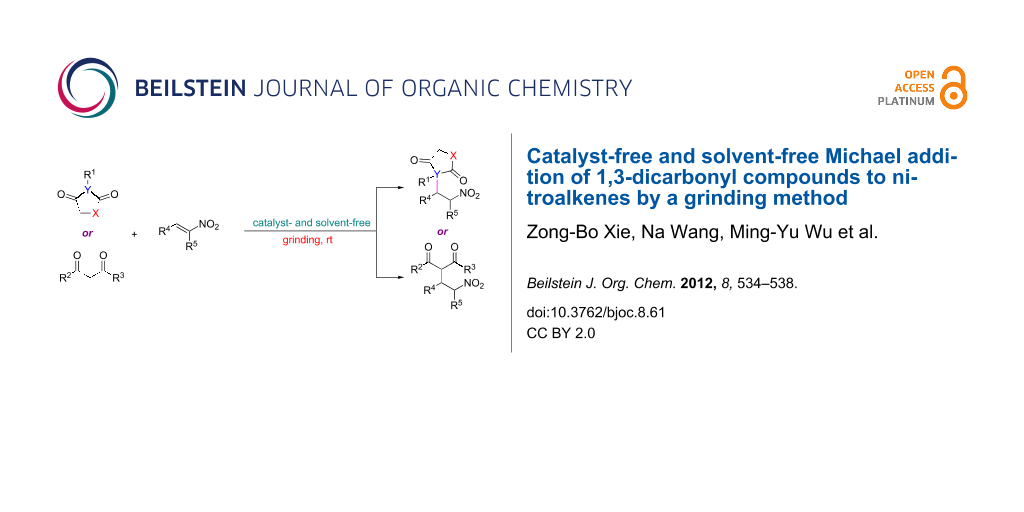
Abstract
An environmentally benign, fast and convenient protocol has been developed for the Michael addition of 1,3-dicarbonyl compounds to β-nitroalkenes in good to excellent yields by a grinding method under catalyst- and solvent-free conditions.
Graphical Abstract
Introduction
Nowadays, chemists are vigorously taking on the challenge of developing green synthetic methodologies to meet the criteria of sustainable, environmentally conscious development. As a result, catalyst- and solvent-free synthetic methods have attracted much interest not only for laboratory synthesis but also in chemical industry, because of reduced pollution, lower costs, mild conditions, and ease of purification. Recently, practical procedures in the absence of solvents and catalysts have been accomplished for greener and cleaner syntheses [1-6]. As the typical representative of solvent-free reactions, the grinding technique has been widely used in organic synthesis [7-13]. Compared to traditional methods, some organic reactions occur more efficiently in the solid state than in solution due to a more tight and regular arrangement of the substrate molecules [14]. Thus, the grinding mode for solid-state reactions had been applied in the Reformatsky reaction [15], Dieckmann condensation [16], Knoevenagel condensation [17], Aldol condensation [18], etc. [1,2,19,20].
The Michael addition is one of the most fundamental and important reactions for the formation of carbon–carbon bonds and carbon–heteroatom bonds in organic synthesis. The conjugate Michael addition of carbon nucleophiles to electron deficient nitroalkenes is particularly interesting and challenging as it involves the generation of a wide range of different functionalized products from Michael adducts [21-24]. In general, Michael addition reactions require basic or acidic catalysts in organic solvents, as well as long reaction times, which may lead to environmentally hazardous residues and undesirable byproducts [25-30].
As one part of our continuing efforts toward the development of green synthesis methods for Michael additions of nitroalkenes, we have previously reported an enzymatic tandem reaction to form 5-hydroxyimino-4,5-dihydrofurans [22], a transition-metal-free process for the synthesis of substituted dihydrofurans [23] and a catalyst-free tandem reaction for the synthesis of 5-hydroxy-1,5-dihydro-2H-pyrrol-2-ones in aqueous medium [24]. Recently, when carrying out the reaction of β-nitrostyrene with 1,3-cyclopentanedione under catalyst- and solvent-free conditions, we were surprised to find that the grinding mode could efficiently promote the reaction, and the corresponding Michael addition product 3a was obtained in nearly 100% yield. Therefore, we were encouraged to research the Michael addition systematically by the grinding method.
