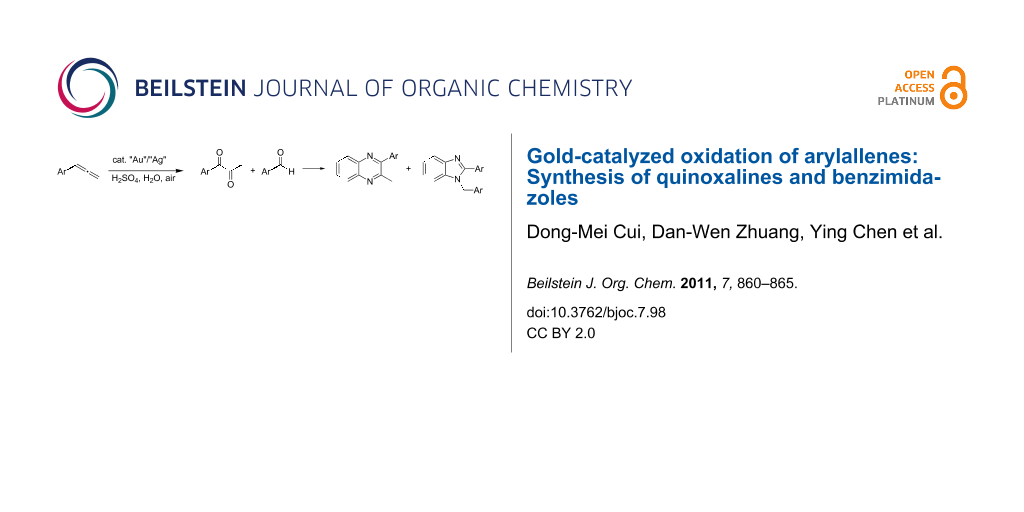
Abstract
A gold-catalyzed oxidation of arylallenes to form α-diketones and aldehydes in good yields is presented. Further directed synthesis of quinoxalines and benzimidazoles, via the condensation of the resulting α-diketones and aldehydes with benzene-1,2-diamine, was achieved in high yields.
Graphical Abstract
Introduction
Recently, several research groups have developed gold-catalyzed homogeneous catalytic reactions [1]. A variety of organic transformations have been shown to be mediated by gold(I) or gold(III) complexes in solution. In addition to its ability to activate unsaturated C–C bonds, the catalysis of nucleophilic addition by gold complexes for the formation of carbon–carbon and carbon–heteroatom bonds has been one of the most investigated reactions in recent organometallic catalysis [1-24]. In particular, water as a nucleophilic reagent has been used in the addition of alkynes and allenes [16-18]. In contrast, gold-catalyzed oxidation chemistry has been less well developed [25-36], although oxidative cleavage of carbon–carbon double bonds and carbon–carbon triple bonds by homogeneous gold catalysts was reported recently [28,29,33]. To the best of our knowledge, gold and other transition metal-catalyzed oxidations of allenes have not been reported [37,38]. In the context of ongoing studies on metal-catalyzed atom-economical reactions, we have been interested in the use of gold for simple and highly efficient transformations. Additionally, quinoxaline and benzimidazole skeletons are common building blocks for the preparation of substances with pronounced biological activities [39-44]. Herein, we report the gold(I)-catalyzed oxidation/hydration and oxidative cleavage of allenes to form α-diketones and aldehydes, and the synthesis of quinoxalines and benzimidazoles via the condensation of the resulting α-diketones and aldehydes with benzene-1,2-diamine [45-56].
Results and Discussion
Our initial explorations focused on the reaction of 4-butylphenylallene (1a) (0.5 mmol) in the presence of a catalytic mixture of (Ph3P)AuCl (2 mol %), AgBF4 (8 mol %), and H2SO4 (0.5 mol %) in dioxane (1.0 mL) and water (10 mmol), at 60 °C for 24 h in air. This proceeded efficiently to form a 44:56 mixture of α-diketone 2a and aldehyde 3a in 70% combined yield (Scheme 1, Table 1, entry 1). The use of either the gold or silver pre-catalyst alone gave lower yields (Table 1, entries 18 and 19). These results indicate that both the Au source and AgBF4 play a crucial role in this oxidation. The superior efficiency of the tetrafluoroborate anion was demonstrated by a comparison with other weakly or non-coordinating counter anions. In addition, a change of the counter anion to OTf−, SbF6−, or NTf2− was also effective (Table 1, entries 2–4). The use of other gold catalysts, e.g., (Ph3P)AuNO3 and IMesAuCl, led to only to combined yields of 2a and 3a of 49% and 60%, respectively (Table 1, entries 16–17). Decreasing the amount of the sulfuric acid also resulted in a lower yield, although the addition of a large amount of the acid did not affect the reaction (Table 1, entries 8–9). Different acids were screened (Table 1, entries 1, 5–7) and sulfuric acid was found to be the most effective. The use of solvents such as THF, toluene, DCE or ether resulted in a lower conversion (Table 1, entries 10–13). Treatment of 1a in an atmosphere of O2 (1 atm) afforded 2a and 3a in a combined yield of 47% (Table 1, entry 20). When the reaction was conducted under a nitrogen atmosphere, only trace of products were observed (Table 1, entry 21).
