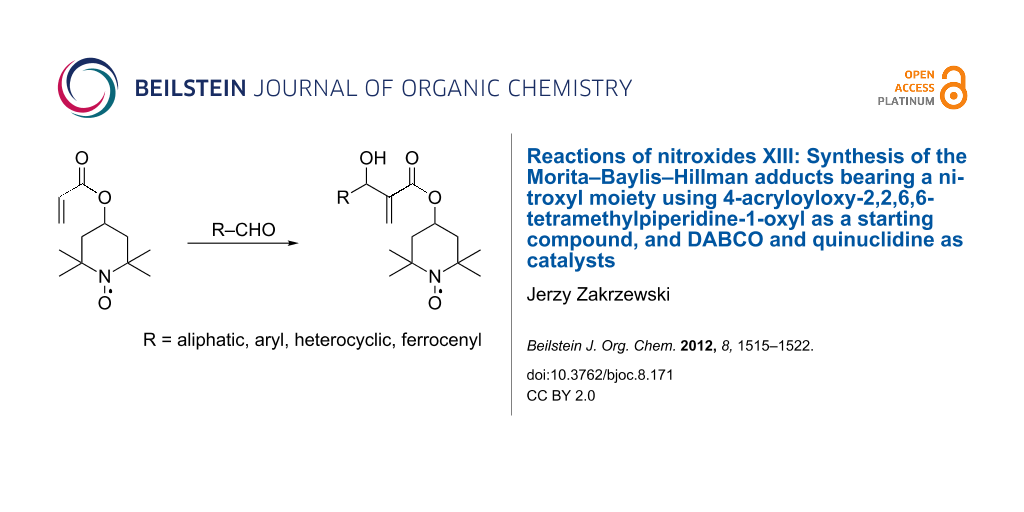
Abstract
The Morita–Baylis–Hillman adducts bearing a nitroxyl moiety were synthesized from 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl and aliphatic, aryl and heterocyclic aldehydes.
Graphical Abstract
Introduction
In the Morita–Baylis–Hillman (MBH) reaction, aldehydes react with a double bond activated by an electron-withdrawing group (EWG). The vinylic carbon bearing an EWG undergoes substitution. The reaction is carried out in the presence of either a tertiary amine (e.g., DABCO [2-6], quinuclidine and its derivatives [7-12], DBU [13,14], DBN [13], DMAP and its derivatives [4,15,16], urotropine [17], brucine N-oxide [18]) or a phosphine [19] as a catalyst. The MBH reaction is a carbon–carbon bond forming process. This is the reason why a huge amount of research devoted to the reaction is reported every year. The application and scope of the reaction has been summarized in many review articles, e.g., [20], as well as the latest ones [21-24]. Some recent results concerning MBH reaction have been presented [6,16,18,25-33]. MBH adducts themselves are reported to be antiproliferative agents [34]; however, they are often applied as a tool for building more complex target structures, usually of biological importance [35-43]. The MBH reaction is a rather slow process (complete reaction can take hundreds of hours), especially when acrylates are used [44]. As has been very well known since the 1960s, stable nitroxides can react without affecting the unpaired electron. However, there are also reactions that do involve the free electron (e.g., many types of reductions, or a disproportionation in an acidic environment). To the best of our knowledge, nitroxyl radicals have not yet been applied in the MBH reaction (to date), and the potential influence of the nitroxide moiety on the MBH reaction is unknown. Herein we present the MBH reaction with a nitroxyl radical, 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl, used as a starting material as an olefin activated with EWG. Acrylates are considered as rather unreactive in the MBH reaction [44]. It was shown that the effects on the reaction of aryl, benzyl, alkyl, and functionalized alkyl acrylic esters with benzaldehyde and furfuraldehyde in the presence of DABCO, strongly depend upon the electronic and steric effects of the ester part. The “unreactivity” of acrylates increases with steric hindrance and with increasing chain length of the alcohol moiety in an acrylate [20,45]. The alcohol moiety in 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl is undoubtly sterically hindered, so the expected reaction times will be long; however, this nitroxide has been chosen based on the availability of such nitroxyl esters. As an electrophilic partner in the MBH reaction, commonly used aldehydes were chosen, whose activity is broadly discussed in the literature. Aromatic aldehydes, especially those containing EWGs, are considered as reactive in the MBH reaction, in contrast to the aliphatic aldehydes (both n-butanal and pivalaldehyde), which are considered to be unreactive, although EWGs on the α-carbon atom (e.g., chloral) enhance their reactivity [20]. 2-Furaldehyde and nicotinic aldehyde were used because they were considered to be especially reactive in the MBH reaction [43].
Results and Discussion
4-Acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl (3) was obtained by esterification of acryloyl chloride (2) with 2,2,6,6-tetramethyl-4-piperidinol-1-oxyl (1) [46,47] in 90–95% yield. To synthesize MBH adducts (5a–o), 3 was reacted with aliphatic (4a–c), aryl (4d–l) and heterocyclic (4m,n) aldehydes, and (to obtain a compound bearing both nitroxyl and ferrocenyl moiety) ferrocenyl aldehyde (4o). Two catalytic systems were tested: DABCO, and quinuclidine with methanol as a cocatalyst. The latter system was chosen because it has been described as an excellent rate enhancer for the MBH reaction [9,11]. Methanol was chosen as an additive bearing a polar O–H bond, which activates both the aldehyde and “Michael” intermediate formed in the first step of the reaction, when an amine catalyst attacks an EWG activated olefin [11]. The reaction was carried out in THF as a solvent or the reagents were stirred neat. The synthesized MBH adducts (5a–o) are summarized in Scheme 1 and Table 1.
Scheme 1: MBH adducts 5a–o from 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl.
Scheme 1: MBH adducts 5a–o from 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl.
Table 1: Yields and melting points (mp) of MBH adducts 5a–o.