Herein, we report a green protocol for the Michael addition of 1,3-dicarbonyl compounds to nitroalkenes under catalyst- and solvent-free conditions (Scheme 1). Utilizing this simple, rapid, low-cost and effective procedure, various nitro diketone derivatives were synthesized in high yields.
Scheme 1: Michael addition under catalyst- and solvent-free conditions.
Scheme 1: Michael addition under catalyst- and solvent-free conditions.
Results and Discussion
In our initial study, equimolar amounts of β-nitrostyrene (1a) and 1,3-cyclopentanedione (2a), as a model reaction (Table 1, entry 1), were mixed and ground in a mortar at room temperature. The mixture became sticky and adhered to the wall of the mortar firmly after a few seconds, which prevented the reactants from mixing thoroughly and coming into sufficient contact. As a result, only a little of the desired product 3a was detected, as monitored by thin-layer chromatography (TLC) after 20 min. In an attempt to improve the grinding process, some silica gel was added in the mortar. Surprisingly, a great quantity of Michael product 3a was obtained after 5 min of grinding. Then a number of powdered substances were screened, such as KBr, quartz sand, Al2O3, kieselguhr, active carbon, and so on. From Figure 1, it was found that all tested grinding aids could promote the reaction to different degrees, and the primary reason may be that the two reactants could come into contact more effectively after dispersion by the grinding aids. It was found that kieselguhr was slightly better than quartz sand in terms of grinding efficiency, and both gave excellent yields. Although kieselguhr is often used as a catalyst in many organic reactions, quartz sand was selected as the suitable grinding aid owing to its low cost and inertness. To the best of our knowledge, this is the first example in which quartz sand has been successfully used as a grinding aid in Michael addition reactions. Next, the same reaction was performed in several organic solvents, as well as in water in the absence of catalysts for the purpose of demonstrating the high efficiency of the grinding method. As shown in Figure 2, after 1 h of magnetic stirring, the best yield of 14% was achieved in polar DMSO, while the other tested solvents gave much lower yields. In fact, there are few reports on the Michael addition of β-nitrostyrene and 1,3-cyclopentanedione. Hrnčiar and Čulák performed the same reaction in methanol using sodium methylate as a catalyst; however, only 85% of product 3a was obtained, and a longer reaction time was required [31].
Figure 1: The grinding effect of different grinding aids. Conditions: β-nitrostyrene (14.9 mg, 0.1 mmol), 1,3-cyclopentanedione (9.8 mg, 0.1 mmol), grinding aid (0.50 g), ground for 10 min and then allowed to stand for a further 10 min. Yields were determined by HPLC.
Figure 1: The grinding effect of different grinding aids. Conditions: β-nitrostyrene (14.9 mg, 0.1 mmol), 1,3...
Figure 2: Yields of the model reaction in different solvents. Conditions: β-nitrostyrene (14.9 mg, 0.1 mmol), 1,3-cyclopentanedione (9.8 mg, 0.1 mmol), solvent 1.0 mL, magnetically stirred for 1 h at rt. Yields were determined by HPLC.
Figure 2: Yields of the model reaction in different solvents. Conditions: β-nitrostyrene (14.9 mg, 0.1 mmol),...
Subsequently, the amount of quartz sand required was investigated to find the optimal amount on a 0.1 mmol scale (Figure 3). The results showed that 0.75 g or more quartz sand was required for an excellent yield. Generally, the reaction rate decreased with the reduction of the reactant concentration when excessive quartz sand was used. However, the yield did not reduce obviously, even when 2.00 g quartz sand was added in the reaction system. Finally, 0.75 g quartz sand was selected as the grinding aid for 0.1 mmol substrates. To optimize the experimental conditions further, we also examined the effects of the molar ratio of reactants on the yield, and a slightly better result was obtained when 1.2 equiv of nitrostyrene was adopted (data not shown). Also considering that nitroalkenes might polymerize by themselves during the grinding process, a 1.2:1 (acceptor/donor) was chosen as the optimal molar ratio. Having established the optimum conditions, the template reaction was enlarged to a gram scale, and a similar result was obtained (data not shown).