Scheme 1: Oxidation of 4-butylphenylallene.
Scheme 1: Oxidation of 4-butylphenylallene.
Table 1: Oxidation of 1a catalyzed by a mixture of (PPh3)AuCl, AgBF4, and H2SO4.a
| Entry |
Au
(2 mol %) |
Ag
(8 mol %) |
H2O
(equiv) |
Acid
(mol %) |
Solvent |
Ratiob
2a:3a |
Yield (%)c of 2a and 3a |
|---|---|---|---|---|---|---|---|
| 1 | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (0.5) | dioxane | 44:56 | 70 |
| 2 | (PPh3)AuCl | AgOTf | 20 | H2SO4 (0.5) | dioxane | 38:62 | 63 |
| 3 | (PPh3)AuCl | AgNTf2 | 20 | H2SO4 (0.5) | dioxane | 55:45 | 43 |
| 4 | (PPh3)AuCl | AgSbF6 | 20 | H2SO4 (0.5) | dioxane | 49:51 | 47 |
| 5 | (PPh3)AuCl | AgBF4 | 20 | F3CCO2H (0.5) | dioxane | 43:57 | 58 |
| 6 | (PPh3)AuCl | AgBF4 | 20 | MsOH (0.5) | dioxane | 39:61 | 68 |
| 7 | (PPh3)AuCl | AgBF4 | 20 | TsOH (0.5) | dioxane | 37:63 | 48 |
| 8 | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (0.25) | dioxane | 49:51 | 40 |
| 9 | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (1.0) | dioxane | 48:52 | 70 |
| 10 | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (0.5) | THF | 47:53 | 19 |
| 11 | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (0.5) | toluene | 39:61 | 49 |
| 12 | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (0.5) | DCE | 43:57 | 60 |
| 13 | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (0.5) | ether | 36:64 | 37 |
| 14 | (PPh3)AuCl | AgBF4 | 10 | H2SO4 (0.5) | dioxane | 46:54 | 32 |
| 15 | (PPh3)AuCl | AgBF4 | 40 | H2SO4 (0.5) | dioxane | 53:47 | 39 |
| 16 | (PPh3)AuNO3 | – | 20 | H2SO4 (0.5) | dioxane | 49:51 | 49 |
| 17 | IMeSAuCl | AgBF4 | 20 | H2SO4 (0.5) | dioxane | 54:46 | 60 |
| 18 | PPh3AuCl | – | 20 | H2SO4 (0.5) | dioxane | 38:62 | 15 |
| 19 | – | AgBF4 | 20 | H2SO4 (0.5) | dioxane | 47:53 | 28 |
| 20d | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (0.5) | dioxane | 50:50 | 47 |
| 21e | (PPh3)AuCl | AgBF4 | 20 | H2SO4 (0.5) | dioxane | – | trace |
aAll reactions were carried out using 1a (0.5 mmol), (Ph3P)AuCl (2 mol %), AgBF4 (8 mol %), and acid (0.25–1.0 mol %) in solvent (1.0 mL) and water (0.5–1.0 mmol) at 60 °C for 24 h. bThe ratio of 2a and 3a was determined by GC. cIsolated and combined yield of 2a and 3a. d Under an atmosphere of O2 (1 atm). eUnder an atmosphere of N2.
