Abstract
A strategy to replace the ethylamine side chain of 2,5-dimethoxy-4-iodoamphetamine (DOI, 1a), and 2,5-dimethoxy-4-bromoamphetamine (DOB, 1b) with a cyclopropylamine moiety was successful in leading to compounds with high affinity at the 5-HT2 family of receptors; and the more potent stereoisomer of the cyclopropane analogues had the expected (−)-(1R,2S)-configuration. Screening for affinity at various serotonin receptor subtypes, however, revealed that the cyclopropane congeners also had increased affinity at several sites in addition to the 5-HT2A and 5-HT2B receptors. Therefore, at appropriate doses – although (−)-4 and (−)-5 may be useful as tools to probe 5-HT2 receptor function – one would need to be mindful that their selectivity for 5-HT2A receptors is somewhat less than for DOI itself.
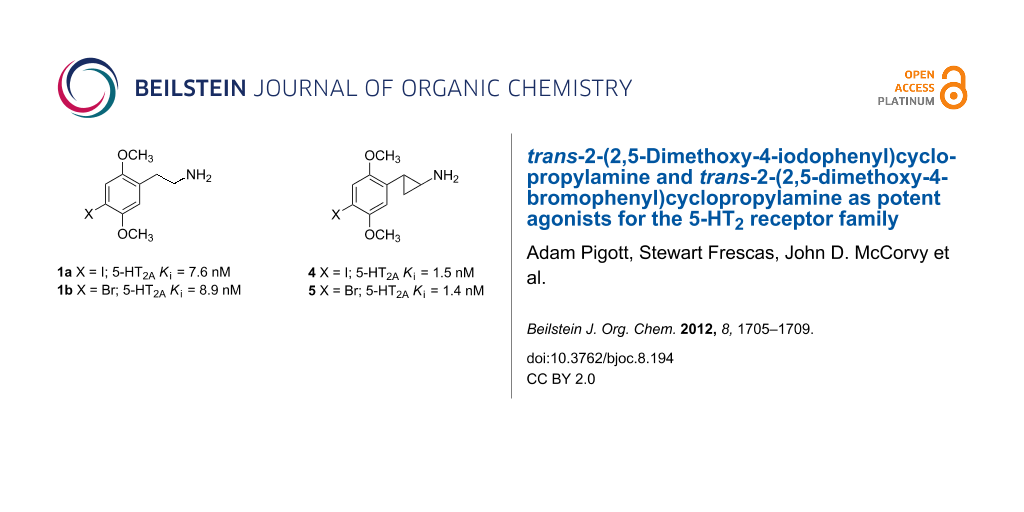
Graphical Abstract
Introduction
Among the molecules that have proven very valuable to neuroscientists studying brain serotonin systems is the substituted phenethylamine derivative 2,5-dimethoxy-4-iodoamphetamine (DOI, 1a, Figure 1), a potent but nonspecific agonist ligand for serotonin 5-HT2A and 5-HT2C receptors. It is relatively inexpensive and has been widely used throughout the neuroscience community to study behaviors mediated by 5-HT2 family receptors. Indeed, as of June 12, 2012, a PubMed search of the terms DOI + 5-HT2 yielded 577 hits, spanning from 1984 to the present. Despite the fact that no significant abuse of DOI has been reported, this substance has been scheduled in a number of countries and has been considered for scheduling by the U.S. Drug Enforcement Administration (DEA). Classification as a controlled substance will be a setback to the neuroscience community because it effectively prevents experiments in any laboratory that does not have a proper license for the use of DOI. To illustrate this point, a related compound – bromo congener 1b (DOB) – presently is a controlled substance and only 52 hits for DOB + 5-HT2 were obtained from PubMed, compared with 577 for DOI over the same period of time.
Figure 1: Structures of well-known serotonin 5-HT2A agonists 1a,b, 2, and 3, and compounds 4 and 5 reported in this paper.
Figure 1: Structures of well-known serotonin 5-HT2A agonists 1a,b, 2, and 3, and compounds 4 and 5 reported i...
Anticipating the potential need for a substance to replace DOI as a research tool, we sought to identify a molecule that might have pharmacological properties which are identical, or at least very similar to those of 1a. We had previously characterized the cyclopropane analogue of a hallucinogenic amphetamine known as DOM (2) and had shown that 3 (DMCPA) had high potency both in vitro and in vivo [1-3]. We thus considered whether the cyclopropane analogues 4 and 5 might be useful research tools. Accordingly, this report details the synthesis of racemic trans-1-(2,5-dimethoxy-4-iodophenyl)-2-aminocyclopropane (4) and its bromo homolog 5, the resolution of 4 into its (−)-(1R,2S)-enantiomer, as well as the resolution of the cyclopropane carboxylic acid precursor and subsequent bromination to provide both enantiomers of 5.