Figure 3: The effect of the amount of quartz sand on the yield. Conditions: β-nitrostyrene (14.9 mg, 0.1 mmol), 1,3-cyclopentanedione (9.8 mg, 0.1 mmol), ground for 10 min and then allowed to stand for a further 10 min. Yields were determined by HPLC.
Figure 3: The effect of the amount of quartz sand on the yield. Conditions: β-nitrostyrene (14.9 mg, 0.1 mmol...
To test the generality of this grinding Michael addition with respect to reactants, different aromatic and heteroaromatic nitroalkenes were used as the acceptors to react with 1,3-dicarbonyl compounds under the optimized conditions. The results are given in Table 1. It can be seen that a wide range of substrates were able to participate in the reaction. A series of substituted β-nitrostyrenes with electron-withdrawing or electron-donating functionalities reacted with 1,3-cyclopentanedione (2a) in good to excellent yields. Similarly, the scope of the donor was expanded to other 1,3-dicarbonyl compounds. The best yield (>99%) was obtained for the reaction of furan-2,4(3H,5H)-dione (2b) with β-nitrostyrene (1a) (Table 1, entry 14). Some aromatic 1,3-dicarbonyl compounds were also successfully used as donors in this Michael reaction in moderate to good yields (Table 1, entries 18–21). In addition, furylnitrostyrene (1m) reacted with 1,3-cyclopentanedione (2a) as well as furan-2,4(3H,5H)-dione (2b) to give the corresponding products in excellent or fair yields (Table 1, entries 13 and 22). But no corresponding products were detected in the reactions of β-nitrostyrene (1a) with 2-methylcyclopentane-1,3-dione (2c) and pyrrolidine-2,5-dione (2d) (Table 1, entries 15 and 16). It is noteworthy that the reaction between β-nitrostyrene (1a) and cyclohexane-1,3-dione (2e) only gave another product through a tandem process (Table 1, entry 17; Supporting Information File 1, Scheme S1). To our delight, Michael products 3n and 3s were formed with excellent diastereoselectivity (dr > 99:1, Table 1, entries 14 and 22), product 3l with moderate diastereoselectivity (dr = 82:18, Table 1, entry 12 ). For the purpose of comparing the reactivity of different nitroalkenes with 1,3-cyclopentanedione, another group of experiments was performed, and a marked difference existed among various nitroalkenes, owing to steric effects or electronic effects (Supporting Information File 1, Table S1).
Table 1: Investigation of the reactant scope in the grinding Michael addition.a
|
|
|||||
| Entry | Acceptor, 1 | Donor, 2 | Product, 3 | Yield [%] | drb |
|---|---|---|---|---|---|
| 1 | 1a: R1 = Ph, R2 = H | 2a: X = CH2, Y = CH, R3 = H | 3a | 99c | – |
| 2 | 1b: R1 = 4-FC6H4, R2 = H | 2a | 3b | >99c | – |
| 3 | 1c: R1 = 4-CF3C6H4, R2 = H | 2a | 3c | >99c | – |
| 4 | 1d: R1 = 4-ClC6H4, R2 = H | 2a | 3d | >99c | – |
| 5 | 1e: R1 = 3-ClC6H4, R2 = H | 2a | 3e | >99c | – |
| 6 | 1f: R1 = 4-BrC6H4, R2 = H | 2a | 3f | 99c | – |
| 7 | 1g: R1 = 2-BrC6H4, R2 = H | 2a | 3g | >99c | – |