In order to assess the scope of this process, we examined the oxidation of several aryallenes under the optimized conditions indicated in entry 1 of Table 1. The results are summarized in Table 2. Phenylallene gave a good isolated yield of 1-phenylpropan-1,2-dione (2c) and benzaldehyde (3c) in a ratio of 43:57 (Table 2, entry 3). With a more electron-donating alkoxy group, the expected products were again obtained in good yields (Table 2, entry 4). In addition, oxidation of arylallene with an electron-withdrawing fluoro or bromo substituent on the benzene ring also took place smoothly (Table 2, entries 5 and 6). Disubstituted allenes were also examined. Thus, the 1,3-disubstituted allene 1g, was oxidized to afford α-diketone 2g and aldehyde 3c in 35% and 32% yields, respectively (Table 2, entry 7). Similarly, oxidation cleavage of 1,1-disubstituted, trisubstituted and tetrasubstituted allenes gave the expected products (Table 2, entries 8–10). In striking contrast to aromatic allenes, aliphatic allenes, such as hepta-1,2-diene and 1-(propa-1,2-dienyl)cyclohex-1-ene failed to undergo Au-catalyzed oxidative transformation under the same reaction conditions.
Table 2: Oxidation of 1 catalyzed by a mixture of (PPh3)AuCl, AgBF4, and H2SO4.
| Entry | Allene 1 | Product 2 | Product 3 | Yield (2 and 3) (%)a |
|---|---|---|---|---|
| 1 |
|
|
|
70 (48:52) |
| 2 |
|
|
|
72 (46:57) |
| 3 |
|
|
|
68 (43:57) |
| 4 |
|
|
|
62 (35:65) |
| 5 |
|
|
|
65 (52:48) |
| 6 |
|
|
|
67 (43:57) |
| 7 |
|
|
|
2g: 35
3c: 32 |
| 8 |
|
|
84 | |
| 9 |
|
|
|
2i: 89
3c: 85 |
| 10 |
|
|
90 | |
aIsolated yield. The ratio of 2 and 3 in the parentheses was determined by GC.
Having prepared a variety of α-diketones and aldehydes successfully, we then undertook the synthesis of quinoxalines and benzimidazoles (Scheme 2). Thus, the treatment of the corresponding mixture of α-diketone 2 and aldehyde 3 with benzene-1,2-diamine in the presence of 20 mol % oxalic acid afforded the desired quinoxalines 4 and benzimidazoles 5 in high yields (Table 3, entries 1–6).
Scheme 2: Preparation of quinoxalines and benzimidazoles.
Scheme 2: Preparation of quinoxalines and benzimidazoles.
Conclusion
We have developed a new gold-catalyzed oxidation of arylallenes to give α-diketones and aldehydes in good yields. In addition, the directed synthesis of quinoxalines and benzimidazoles via the condensation of the resulting α-diketones and aldehydes with benzene-1,2-diamine was achieved in high yields. This reaction appears to proceed via oxidation/hydration and oxidative cleavage of the allene, and investigations into the mechanism of this reaction are underway in our laboratory.
Experimental
General methods: Unless otherwise noted, materials were obtained from commercial suppliers and used without further purification. Thin layer chromatography (TLC) was performed on silica gel 60 F254 and visualized with UV light. Column chromatography was performed with silica gel (mesh 300–400). 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 500 MHz spectrometer in CDCl3 with Me4Si as internal standard. Data are reported as follows: Chemical shift in ppm (δ), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, br = broad and m = multiplet), coupling constant in Hertz (Hz) and signal integration. Infrared spectra (IR) were obtained on 370 FT-IR spectrometer; absorptions are reported in cm−1. Mass spectra were obtained under electron impact mode (EI) and high resolution mass spectra were measured on a high resolution mass spectrometer (GCT Premier).
General procedure
Step A (a typical procedure): Sulfuric acid (0.5 mol %) was added to a mixture of 4-butylphenylallene (0.5 mmol), water (10 mmol), (PPh3)AuCl (2 mol %), AgBF4 (8 mol %), and dioxane (1 mL). The mixture was stirred at 60 °C for 24 , the reaction quenched with a saturated solution of NaHCO3 and extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine, dried over Na2SO4 and concentrated in vacuo. The residue was purified by flash chromatography to give the desired products 2a and 3a (48:52, 63.75 mg, 70%).
Step B (a typical procedure): A mixture of 2a and 3a (63.75 mg), benzene-1,2-diamine (28 mg, 0.259 mmol), oxalic acid (6.3 mg, 0.07 mmol, 20 mol %), water (1 mL) was dissolved in ethanol (1 mL). The mixture was heated under reflux for 4 h. The reaction was quenched with a saturated solution of NaHCO3 and then extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine, dried over Na2SO4 and concentrated in vacuo. The residue was purified by flash chromatography to give the desired products 4a (45.0 mg, 97%) and 5a (33.9 mg, 94%).
