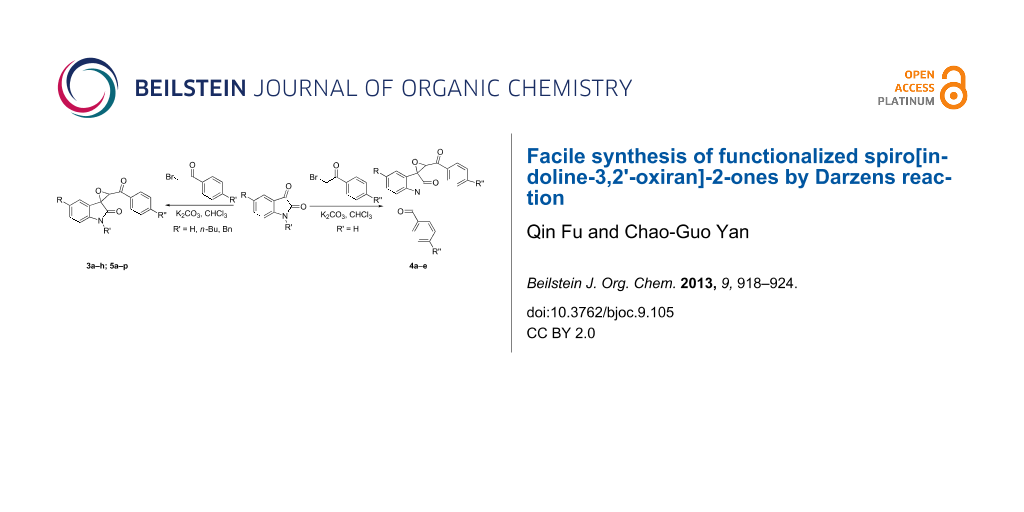
Abstract
A series of functionalized spiro[indoline-3,2'-oxiran]-2-ones was efficiently synthesized by Darzens reaction of phenacyl bromides with isatins both with N-alkyl groups and without N-substituent in the presence of potassium carbonate as a base catalyst. When two equivalents phenacyl bromides were used in the reaction, the N-substitution reaction of isatin also finished with the formation of spiro-oxirane-oxindoles.
Graphical Abstract
Introduction
The spirooxindole unit is a privileged heterocyclic motif that forms the core structure of a large family of natural alkaloids and many pharmacological agents with important bioactivity and interesting structural properties [1-5]. The unique structures and the highly pronounced pharmacological activity displayed by the spirooxindoles have made them attractive synthetic targets [6-9]. In various heterocyclic and carbocyclic spirooxindoles, the spiro-oxirane-oxindoles are a particular class of compounds with both spiro-carbon and unstable oxirane features in the molecule. These fascinating spiranic frameworks can serve as important building blocks in organic synthesis for the synthesis of large-ring heterocycles [10-13]. As a consequence, in recent years much attention has been paid to the diastereoselective and enantioselective synthesis of versatile spiro-oxirane-oxindoles [14-19]. With the aim of expanding our previous studies on the synthesis of various spirooxindoles [20-25], we decided to systematically investigate the Darzens reactions of a series of isatins with phenacyl bromides and report the facile synthesis of versatile spiro[indoline-3,2'-oxiran]-2-ones.
Results and Discussion
In recent years we have found that pyridinium salts react with versatile reactive methylene compounds to give different kinds of products, including functionalized cyclopropanes, 2,3-dihydrofurans, polysubstituted pyridines, pyrido[1,2-a]benzimidazoles and charge-separated zwitterionic salts [26-31]. We envisaged that in situ generated pyridinium ylide might react with the reactive carbonyl group of isatins to afford spiro epoxyoxindoles (Scheme 1). To test this feasibility, the reactions of various substituted isatins with pyridinium salt in the presence of base were examined under different conditions. We were disappointed that the reactions produced much complicated mixtures and no acceptable results were obtained. Thus, our attention was turned toward the development of straightforward reactions of phenacyl bromides with isatins.
Scheme 1: Synthesis of spiro-epoxyoxindole with pyridinium ylide.
Scheme 1: Synthesis of spiro-epoxyoxindole with pyridinium ylide.