| 5 | R |
DABCO
t [h] |
DABCO
yield [%] |
quinuclidine/methanol
t [h] |
quinuclidine/methanol
yield [%] |
mp [°C] |
|---|---|---|---|---|---|---|
| a | n-C4H9 | 333 | 36 | 96 | 81 | 83–85 |
| b | (CH3)3C | 330 | — | 480 | 33 | red oil |
| c | CCl3 | 91 | 48 | 26 | 99 | 154–156 |
| d | C6H5 | 115 | 78 | 48 | 92 | 116–118 |
| e | 4-CH3C6H4 | 672 | — | 336 | 42 | 130–133 |
| f | 4-CH3OC6H4 | 984 | — | 1112 | 27 | 121–124 |
| g | 4-FC6H4 | 1008 | 27 | 336 | 23 | 105–109 |
| h | 4-BrC6H4 | 672 | 24 | 168 | 56 | 127–130 |
| i | 4-CF3C6H4 | 768 | 76 | 72 | 77 | 132–135 |
| j | 3,5-(CF3)2C6H3 | 528 | 59 | 432 | 47 | 112–115 |
| k | 4-NO2C6H4 | 376 | 22 | 96 | 79 | 102–104 |
| l | 2,4-(NO2)2C6H3 | 90 | 69 | 30 | 56 | 50–57 (glass) |
| m | 3-pyridyl | 592 | 99 | 72 | 65 | 92–97 |
| n | 2-furyl | 664 | 24 | 144 | 48 | 114–118 |
| o | ferrocenyl | 1128 | — | 1680 | 2 | 118–124 |
MBH adducts (5a–o) were obtained with very diverse results. Reaction times varied from one day to tens of days (5f,g,o). The reaction was significantly faster in the presence of quinuclidine/methanol system as a catalyst than in the presence of DABCO. The yields of the MBH adducts successfully obtained varied from negligible (5o, see below) to almost quantitative (5c,m). The use of 4-hydroxybenzaldehyde, 2-bromonicotinic aldehyde and 3-ferrocenylpropenal [48] did not result in any detectable MBH adducts in either catalytic system. The reaction with 2,4-dinitrobenzaldehyde (4l) provides some atypical intensely blue-colored side-products. The appropriate blue zones were isolated during column chromatography (Supporting Information File 1); however, attempts to obtain their physicochemical and spectral data were unsuccessful. The blue color of the side-products suggests that a nitroso group may be formed as a result of the reduction of the nitro group. Such transformations of 2,4-dinitrobenzaldehyde are well known. Under light, 2-nitroso-4-nitrobenzoic acid (characterized as its methyl ester) is formed [49]. In dilute aqueous sodium hydroxide solution, 4-nitroso-2-nitrophenol and formic acid are formed [50-52]. Ferrocenyl aldehyde (4o) as the starting compound [53] did not afford any product when DABCO was used as a catalyst. Use of the quinuclidine/methanol system and thorough searching of a potential product by TLC, resulted in the separation of the expected adduct 5o in 2% yield. However, its identity was confirmed unambiguously by (HR-)MS (both EI and ESI) and IR spectroscopy. This poor result is probably caused by the cumulative steric hindrance of both ferrocenyl and nitroxyl moieties (the sensitivity of the MBH reaction to the steric hindrance of the alcohol moiety in an acrylate was commented on in the introduction [20,45]). Due to the radical nature of the adducts 5a–o, their structures were confirmed by (HR-)MS (both EI and ESI), and IR spectroscopy (see Supporting Information File 1 for EIMS and IR spectra). Most of the EIMS spectra show the m/z 124 peak as the base one (except 5k,m,o), and the abundant intensity of m/z 109 peak (except 5c,f,l,o). The both fragments: m/z 124 and 109 are originated from the nitroxyl moiety of the investigated compounds 5. HRMS–EI for m/z 124: calcd for C9H16: 124.12520; found: 124.12515; for m/z 109: calcd for C8H13: 109.10173; found: 109.10079. However, direct characterization of the adducts 5a–o by NMR is impossible, one of the adducts (5d, R = C6H5) was subjected to the exemplary experiment developed for nitroxides by Keana and coworkers in 1975 [54]. 5d was reduced in situ with phenylhydrazine directly in an NMR tube to a nonradical, corresponding N-hydroxylamine. The 1H NMR and 13C NMR spectra were recorded, but only their aliphatic part was found to be valuable due to the signals belonging to phenylhydrazine itself. The recorded spectral data are presented in the experimental part and the Supporting Information File 1 (together with spectra of phenylhydrazine itself, as a background) as well. The observed singlet in 1H NMR at δ 5.54, and symmetric narrow multiplets at 5.76–5.90 and 6.28–6.45 assigned to the C6H5–CH(OH)–C(=CH2)- fragment are well consistent with the spectra of the typical series of the MBH adducts of common aldehydes and methyl acrylate, presented in [32]. Synthesized compounds 5a–o showed a weak antifungal activity. No insecticidal, acaricidal and herbicidal activity were shown.
Conclusion
In conclusion, it has been demonstrated that the use of 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl (3) as a starting compound allows us to obtain new MBH adducts 5a–o bearing a nitroxyl moiety. The use of quinuclidine with methanol as a catalyst instead of DABCO decreases the time of the reaction. No influence of the radical nature of 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl (3) on the reaction course was observed.
Experimental
General: 2,2,6,6-Tetramethyl-4-hydroxypiperidin-1-oxyl (1) was synthesized by the oxidation of 2,2,6,6-tetramethyl-4-piperidinol with 30% hydrogen peroxide (76.5% yield, mp 71–73 °C), according to [55-57]. Liquid aldehydes were purified by using vacuum distillation. THF was distilled over sodium under argon in the presence of benzophenone as an indicator. The experiments were performed in a 5 mL round-bottom flask, equipped with a magnetic stirrer. Most of the MBH adducts were obtained as red solids. TLC was carried out on silica gel Merck Alurolle 5562, Alufolien 5554. Column chromatography was performed by using Merck 1.09385.1000 or Zeochem 60 hyd 40–63 μm (0.040–0.063 mm, 230–400 mesh). TLC visualisation: UV 254 nm light and/or iodine vapours. EIMS data were recorded by using AMD 604 and Agilent Technologies 5975 B mass spectrometers. HRMS–EI data were recorded by using an AMD 604 mass spectrometer. ESIMS and HRMS–ESI (positive ions, CH3OH as solvent) were recorded by using a Micromass LCT apparatus. IR (ν, cm−1) data were recorded by using an FT/IR Jasco 420 spectrophotometer. 1H and 13C NMR data were collected by using a Varian UNITYplus 200 spectrophotometer.
4-Acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl (3): 2,2,6,6-Tetramethyl-4-piperidinol-1-oxyl (1, 0.344 g, 2 mmol) in benzene (≈5 mL) was placed in a round bottomed flask of 50 mL capacity equipped with a magnetic stirrer. A solution of triethylamine (1.54 g, 15.3 mmol, 2.1–2.2 mL) in benzene (6 mL) was added dropwise from a pipette. A solution of acryloyl chloride (2, 0.187 g, 2.08 mmol, 170 μL) in benzene (3.5 mL) was added dropwise from a syringe at room temperature. The progress of the reaction was monitored by TLC (benzene/ethyl acetate 9:1, benzene/methanol 9:1). The reaction mixture was stirred at room temperature for 20 h, then the second portion of 2 (0.094 g, 1.04 mmol, 85 μL) in benzene (1.7 mL) was added dropwise from a syringe. The reaction mixture was stirred at room temperature for 1 h. Depending on the results of the TLC control of the progress of the reaction the third portion of 2 may be added (≈50 μL). After the reaction had been terminated, the precipitate of triethylamine hydrochloride was filtered off and the filtrate was concentrated under reduced pressure. The red residue was subjected to column chromatography (hexane/ethyl acetate 9:1 as a mobile phase). The red eluent was collected to give red crystals of 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl (3), yield: 0.41–0.43 g (90–95%); mp 102–104 °C (lit. [46]: 102.5–103 °C, [58]: 102 °C, [59]: 99 °C ); EIMS m/z (% relative intensity): M+ 226 (16), 194 (10), 154 (15), 141 (7), 140 (17), 139 (21), 124 (84), 109 (95), 98 (6), 95 (8), 82 (21), 81 (20), 69 (11), 68 (20), 67 (19), 55 (100), 41 (34); HRMS–EI (m/z): calcd for C12H20NO3, 226.14432; found, 226.14401; IR (KBr): 1720 (C=O), 1635 (C=C) cm−1.