Supporting Information
| Supporting Information File 1: Analytical and spectroscopic data for new compounds. | ||
| Format: PDF | Size: 1.9 MB | Download |
References
-
Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351. doi:10.1021/cr068430g
Return to citation in text: [1] [2] -
Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326. doi:10.1021/cr0684319
Return to citation in text: [1] -
Arcadi, A. Chem. Rev. 2008, 108, 3266. doi:10.1021/cr068435d
Return to citation in text: [1] -
Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239. doi:10.1021/cr068434l
Return to citation in text: [1] -
Skouta, R.; Li, C.-J. Tetrahedron 2008, 64, 4917. doi:10.1016/j.tet.2008.03.083
Return to citation in text: [1] -
Shen, H. C. Tetrahedron 2008, 64, 3885. doi:10.1016/j.tet.2008.01.081
Return to citation in text: [1] -
Muzart, J. Tetrahedron 2008, 64, 5815. doi:10.1016/j.tet.2008.04.018
Return to citation in text: [1] -
Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180. doi:10.1021/cr000436x
Return to citation in text: [1] -
Hashmi, A. S. K. Gold Bull. 2004, 37, 51. doi:10.1007/BF03215517
Return to citation in text: [1] -
Arcadi, A.; Giuseppe, S. D. Curr. Org. Chem. 2004, 8, 795. doi:10.2174/1385272043370564
Return to citation in text: [1] -
Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896. doi:10.1002/anie.200602454
Return to citation in text: [1] -
Shapiro, N. D.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 4160. doi:10.1021/ja070789e
Return to citation in text: [1] -
Dubé, P.; Toste, F. D. J. Am. Chem. Soc. 2006, 128, 12062. doi:10.1021/ja064209+
Return to citation in text: [1] -
Marion, N.; Díez-González, S.; de Frémont, P.; Noble, A. R.; Nolan, S. P. Angew. Chem., Int. Ed. 2006, 45, 3647. doi:10.1002/anie.200600571
Return to citation in text: [1] -
Zhang, Y.; Donahue, J. P.; Li, C.-J. Org. Lett. 2007, 9, 627. doi:10.1021/ol062918m
Return to citation in text: [1] -
Mizushima, E.; Sato, K.; Hayashi, T.; Tanaka, M. Angew. Chem., Int. Ed. 2002, 41, 4563. doi:10.1002/1521-3773(20021202)41:23<4563::AID-ANIE4563>3.0.CO;2-U
Return to citation in text: [1] [2] -
Casado, R.; Contel, M.; Laguna, M.; Romero, P.; Sanz, S. J. Am. Chem. Soc. 2003, 125, 11925. doi:10.1021/ja036049x
Return to citation in text: [1] [2] -
Zhang, Z.-B.; Lee, S. D.; Fisher, A. S.; Widenhoefer, R. A. Tetrahedron 2009, 65, 1794. doi:10.1016/j.tet.2008.10.113
Return to citation in text: [1] [2] -
Liu, B.; De Brabander, J. K. Org. Lett. 2006, 8, 4907. doi:10.1021/ol0619819
Return to citation in text: [1] -
Mizushima, E.; Hayashi, T.; Tanaka, M. Org. Lett. 2003, 5, 3349. doi:10.1021/ol0353159
Return to citation in text: [1] -
Arcadi, A.; Di Giuseppe, S.; Marinelli, F.; Rossi, E. Adv. Synth. Catal. 2001, 343, 443. doi:10.1002/1615-4169(200107)343:5<443::AID-ADSC443>3.0.CO;2-#
Return to citation in text: [1] -
