to citation in text: [1] -
Armstrong, A.; Emmerson, D. P. G. Org. Lett. 2009, 11, 1547. doi:10.1021/ol900146s
Return to citation in text: [1] -
Beccalli, E. M.; Broggini, G.; Clerici, F.; Galli, S.; Kammerer, C.; Rigamonti, M.; Sottocornola, S. Org. Lett. 2009, 11, 1563. doi:10.1021/ol900171g
Return to citation in text: [1] -
Broggini, G.; Galli, S.; Rigamonti, M.; Sottocornola, S.; Zecchi, G. Tetrahedron Lett. 2009, 50, 1447. doi:10.1016/j.tetlet.2009.01.074
Return to citation in text: [1] -
Lohse, A. G.; Hsung, R. P. Org. Lett. 2009, 11, 3430. doi:10.1021/ol901283m
Return to citation in text: [1] -
Lu, T.; Hayashi, R.; Hsung, R. P.; DeKorver, K. A.; Lohse, A. G.; Song, Z.; Tang, Y. Org. Biomol. Chem. 2009, 7, 3331. doi:10.1039/b908205k
Return to citation in text: [1] -
Overman, L. E.; Clizbe, L. A.; Freerks, R. L.; Marlowe, C. K. J. Am. Chem. Soc. 1981, 103, 2807. doi:10.1021/ja00400a053
Return to citation in text: [1] -
Farmer, M. L.; Billups, W. E.; Greenlee, R. B.; Kurtz, A. N. J. Org. Chem. 1966, 31, 2885. doi:10.1021/jo01347a035
Return to citation in text: [1] -
Kinderman, S. S.; van Maarseveen, J. H.; Schoemaker, H. E.; Hiemstra, H.; Rutjes, F. P. J. T. Org. Lett. 2001, 3, 2045. doi:10.1021/ol016013e
Return to citation in text: [1] -
Tracey, M. R.; Hsung, R. P.; Antoline, J.; Kurtz, K. C. M.; Shen, L.; Slafer, B. W.; Zhang, Y. Product Class 4: N-Arylalkanamides, Ynamides, Enamides, Dienamides, and Allenamides. In Science of Synthesis, Houben-Weyl Methods of Molecular Transformations; Weinreb, S. M., Ed.; Thieme: Stuttgart, 2005; pp 387–476.
Return to citation in text: [1] [2] [3] -
Overman, L. E. Acc. Chem. Res. 1980, 13, 218. doi:10.1021/ar50151a005
Return to citation in text: [1] -
Petrzilka, M.; Grayson, J. I. Synthesis 1981, 753. doi:10.1055/s-1981-29592
Return to citation in text: [1] -
Campbell, A. L.; Lenz, G. R. Synthesis 1987, 421. doi:10.1055/s-1987-27972
Return to citation in text: [1] -
Krohn, K. Angew. Chem., Int. Ed. Engl. 1993, 32, 1582. doi:10.1002/anie.199315821
Return to citation in text: [1] -
Enders, D.; Meyer, O. Liebigs Ann. 1996, 1023. doi:10.1002/jlac.199619960702
Return to citation in text: [1] -
Lindley, J. Tetrahedron 1984, 40, 1433. doi:10.1016/S0040-4020(01)91791-0
Return to citation in text: [1] -
Ley, S. V.; Thomas, A. W. Angew. Chem., Int. Ed. 2003, 42, 5400. doi:10.1002/anie.200300594
Return to citation in text: [1] -
Dehli, J. R.; Legros, J.; Bolm, C. Chem. Commun. 2005, 973. doi:10.1039/b415954c
Return to citation in text: [1] -
Klapper, A.; Huang, X.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 7421. doi:10.1021/ja0260465
Return to citation in text: [1] -
Han, C.; Shen, R.; Su, S.; Porco, J. A., Jr. Org. Lett. 2004, 6, 27. doi:10.1021/ol0360041
Return to citation in text: [1] -
Shen, R.; Lin, C. T.; Bowman, E. J.; Bowman, B. J.; Porco, J. A., Jr. J. Am. Chem. Soc. 2003, 125, 7889. doi:10.1021/ja0352350
Return to citation in text: [1] -
Xiong, H.; Hsung, R. P.; Wei, L.-L.; Berry, C. R.; Mulder, J. A.; Stockwell, B. Org. Lett. 2000, 2, 2869. doi:10.1021/ol000181+
Return to citation in text: [1] -
Wei, L.-L.; Mulder, J. A.; Xiong, H.; Zificsak, C. A.; Douglas, C. J.; Hsung, R. P. Tetrahedron 2001, 57, 459. doi:10.1016/S0040-4020(00)01014-0