MBH adducts 5; general procedure without solvent with an excess of aldehyde (5a,b,d,n): 4-Acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl (3, 0.4 mmol), and a catalyst (about 10–20 mol % DABCO or quinuclidine), methanol (only if quinuclidine is used as catalyst) (10 μL), and an excess of freshly distilled aldehyde (4a, 4b, 4d, 4n, ≈1 mL) were stirred under argon at room temperature for the time mentioned in Table 1. The progress of the reaction was monitored by TLC (hexane/ethyl acetate 9:1, benzene/ethyl acetate 9:1, benzene/methanol 9:1). The MBH adduct 5 was isolated by direct column chromatography of the reaction mixture by using an appropriate mobile phase (hexane/ethyl acetate 9:1, benzene/ethyl acetate 95:5, benzene/methanol 95:5) to afford the desired adduct 5.
MBH adducts 5; general procedure in THF (5c, e–m, o): An appropriate aldehyde, freshly distilled and added with a syringe if liquid (0.8 mmol (4e, 4f, 4g, 4h, 4i, 4j, 4m), 0.6 mmol (4c), or 0.4 mmol (4k, 4l, 4o)), 4-acryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl (3, 0.4 mmol), a catalyst (about 10–20 mol % DABCO or quinuclidine), methanol (only if quinuclidine is used as catalyst, 10 μL), and anhydrous THF (1.0–1.5 mL), were stirred under argon at room temperature for the time mentioned in Table 1. The progress of the reaction was monitored by TLC (hexane/ethyl acetate 9:1, benzene/ethyl acetate 9:1, benzene/methanol 9:1). THF was evaporated under reduced pressure. The residue was subjected to column chromatography by using an appropriate mobile phase (hexane/ethyl acetate 9:1, benzene/ethyl acetate 95:5, benzene/methanol 95:5) to afford the desired adduct 5.
4-(2-((n-Butyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5a): Yield (DABCO): 46 mg (36%); Yield (quinuclidine): 101 mg (81%); orange solid; mp 83–85 °C; EIMS m/z (% relative intensity): M+ 312 (10), 255 (7), 240 (10), 155 (45), 154 (23), 141 (11), 140 (35), 139 (17), 124 (100), 109 (69), 100 (18), 98 (9), 95 (30), 85 (10), 83 (44), 82 (19), 81 (16), 74 (14), 69 (31), 67 (17), 56 (18), 55 (29), 41 (32); HRMS–EI (m/z): calcd for C17H30NO4: 312.21748; found: 312.21674; mass spectrum (ESI, m/z, %) 335 (100, [M + Na]+) 140 (46); HRMS–ESI (m/z): calcd for C19H26NO4Na, 335.2073; found, 335.2067; IR (KBr): 1701 (C=O), 1633 (C=C) cm−1.
4-(2-((tert-Butyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5b): Yield (quinuclidine): 41 mg (33%); red oil; EIMS m/z (% relative intensity): M+ 312 (11), 241 (6), 172 (7), 156 (44), 155 (64), 140 (73), 124 (100), 109 (65), 100 (49), 98 (17), 95 (22), 85 (13), 83 (22), 82 (27), 81 (24), 74 (55), 69 (46), 67 (23), 58 (17), 57 (98), 56 (38), 55 (42), 41 (65); HRMS–ESI (m/z): calcd for C17H30NO4, 312.21748; found, 312.21818; ESIMS m/z (% relative intensity): [M + Na]+ 335 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C17H30NO4Na, 335.2073; found, 335.2042; IR (KBr): 1706 (C=O) 1627 (C=C) cm−1.
4-(2-((Trichloromethyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5c): Yield (DABCO): 72 mg (48%); yield (quinuclidine): 148 mg (99%); yellow powder; mp 154–156 °C; EIMS m/z (% relative intensity): 374 (5), M+ 372 (5), 240 (9), 203 (4), 201 (5), 167 (9), 165 (15), 155 (24), 154 (20), 140 (17), 139 (19), 124 (100), 110 (6), 100 (10), 98 (6), 95 (5), 85 (11), 83 (7), 82 (17), 81 (14), 69 (22), 68 (11), 67 (13), 56 (14), 55 (20); HRMS–EI (m/z): calcd for C14H21NO4Cl3, 372.05362; found, 372.05425; ESIMS m/z (% relative intensity): 397 (86), [M + Na]+ 395 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C14H21Cl3NO4Na, 395.0434; found, 395.0429; IR (KBr): 1710 (C=O), 1633 (C=C) cm−1.
4-(2-((Phenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5d): Yield (DABCO): 104 mg (78%); yield (quinuclidine): 122 mg (92%); orange crystals; mp 116–118 °C; EIMS m/z (% relative intensity): M+ 332 (9), 302 (3), 284 (2), 154 (16), 140 (11), 133 (8), 124 (100), 117 (28), 116 (14), 115 (27), 109 (82), 79 (21); HRMS–EI calcd for C19H26NO4, 332.1862; found, 332.1856; ESIMS m/z (% relative intensity): [M + Na]+ 355 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C19H26NO4Na, 355.1760; found, 355.1742; IR (KBr): 1707 (C=O), 1628 (C=C) cm−1; 1H NMR (200 MHz, CDCl3, after reduction with phenylhydrazine in situ, in an NMR tube, in CDCl3, aliphatic part of the spectrum, in fact the spectrum of the corresponding hydroxylamine [54]) δ 1.16, 1.18, 1.19, 1.21 (4s, 12H, 4CH3), 1.40–1.64 (m, 2H, CH2), 1.75–1.95 (m, 2H, CH2), 4.96–5.18 (m, 1H, CH–OC(=O)), 5.54 (s, 1H, CH–OH), 5.76–5.90 (m, 1H, =CHH), 6.28–6.45 (m, 1H, =CHH) ppm; 13C NMR (50 MHz, CDCl3, after reduction with phenylhydrazine in situ, in an NMR tube, in CDCl3, aliphatic part of the spectrum, in fact the spectrum of the corresponding hydroxylamine [54]) δ 20.65 (2CH3), 32.00 (2CH3), 43.75 (CH2, confirmed by DEPT 135, piperidine ring), 43.81 (CH2, confirmed by DEPT 135, piperidine ring), 43.97 (CH2, confirmed by DEPT 135, piperidine ring), 59.48 (2C, absent in DEPT 135, piperidine ring), 67.68 (CHOC=O), 73.32 (CHOH), 126.12 (C=CH2, confirmed by DEPT 135), 126.84 (2CHar), 128.03 (CHar), 128.64 (2CHar), 141.63 (Car–CHOH, absent in DEPT 135), 142.50 (C=CH2, absent in DEPT 135), 165.98 (C=O, absent in DEPT 135) ppm.