Hashmi, A. S. K.; Weyrauch, J. P.; Frey, W.; Bats, J. W. Org. Lett. 2004, 6, 4391. doi:10.1021/ol0480067
Return to citation in text: [1] -
Luo, Y. M.; Li, Z.; Li, C. J. Org. Lett. 2005, 7, 2675. doi:10.1021/ol050826b
Return to citation in text: [1] -
Lavallo, V.; Frey, G. D.; Donnadieu, B.; Soleilhavoup, M.; Bertrand, G. Angew. Chem., Int. Ed. 2008, 47, 5224. doi:10.1002/anie.200801136
Return to citation in text: [1] -
Deng, X.-Y.; Friend, C. M. J. Am. Chem. Soc. 2005, 127, 17178. doi:10.1021/ja0557031
Return to citation in text: [1] -
Guzman, J.; Carrettin, S.; Corma, A. J. Am. Chem. Soc. 2005, 127, 3286. doi:10.1021/ja043752s
Return to citation in text: [1] -
Guan, B.; Xing, D.; Cai, G.; Wan, X.; Yu, N.; Fang, Z.; Yang, L.; Shi, Z. J. Am. Chem. Soc. 2005, 127, 18004. doi:10.1021/ja055398j
Return to citation in text: [1] -
Xing, D.; Guan, B.; Cai, G.; Fang, Z.; Yang, L.; Shi, Z. Org. Lett. 2006, 8, 693. doi:10.1021/ol052830t
Return to citation in text: [1] [2] -
Liu, Y.; Song, F.; Guo, S. J. Am. Chem. Soc. 2006, 128, 11332. doi:10.1021/ja062610q
Return to citation in text: [1] [2] -
Taduri, B. P.; Sohel, S. M. A.; Cheng, H.-M.; Lin, G.-Y.; Liu, R.-S. Chem. Commun. 2007, 2530. doi:10.1039/b700659d
Return to citation in text: [1] -
Witham, C. A.; Mauleon, P.; Shapiro, N. D.; Sherry, B. D.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 5838. doi:10.1021/ja071231+
Return to citation in text: [1] -
Murakami, Y.; Konishi, K. J. Am. Chem. Soc. 2007, 129, 14401. doi:10.1021/ja075051b
Return to citation in text: [1] -
Das, A.; Chaudhuri, R.; Liu, R.-S. Chem. Commun. 2009, 4046. doi:10.1039/b908338c
Return to citation in text: [1] [2] -
Ye, L.; Cui, L.; Zhang, G.; Zhang, L. J. Am. Chem. Soc. 2010, 132, 3258. doi:10.1021/ja100041e
Return to citation in text: [1] -
Lu, B.; Li, C.; Zhang, L. J. Am. Chem. Soc. 2010, 132, 14070. doi:10.1021/ja1072614
Return to citation in text: [1] -
Liu, Y.; Song, F.; Song, Z.; Liu, M.; Yan, B. Org. Lett. 2005, 7, 5409. doi:10.1021/ol052160r
Return to citation in text: [1] -
Erden, I.; Martinez, T. R. Tetrahedron Lett. 1991, 32, 1859. doi:10.1016/S0040-4039(00)85981-X
Return to citation in text: [1] -
Fleming, S. A.; Liu, R.; Redd, J. T. Tetrahedron Lett. 2005, 46, 8095. doi:10.1016/j.tetlet.2005.09.138
Return to citation in text: [1] -
Seitz, L. E.; Suling, W. J.; Reynolds, R. C. J. Med. Chem. 2002, 45, 5604. doi:10.1021/jm020310n
Return to citation in text: [1] -
Hazeldine, S. T.; Polin, L.; Kushner, J.; Paluch, J.; White, K.; Edelstein, M.; Palomino, E.; Corbett, T. H.; Horwitz, J. P. J. Med. Chem. 2001, 44, 1758. doi:10.1021/jm0005149
Return to citation in text: [1] -
Bailly, C.; Echepare, S.; Gago, F.; Waring, M. J. Anti-cancer Drug Des. 1999, 14, 291.
Return to citation in text: [1] -
Sato, K.; Shiratori, O.; Katagiri, K. J. Antibiot. 1967, 20, 270.
Return to citation in text: [1] -
Tamm, I. Science 1957, 126, 1235.