Return to citation in text: [1] -
Trost, B. M.; Stiles, D. T. Org. Lett. 2005, 7, 2117. doi:10.1021/ol050395x
Return to citation in text: [1] -
Shen, L.; Hsung, R. P.; Zhang, Y.; Antoline, J. E.; Zhang, X. Org. Lett. 2005, 7, 3081. doi:10.1021/ol051094q
Return to citation in text: [1] -
Hayashi, R.; Hsung, R. P.; Feltenberger, J. B.; Lohse, A. G. Org. Lett. 2009, 11, 2125. doi:10.1021/ol900647s
Return to citation in text: [1] -
Hayashi, R.; Feltenberger, J. B.; Hsung, R. P. Org. Lett. 2010, 12, 1152. doi:10.1021/ol902821w
Return to citation in text: [1] -
Terada, A.; Murata, K. Bull. Chem. Soc. Jpn. 1967, 40, 1644. doi:10.1246/bcsj.40.1644
Return to citation in text: [1] -
Overman, L. E.; Clizbe, L. A. J. Am. Chem. Soc. 1976, 98, 2352. doi:10.1021/ja00424a068
Return to citation in text: [1] -
Oppolzer, W.; Bieber, L.; Francotte, E. Tetrahedron Lett. 1979, 20, 4537. doi:10.1016/S0040-4039(01)86643-0
Return to citation in text: [1] -
Smith, A. B., III; Wexler, B. A.; Tu, C.-Y.; Konopelski, J. P. J. Am. Chem. Soc. 1985, 107, 1308. doi:10.1021/ja00291a034
Return to citation in text: [1] -
Schlessinger, R. H.; Pettus, T. R. R.; Springer, J. P.; Hoogsteen, K. J. Org. Chem. 1994, 59, 3246. doi:10.1021/jo00091a002
Return to citation in text: [1] -
Kozmin, S. A.; Rawal, V. H. J. Am. Chem. Soc. 1997, 119, 7165. doi:10.1021/ja971272d
Return to citation in text: [1] -
Huang, Y.; Iwama, T.; Rawal, V. H. J. Am. Chem. Soc. 2002, 124, 5950. doi:10.1021/ja026088t
Return to citation in text: [1] -
Robiette, R.; Cheboub-Benchaba, K.; Peeters, D.; Marchand-Brynaert, J. J. Org. Chem. 2003, 68, 9809. doi:10.1021/jo0302049
Return to citation in text: [1] -
Ha, J. D.; Kang, C. H.; Belmore, K. A.; Cha, J. K. J. Org. Chem. 1998, 63, 3810. doi:10.1021/jo980593k
Return to citation in text: [1] -
González-Romero, C.; Bernal, P.; Jiménez, F.; Cruz, M. C.; Fuentes-Benites, A.; Benavides, A.; Bautista, R.; Tamariz, J. Pure Appl. Chem. 2007, 79, 181.
Return to citation in text: [1] -
Movassaghi, M.; Hunt, D. K.; Tjandra, M. J. Am. Chem. Soc. 2006, 128, 8126. doi:10.1021/ja0626180
Return to citation in text: [1] -
Enders, D.; Meyer, O.; Raabe, G. Synthesis 1992, 1242. doi:10.1055/s-1992-26349
Return to citation in text: [1] -
Barluenga, J.; Canteli, R.-M.; Flórez, J.; García-Granda, S.; Gutiérrez-Rodríguez, A.; Martín, E. J. Am. Chem. Soc. 1998, 120, 2514. doi:10.1021/ja972588o
Return to citation in text: [1] -
Siebert, M.; Osbourn, J. M.; Brummond, K. M.; Tantillo, D. J. J. Am. Chem. Soc. 2010, 132, 11952. doi:10.1021/ja102848z
Return to citation in text: [1] -
Kerr, D. J.; Willis, A. C.; Flynn, B. L. Org. Lett. 2004, 6, 457. doi:10.1021/ol035822q
Return to citation in text: [1] -
Mousavipour, S. H.; Fernández-Ramos, A.; Meana-Pañeda, R.; Martínez-Núñez, E.; Vázquez, S. A.; Ríos, M. A. J. Phys. Chem. A 2007, 111, 719. doi:10.1021/jp0665269
Return to citation in text: [1] -
Gu, Z.; Ma, S. Chem.–Eur. J. 2008, 14, 2453. doi:10.1002/chem.200701171
Return to citation in text: [1] -
Shu, X.-Z.; Ji, K.-G.; Zhao, S.-C.; Zheng, Z.-J.; Chen, J.; Lu, L.; Liu, X.-Y.; Liang, Y.-M. Chem.–Eur. J. 2008, 14, 10556. doi:10.1002/chem.200801591
Return to citation in text: [1] -
Marvell, E. N. Thermal Electrocyclic Reactions; Academic Press: New York, 1980.