4-(2-((4-Methylphenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5e): Yield (quinuclidine): 58 mg (42%); orange solid; mp 130–133 °C; EIMS m/z (% relative intensity): M+ 346 (22), 191 (13), 175 (15), 173 (12), 156 (11), 155 (10), 154 (22), 147 (9), 140 (18), 139 (16), 131 (25), 130 (9), 129 (15), 124 (100), 109 (67), 93 (11), 91 (19), 82 (13), 81 (14), 77 (9), 74 (8), 69 (13), 68 (7), 69 (13), 56 (8), 55 (13), 41 (14); HRMS–EI (m/z): calcd for C20H28NO4, 346.20183; found, 346.20093; ESIMS m/z (% relative intensity): [2M + Na] 715 (3), [M + Na]+ 369 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C20H28NO4Na, 369.1905; found, 369.1916; IR (KBr): 1709 (C=O), 1630 (C=C) cm−1.
4-(2-((4-Methoxyphenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5f): Yield (quinuclidine): 39 mg (27%); orange solid; mp 121–124 °C; EIMS m/z (% relative intensity): M+ 362 (23), 208 (13), 207 (17), 191 (23), 190 (14), 189 (19), 173 (12), 163 (9), 162 (8), 154 (42), 147 (10), 146 (14), 145 (20), 140 (31), 139 (19), 137 (23), 135 (36), 124 (100), 110 (7), 69 (23), 57 (6), 56 (11), 41 (21); HRMS–EI (m/z): calcd for C20H28NO5, 362.19675; found, 362.19501; EIMS m/z (% relative intensity): [M + Na]+ 385 (100), 119 (15); HRMS–ESI (m/z): [M + Na]+ calcd for C20H28NO5Na, 385.1865; found, 385.1841; IR (KBr): 1707 (C=O), 1611 (C=C) cm−1.
4-(2-((4-Fluorophenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5g): Yield (DABCO): 38 mg (27%); Yield (quinuclidine): 33 mg (23%); yellow powder; mp 105–109 °C; EIMS m/z (% relative intensity): M+ 350 (13), 195 (5), 180 (5), 179 (7), 177 (4), 154 (33), 140 (22), 139 (16), 135 (25), 134 (12), 133 (20), 124 (100), 109 (83), 97 (15), 83 (6), 82 (16), 69 (25), 67 (13), 56 (11), 55 (18), 41 (22); HRMS–EI (m/z): calcd for C19H25NO4F, 350.17676; found, 350.17570; ESIMS m/z (% relative intensity): [M + Na]+ 373 (20), 288 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C19H25FNO4Na, 373.1665; found: 373.1652; IR (KBr): 1712 (C=O), 1631 (C=C) cm−1.
4-(2-((4-Bromophenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5h): Yield (DABCO): 39 mg (24%); yield (quinuclidine): 92 mg (56%); orange powder; mp 127–130 °C; EIMS m/z (% relative intensity): 412 (12), M+ 410 (11), 255 (5), 239 (7), 185 (6), 160 (23), 154 (43), 140 (15), 139 (17), 124 (100), 116 (21), 109 (66), 69 (18), 55 (14), 41 (16); HRMS–EI (m/z): calcd for C19H25NO4Br, 410.09669; found, 410.09745; ESIMS m/z (% relative intensity): 435 (80), [M + Na]+ 433 (80), 414 (95), [M + 2H]+ 412 (100); HRMS–ESI (m/z): [M + 2H]+ calcd for C19H27NO4Br, 412.1123, found, 412.1109; HRMS–ESI (m/z): [M + Na]+ calcd for C19H25NO4BrNa, 433.0865; found, 433.0868; IR (KBr): 1708 (C=O), 1630 (C=C) cm−1.
4-(2-((4-Trifluoromethylphenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5i): Yield (DABCO): 122 mg (76%); yield (quinuclidine): 123 mg (77%); orange-yellow powder; mp 132–135 °C; EIMS m/z (% relative intensity): M+ 400 (13), 229 (6), 201 (10), 185 (15), 184 (8), 183 (12), 154 (39), 141 (7), 139 (16), 127 (16), 125 (15), 124 (100), 109 (82), 85 (10), 83 (6), 82 (14), 81 (16), 74 (9), 69 (22), 68 (10), 67 (12), 57 (7), 56 (11), 55 (17), 41 (22); HRMS–EI (m/z): calcd for C20H25NO4F3, 400.17357; found, 400.17225; ESIMS m/z (% relative intensity): [M + Na]+ 423 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C20H25F3NO4Na, 423.1633; found, 423.1629; IR (KBr): 1712 (C=O), 1630 (C=C) cm−1.
4-(2-((3,5-Bis(trifluoromethyl)phenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5j): Yield (DABCO): 111 mg (59%); yield (quinuclidine): 88 mg (47%); light-orange crystals; mp 112–115 °C; EIMS m/z (% relative intensity): M+ 468 (16), 454 (4), 434 (10), 253 (10), 252 (5), 251 (4), 249 (6), 243 (6), 241 (9), 233 (10), 229 (8), 213 (7), 195 (13), 154 (36), 140 (22), 139 (17), 124 (100), 109 (74), 85 (12), 82 (14), 81 (14), 69 (15), 68 (9), 67 (11), 57 (6), 56 (10), 55 (14), 41 (16); HRMS–EI (m/z): calcd for C21H24NO4F6, 468.16095; found, 468.16152; ESIMS m/z (% relative intensity): [M + Na]+ 491 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C21H24F6NO4Na, 491.1507; found: 491.1459; IR (KBr): 1714 (C=O), 1638 (C=C) cm−1.
4-(2-((4-Nitrophenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5k): Yield (DABCO): 33 mg (22%); yield (quinuclidine): 120 mg (79%); yellow powder; mp 102–104 °C; EIMS m/z (% relative intensity): M+ 377 (15), 363 (12), 189 (8), 160 (25), 154 (51), 140 (67), 139 (19), 124 (76), 109 (100), 85 (13), 82 (22), 81 (21), 69 (29), 68 (13), 67 (16), 57 (9), 56 (16), 55 (23), 41 (28); HRMS–EI (m/z): calcd for C19H25N2O6, 377.17126; found, 377.17090; ESIMS m/z (% relative intensity): [M + Na]+ 400 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C19H25N2O6Na, 400.1610; found, 400.1603; IR (KBr): 1713 (C=O), 1636 (C=C), 1521 (NO2), 1351 (NO2) cm−1.
4-(2-((2,4-Dinitrophenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5l): Yield (DABCO): 117 mg (69%); yield (quinuclidine): 95 mg (56%); red glass; mp 50–67 °C; EIMS m/z (% relative intensity): M+ 422 (8), 408 (5), 179 (8), 154 (34), 140 (34), 139 (15), 124 (100), 85 (10), 82 (15), 81 (15), 69 (21), 68 (9), 67 (13), 57 (10), 56 (13), 55 (21), 41 (22); HRMS–EI (m/z): calcd for C19H24N3O8, 422.15634; found, 422.15561; ESIMS m/z (% relative intensity): [M + 2H]+ 424 (100); HRMS–ESI (m/z): [M + 2H]+ calcd for C19H26N3O8, 424.1720; found, 424.1729; IR (KBr): 1717 (C=O), 1628, (C=C), 1537 (NO2), 1347 (NO2) cm−1.