Return to citation in text: [1] -
Roth, T.; Morningstar, M. L.; Boyer, P. L.; Hughes, S. H.; Buckheit, R. W., Jr.; Michejda, C. J. J. Med. Chem. 1997, 40, 4199. doi:10.1021/jm970096g
Return to citation in text: [1] -
Dayan, S.; Ben-David, I.; Rozen, S. J. Org. Chem. 2000, 65, 8816. doi:10.1021/jo001101i
Return to citation in text: [1] -
Wan, Z.; Jones, C. D.; Mitchell, D.; Pu, J. Y.; Zhang, T. Y. J. Org. Chem. 2006, 71, 826. doi:10.1021/jo051793g
Return to citation in text: [1] -
Yusubov, M. S.; Zholobova, G. A.; Vasilevsky, S. F.; Tretyakov, E. V.; Knight, D. W. Tetrahedron 2002, 58, 1607. doi:10.1016/S0040-4020(02)00025-X
Return to citation in text: [1] -
Giraud, A.; Provot, O.; Peyrat, J.-F.; Alami, M.; Brion, J.-D. Tetrahedron 2006, 62, 7667. doi:10.1016/j.tet.2006.05.072
Return to citation in text: [1] -
Mousset, C.; Provot, O.; Hamze, A.; Bignon, J.; Brion, J.-D.; Alami, M. Tetrahedron 2008, 64, 4287. doi:10.1016/j.tet.2008.02.081
Return to citation in text: [1] -
Niu, M.; Fu, H.; Jiang, Y.; Zhao, Y. Synthesis 2008, 2879. doi:10.1055/s-2008-1067240
Return to citation in text: [1] -
Tan, K. J.; Wille, U. Chem. Commun. 2008, 6239. doi:10.1039/B815358B
Return to citation in text: [1] -
Ren, W.; Xia, Y.; Ji, S.-J.; Zhang, Y.; Wan, X.; Zhao, J. Org. Lett. 2009, 11, 1841. doi:10.1021/ol900344g
Return to citation in text: [1] -
Al-Rashid, Z. F.; Johnson, W. L.; Hsung, R. P.; Wei, Y.; Yao, P.-Y.; Liu, R.; Zhao, K. J. Org. Chem. 2008, 73, 8780. doi:10.1021/jo8015067
Return to citation in text: [1] -
Ren, W.; Liu, J.; Chen, L.; Wan, X. Adv. Synth. Catal. 2010, 352, 1424. doi:10.1002/adsc.201000250
Return to citation in text: [1] -
Mori, S.; Takubo, M.; Yanase, T.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Adv. Synth. Catal. 2010, 352, 1630. doi:10.1002/adsc.201000173
Return to citation in text: [1] -
Hasaninejad, A.; Zare, A.; Mohammadizadeh, M. R.; Shekouhy, M. ARKIVOC 2008, (xiii), 28.
Return to citation in text: [1]
| 1. | Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351. doi:10.1021/cr068430g |
| 28. | Xing, D.; Guan, B.; Cai, G.; Fang, Z.; Yang, L.; Shi, Z. Org. Lett. 2006, 8, 693. doi:10.1021/ol052830t |
| 29. | Liu, Y.; Song, F.; Guo, S. J. Am. Chem. Soc. 2006, 128, 11332. doi:10.1021/ja062610q |
| 33. | Das, A.; Chaudhuri, R.; Liu, R.-S. Chem. Commun. 2009, 4046. doi:10.1039/b908338c |
| 25. | Deng, X.-Y.; Friend, C. M. J. Am. Chem. Soc. 2005, 127, 17178. doi:10.1021/ja0557031 |
| 26. | Guzman, J.; Carrettin, S.; Corma, A. J. Am. Chem. Soc. 2005, 127, 3286. doi:10.1021/ja043752s |
| 27. | Guan, B.; Xing, D.; Cai, G.; Wan, X.; Yu, N.; Fang, Z.; Yang, L.; Shi, Z. J. Am. Chem. Soc. 2005, 127, 18004. doi:10.1021/ja055398j |
| 28. | Xing, D.; Guan, B.; Cai, G.; Fang, Z.; Yang, L.; Shi, Z. Org. Lett. 2006, 8, 693. doi:10.1021/ol052830t |
| 29. | Liu, Y.; Song, F.; Guo, S. J. Am. Chem. Soc. 2006, 128, 11332. doi:10.1021/ja062610q |
| 30. | Taduri, B. P.; Sohel, S. M. A.; Cheng, H.-M.; Lin, G.-Y.; Liu, R.-S. Chem. Commun. 2007, 2530. doi:10.1039/b700659d |