Return to citation in text: [1] -
Okamura, W. H.; de Lera, A. R. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I.; Paquette, L. A., Eds.; Pergamon Press: New York, 1991; Vol. 5, pp 699–750. doi:10.1016/B978-0-08-052349-1.00137-2
Return to citation in text: [1] -
Pindur, U.; Schneider, G. H. Chem. Soc. Rev. 1994, 23, 409. doi:10.1039/cs9942300409
Return to citation in text: [1] -
Beaudry, C. M.; Malerich, J. P.; Trauner, D. Chem. Rev. 2005, 105, 4757. doi:10.1021/cr0406110
Return to citation in text: [1] -
Bishop, L. M.; Barbarow, J. E.; Bergmen, R. G.; Trauner, D. Angew. Chem., Int. Ed. 2008, 47, 8100. doi:10.1002/anie.200803336
Return to citation in text: [1] -
Sofiyev, V.; Navarro, G.; Trauner, D. Org. Lett. 2008, 10, 149. doi:10.1021/ol702806v
Return to citation in text: [1] -
Kan, S. B. J.; Anderson, E. A. Org. Lett. 2008, 10, 2323. doi:10.1021/ol8007952
Return to citation in text: [1] -
Hulot, C.; Blond, G.; Suffert, J. J. Am. Chem. Soc. 2008, 130, 5046. doi:10.1021/ja800691c
Return to citation in text: [1] -
Benson, C. L.; West, F. G. Org. Lett. 2007, 9, 2545. doi:10.1021/ol070924s
Return to citation in text: [1] -
Pouwer, R. H.; Schill, H.; Williams, C. M.; Bernhardt, P. V. Eur. J. Org. Chem. 2007, 4699. doi:10.1002/ejoc.200700367
Return to citation in text: [1] -
Jung, M. E.; Min, S.-J. Tetrahedron 2007, 63, 3682. doi:10.1016/j.tet.2007.02.085
Return to citation in text: [1] -
Sünnemann, H. W.; Banwell, M. G.; de Meijere, A. Eur. J. Org. Chem. 2007, 3879. doi:10.1002/ejoc.200700201
Return to citation in text: [1] [2] -
Tessier, P. E.; Nguyen, N.; Clay, M. D.; Fallis, A. G. Org. Lett. 2005, 7, 767. doi:10.1021/ol047602y
Return to citation in text: [1] [2] -
Greshock, T. J.; Funk, R. L. J. Am. Chem. Soc. 2006, 128, 4946. doi:10.1021/ja060282o
Return to citation in text: [1] [2] -
Yu, T.-Q.; Fu, Y.; Liu, L.; Guo, Q.-X. J. Org. Chem. 2006, 71, 6157. doi:10.1021/jo060885i
Return to citation in text: [1] [2] [3] -
Magomedov, N. A.; Ruggiero, P. L.; Tang, Y. J. Am. Chem. Soc. 2004, 126, 1624. doi:10.1021/ja0399066
Return to citation in text: [1] [2] -
Barluenga, J.; Merino, I.; Palacios, F. Tetrahedron Lett. 1990, 31, 6713. doi:10.1016/S0040-4039(00)97155-7
Return to citation in text: [1] [2] -
Martínez, R.; Jiménez-Vázquez, H. A.; Delgado, F.; Tamariz, J. Tetrahedron 2003, 59, 481. doi:10.1016/S0040-4020(02)01536-3
Return to citation in text: [1] [2] -
Wallace, D. J.; Klauber, D. J.; Chen, C. Y.; Volante, R. P. Org. Lett. 2003, 5, 4749. doi:10.1021/ol035959g
Return to citation in text: [1] [2] -
Wabnitz, T. C.; Yu, J.-Q.; Spencer, J. B. Chem.–Eur. J. 2004, 10, 484. doi:10.1002/chem.200305407
Return to citation in text: [1] [2] -
Spangler, C. W.; Jondahl, T. P.; Spangler, B. J. Org. Chem. 1973, 38, 2478. doi:10.1021/jo00954a013
Return to citation in text: [1] -
Guner, V. A.; Houk, K. N.; Davies, I. A. J. Org. Chem. 2004, 69, 8024. doi:10.1021/jo048540s
Return to citation in text: [1] -
Duncan, J. A.; Calkins, D. E. G.; Chavarha, M. J. Am. Chem. Soc. 2008, 130, 6740. doi:10.1021/ja074402j