Results and Discussion
Racemic 4 and 5 were compared in radioligand competition assays against radiolabeled antagonists defined at the human 5-HT2A and 5-HT2C receptors and compared with racemic 1a and 1b. The results are shown in Table 1. As can be seen, the cyclopropane analogues 4 and 5 showed affinities for the 5-HT2A receptor 5–6-fold greater than 1a and 1b. Affinities at the 5-HT2C receptor were about two-fold higher than for 1a and 1b.
Table 1: Affinity values (Ki in nM) at human 5-HT2A and 5-HT2C receptors. All values represent mean and SEM from at least three independent experiments.
| 3H-ketanserin | 3H-mesulergine | |
|---|---|---|
| Compound |
5-HT2A
Ki in (nM) |
5-HT2C
Ki in (nM) |
| (±)-1a | 7.6 ± 0.9 | 35 ± 6 |
| (±)-1b | 8.9 ± 0.5 | 31 ± 5 |
| (±)-4 | 1.5 ± 0.1 | 17 ± 3 |
| (±)-5 | 1.4 ± 0.3 | 7.5 ± 1.1 |
The more potent (−)-enantiomers were then tested for functional potency using a calcium release assay. The EC50 values and maximal effect at the 5-HT2A receptor were virtually identical for 1a and 4, and for 1b and 5 (Table 2).
Table 2: Potency and percent max values for calcium release at 5-HT2A and 5-HT2C receptors. All values represent mean and SEM from at least three independent experiments.
| 5-HT2A | 5-HT2C | |||
|---|---|---|---|---|
| Compound |
EC50
(nM) |
%max |
EC50
(nM) |
%max |
| (−)-1a | 3.3 ± 0.7 | 87 ± 1 | 8.7 ± 0.2 | 50 ± 5 |
| (−)-1b | 5.8 ± 1.3 | 75 ± 7 | 28 ± 4 | 59 ± 7 |
| (−)-4 | 2.0 ± 0.3 | 89 ± 4 | 21 ± 4 | 63 ± 6 |
| (−)-5 | 6.3 ± 1.6 | 76 ± 10 | 32 ± 8 | 77 ± 6 |
At the 5-HT2C receptor 1a was the most potent, with an EC50 that was about three times lower than for 1b, 4, or 5. In functional assays, therefore, the cyclopropane analogues 4 and 5 compared to 1a or 1b appeared as potent and had a similar degree of maximal stimulation at each of the respective 5-HT2 receptors.
We then carried out a broader screen of 4 and 5 for affinities at a range of other 5-HT receptor isoforms (Table 3). Their affinities at other 5-HT receptors, however, were higher than for 1a. In particular, the introduction of the cyclopropane appears to increase significantly affinities at the 5-HT1A, 5HT1B, and 5-HT1D receptors. In that regard, although (−)-4 and (−)-5 have affinities at the 5-HT2A receptor somewhat higher than 1a, their selectivity over the 5-HT1A receptor is less than 100-fold. As shown in Table 1, both 4 and 5 are extremely potent ligands in vitro. Furthermore, as anticipated, it was the (−)-enantiomers that proved to have highest affinity. We included (+)-5 in Table 3 simply to illustrate the difference in affinity between the two enantiomers. We assume that the final compounds have the (−)-(1R,2S) and (+)-(1S,2R) absolute configurations based on our earlier work establishing the absolute configuration of 3 [2], and the fact that substitutions at the 4-position of the aromatic ring in chiral substituted amphetamines do not change the sign of optical rotation [4]. The biological data are consistent with those configuration assignments.
Table 3: Affinity values (Ki in nM) at selected serotonin receptor isoforms.
| Cmpd | 5-HT1A | 5-HT1B | 5-HT1D | 5-HT1E | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT6 | 5-HT7 |
|---|---|---|---|---|---|---|---|---|---|
| (±)-1a | <50%a | <50%a | <50%a | 1090 | 9 | 3 | 19 | 1380 | 850 |
| (±)-4 | 410 | 290 | 535 | 1660 | 9 | 10 | 17 | 100 | 580 |
| (−)-4 | 150 | 230 | 90 | 1380 | 2.4 | 6 | 7.4 | 70 | 260 |
| (+)-5 | 210 | <50%a | <50%a | <50%a | 540 | 20 | 130 | NA | 170 |
| (−)-5 | 220 | 375 | 390 | 890 | 3 | 4 | 9 | 45 | 120 |
a<50% displacement at 10−6 M.