| 31. | Witham, C. A.; Mauleon, P.; Shapiro, N. D.; Sherry, B. D.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 5838. doi:10.1021/ja071231+ |
| 32. | Murakami, Y.; Konishi, K. J. Am. Chem. Soc. 2007, 129, 14401. doi:10.1021/ja075051b |
| 33. | Das, A.; Chaudhuri, R.; Liu, R.-S. Chem. Commun. 2009, 4046. doi:10.1039/b908338c |
| 34. | Ye, L.; Cui, L.; Zhang, G.; Zhang, L. J. Am. Chem. Soc. 2010, 132, 3258. doi:10.1021/ja100041e |
| 35. | Lu, B.; Li, C.; Zhang, L. J. Am. Chem. Soc. 2010, 132, 14070. doi:10.1021/ja1072614 |
| 36. | Liu, Y.; Song, F.; Song, Z.; Liu, M.; Yan, B. Org. Lett. 2005, 7, 5409. doi:10.1021/ol052160r |
| 16. | Mizushima, E.; Sato, K.; Hayashi, T.; Tanaka, M. Angew. Chem., Int. Ed. 2002, 41, 4563. doi:10.1002/1521-3773(20021202)41:23<4563::AID-ANIE4563>3.0.CO;2-U |
| 17. | Casado, R.; Contel, M.; Laguna, M.; Romero, P.; Sanz, S. J. Am. Chem. Soc. 2003, 125, 11925. doi:10.1021/ja036049x |
| 18. | Zhang, Z.-B.; Lee, S. D.; Fisher, A. S.; Widenhoefer, R. A. Tetrahedron 2009, 65, 1794. doi:10.1016/j.tet.2008.10.113 |
| 1. | Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351. doi:10.1021/cr068430g |
| 2. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326. doi:10.1021/cr0684319 |
| 3. | Arcadi, A. Chem. Rev. 2008, 108, 3266. doi:10.1021/cr068435d |
| 4. | Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239. doi:10.1021/cr068434l |
| 5. | Skouta, R.; Li, C.-J. Tetrahedron 2008, 64, 4917. doi:10.1016/j.tet.2008.03.083 |
| 6. | Shen, H. C. Tetrahedron 2008, 64, 3885. doi:10.1016/j.tet.2008.01.081 |
| 7. | Muzart, J. Tetrahedron 2008, 64, 5815. doi:10.1016/j.tet.2008.04.018 |
| 8. | Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180. doi:10.1021/cr000436x |
| 9. | Hashmi, A. S. K. Gold Bull. 2004, 37, 51. doi:10.1007/BF03215517 |
| 10. | Arcadi, A.; Giuseppe, S. D. Curr. Org. Chem. 2004, 8, 795. doi:10.2174/1385272043370564 |
| 11. | Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896. doi:10.1002/anie.200602454 |
| 12. | Shapiro, N. D.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 4160. doi:10.1021/ja070789e |
| 13. | Dubé, P.; Toste, F. D. J. Am. Chem. Soc. 2006, 128, 12062. doi:10.1021/ja064209+ |
| 14. | Marion, N.; Díez-González, S.; de Frémont, P.; Noble, A. R.; Nolan, S. P. Angew. Chem., Int. Ed. 2006, 45, 3647. doi:10.1002/anie.200600571 |
| 15. | Zhang, Y.; Donahue, J. P.; Li, C.-J. Org. Lett. 2007, 9, 627. doi:10.1021/ol062918m |
| 16. | Mizushima, E.; Sato, K.; Hayashi, T.; Tanaka, M. Angew. Chem., Int. Ed. 2002, 41, 4563. doi:10.1002/1521-3773(20021202)41:23<4563::AID-ANIE4563>3.0.CO;2-U |
| 17. | Casado, R.; Contel, M.; Laguna, M.; Romero, P.; Sanz, S. J. Am. Chem. Soc. 2003, 125, 11925. doi:10.1021/ja036049x |
| 18. | Zhang, Z.-B.; Lee, S. D.; Fisher, A. S.; Widenhoefer, R. A. Tetrahedron 2009, 65, 1794. doi:10.1016/j.tet.2008.10.113 |
| 19. | Liu, B.; De Brabander, J. K. Org. Lett. 2006, 8, 4907. doi:10.1021/ol0619819 |