Return to citation in text: [1]
| 85. | Pindur, U.; Schneider, G. H. Chem. Soc. Rev. 1994, 23, 409. doi:10.1039/cs9942300409 |
| 86. | Beaudry, C. M.; Malerich, J. P.; Trauner, D. Chem. Rev. 2005, 105, 4757. doi:10.1021/cr0406110 |
| 87. | Bishop, L. M.; Barbarow, J. E.; Bergmen, R. G.; Trauner, D. Angew. Chem., Int. Ed. 2008, 47, 8100. doi:10.1002/anie.200803336 |
| 88. | Sofiyev, V.; Navarro, G.; Trauner, D. Org. Lett. 2008, 10, 149. doi:10.1021/ol702806v |
| 89. | Kan, S. B. J.; Anderson, E. A. Org. Lett. 2008, 10, 2323. doi:10.1021/ol8007952 |
| 90. | Hulot, C.; Blond, G.; Suffert, J. J. Am. Chem. Soc. 2008, 130, 5046. doi:10.1021/ja800691c |
| 91. | Benson, C. L.; West, F. G. Org. Lett. 2007, 9, 2545. doi:10.1021/ol070924s |
| 92. | Pouwer, R. H.; Schill, H.; Williams, C. M.; Bernhardt, P. V. Eur. J. Org. Chem. 2007, 4699. doi:10.1002/ejoc.200700367 |
| 93. | Jung, M. E.; Min, S.-J. Tetrahedron 2007, 63, 3682. doi:10.1016/j.tet.2007.02.085 |
| 94. | Sünnemann, H. W.; Banwell, M. G.; de Meijere, A. Eur. J. Org. Chem. 2007, 3879. doi:10.1002/ejoc.200700201 |
| 95. | Tessier, P. E.; Nguyen, N.; Clay, M. D.; Fallis, A. G. Org. Lett. 2005, 7, 767. doi:10.1021/ol047602y |
| 96. | Greshock, T. J.; Funk, R. L. J. Am. Chem. Soc. 2006, 128, 4946. doi:10.1021/ja060282o |
| 97. | Yu, T.-Q.; Fu, Y.; Liu, L.; Guo, Q.-X. J. Org. Chem. 2006, 71, 6157. doi:10.1021/jo060885i |
| 98. | Magomedov, N. A.; Ruggiero, P. L.; Tang, Y. J. Am. Chem. Soc. 2004, 126, 1624. doi:10.1021/ja0399066 |
| 99. | Barluenga, J.; Merino, I.; Palacios, F. Tetrahedron Lett. 1990, 31, 6713. doi:10.1016/S0040-4039(00)97155-7 |
| 1. | Woodward, R. B.; Hoffmann, R. Angew. Chem., Int. Ed. Engl. 1969, 8, 781. doi:10.1002/anie.196907811 |
| 4. | Crandall, J. K.; Paulson, D. R. J. Am. Chem. Soc. 1966, 88, 4302. doi:10.1021/ja00970a063 |
| 5. | Paulson, D. R.; Crandall, J. K.; Bunnell, C. A. J. Org. Chem. 1970, 35, 3708. doi:10.1021/jo00836a027 |
| 6. | Bloch, R.; Perchec, P. L.; Conia, J.-M. Angew. Chem., Int. Ed. Engl. 1970, 9, 798. doi:10.1002/anie.197007981 |
| 7. | Jones, M.; Hendrick, M. E.; Hardie, J. A. J. Org. Chem. 1971, 36, 3061. doi:10.1021/jo00819a042 |
| 8. | Patrick, T. B.; Haynie, E. C.; Probst, W. J. Tetrahedron Lett. 1971, 27, 423. doi:10.1016/S0040-4039(01)96457-3 |
| 9. | Lenk, W.; Hopf, H. Tetrahedron Lett. 1982, 23, 4073. doi:10.1016/S0040-4039(00)88350-1 |
| 10. | Hopf, H.; Gottschild, D.; Lenk, W. Isr. J. Chem. 1985, 26, 79. |
| 11. | Lehrich, F.; Hopf, H. Tetrahedron Lett. 1987, 28, 2697. doi:10.1016/S0040-4039(00)96184-7 |
| 12. | Meier, H.; Schmitt, M. Tetrahedron Lett. 1989, 30, 5873. doi:10.1016/S0040-4039(01)93493-8 |
| 53. | Lindley, J. Tetrahedron 1984, 40, 1433. doi:10.1016/S0040-4020(01)91791-0 |
| 54. | Ley, S. V.; Thomas, A. W. Angew. Chem., Int. Ed. 2003, 42, 5400. doi:10.1002/anie.200300594 |
| 55. | Dehli, J. R.; Legros, J.; Bolm, C. Chem. Commun. 2005, 973. doi:10.1039/b415954c |
| 3. | Krause, N.; Hashmi, A. S. K., Eds. Modern allene chemistry; Wiley-VCH: Weinheim, Germany, 2004. |