4-(2-((3-Pyridyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5m): Yield (DABCO): 132 mg (99%); yield (quinuclidine): 87 mg (65%); orange-yellow powder; mp 92–97 °C; EIMS m/z (% relative intensity): 334 (12), M+ 333 (17), 303 (6), 301 (7), 247 (9), 180 (100), 163 (7), 162 (13), 154 (15), 144 (8), 140 (24), 135 (17), 124 (59), 118 (23), 117 (18), 109 (73), 82 (11), 81 (13), 80 (13), 79 (6), 78 (7), 69 (19), 68 (8), 67 (14), 56 (11), 55 (20), 53 (9), 41 (26); HRMS–EI (m/z): calcd for C18H25N2O4, 333.18143; found, 333.18050; ESIMS m/z (% relative intensity): [M + Na]+ 356 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C18H25N2O4Na, 356.1712; found, 356.1708; IR (film) 1714 (C=O), 1632 (C=C) cm−1.
4-(2-((2-Furyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5n): Yield (DABCO): 31 mg (24%); yield (quinuclidine): 62 mg (48%); dark orange-yellow powder; mp 114–118 °C; EIMS m/z (% relative intensity): M+ 322 (20), 305 (4), 154 (25), 151 (29), 140 (30), 124 (100), 109 (84), 97 (24), 83 (9), 82 (17), 81 (17), 69 (43), 67 (19), 56 (13), 55 (19), 41 (36); HRMS–EI (m/z): calcd for C17H24NO5, 322.16545; found, 322.16457; ESIMS m/z (% relative intensity): 346 (50), [M + Na]+ 345 (100); HRMS–ESI (m/z): [M + Na]+ calcd for C17H24NO5Na, 345.1552; found, 345.1554; IR (KBr): 1706 (C=O), 1633 (C=C) cm−1.
4-(2-((Ferrocenyl)hydroxymethyl)acryloyloxy)-2,2,6,6-tetramethylpiperidine-1-oxyl (5o): Yield (quinuclidine): 4 mg (2%); orange solid; mp 118–124 °C; EIMS m/z (% relative intensity): 441 (8), M+ 440 (16), 439 (1), 438 (2), 426 (6), 425 (14), 424 (2), 423 (7), 410 (2), 409 (4), 392 (3), 286 (64), 284 (9), 270 (14), 269 (6), 268 (7), 243 (8), 241 (5), 224 (25), 186 (7), 185 (9), 149 (18), 98 (35), 97 (22), 80 (78), 71 (34), 70 (15), 69 (31), 58 (39), 57 (64), 56 (18), 55 (52), 45 (13), 44 (67), 43 (36), 42 (22), 41 (48), 40 (100); HRMS–EI (m/z): calcd for C23H30NO4Fe; 440.15242; found, 440.15327; ESIMS m/z (% relative intensity): [M + Na]+ 463 (95), M+ 440 (100); HRMS–ESI (m/z): calcd for C23H30NO4Fe, 440.1524; found, 440.1495; IR (KBr): 1701 (C=O), 1633 (C=C) cm−1.
Supporting Information
Supporting Information features EIMS and IR spectra of the synthesized compounds 5a–o, 1H and 13C NMR of 5d with phenylhydrazine, and the chromatographic separation of 5l.
| Supporting Information File 1: Detailed spectrographic data. | ||
| Format: PDF | Size: 3.0 MB | Download |
References
-
Zakrzewski, J.; Krawczyk, M. Z. Naturforsch., B 2011, 66b, 493–498.
-
Kannan, V. Synlett 2004, 1120–1121. doi:10.1055/s-2004-822909
Return to citation in text: [1] -
Rosa, J. N.; Afonso, C. A. M.; Santos, A. G. Tetrahedron 2001, 57, 4189–4193. doi:10.1016/S0040-4020(01)00316-7
Return to citation in text: [1] -
Liu, H.-J.; Wynn, H. Can. J. Chem. 1986, 64, 649–657. doi:10.1139/v86-105
Return to citation in text: [1] [2] -
Baylis, A. B.; Hillman, M. E. D. Verfahren zur Herstellung von Acrylverbindungen. DE2155113, Nov 6, 1972.
Return to citation in text: [1] -
Hillman, M. E. D.; Baylis, A. B. Reaction of acrylic types compounds with aldehydes and certain ketones. US3743669, July 3, 1973.
Return to citation in text: [1] [2] -
Bobbitt, J. personal communication.
Return to citation in text: [1] -
Mi, X.; Luo, S.; Xu, H.; Zhang, L.; Cheng, J.-P. Tetrahedron 2006, 62, 2537–2544. doi:10.1016/j.tet.2005.12.045
Return to citation in text: [1] -
Krafft, M. E.; Song, E.-H.; Davoile, R. J. Tetrahedron Lett. 2005, 46, 6359–6362. doi:10.1016/j.tetlet.2005.07.066
Return to citation in text: [1] [2] -
Mi, X.; Luo, S.; Cheng, J.-P. J. Org. Chem. 2005, 70, 2338–2341. doi:10.1021/jo048391d
Return to citation in text: [1] -
Aggarwal, V. K.; Emme, I.; Fulford, S. Y. J. Org. Chem. 2003, 68, 692–700. doi:10.1021/jo026671s
Return to citation in text: [1] [2] [3] -
Aggarwal, V. K.; Dean, D. K.; Mereu, A.; Williams, R. J. Org. Chem. 2002, 67, 510–514. doi:10.1021/jo016073y
Return to citation in text: [1] -
Im, Y. J.; Gong, J. H.; Kim, H. J.; Kim, J. N. Bull. Korean Chem. Soc. 2001, 22, 1053–1055.