| 8 | 1h: R1 = 4-NO2C6H4, R2 = H | 2a | 3h | 99c | – |
| 9 | 1i: R1 = 2-NO2C6H4, R2 = H | 2a | 3i | 91d | – |
| 10 | 1j: R1 = 4-MeC6H4, R2 = H | 2a | 3j | >99c | – |
| 11 | 1k: R1 = 4-MeOC6H4, R2 = H | 2a | 3k | >99c | – |
| 12 | 1l: R1 = Ph, R2 = Me | 2a | 3l | 83d | 82:18 |
| 13 | 1m: R1 = 2-furanyl, R2 = H | 2a | 3m | >99c | – |
| 14 | 1a | 2b: X = O, Y = CH, R3 = H | 3n | >99c | >99:1 |
| 15 | 1a | 2c: X = CH2, Y = CH, R3 = Me | – | n.d.e | – |
| 16 | 1a | 2d: X =CH2, Y = N, R3 = H | – | n.d.e | – |
| 17 | 1a | 2e: X = CH2CH2, Y = CH, R3 = H | – | n.d.f | – |
| 18 | 1a | 2f: R4 = Ph, R5 = Me | 3o | 70d | 53:47 |
| 19 | 1a | 2g: R4 = Ph, R5 = OEt | 3p | 81d | 55:45 |
| 20 | 1a | 2h: R4 = 4-MeOC6H4, R5 = OEt | 3q | 66d | 59:41 |
| 21 | 1a | 2i: R4 = R5 = Ph | 3r | 58d | – |
| 22 | 1m | 2b | 3s | 63d | >99:1 |
aConditions: acceptor (0.36 mmol), donor (0.30 mmol), quartz sand (2.25 g), ground occasionally at room temperature. Reaction time: the exact reaction time was not determined owing to the discontinuous grinding process, but most reactions were complete in 3 h. However, a longer time was required for some reactions (e.g., entries 9 and 12), and good yields were obtained after overnight standing. bdr was determined by 1H NMR. cYields of the isolated product after elution from a sand core funnel. dYields of the isolated product after chromatography on silica gel. en.d. = not detected. fNo Michael product was detected, and another product was only obtained by tandem coupling.
After completion of the reaction, the mixture was purified directly by flash column chromatography to give the product without the need for any pretreatment. However, a more efficient and convenient purification procedure was developed in our research lab in order to meet the requirement of green chemistry. Namely, the reaction mixture was filtered through a sand core funnel containing a thin layer of silica gel and the pure product was obtained. More than half of the products could be rapidly purified in this way with similar or higher yields and only a little eluent was needed.
Conclusion
In summary, a convenient, efficient and rapid method was developed for the Michael addition of 1,3-dicarbonyl compounds to β-nitroalkenes in good to excellent yields by a grinding method under catalyst- and solvent-free conditions. It was more meaningful to find that quartz sand could effectively promote this reaction by acting as a grinding aid. The reactions could be performed smoothly between solid–solid or solid–liquid materials at room temperature with a wide range of reactants. Moreover, a much simpler purification procedure was developed, in place of column chromatography.
Supporting Information
| Supporting Information File 1: General procedures and analytical data. | ||
| Format: PDF | Size: 2.1 MB | Download |
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (No. 21001077 and 20121001), Program for Changjiang Scholars and Natural Science Foundation of Jiangxi (2009GQH0007) for financial support. We also thank the Sichuan University Analytical & Testing Center for NMR analysis.