| 56. | Klapper, A.; Huang, X.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 7421. doi:10.1021/ja0260465 |
| 57. | Han, C.; Shen, R.; Su, S.; Porco, J. A., Jr. Org. Lett. 2004, 6, 27. doi:10.1021/ol0360041 |
| 58. | Shen, R.; Lin, C. T.; Bowman, E. J.; Bowman, B. J.; Porco, J. A., Jr. J. Am. Chem. Soc. 2003, 125, 7889. doi:10.1021/ja0352350 |
| 51. | Krohn, K. Angew. Chem., Int. Ed. Engl. 1993, 32, 1582. doi:10.1002/anie.199315821 |
| 52. | Enders, D.; Meyer, O. Liebigs Ann. 1996, 1023. doi:10.1002/jlac.199619960702 |
| 47. | Tracey, M. R.; Hsung, R. P.; Antoline, J.; Kurtz, K. C. M.; Shen, L.; Slafer, B. W.; Zhang, Y. Product Class 4: N-Arylalkanamides, Ynamides, Enamides, Dienamides, and Allenamides. In Science of Synthesis, Houben-Weyl Methods of Molecular Transformations; Weinreb, S. M., Ed.; Thieme: Stuttgart, 2005; pp 387–476. |
| 24. | Zhu, Y.; Yin, G.; Hong, D.; Lu, P.; Wang, Y. Org. Lett. 2011, 13, 1024. doi:10.1021/ol103074d |
| 25. | Yin, G.; Zhu, Y.; Zhang, L.; Lu, P.; Wang, Y. Org. Lett. 2011, 13, 940. doi:10.1021/ol102992n |
| 26. | Hayashi, R.; Walton, M. C.; Hsung, R. P.; Schwab, J. H.; Yu, X. Org. Lett. 2010, 12, 5768. doi:10.1021/ol102693e |
| 27. | Lohse, A. G.; Krenske, E. H.; Antoline, J. E.; Houk, K. N.; Hsung, R. P. Org. Lett. 2010, 12, 5506. doi:10.1021/ol1023745 |
| 28. | Beccalli, E. M.; Bernasconi, A.; Borsini, E.; Broggini, G.; Rigamonti, M.; Zecchi, G. J. Org. Chem. 2010, 75, 6923. doi:10.1021/jo101501u |
| 29. | Hill, A. W.; Elsegood, M. R. J.; Kimber, M. C. J. Org. Chem. 2010, 75, 5406. doi:10.1021/jo101035n |
| 30. | Persson, A. K. Å.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2010, 49, 4624. doi:10.1002/anie.201000726 |
| 31. | Krenske, E. H.; Houk, K. N.; Lohse, A. G.; Antoline, J. E.; Hsung, R. P. Chem. Sci. 2010, 1, 387. doi:10.1039/c0sc00280a |
| 32. | Danowitz, A. M.; Taylor, C. E.; Shrikian, T. M.; Mapp, A. K. Org. Lett. 2010, 12, 2574. doi:10.1021/ol1007845 |
| 33. | Zbieg, J. R.; McInturff, E. L.; Krische, M. J. Org. Lett. 2010, 12, 2514. doi:10.1021/ol1007235 |
| 34. | Cordier, P.; Aubert, C.; Malacria, M.; Gandon, V.; Lacôte, E. Chem.–Eur. J. 2010, 16, 9973. doi:10.1002/chem.201000914 |
| 35. | Kimber, M. C. Org. Lett. 2010, 12, 1128. doi:10.1021/ol1001494 |
| 36. | Hashimoto, K.; Horino, Y.; Kuroda, S. Heterocycles 2010, 80, 187. doi:10.3987/COM-09-S(S)54 |
| 37. | Persson, A. K. Å.; Johnston, E. V.; Bäckvall, J.-E. Org. Lett. 2009, 11, 3814. doi:10.1021/ol901294j |
| 38. | Skucas, E.; Zbieg, J. R.; Krische, M. J. J. Am. Chem. Soc. 2009, 131, 5054. doi:10.1021/ja900827p |
| 39. | Armstrong, A.; Emmerson, D. P. G. Org. Lett. 2009, 11, 1547. doi:10.1021/ol900146s |
| 40. | Beccalli, E. M.; Broggini, G.; Clerici, F.; Galli, S.; Kammerer, C.; Rigamonti, M.; Sottocornola, S. Org. Lett. 2009, 11, 1563. doi:10.1021/ol900171g |
| 41. | Broggini, G.; Galli, S.; Rigamonti, M.; Sottocornola, S.; Zecchi, G. Tetrahedron Lett. 2009, 50, 1447. doi:10.1016/j.tetlet.2009.01.074 |
| 42. | Lohse, A. G.; Hsung, R. P. Org. Lett. 2009, 11, 3430. doi:10.1021/ol901283m |