In a preliminary experiment, a model between 5-methylisatin (1) and phenacyl bromide (2) was examined under a broad set of conditions (Table 1). A careful screening of bases revealed that potassium afforded the product in better yields. The main problem is that the N-alkylated spiro epoxyoxindole 4 is accompanied by the formation of spiro epoxyoxindole 3 even if equivalent reactants are used, which is consistent with the recently reported reactions of isatins with alkylating agents having an acidic methylene group by Blanco et al. [19]. To our delight, the spiro epoxyoxindole 3 could be selectively obtained in 85% yield when the reaction was carried out in the system of K2CO3/CHCl3 at about 50 °C for 10 h. On the other hand the N-alkylated spiro epoxyoxindole 4 was also successfully prepared in 90% yield in this system when more than two equivalents of phenacyl bromide were utilized.
Table 1: Reaction of 5-methylisatin (1) with phenacyl bromide (2).
|
|
|||||||
| Entry | Base | Ratios of 1/2 | Solvent | Temp. (°C) | Time (h) | Yield (%) | |
|---|---|---|---|---|---|---|---|
| 3 | 4 | ||||||
| 1 | NEt3 | 1:1 | EtOH | 10–15 | 24 | — | — |
| 2 | DABCO | 1:1 | EtOH | 10–15 | 18 | 40 | — |
| 3 | DBU | 1:1 | EtOH | 10–15 | 18 | 62 | — |
| 4 | K2CO3 | 1:1 | EtOH | 10–15 | 18 | 70 | — |
| 5 | K2CO3 | 1:1 | CHCl3 | 10–15 | 18 | 78 | — |
| 6 | K2CO3 | 1:1 | CHCl3 | 50 | 10 | 85 | — |
| 7 | K2CO3 | 1:1 | MeCN | 50 | 10 | 60 | — |
| 8 | K2CO3 | 1:1 | EtOH | 50 | 10 | 56 | 18 |
| 9 | K2CO3 | 1:1 | toluene | 50 | 10 | 87 | — |
| 10 | K2CO3 | 1:1 | DMF | 50 | 10 | 28 | 46 |
| 11 | K2CO3 | 1:1.2 | CHCl3 | 50 | 10 | 66 | 15 |
| 12 | K2CO3 | 1:2 | CHCl3 | 50 | 10 | 10 | 77 |
| 13 | K2CO3 | 1:2.2 | CHCl3 | 50 | 10 | — | 90 |
| 14 | K2CO3 | 1:2.2 | DMF | 50 | 10 | — | 89 |
After obtaining the optimized reaction conditions, various substituted isatins and phenacyl bromides were employed in the reaction. The results are summarized in Table 2. All reactions proceeded very smoothly and eight spiro epoxyoxindoles 3a–h were obtained in satisfactory yields. Similarly the N-substituted spiro epoxyoxindoles 4a–e were synthesized in high yields by using an excess of phenacyl bromide. The results are summarized in Table 3. The structures of compounds 3a–h and 4a–e were characterized by IR, 1H, 13C NMR, and HRMS spectra and were further confirmed by single-crystal X-ray diffraction determination of the compound 3c (Figure 1) and 4a (Figure 2). It should be pointed out that some of the spiro epoxyoxindoles 3a–h have been previously prepared by other methods and the related references are also listed in Table 2. The 1H NMR spectra of compounds 3a–h and 4a–e usually show one set of characteristic peaks for each group, especially one singlet at about 5.10 ppm for one proton of the epoxy unit, which clearly indicates that only one isomer exists in each sample. However, 1H NMR spectra of compounds 3a and 3f clearly displayed that the trans-isomer existed mainly with ratios of cis/trans isomers of 1:14 and 1:8, respectively. From Figure 1 and Figure 2 it is seen that the phenyl group of the oxindole unit and the benzoyl group existed in the cis-position. This result also indicated that the thermodynamic reaction produces a more stable trans-isomer.
![[1860-5397-9-105-1]](/bjoc/content/figures/1860-5397-9-105-1.png?scale=1.5&max-width=1024&background=FFFFFF)
Figure 1: Molecular structure of spiro compound 3c.
Figure 1: Molecular structure of spiro compound 3c.
![[1860-5397-9-105-2]](/bjoc/content/figures/1860-5397-9-105-2.png?scale=1.5&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure of spiro compound 4a.
Figure 2: Molecular structure of spiro compound 4a.
To further illustrate the power of this reaction procedure, the N-substituted isatins were also employed to react with phenacyl bromides under similar reaction conditions. A series of new spiro epoxyoxindoles 5a–p were prepared in high yields (Table 4). The structures of the spiro compounds 5a–p were also established by spectroscopic methods and were confirmed by single-crystal X-ray structure determination of compound 5o (Figure 3). 1H NMR spectra showed that a mixture of cis/trans isomers existed in most samples with a large range of different cis/trans ratios. It is known that the closure of the epoxy ring would form cis/trans isomers in the Darzens reaction process. Here the N-benzyl and the N-butyl group in the oxindole moiety may decrease the steric effect of formation of the epoxy ring and lead to the easier formation of the relatively unstable cis-isomer.