Chemistry
We reasoned that a palladium-mediated cyclopropanation of the corresponding cinnamic acids would provide the required cyclopropanecarboxylic acid (Scheme 1); which could be readily converted to the amine by a Curtius type rearrangement (Scheme 2). In our previous synthesis [2] we had employed an N-carbobenzoxy intermediate, followed by catalytic debenzylation over Pd(C); but those conditions would lead to dehalogenation in the present series, so we instead employed acid-catalyzed removal of a BOC protecting group (Scheme 2).
Scheme 1: Synthesis of arylcyclopropane carboxylic acids from the corresponding cinnamic acids, followed by halogenation.
Scheme 1: Synthesis of arylcyclopropane carboxylic acids from the corresponding cinnamic acids, followed by h...
Scheme 2: Conversion of arylcyclopropane carboxylic acids 10a,b to the amines 4 and 5, and chemical resolution of 4 into its enantiomers.
Scheme 2: Conversion of arylcyclopropane carboxylic acids 10a,b to the amines 4 and 5, and chemical resolutio...
Thus, we first prepared 2-(2,5-dimethoxyphenyl)cyclopropanecarboxylic acid methyl ester (7) from the corresponding cinnamic ester 6 [5], followed by I2/AgNO3 iodination (Scheme 1), and base hydrolysis of the resulting ester to provide iodo acid 10a. Hydrolysis of ester 7 followed by bromination of acid 9 using Br2-dioxane complex gave a good yield of bromo acid 10b.
These acids were readily converted to their isocyanates using the Weinstock modification of the Curtius rearrangement [6]. Those isocyanates were heated with tert-butanol to afford the corresponding carbamates 11a and 11b (Scheme 2). A brief treatment of these with 3 M HCl at 45 °C cleanly affected N-deprotection and afforded the desired final amines 4 and 5.
The stereochemistry of the more potent enantiomer of DOI is (−)-(R) [7], and of 3 is (−)-(1R,2S) [2]. We therefore undertook the resolution of the enantiomers of DOI by fractional crystallization of the diastereomeric salts prepared with di-O,O-benzoyltartaric acid. Unfortunately, we discovered that heating solutions of the dibenzoyltartrate salt of 4 in EtOH or iPrOH led to nearly complete decomposition, presumably through a cyclopropane ring-opening pathway. The apparent need to recrystallize the O,O-dibenzoyltartrate salts from a nonprotic solvent led us to employ warm acetone, which proved satisfactory. The resolution went well, achieving constant optical rotation after only three crystallizations. The salt was converted to the free base, which was dissolved in dry Et2O, followed by addition of the stoichiometric amount of ethereal HCl. The salt precipitated out of solution and could be used directly for pharmacological experiments. Attempts to recrystallize the HCl salt from protic solvents also led to nearly complete decomposition, although salts of bromo compound 5 appeared somewhat more stable.
We then followed a more efficient divergent approach to obtain the enantiomers of 5 that employed resolution of the cyclopropane carboxylic acid, followed by bromination, and then conversion to the cyclopropylamine (Scheme 3). We are aware that the use of chiral auxiliaries in the cyclopropanation step could directly afford the chiral cyclopropane acids [8], but time and resources did not allow us to pursue that approach.
Scheme 3: Chemical resolution of arylcyclopropane carboxylic acid 9 followed by bromination.
Scheme 3: Chemical resolution of arylcyclopropane carboxylic acid 9 followed by bromination.
Conclusion
In conclusion, our strategy to replace the ethylamine side chain of 1a (or 1b) with a cyclopropylamine moiety was successful in leading to compounds with high affinity at the 5-HT2 family of receptors; and the more potent stereoisomer of the cyclopropane analogues had the expected (−)-1R,2S-configuration. However, at appropriate doses, although (−)-4 and (−)-5 may be useful as tools to probe 5-HT2 receptor function, one would also need to be mindful that their selectivity for 5-HT2A over 5-HT1A is only about 70-fold.
The most efficient approach appears to be the synthesis of the chiral cyclopropane carboxylic acids, followed by derivatization at the 4-position. This approach would be most appealing if a chiral auxiliary was used in the cyclopropanation step [8]. We also note that compound 4 was less stable than 5 under recrystallization conditions, an instability we did not observe during our earlier work with 3. We have observed even greater instability in 2-(indol-3-yl)cyclopropylamines [8,9], suggesting that electron “excessive” π-systems, or the ability to “donate” electrons through resonance (i.e. Br and I), leads to cyclopropane ring instability in 2-arylcyclopropylamines.