References
-
Choudhary, G.; Peddinti, R. K. Green Chem. 2011, 13, 276–282. doi:10.1039/c0gc00830c
Return to citation in text: [1] [2] -
Kumar, S.; Sharma, P.; Kapoor, K. K.; Hundal, M. S. Tetrahedron 2008, 64, 536–542. doi:10.1016/j.tet.2007.11.008
Return to citation in text: [1] [2] -
Ranu, B. C.; Dey, S. S.; Hajra, A. Green Chem. 2003, 5, 44–46. doi:10.1039/B211238H
Return to citation in text: [1] -
Halimehjani, A. Z.; Pourshojaei, Y.; Saidi, M. R. Tetrahedron Lett. 2009, 50, 32–34. doi:10.1016/j.tetlet.2008.10.063
Return to citation in text: [1] -
Habib, P. M.; Kavala, V.; Kuo, C.-W.; Raihan, M. J.; Yao, C.-F. Tetrahedron 2010, 66, 7050–7056. doi:10.1016/j.tet.2010.05.104
Return to citation in text: [1] -
Zhang, J.; Cui, Z.; Wang, F.; Wang, Y.; Miao, Z.; Chen, R. Green Chem. 2007, 9, 1341–1345. doi:10.1039/B710008F
Return to citation in text: [1] -
Li, J.; Jiang, D.-N.; Chen, J.-X.; Liu, M.-C.; Ding, J.-C.; Wu, H.-Y. J. Heterocycl. Chem. 2011, 48, 403–406. doi:10.1002/jhet.597
Return to citation in text: [1] -
Heravi, M. M.; Poormohammad, N.; Beheshtiha, Y. S.; Baghernejad, B. Synth. Commun. 2011, 41, 579–582. doi:10.1080/00397911003629440
Return to citation in text: [1] -
Kumar, A.; Makrandi, J. K. Green Chem. Lett. Rev. 2011, 4, 87–89. doi:10.1080/17518253.2010.502909
Return to citation in text: [1] -
Sharma, D.; Kumar, S.; Makrandi, J. K. Green Chem. Lett. Rev. 2011, 4, 127–129. doi:10.1080/17518253.2010.517785
Return to citation in text: [1] -
Zonouz, A. M.; Moghani, D. Synth. Commun. 2011, 41, 2152–2160. doi:10.1080/00397911.2010.499488
Return to citation in text: [1] -
Rong, L.; Han, H.; Jiang, H.; Tu, S. Synth. Commun. 2008, 38, 3530–3542. doi:10.1080/00397910802164724
Return to citation in text: [1] -
Wu, D.; Ren, Z.; Cao, W.; Tong, W. Synth. Commun. 2005, 35, 3157–3162. doi:10.1080/00397910500282968
Return to citation in text: [1] -
Toda, F.; Tanaka, K. Chem. Rev. 2000, 100, 1025–1074. doi:10.1021/cr940089p
Return to citation in text: [1] -
Tanaka, K.; Kishigami, S.; Toda, F. J. Org. Chem. 1991, 56, 4333–4334. doi:10.1021/jo00013a055
Return to citation in text: [1] -
Toda, F.; Suzuki, T.; Higa, S. J. Chem. Soc., Perkin Trans. 1 1998, 3521–3522. doi:10.1039/A805884I
Return to citation in text: [1] -
Ren, Z.; Cao, W.; Tong, W. Synth. Commun. 2002, 32, 3475–3479. doi:10.1081/SCC-120014780
Return to citation in text: [1] -
Toda, F.; Tanaka, K.; Hamai, K. J. Chem. Soc., Perkin Trans. 1 1990, 3207–3209. doi:10.1039/P19900003207
Return to citation in text: [1] -
Bernhardt, F.; Trotzki, R.; Szuppa, T.; Stolle, A.; Ondruschka, B. Beilstein J. Org. Chem. 2010, 6, No. 7. doi:10.3762/bjoc.6.7
Return to citation in text: [1] -
Moon, M. E.; Choi, Y.; Lee, Y. M.; Vajpayee, V.; Trusova, M.; Filimonov, V. D.; Chi, K.-W. Tetrahedron Lett. 2010, 51, 6769–6771. doi:10.1016/j.tetlet.2010.10.099