| 20. | Mizushima, E.; Hayashi, T.; Tanaka, M. Org. Lett. 2003, 5, 3349. doi:10.1021/ol0353159 |
| 21. | Arcadi, A.; Di Giuseppe, S.; Marinelli, F.; Rossi, E. Adv. Synth. Catal. 2001, 343, 443. doi:10.1002/1615-4169(200107)343:5<443::AID-ADSC443>3.0.CO;2-# |
| 22. | Hashmi, A. S. K.; Weyrauch, J. P.; Frey, W.; Bats, J. W. Org. Lett. 2004, 6, 4391. doi:10.1021/ol0480067 |
| 23. | Luo, Y. M.; Li, Z.; Li, C. J. Org. Lett. 2005, 7, 2675. doi:10.1021/ol050826b |
| 24. | Lavallo, V.; Frey, G. D.; Donnadieu, B.; Soleilhavoup, M.; Bertrand, G. Angew. Chem., Int. Ed. 2008, 47, 5224. doi:10.1002/anie.200801136 |
| 45. | Dayan, S.; Ben-David, I.; Rozen, S. J. Org. Chem. 2000, 65, 8816. doi:10.1021/jo001101i |
| 46. | Wan, Z.; Jones, C. D.; Mitchell, D.; Pu, J. Y.; Zhang, T. Y. J. Org. Chem. 2006, 71, 826. doi:10.1021/jo051793g |
| 47. | Yusubov, M. S.; Zholobova, G. A.; Vasilevsky, S. F.; Tretyakov, E. V.; Knight, D. W. Tetrahedron 2002, 58, 1607. doi:10.1016/S0040-4020(02)00025-X |
| 48. | Giraud, A.; Provot, O.; Peyrat, J.-F.; Alami, M.; Brion, J.-D. Tetrahedron 2006, 62, 7667. doi:10.1016/j.tet.2006.05.072 |
| 49. | Mousset, C.; Provot, O.; Hamze, A.; Bignon, J.; Brion, J.-D.; Alami, M. Tetrahedron 2008, 64, 4287. doi:10.1016/j.tet.2008.02.081 |
| 50. | Niu, M.; Fu, H.; Jiang, Y.; Zhao, Y. Synthesis 2008, 2879. doi:10.1055/s-2008-1067240 |
| 51. | Tan, K. J.; Wille, U. Chem. Commun. 2008, 6239. doi:10.1039/B815358B |
| 52. | Ren, W.; Xia, Y.; Ji, S.-J.; Zhang, Y.; Wan, X.; Zhao, J. Org. Lett. 2009, 11, 1841. doi:10.1021/ol900344g |
| 53. | Al-Rashid, Z. F.; Johnson, W. L.; Hsung, R. P.; Wei, Y.; Yao, P.-Y.; Liu, R.; Zhao, K. J. Org. Chem. 2008, 73, 8780. doi:10.1021/jo8015067 |
| 54. | Ren, W.; Liu, J.; Chen, L.; Wan, X. Adv. Synth. Catal. 2010, 352, 1424. doi:10.1002/adsc.201000250 |
| 55. | Mori, S.; Takubo, M.; Yanase, T.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Adv. Synth. Catal. 2010, 352, 1630. doi:10.1002/adsc.201000173 |
| 56. | Hasaninejad, A.; Zare, A.; Mohammadizadeh, M. R.; Shekouhy, M. ARKIVOC 2008, (xiii), 28. |
| 39. | Seitz, L. E.; Suling, W. J.; Reynolds, R. C. J. Med. Chem. 2002, 45, 5604. doi:10.1021/jm020310n |
| 40. | Hazeldine, S. T.; Polin, L.; Kushner, J.; Paluch, J.; White, K.; Edelstein, M.; Palomino, E.; Corbett, T. H.; Horwitz, J. P. J. Med. Chem. 2001, 44, 1758. doi:10.1021/jm0005149 |
| 41. | Bailly, C.; Echepare, S.; Gago, F.; Waring, M. J. Anti-cancer Drug Des. 1999, 14, 291. |
| 42. | Sato, K.; Shiratori, O.; Katagiri, K. J. Antibiot. 1967, 20, 270. |
| 43. | Tamm, I. Science 1957, 126, 1235. |
| 44. | Roth, T.; Morningstar, M. L.; Boyer, P. L.; Hughes, S. H.; Buckheit, R. W., Jr.; Michejda, C. J. J. Med. Chem. 1997, 40, 4199. doi:10.1021/jm970096g |
| 37. | Erden, I.; Martinez, T. R. Tetrahedron Lett. 1991, 32, 1859. doi:10.1016/S0040-4039(00)85981-X |
| 38. | Fleming, S. A.; Liu, R.; Redd, J. T. Tetrahedron Lett. 2005, 46, 8095. doi:10.1016/j.tetlet.2005.09.138 |
© 2011 Cui et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)