| 43. | Lu, T.; Hayashi, R.; Hsung, R. P.; DeKorver, K. A.; Lohse, A. G.; Song, Z.; Tang, Y. Org. Biomol. Chem. 2009, 7, 3331. doi:10.1039/b908205k |
| 47. | Tracey, M. R.; Hsung, R. P.; Antoline, J.; Kurtz, K. C. M.; Shen, L.; Slafer, B. W.; Zhang, Y. Product Class 4: N-Arylalkanamides, Ynamides, Enamides, Dienamides, and Allenamides. In Science of Synthesis, Houben-Weyl Methods of Molecular Transformations; Weinreb, S. M., Ed.; Thieme: Stuttgart, 2005; pp 387–476. |
| 97. | Yu, T.-Q.; Fu, Y.; Liu, L.; Guo, Q.-X. J. Org. Chem. 2006, 71, 6157. doi:10.1021/jo060885i |
| 103. | Spangler, C. W.; Jondahl, T. P.; Spangler, B. J. Org. Chem. 1973, 38, 2478. doi:10.1021/jo00954a013 |
| 104. | Guner, V. A.; Houk, K. N.; Davies, I. A. J. Org. Chem. 2004, 69, 8024. doi:10.1021/jo048540s |
| 105. | Duncan, J. A.; Calkins, D. E. G.; Chavarha, M. J. Am. Chem. Soc. 2008, 130, 6740. doi:10.1021/ja074402j |
| 21. | Wei, L.-L.; Xiong, H.; Hsung, R. P. Acc. Chem. Res. 2003, 36, 773. doi:10.1021/ar030029i |
| 22. | Standen, P. E.; Kimber, M. C. Curr. Opin. Drug Discovery Dev. 2010, 13, 645. |
| 23. | Deagostino, A.; Prandi, C.; Tabasso, S.; Venturello, P. Molecules 2010, 15, 2667. doi:10.3390/molecules15042667 |
| 48. | Overman, L. E. Acc. Chem. Res. 1980, 13, 218. doi:10.1021/ar50151a005 |
| 49. | Petrzilka, M.; Grayson, J. I. Synthesis 1981, 753. doi:10.1055/s-1981-29592 |
| 50. | Campbell, A. L.; Lenz, G. R. Synthesis 1987, 421. doi:10.1055/s-1987-27972 |
| 100. | Martínez, R.; Jiménez-Vázquez, H. A.; Delgado, F.; Tamariz, J. Tetrahedron 2003, 59, 481. doi:10.1016/S0040-4020(02)01536-3 |
| 101. | Wallace, D. J.; Klauber, D. J.; Chen, C. Y.; Volante, R. P. Org. Lett. 2003, 5, 4749. doi:10.1021/ol035959g |
| 102. | Wabnitz, T. C.; Yu, J.-Q.; Spencer, J. B. Chem.–Eur. J. 2004, 10, 484. doi:10.1002/chem.200305407 |
| 13. | Tsuboi, S.; Masuda, T.; Takeda, A. J. Org. Chem. 1982, 47, 4478. doi:10.1021/jo00144a015 |
| 14. | Peng, W.; Zhu, S. Tetrahedron 2003, 59, 4641. doi:10.1016/S0040-4020(03)00663-X |
| 15. | Al-Masum, M.; Yamamoto, Y. J. Am. Chem. Soc. 1998, 120, 3809. doi:10.1021/ja974223+ |
| 16. | Bibas, H.; Koch, R.; Wentrup, C. J. Org. Chem. 1998, 63, 2619. doi:10.1021/jo972137m |
| 17. | Jacobs, T. L.; Johnson, R. N. J. Am. Chem. Soc. 1960, 82, 6397. doi:10.1021/ja01509a050 |
| 18. | Buzas, A. K.; Istrate, F. M.; Gagosz, F. Org. Lett. 2007, 9, 985. doi:10.1021/ol063031t |
| 19. | Trost, B. M.; Kazmaier, U. J. Am. Chem. Soc. 1992, 114, 7933. doi:10.1021/ja00046a062 |
| 20. | Guo, C.; Lu, X. J. Chem. Soc., Perkin Trans. 1 1993, 1921. doi:10.1039/P19930001921 |
| 100. | Martínez, R.; Jiménez-Vázquez, H. A.; Delgado, F.; Tamariz, J. Tetrahedron 2003, 59, 481. doi:10.1016/S0040-4020(02)01536-3 |
| 101. | Wallace, D. J.; Klauber, D. J.; Chen, C. Y.; Volante, R. P. Org. Lett. 2003, 5, 4749. doi:10.1021/ol035959g |
| 102. | Wabnitz, T. C.; Yu, J.-Q.; Spencer, J. B. Chem.–Eur. J. 2004, 10, 484. doi:10.1002/chem.200305407 |
| 3. | Krause, N.; Hashmi, A. S. K., Eds. Modern allene chemistry; Wiley-VCH: Weinheim, Germany, 2004. |