Return to citation in text: [1] [2] -
Aggarwal, V. K.; Mereu, A. Chem. Commun. 1999, 2311–2312. doi:10.1039/a907754e
Return to citation in text: [1] -
Dálaigh, C. Ó.; Connon, S. J. J. Org. Chem. 2007, 72, 7066–7069. doi:10.1021/jo071223b
Return to citation in text: [1] -
Lee, K.-Y.; Gong, J.-H.; Kim, J.-N. Bull. Korean Chem. Soc. 2002, 23, 659–660. doi:10.5012/bkcs.2002.23.5.659
Return to citation in text: [1] [2] -
de Souza, R. O. M. A.; Meireles, B. A.; Aguiar, L. C. S.; Vasconcellos, M. L. A. A. Synthesis 2004, 1595–1600. doi:10.1055/s-2004-822409
Return to citation in text: [1] -
Oh, K.; Li, J.-Y.; Ryu, J. Org. Biomol. Chem. 2010, 8, 3015–3024. doi:10.1039/c003667f
Return to citation in text: [1] [2] -
Morita, K.-i.; Suzuki, Z.; Hirose, H. Bull. Chem. Soc. Jpn. 1968, 41, 2815. doi:10.1246/bcsj.41.2815
Return to citation in text: [1] -
Ciganek, E. Org. React. 1997, 51, 201–350. doi:10.1002/0471264180.or051.02
Return to citation in text: [1] [2] [3] [4] -
Ma, G.-N.; Jiang, J.-J.; Shi, M.; Wei, Y. Chem. Commun. 2009, 5496–5514. doi:10.1039/b909405a
Return to citation in text: [1] -
Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447–5674. doi:10.1021/cr900291g
Return to citation in text: [1] -
Shi, M.; Wang, F.; Zhao, M.-X.; Wei, Y. The Chemistry of the Morita–Baylis–Hillman Reaction; RSC Publishing: London, Cambridge, 2011. doi:10.1039/9781849732659
Return to citation in text: [1] -
Basavaiah, D.; Veeraraghavaiah, G. Chem. Soc. Rev. 2012, 41, 68–78. doi:10.1039/c1cs15174f
Return to citation in text: [1] -
Dordonne, S.; Crousse, B.; Bonnet-Delpon, D.; Legros, J. Chem. Commun. 2011, 47, 5855–5857. doi:10.1039/c1cc10869g
Return to citation in text: [1] -
Han, X.; Wang, Y.; Zhong, F.; Lu, Y. Org. Biomol. Chem. 2011, 9, 6734–6740. doi:10.1039/c1ob05881a
Return to citation in text: [1] -
Abaee, M. S.; Mojtahedi, M. M.; Pasha, G. F.; Akbarzadeh, E.; Shockravi, A.; Mesbah, A. W.; Massa, W. Org. Lett. 2011, 13, 5282–5285. doi:10.1021/ol202145w
Return to citation in text: [1] -
Ji, S.; Bruchmann, B.; Klok, H.-A. Macromolecules 2011, 44, 5218–5226. doi:10.1021/ma2006238
Return to citation in text: [1] -
Pawar, B.; Padalkar, V.; Phatangare, K.; Nirmalkar, S.; Chaskar, A. Catal. Sci. Technol. 2011, 1, 1641–1644. doi:10.1039/c1cy00278c
Return to citation in text: [1] -
Zhong, F.; Chen, G.-Y.; Lu, Y. Org. Lett. 2011, 13, 82–85. doi:10.1021/ol102597s
Return to citation in text: [1] -
Song, H.-L.; Yuan, K.; Wu, X.-Y. Chem. Commun. 2011, 47, 1012–1014. doi:10.1039/c0cc03187a
Return to citation in text: [1] -
Jeong, Y.; Ryu, J.-S. J. Org. Chem. 2010, 75, 4183–4191. doi:10.1021/jo100618d
Return to citation in text: [1] [2] -
Anstiss, C.; Liu, F. Tetrahedron 2010, 66, 5486–5491. doi:10.1016/j.tet.2010.05.007
Return to citation in text: [1] -
Kohn, L. K.; Pavam, C. H.; Veronese, D.; Coelho, F.; De Carvalho, J. E.; Almeida, W. P. Eur. J. Med. Chem. 2006, 41, 738–744. doi:10.1016/j.ejmech.2006.03.006
Return to citation in text: [1] -
Cao, H.; Vieira, T. O.; Alper, H. Org. Lett. 2011, 13, 11–13. doi:10.1021/ol102699a
Return to citation in text: [1] -
Wu, C.; Liu, Y.; Zeng, H.; Liu, L.; Wang, D.; Chen, Y. Org. Biomol. Chem. 2011, 9, 253–256. doi:10.1039/c0ob00604a
Return to citation in text: [1] -
Sharma, V.; McLaughlin, M. L. J. Comb. Chem. 2010, 12, 327–331. doi:10.1021/cc100001e
Return to citation in text: [1] -
Clary, K. N.; Parvez, M.; Back, T. G. J. Org. Chem. 2010, 75, 3751–3760. doi:10.1021/jo1005087
Return to citation in text: [1] -
Ishikawa, S.; Noguchi, F.; Kamimura, A. J. Org. Chem. 2010, 75, 3578–3586. doi:10.1021/jo100315j
Return to citation in text: [1] -
Shahrisa, A.; Ghasemi, Z. Chem. Heterocycl. Compd. 2010, 46, 30–36. doi:10.1007/s10593-010-0466-5
Translated from Khim. Geterotsikl. Soedin. 2010, 46, 37–43.
Return to citation in text: [1] -
Jiang, K.; Peng, J.; Cui, H.-L.; Chen, Y.-C. Chem. Commun. 2009, 3955–3957. doi:10.1039/b905177e
Return to citation in text: [1] -
Nikpassand, M.; Mamaghani, M.; Tabatabaeian, K.; Kupaei Abiazi, M. Mol. Diversity 2009, 13, 389–393. doi:10.1007/s11030-009-9123-2
Return to citation in text: [1] -
Hoffmann, H. M. R.; Rabe, J. Angew. Chem., Int. Ed. Engl. 1983, 22, 795–796. doi:10.1002/anie.198307951
Return to citation in text: [1] [2] -
Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W. W.-L. Chem. Rev. 2002, 102, 2227–2302. doi:10.1021/cr010289i
Return to citation in text: [1] [2] -
Fort, Y.; Berthe, M. C.; Caubere, P. Tetrahedron 1992, 48, 6371–6384. doi:10.1016/S0040-4020(01)88227-2
Return to citation in text: [1] [2] -
Rozantsev, E. G.; Suskina, V. J. Izv. Akad. Nauk SSSR, Ser. Khim. 1968, 2106.
Return to citation in text: [1] [2] -
Kurosaki, T.; Lee, K. W.; Okawara, M. J. Polym. Sci., Part A-1 1972, 10, 3295–3310. doi:10.1002/pol.1972.170101116
Return to citation in text: [1] -
Grodner, J.; Sałaciński, T. Synthesis 2012, in press. doi:10.1055/s-0032-1316736
Return to citation in text: [1] -
Cohn, P.; Friedländer, P. Ber. Dtsch. Chem. Ges. 1902, 35, 1265–1267. doi:10.1002/cber.19020350210
Return to citation in text: [1] -
Crampton, M. R. Nucleophilic Aromatic Substitution. In Organic Reaction Mechanisms 1995; Knipe, A. C.; Watts, W. E., Eds.; John Wiley and Sons: Chichester, UK, 1995; pp 231–245. doi:10.1002/9780470066935.ch7
Return to citation in text: [1] -
Macháček, V.; Manová, J.; Sedlák, M.; Štěrba, V. Collect. Czech. Chem. Commun. 1994, 59, 2262–2268. doi:10.1135/cccc19942262
Return to citation in text: [1] -
Forbes, E. J.; Gregory, M. J. J. Chem. Soc. B 1968, 207–209. doi:10.1039/j29680000207
Return to citation in text: [1] -
Madhavan, S.; Shanmugam, P. Org. Lett. 2011, 13, 1590–1593. doi:10.1021/ol200215d
Return to citation in text: [1] -
Lee, T. D.; Keana, J. F. W. J. Org. Chem. 1975, 40, 3145–3147. doi:10.1021/jo00909a033
Return to citation in text: [1] [2] [3] -
Brière, R.; Lemaire, H.; Rassat, A. Bull. Soc. Chim. Fr. 1965, 3273–3283.