Return to citation in text: [1] -
Mendler, B.; Kazmaier, U. Org. Lett. 2005, 7, 1715–1718. doi:10.1021/ol050129j
Return to citation in text: [1] -
Wu, M.-Y.; Li, K.; He, T.; Feng, X.-W.; Wang, N.; Wang, X.-Y.; Yu, X.-Q. Tetrahedron 2011, 67, 2681–2688. doi:10.1016/j.tet.2011.01.060
Return to citation in text: [1] [2] -
Wu, M.-Y.; Wang, M.-Q.; Li, K.; Feng, X.-W.; He, T.; Wang, N.; Yu, X.-Q. Tetrahedron Lett. 2011, 52, 679–683. doi:10.1016/j.tetlet.2010.11.151
Return to citation in text: [1] [2] -
Wu, M.-Y.; Li, K.; Wang, N.; He, T.; Yu, X.-Q. Synthesis 2011, 1831–1839. doi:10.1055/s-0030-1260049
Return to citation in text: [1] [2] -
Kabashima, H.; Tsuji, H.; Shibuya, T.; Hattori, H. J. Mol. Catal. A: Chem. 2000, 155, 23–29. doi:10.1016/S1381-1169(99)00316-7
Return to citation in text: [1] -
Bandini, M.; Fagioli, M.; Umani-Ronchi, A. Adv. Synth. Catal. 2004, 346, 545–548. doi:10.1002/adsc.200303213
Return to citation in text: [1] -
Veldurthy, B.; Clacens, J. M.; Figueras, F. Adv. Synth. Catal. 2005, 347, 767–771. doi:10.1002/adsc.200404371
Return to citation in text: [1] -
Choudary, B. M.; Rajasekhar, C. V.; Krishna, G. G.; Reddy, K. R. Synth. Commun. 2007, 37, 91–98. doi:10.1080/00397910600978218
Return to citation in text: [1] -
Gu, Q.; Guo, X.-T.; Wu, X.-Y. Tetrahedron 2009, 65, 5265–5270. doi:10.1016/j.tet.2009.04.087
Return to citation in text: [1] -
Yoshida, M.; Ohno, Y.; Hara, S. Tetrahedron Lett. 2010, 51, 5134–5136. doi:10.1016/j.tetlet.2010.07.089
Return to citation in text: [1] -
Hrnčiar, P.; Čulák, I. Collect. Czech. Chem. Commun. 1984, 49, 1421–1431. doi:10.1135/cccc19841421
Return to citation in text: [1]
| 1. | Choudhary, G.; Peddinti, R. K. Green Chem. 2011, 13, 276–282. doi:10.1039/c0gc00830c |
| 2. | Kumar, S.; Sharma, P.; Kapoor, K. K.; Hundal, M. S. Tetrahedron 2008, 64, 536–542. doi:10.1016/j.tet.2007.11.008 |
| 3. | Ranu, B. C.; Dey, S. S.; Hajra, A. Green Chem. 2003, 5, 44–46. doi:10.1039/B211238H |
| 4. | Halimehjani, A. Z.; Pourshojaei, Y.; Saidi, M. R. Tetrahedron Lett. 2009, 50, 32–34. doi:10.1016/j.tetlet.2008.10.063 |
| 5. | Habib, P. M.; Kavala, V.; Kuo, C.-W.; Raihan, M. J.; Yao, C.-F. Tetrahedron 2010, 66, 7050–7056. doi:10.1016/j.tet.2010.05.104 |
| 6. | Zhang, J.; Cui, Z.; Wang, F.; Wang, Y.; Miao, Z.; Chen, R. Green Chem. 2007, 9, 1341–1345. doi:10.1039/B710008F |
| 16. | Toda, F.; Suzuki, T.; Higa, S. J. Chem. Soc., Perkin Trans. 1 1998, 3521–3522. doi:10.1039/A805884I |
| 15. | Tanaka, K.; Kishigami, S.; Toda, F. J. Org. Chem. 1991, 56, 4333–4334. doi:10.1021/jo00013a055 |
| 24. | Wu, M.-Y.; Li, K.; Wang, N.; He, T.; Yu, X.-Q. Synthesis 2011, 1831–1839. doi:10.1055/s-0030-1260049 |
| 7. | Li, J.; Jiang, D.-N.; Chen, J.-X.; Liu, M.-C.; Ding, J.-C.; Wu, H.-Y. J. Heterocycl. Chem. 2011, 48, 403–406. doi:10.1002/jhet.597 |
| 8. | Heravi, M. M.; Poormohammad, N.; Beheshtiha, Y. S.; Baghernejad, B. Synth. Commun. 2011, 41, 579–582. doi:10.1080/00397911003629440 |