| 44. | Overman, L. E.; Clizbe, L. A.; Freerks, R. L.; Marlowe, C. K. J. Am. Chem. Soc. 1981, 103, 2807. doi:10.1021/ja00400a053 |
| 45. | Farmer, M. L.; Billups, W. E.; Greenlee, R. B.; Kurtz, A. N. J. Org. Chem. 1966, 31, 2885. doi:10.1021/jo01347a035 |
| 46. | Kinderman, S. S.; van Maarseveen, J. H.; Schoemaker, H. E.; Hiemstra, H.; Rutjes, F. P. J. T. Org. Lett. 2001, 3, 2045. doi:10.1021/ol016013e |
| 94. | Sünnemann, H. W.; Banwell, M. G.; de Meijere, A. Eur. J. Org. Chem. 2007, 3879. doi:10.1002/ejoc.200700201 |
| 95. | Tessier, P. E.; Nguyen, N.; Clay, M. D.; Fallis, A. G. Org. Lett. 2005, 7, 767. doi:10.1021/ol047602y |
| 96. | Greshock, T. J.; Funk, R. L. J. Am. Chem. Soc. 2006, 128, 4946. doi:10.1021/ja060282o |
| 97. | Yu, T.-Q.; Fu, Y.; Liu, L.; Guo, Q.-X. J. Org. Chem. 2006, 71, 6157. doi:10.1021/jo060885i |
| 98. | Magomedov, N. A.; Ruggiero, P. L.; Tang, Y. J. Am. Chem. Soc. 2004, 126, 1624. doi:10.1021/ja0399066 |
| 99. | Barluenga, J.; Merino, I.; Palacios, F. Tetrahedron Lett. 1990, 31, 6713. doi:10.1016/S0040-4039(00)97155-7 |
| 61. | Trost, B. M.; Stiles, D. T. Org. Lett. 2005, 7, 2117. doi:10.1021/ol050395x |
| 62. | Shen, L.; Hsung, R. P.; Zhang, Y.; Antoline, J. E.; Zhang, X. Org. Lett. 2005, 7, 3081. doi:10.1021/ol051094q |
| 47. | Tracey, M. R.; Hsung, R. P.; Antoline, J.; Kurtz, K. C. M.; Shen, L.; Slafer, B. W.; Zhang, Y. Product Class 4: N-Arylalkanamides, Ynamides, Enamides, Dienamides, and Allenamides. In Science of Synthesis, Houben-Weyl Methods of Molecular Transformations; Weinreb, S. M., Ed.; Thieme: Stuttgart, 2005; pp 387–476. |
| 59. | Xiong, H.; Hsung, R. P.; Wei, L.-L.; Berry, C. R.; Mulder, J. A.; Stockwell, B. Org. Lett. 2000, 2, 2869. doi:10.1021/ol000181+ |
| 60. | Wei, L.-L.; Mulder, J. A.; Xiong, H.; Zificsak, C. A.; Douglas, C. J.; Hsung, R. P. Tetrahedron 2001, 57, 459. doi:10.1016/S0040-4020(00)01014-0 |
| 79. | Kerr, D. J.; Willis, A. C.; Flynn, B. L. Org. Lett. 2004, 6, 457. doi:10.1021/ol035822q |
| 80. | Mousavipour, S. H.; Fernández-Ramos, A.; Meana-Pañeda, R.; Martínez-Núñez, E.; Vázquez, S. A.; Ríos, M. A. J. Phys. Chem. A 2007, 111, 719. doi:10.1021/jp0665269 |
| 81. | Gu, Z.; Ma, S. Chem.–Eur. J. 2008, 14, 2453. doi:10.1002/chem.200701171 |
| 82. | Shu, X.-Z.; Ji, K.-G.; Zhao, S.-C.; Zheng, Z.-J.; Chen, J.; Lu, L.; Liu, X.-Y.; Liang, Y.-M. Chem.–Eur. J. 2008, 14, 10556. doi:10.1002/chem.200801591 |
| 83. | Marvell, E. N. Thermal Electrocyclic Reactions; Academic Press: New York, 1980. |
| 84. | Okamura, W. H.; de Lera, A. R. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I.; Paquette, L. A., Eds.; Pergamon Press: New York, 1991; Vol. 5, pp 699–750. doi:10.1016/B978-0-08-052349-1.00137-2 |
| 78. | Siebert, M.; Osbourn, J. M.; Brummond, K. M.; Tantillo, D. J. J. Am. Chem. Soc. 2010, 132, 11952. doi:10.1021/ja102848z |
| 2. | Jensen, F. J. Am. Chem. Soc. 1995, 117, 7487. doi:10.1021/ja00133a021 |
| 4. | Crandall, J. K.; Paulson, D. R. J. Am. Chem. Soc. 1966, 88, 4302. doi:10.1021/ja00970a063 |