Return to citation in text: [1] -
Sosnovsky, G.; Konieczny, M. Z. Naturforsch., B 1976, 31b, 1376–1378.
Return to citation in text: [1] -
Zakrzewski, J. Monatsh. Chem. 1990, 121, 803–808. doi:10.1007/BF00808373
Return to citation in text: [1] -
Litvin, E. F.; Kozlova, L. M.; Shapiro, A. B.; Rozantsev, E. G.; Freidlin, L. K. Izv. Akad. Nauk SSSR, Ser. Khim. 1975, 1353.
Return to citation in text: [1] -
Yan, L.; Fang, Y.; Fang, M.; Zhu, L.; Shen, D.; Liang, X. Org. Mass Spectrom. 1989, 24, 303–308. doi:10.1002/oms.1210240503
Return to citation in text: [1]
| 32. | Jeong, Y.; Ryu, J.-S. J. Org. Chem. 2010, 75, 4183–4191. doi:10.1021/jo100618d |
| 55. | Brière, R.; Lemaire, H.; Rassat, A. Bull. Soc. Chim. Fr. 1965, 3273–3283. |
| 56. | Sosnovsky, G.; Konieczny, M. Z. Naturforsch., B 1976, 31b, 1376–1378. |
| 57. | Zakrzewski, J. Monatsh. Chem. 1990, 121, 803–808. doi:10.1007/BF00808373 |
| 46. | Rozantsev, E. G.; Suskina, V. J. Izv. Akad. Nauk SSSR, Ser. Khim. 1968, 2106. |
| 2. | Kannan, V. Synlett 2004, 1120–1121. doi:10.1055/s-2004-822909 |
| 3. | Rosa, J. N.; Afonso, C. A. M.; Santos, A. G. Tetrahedron 2001, 57, 4189–4193. doi:10.1016/S0040-4020(01)00316-7 |
| 4. | Liu, H.-J.; Wynn, H. Can. J. Chem. 1986, 64, 649–657. doi:10.1139/v86-105 |
| 5. | Baylis, A. B.; Hillman, M. E. D. Verfahren zur Herstellung von Acrylverbindungen. DE2155113, Nov 6, 1972. |
| 6. | Hillman, M. E. D.; Baylis, A. B. Reaction of acrylic types compounds with aldehydes and certain ketones. US3743669, July 3, 1973. |
| 4. | Liu, H.-J.; Wynn, H. Can. J. Chem. 1986, 64, 649–657. doi:10.1139/v86-105 |
| 15. | Dálaigh, C. Ó.; Connon, S. J. J. Org. Chem. 2007, 72, 7066–7069. doi:10.1021/jo071223b |
| 16. | Lee, K.-Y.; Gong, J.-H.; Kim, J.-N. Bull. Korean Chem. Soc. 2002, 23, 659–660. doi:10.5012/bkcs.2002.23.5.659 |
| 44. | Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W. W.-L. Chem. Rev. 2002, 102, 2227–2302. doi:10.1021/cr010289i |
| 13. | Im, Y. J.; Gong, J. H.; Kim, H. J.; Kim, J. N. Bull. Korean Chem. Soc. 2001, 22, 1053–1055. |
| 20. | Ciganek, E. Org. React. 1997, 51, 201–350. doi:10.1002/0471264180.or051.02 |
| 45. | Fort, Y.; Berthe, M. C.; Caubere, P. Tetrahedron 1992, 48, 6371–6384. doi:10.1016/S0040-4020(01)88227-2 |
| 13. | Im, Y. J.; Gong, J. H.; Kim, H. J.; Kim, J. N. Bull. Korean Chem. Soc. 2001, 22, 1053–1055. |
| 14. | Aggarwal, V. K.; Mereu, A. Chem. Commun. 1999, 2311–2312. doi:10.1039/a907754e |
| 35. | Cao, H.; Vieira, T. O.; Alper, H. Org. Lett. 2011, 13, 11–13. doi:10.1021/ol102699a |
| 36. | Wu, C.; Liu, Y.; Zeng, H.; Liu, L.; Wang, D.; Chen, Y. Org. Biomol. Chem. 2011, 9, 253–256. doi:10.1039/c0ob00604a |
| 37. | Sharma, V.; McLaughlin, M. L. J. Comb. Chem. 2010, 12, 327–331. doi:10.1021/cc100001e |
| 38. | Clary, K. N.; Parvez, M.; Back, T. G. J. Org. Chem. 2010, 75, 3751–3760. doi:10.1021/jo1005087 |
| 39. | Ishikawa, S.; Noguchi, F.; Kamimura, A. J. Org. Chem. 2010, 75, 3578–3586. doi:10.1021/jo100315j |
| 40. |
Shahrisa, A.; Ghasemi, Z. Chem. Heterocycl. Compd. 2010, 46, 30–36. doi:10.1007/s10593-010-0466-5
Translated from Khim. Geterotsikl. Soedin. 2010, 46, 37–43. |
| 41. | Jiang, K.; Peng, J.; Cui, H.-L.; Chen, Y.-C. Chem. Commun. 2009, 3955–3957. doi:10.1039/b905177e |
| 42. | Nikpassand, M.; Mamaghani, M.; Tabatabaeian, K.; Kupaei Abiazi, M. Mol. Diversity 2009, 13, 389–393. doi:10.1007/s11030-009-9123-2 |
| 43. | Hoffmann, H. M. R.; Rabe, J. Angew. Chem., Int. Ed. Engl. 1983, 22, 795–796. doi:10.1002/anie.198307951 |
| 7. | Bobbitt, J. personal communication. |
| 8. | Mi, X.; Luo, S.; Xu, H.; Zhang, L.; Cheng, J.-P. Tetrahedron 2006, 62, 2537–2544. doi:10.1016/j.tet.2005.12.045 |
| 9. | Krafft, M. E.; Song, E.-H.; Davoile, R. J. Tetrahedron Lett. 2005, 46, 6359–6362. doi:10.1016/j.tetlet.2005.07.066 |
| 10. | Mi, X.; Luo, S.; Cheng, J.-P. J. Org. Chem. 2005, 70, 2338–2341. doi:10.1021/jo048391d |
| 11. | Aggarwal, V. K.; Emme, I.; Fulford, S. Y. J. Org. Chem. 2003, 68, 692–700. doi:10.1021/jo026671s |
| 12. | Aggarwal, V. K.; Dean, D. K.; Mereu, A.; Williams, R. J. Org. Chem. 2002, 67, 510–514. doi:10.1021/jo016073y |
| 44. | Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W. W.-L. Chem. Rev. 2002, 102, 2227–2302. doi:10.1021/cr010289i |
| 6. | Hillman, M. E. D.; Baylis, A. B. Reaction of acrylic types compounds with aldehydes and certain ketones. US3743669, July 3, 1973. |