| 9. | Kumar, A.; Makrandi, J. K. Green Chem. Lett. Rev. 2011, 4, 87–89. doi:10.1080/17518253.2010.502909 |
| 10. | Sharma, D.; Kumar, S.; Makrandi, J. K. Green Chem. Lett. Rev. 2011, 4, 127–129. doi:10.1080/17518253.2010.517785 |
| 11. | Zonouz, A. M.; Moghani, D. Synth. Commun. 2011, 41, 2152–2160. doi:10.1080/00397911.2010.499488 |
| 12. | Rong, L.; Han, H.; Jiang, H.; Tu, S. Synth. Commun. 2008, 38, 3530–3542. doi:10.1080/00397910802164724 |
| 13. | Wu, D.; Ren, Z.; Cao, W.; Tong, W. Synth. Commun. 2005, 35, 3157–3162. doi:10.1080/00397910500282968 |
| 31. | Hrnčiar, P.; Čulák, I. Collect. Czech. Chem. Commun. 1984, 49, 1421–1431. doi:10.1135/cccc19841421 |
| 21. | Mendler, B.; Kazmaier, U. Org. Lett. 2005, 7, 1715–1718. doi:10.1021/ol050129j |
| 22. | Wu, M.-Y.; Li, K.; He, T.; Feng, X.-W.; Wang, N.; Wang, X.-Y.; Yu, X.-Q. Tetrahedron 2011, 67, 2681–2688. doi:10.1016/j.tet.2011.01.060 |
| 23. | Wu, M.-Y.; Wang, M.-Q.; Li, K.; Feng, X.-W.; He, T.; Wang, N.; Yu, X.-Q. Tetrahedron Lett. 2011, 52, 679–683. doi:10.1016/j.tetlet.2010.11.151 |
| 24. | Wu, M.-Y.; Li, K.; Wang, N.; He, T.; Yu, X.-Q. Synthesis 2011, 1831–1839. doi:10.1055/s-0030-1260049 |
| 22. | Wu, M.-Y.; Li, K.; He, T.; Feng, X.-W.; Wang, N.; Wang, X.-Y.; Yu, X.-Q. Tetrahedron 2011, 67, 2681–2688. doi:10.1016/j.tet.2011.01.060 |
| 1. | Choudhary, G.; Peddinti, R. K. Green Chem. 2011, 13, 276–282. doi:10.1039/c0gc00830c |
| 2. | Kumar, S.; Sharma, P.; Kapoor, K. K.; Hundal, M. S. Tetrahedron 2008, 64, 536–542. doi:10.1016/j.tet.2007.11.008 |
| 19. | Bernhardt, F.; Trotzki, R.; Szuppa, T.; Stolle, A.; Ondruschka, B. Beilstein J. Org. Chem. 2010, 6, No. 7. doi:10.3762/bjoc.6.7 |
| 20. | Moon, M. E.; Choi, Y.; Lee, Y. M.; Vajpayee, V.; Trusova, M.; Filimonov, V. D.; Chi, K.-W. Tetrahedron Lett. 2010, 51, 6769–6771. doi:10.1016/j.tetlet.2010.10.099 |
| 23. | Wu, M.-Y.; Wang, M.-Q.; Li, K.; Feng, X.-W.; He, T.; Wang, N.; Yu, X.-Q. Tetrahedron Lett. 2011, 52, 679–683. doi:10.1016/j.tetlet.2010.11.151 |
| 18. | Toda, F.; Tanaka, K.; Hamai, K. J. Chem. Soc., Perkin Trans. 1 1990, 3207–3209. doi:10.1039/P19900003207 |
| 17. | Ren, Z.; Cao, W.; Tong, W. Synth. Commun. 2002, 32, 3475–3479. doi:10.1081/SCC-120014780 |
| 25. | Kabashima, H.; Tsuji, H.; Shibuya, T.; Hattori, H. J. Mol. Catal. A: Chem. 2000, 155, 23–29. doi:10.1016/S1381-1169(99)00316-7 |
| 26. | Bandini, M.; Fagioli, M.; Umani-Ronchi, A. Adv. Synth. Catal. 2004, 346, 545–548. doi:10.1002/adsc.200303213 |
| 27. | Veldurthy, B.; Clacens, J. M.; Figueras, F. Adv. Synth. Catal. 2005, 347, 767–771. doi:10.1002/adsc.200404371 |
| 28. | Choudary, B. M.; Rajasekhar, C. V.; Krishna, G. G.; Reddy, K. R. Synth. Commun. 2007, 37, 91–98. doi:10.1080/00397910600978218 |
| 29. | Gu, Q.; Guo, X.-T.; Wu, X.-Y. Tetrahedron 2009, 65, 5265–5270. doi:10.1016/j.tet.2009.04.087 |
| 30. | Yoshida, M.; Ohno, Y.; Hara, S. Tetrahedron Lett. 2010, 51, 5134–5136. doi:10.1016/j.tetlet.2010.07.089 |
© 2012 Xie et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)