| 24. | Zhu, Y.; Yin, G.; Hong, D.; Lu, P.; Wang, Y. Org. Lett. 2011, 13, 1024. doi:10.1021/ol103074d |
| 73. | Ha, J. D.; Kang, C. H.; Belmore, K. A.; Cha, J. K. J. Org. Chem. 1998, 63, 3810. doi:10.1021/jo980593k |
| 74. | González-Romero, C.; Bernal, P.; Jiménez, F.; Cruz, M. C.; Fuentes-Benites, A.; Benavides, A.; Bautista, R.; Tamariz, J. Pure Appl. Chem. 2007, 79, 181. |
| 75. | Movassaghi, M.; Hunt, D. K.; Tjandra, M. J. Am. Chem. Soc. 2006, 128, 8126. doi:10.1021/ja0626180 |
| 76. | Enders, D.; Meyer, O.; Raabe, G. Synthesis 1992, 1242. doi:10.1055/s-1992-26349 |
| 77. | Barluenga, J.; Canteli, R.-M.; Flórez, J.; García-Granda, S.; Gutiérrez-Rodríguez, A.; Martín, E. J. Am. Chem. Soc. 1998, 120, 2514. doi:10.1021/ja972588o |
| 2. | Jensen, F. J. Am. Chem. Soc. 1995, 117, 7487. doi:10.1021/ja00133a021 |
| 4. | Crandall, J. K.; Paulson, D. R. J. Am. Chem. Soc. 1966, 88, 4302. doi:10.1021/ja00970a063 |
| 5. | Paulson, D. R.; Crandall, J. K.; Bunnell, C. A. J. Org. Chem. 1970, 35, 3708. doi:10.1021/jo00836a027 |
| 6. | Bloch, R.; Perchec, P. L.; Conia, J.-M. Angew. Chem., Int. Ed. Engl. 1970, 9, 798. doi:10.1002/anie.197007981 |
| 7. | Jones, M.; Hendrick, M. E.; Hardie, J. A. J. Org. Chem. 1971, 36, 3061. doi:10.1021/jo00819a042 |
| 8. | Patrick, T. B.; Haynie, E. C.; Probst, W. J. Tetrahedron Lett. 1971, 27, 423. doi:10.1016/S0040-4039(01)96457-3 |
| 9. | Lenk, W.; Hopf, H. Tetrahedron Lett. 1982, 23, 4073. doi:10.1016/S0040-4039(00)88350-1 |
| 10. | Hopf, H.; Gottschild, D.; Lenk, W. Isr. J. Chem. 1985, 26, 79. |
| 11. | Lehrich, F.; Hopf, H. Tetrahedron Lett. 1987, 28, 2697. doi:10.1016/S0040-4039(00)96184-7 |
| 12. | Meier, H.; Schmitt, M. Tetrahedron Lett. 1989, 30, 5873. doi:10.1016/S0040-4039(01)93493-8 |
| 63. | Hayashi, R.; Hsung, R. P.; Feltenberger, J. B.; Lohse, A. G. Org. Lett. 2009, 11, 2125. doi:10.1021/ol900647s |
| 64. | Hayashi, R.; Feltenberger, J. B.; Hsung, R. P. Org. Lett. 2010, 12, 1152. doi:10.1021/ol902821w |
| 65. | Terada, A.; Murata, K. Bull. Chem. Soc. Jpn. 1967, 40, 1644. doi:10.1246/bcsj.40.1644 |
| 66. | Overman, L. E.; Clizbe, L. A. J. Am. Chem. Soc. 1976, 98, 2352. doi:10.1021/ja00424a068 |
| 67. | Oppolzer, W.; Bieber, L.; Francotte, E. Tetrahedron Lett. 1979, 20, 4537. doi:10.1016/S0040-4039(01)86643-0 |
| 68. | Smith, A. B., III; Wexler, B. A.; Tu, C.-Y.; Konopelski, J. P. J. Am. Chem. Soc. 1985, 107, 1308. doi:10.1021/ja00291a034 |
| 69. | Schlessinger, R. H.; Pettus, T. R. R.; Springer, J. P.; Hoogsteen, K. J. Org. Chem. 1994, 59, 3246. doi:10.1021/jo00091a002 |
| 70. | Kozmin, S. A.; Rawal, V. H. J. Am. Chem. Soc. 1997, 119, 7165. doi:10.1021/ja971272d |
| 71. | Huang, Y.; Iwama, T.; Rawal, V. H. J. Am. Chem. Soc. 2002, 124, 5950. doi:10.1021/ja026088t |
| 72. | Robiette, R.; Cheboub-Benchaba, K.; Peeters, D.; Marchand-Brynaert, J. J. Org. Chem. 2003, 68, 9809. doi:10.1021/jo0302049 |
© 2011 Hayashi et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)




![[1860-5397-7-53-1]](/bjoc/content/figures/1860-5397-7-53-1.png?scale=2.0&max-width=1024&background=FFFFFF)