Supporting Information
| Supporting Information File 1: Experimental details for all new compounds as well as the pharmacological methods used to measure receptor affinity and functional activity. | ||
| Format: PDF | Size: 206.7 KB | Download |
References
-
Nichols, D. E.; Pfister, W. R.; Yim, G. K. W. Life Sci. 1978, 22, 2165–2170. doi:10.1016/0024-3205(78)90567-2
Return to citation in text: [1] -
Nichols, D. E.; Woodard, R.; Hathaway, B. A.; Lowy, M. T.; Yim, G. K. W. J. Med. Chem. 1979, 22, 458–460. doi:10.1021/jm00190a021
Return to citation in text: [1] [2] [3] [4] -
Johnson, M. P.; Mathis, C. A.; Shulgin, A. T.; Hoffman, A. J.; Nichols, D. E. Pharmacol. Biochem. Behav. 1990, 35, 211–217. doi:10.1016/0091-3057(90)90228-A
Return to citation in text: [1] -
Nichols, D. E.; Barfknecht, C. F.; Rusterholz, D. B.; Benington, F.; Morin, R. D. J. Med. Chem. 1973, 16, 480–483. doi:10.1021/jm00263a013
Return to citation in text: [1] -
Peterson, J. R.; Russell, M. E.; Surjasasmita, I. B. J. Chem. Eng. Data 1988, 33, 534–537. doi:10.1021/je00054a042
Return to citation in text: [1] -
Weinstock, J. J. Org. Chem. 1961, 26, 3511. doi:10.1021/jo01067a604
Return to citation in text: [1] -
Johnson, M. P.; Hoffman, A. J.; Nichols, D. E.; Mathis, C. A. Neuropharmacology 1987, 26, 1803–1806. doi:10.1016/0028-3908(87)90138-9
Return to citation in text: [1] -
Vangveravong, S.; Nichols, D. E. J. Org. Chem. 1995, 60, 3409–3413. doi:10.1021/jo00116a028
Return to citation in text: [1] [2] [3] -
Vangveravong, S.; Kanthasamy, A.; Lucaites, V. L.; Nelson, D. L.; Nichols, D. E. J. Med. Chem. 1998, 41, 4995–5001. doi:10.1021/jm980318q
Return to citation in text: [1]
| 1. | Nichols, D. E.; Pfister, W. R.; Yim, G. K. W. Life Sci. 1978, 22, 2165–2170. doi:10.1016/0024-3205(78)90567-2 |
| 2. | Nichols, D. E.; Woodard, R.; Hathaway, B. A.; Lowy, M. T.; Yim, G. K. W. J. Med. Chem. 1979, 22, 458–460. doi:10.1021/jm00190a021 |
| 3. | Johnson, M. P.; Mathis, C. A.; Shulgin, A. T.; Hoffman, A. J.; Nichols, D. E. Pharmacol. Biochem. Behav. 1990, 35, 211–217. doi:10.1016/0091-3057(90)90228-A |
| 5. | Peterson, J. R.; Russell, M. E.; Surjasasmita, I. B. J. Chem. Eng. Data 1988, 33, 534–537. doi:10.1021/je00054a042 |
| 2. | Nichols, D. E.; Woodard, R.; Hathaway, B. A.; Lowy, M. T.; Yim, G. K. W. J. Med. Chem. 1979, 22, 458–460. doi:10.1021/jm00190a021 |
| 4. | Nichols, D. E.; Barfknecht, C. F.; Rusterholz, D. B.; Benington, F.; Morin, R. D. J. Med. Chem. 1973, 16, 480–483. doi:10.1021/jm00263a013 |
| 2. | Nichols, D. E.; Woodard, R.; Hathaway, B. A.; Lowy, M. T.; Yim, G. K. W. J. Med. Chem. 1979, 22, 458–460. doi:10.1021/jm00190a021 |
| 8. | Vangveravong, S.; Nichols, D. E. J. Org. Chem. 1995, 60, 3409–3413. doi:10.1021/jo00116a028 |
| 8. | Vangveravong, S.; Nichols, D. E. J. Org. Chem. 1995, 60, 3409–3413. doi:10.1021/jo00116a028 |
| 9. | Vangveravong, S.; Kanthasamy, A.; Lucaites, V. L.; Nelson, D. L.; Nichols, D. E. J. Med. Chem. 1998, 41, 4995–5001. doi:10.1021/jm980318q |
| 2. | Nichols, D. E.; Woodard, R.; Hathaway, B. A.; Lowy, M. T.; Yim, G. K. W. J. Med. Chem. 1979, 22, 458–460. doi:10.1021/jm00190a021 |
| 7. | Johnson, M. P.; Hoffman, A. J.; Nichols, D. E.; Mathis, C. A. Neuropharmacology 1987, 26, 1803–1806. doi:10.1016/0028-3908(87)90138-9 |
| 8. | Vangveravong, S.; Nichols, D. E. J. Org. Chem. 1995, 60, 3409–3413. doi:10.1021/jo00116a028 |
© 2012 Pigott et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)