| 16. | Lee, K.-Y.; Gong, J.-H.; Kim, J.-N. Bull. Korean Chem. Soc. 2002, 23, 659–660. doi:10.5012/bkcs.2002.23.5.659 |
| 18. | Oh, K.; Li, J.-Y.; Ryu, J. Org. Biomol. Chem. 2010, 8, 3015–3024. doi:10.1039/c003667f |
| 25. | Dordonne, S.; Crousse, B.; Bonnet-Delpon, D.; Legros, J. Chem. Commun. 2011, 47, 5855–5857. doi:10.1039/c1cc10869g |
| 26. | Han, X.; Wang, Y.; Zhong, F.; Lu, Y. Org. Biomol. Chem. 2011, 9, 6734–6740. doi:10.1039/c1ob05881a |
| 27. | Abaee, M. S.; Mojtahedi, M. M.; Pasha, G. F.; Akbarzadeh, E.; Shockravi, A.; Mesbah, A. W.; Massa, W. Org. Lett. 2011, 13, 5282–5285. doi:10.1021/ol202145w |
| 28. | Ji, S.; Bruchmann, B.; Klok, H.-A. Macromolecules 2011, 44, 5218–5226. doi:10.1021/ma2006238 |
| 29. | Pawar, B.; Padalkar, V.; Phatangare, K.; Nirmalkar, S.; Chaskar, A. Catal. Sci. Technol. 2011, 1, 1641–1644. doi:10.1039/c1cy00278c |
| 30. | Zhong, F.; Chen, G.-Y.; Lu, Y. Org. Lett. 2011, 13, 82–85. doi:10.1021/ol102597s |
| 31. | Song, H.-L.; Yuan, K.; Wu, X.-Y. Chem. Commun. 2011, 47, 1012–1014. doi:10.1039/c0cc03187a |
| 32. | Jeong, Y.; Ryu, J.-S. J. Org. Chem. 2010, 75, 4183–4191. doi:10.1021/jo100618d |
| 33. | Anstiss, C.; Liu, F. Tetrahedron 2010, 66, 5486–5491. doi:10.1016/j.tet.2010.05.007 |
| 54. | Lee, T. D.; Keana, J. F. W. J. Org. Chem. 1975, 40, 3145–3147. doi:10.1021/jo00909a033 |
| 19. | Morita, K.-i.; Suzuki, Z.; Hirose, H. Bull. Chem. Soc. Jpn. 1968, 41, 2815. doi:10.1246/bcsj.41.2815 |
| 34. | Kohn, L. K.; Pavam, C. H.; Veronese, D.; Coelho, F.; De Carvalho, J. E.; Almeida, W. P. Eur. J. Med. Chem. 2006, 41, 738–744. doi:10.1016/j.ejmech.2006.03.006 |
| 54. | Lee, T. D.; Keana, J. F. W. J. Org. Chem. 1975, 40, 3145–3147. doi:10.1021/jo00909a033 |
| 18. | Oh, K.; Li, J.-Y.; Ryu, J. Org. Biomol. Chem. 2010, 8, 3015–3024. doi:10.1039/c003667f |
| 58. | Litvin, E. F.; Kozlova, L. M.; Shapiro, A. B.; Rozantsev, E. G.; Freidlin, L. K. Izv. Akad. Nauk SSSR, Ser. Khim. 1975, 1353. |
| 17. | de Souza, R. O. M. A.; Meireles, B. A.; Aguiar, L. C. S.; Vasconcellos, M. L. A. A. Synthesis 2004, 1595–1600. doi:10.1055/s-2004-822409 |
| 21. | Ma, G.-N.; Jiang, J.-J.; Shi, M.; Wei, Y. Chem. Commun. 2009, 5496–5514. doi:10.1039/b909405a |
| 22. | Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447–5674. doi:10.1021/cr900291g |
| 23. | Shi, M.; Wang, F.; Zhao, M.-X.; Wei, Y. The Chemistry of the Morita–Baylis–Hillman Reaction; RSC Publishing: London, Cambridge, 2011. doi:10.1039/9781849732659 |
| 24. | Basavaiah, D.; Veeraraghavaiah, G. Chem. Soc. Rev. 2012, 41, 68–78. doi:10.1039/c1cs15174f |
| 59. | Yan, L.; Fang, Y.; Fang, M.; Zhu, L.; Shen, D.; Liang, X. Org. Mass Spectrom. 1989, 24, 303–308. doi:10.1002/oms.1210240503 |
| 46. | Rozantsev, E. G.; Suskina, V. J. Izv. Akad. Nauk SSSR, Ser. Khim. 1968, 2106. |
| 47. | Kurosaki, T.; Lee, K. W.; Okawara, M. J. Polym. Sci., Part A-1 1972, 10, 3295–3310. doi:10.1002/pol.1972.170101116 |
| 43. | Hoffmann, H. M. R.; Rabe, J. Angew. Chem., Int. Ed. Engl. 1983, 22, 795–796. doi:10.1002/anie.198307951 |
| 20. | Ciganek, E. Org. React. 1997, 51, 201–350. doi:10.1002/0471264180.or051.02 |
| 45. | Fort, Y.; Berthe, M. C.; Caubere, P. Tetrahedron 1992, 48, 6371–6384. doi:10.1016/S0040-4020(01)88227-2 |
| 54. | Lee, T. D.; Keana, J. F. W. J. Org. Chem. 1975, 40, 3145–3147. doi:10.1021/jo00909a033 |
| 50. | Crampton, M. R. Nucleophilic Aromatic Substitution. In Organic Reaction Mechanisms 1995; Knipe, A. C.; Watts, W. E., Eds.; John Wiley and Sons: Chichester, UK, 1995; pp 231–245. doi:10.1002/9780470066935.ch7 |
| 51. | Macháček, V.; Manová, J.; Sedlák, M.; Štěrba, V. Collect. Czech. Chem. Commun. 1994, 59, 2262–2268. doi:10.1135/cccc19942262 |
| 52. | Forbes, E. J.; Gregory, M. J. J. Chem. Soc. B 1968, 207–209. doi:10.1039/j29680000207 |
| 53. | Madhavan, S.; Shanmugam, P. Org. Lett. 2011, 13, 1590–1593. doi:10.1021/ol200215d |
| 48. | Grodner, J.; Sałaciński, T. Synthesis 2012, in press. doi:10.1055/s-0032-1316736 |
| 49. | Cohn, P.; Friedländer, P. Ber. Dtsch. Chem. Ges. 1902, 35, 1265–1267. doi:10.1002/cber.19020350210 |
| 9. | Krafft, M. E.; Song, E.-H.; Davoile, R. J. Tetrahedron Lett. 2005, 46, 6359–6362. doi:10.1016/j.tetlet.2005.07.066 |
| 11. | Aggarwal, V. K.; Emme, I.; Fulford, S. Y. J. Org. Chem. 2003, 68, 692–700. doi:10.1021/jo026671s |
| 11. | Aggarwal, V. K.; Emme, I.; Fulford, S. Y. J. Org. Chem. 2003, 68, 692–700. doi:10.1021/jo026671s |
© 2012 Zakrzewski; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)