Table 4: Synthesis of spiro[indoline-3,2'-oxiran]-2-ones 5a–p.
|
|
|||||
| Entry | Compound | R | R’ | R” | Yield (%, cis/trans ratio) |
|---|---|---|---|---|---|
| 1 | 5a | H | CH2Ph | H | 92 [35] |
| 2 | 5b | H | CH2Ph | Cl | 80 (12:1) |
| 3 | 5c | H | n-Bu | H | 88 |
| 4 | 5d | H | n-Bu | Cl | 76 (1:1) |
| 5 | 5e | CH3 | CH2Ph | H | 90 (2:1) |
| 6 | 5f | CH3 | CH2Ph | Cl | 86 (3:1) |
| 7 | 5g | CH3 | n-Bu | H | 82 (4:1) |
| 8 | 5h | CH3 | n-Bu | Cl | 86 (4:1) |
| 9 | 5i | F | CH2Ph | H | 80 (5:4) |
| 10 | 5j | F | CH2Ph | Cl | 89 (5:2) |
| 11 | 5k | F | n-Bu | H | 83 (11:1) |
| 12 | 5l | F | n-Bu | Cl | 92 (3:1) |
| 13 | 5m | Cl | CH2Ph | H | 84 (5:1) |
| 14 | 5n | Cl | CH2Ph | Cl | 91 |
| 15 | 5o | Cl | n-Bu | H | 81 |
| 16 | 5p | Cl | n-Bu | Cl | 88 (10:1) |
![[1860-5397-9-105-3]](/bjoc/content/figures/1860-5397-9-105-3.png?scale=1.5&max-width=1024&background=FFFFFF)
Figure 3: Molecular structure of spiro compound 5o.
Figure 3: Molecular structure of spiro compound 5o.
Conclusion
In summary, we have systematically investigated the Darzens reaction of phenacyl bromides with isatins for the efficient synthesis of the functionalized spiro epoxyoxindoles. Both the nonsubstituted isatins and N-alkylated isatins were successfully used in the reactions. The scope and limitation of the reaction was established. The N-alkylation of the isatins is usually accompanied by the formation of spiro epoxyoxindoles.
Experimental
Reagents and apparatus: Melting points were taken on a hot-plate microscope apparatus. IR spectra were obtained on a Bruker Tensor 27 spectrometer (KBr disc). NMR spectra were recorded with a Bruker AV-600 spectrometer with DMSO-d6 as solvent and TMS as internal standard (600 and 150 MHz for 1H and 13C NMR spectra, respectively). HRMS were measured on a UHR-TOF maXis instrument. X-ray data were collected on a Bruker Smart APEX-2 diffractometer. Isatins, phenacyl bromide and other reagents are commercial reagents and were used as received. Solvents were purified by standard techniques. All reactions were monitored by TLC.
Typical procedure for the preparation of spiro[indoline-3,2'-oxiran]-2-ones 3a–h: A mixture of isatin (1.0 mmol), phenacyl bromide (1.0 mmol) and potassium carbonate (1.2 mmol) in 20.0 mL chloroform was stirred at 50 °C for 10–24 h (TLC analysis). After cooling the reaction was quenched with water. The solvent was evaporated under reduced pressure. The residue was recrystallized from ethanol to give the pure product for analysis.
3'-benzoylspiro[indoline-3,2'-oxiran]-2-one (3a) [32]: White solid, yield: 83%; mp 158–159 °C; IR (KBr) ν: 3457, 3180, 2972, 1735, 1676, 1620, 1597, 1469, 1335, 1219, 1041, 1013, 927, 850, 794 cm−1; 1H NMR (600 MHz, DMSO-d6) δ cis-isomer: 11.04 (s, 1H, NH), 7.88 (d, J = 7.8 Hz, 2H, ArH), 7.66 (t, J = 7.2 Hz, 1H, ArH), 7.53 (t, J = 7.8 Hz, 2H, ArH), 7.28 (t, J = 7.2 Hz, 1H, ArH), 6.93 (d, J = 7.8 Hz, 1H, ArH), 6.88 (t, J = 7.2 Hz, 1H, ArH), 6.82 (d, J = 7.8 Hz, 1H, ArH), 5.15 (s, 1H, CH); trans-isomer: 7.96 (d, J = 7.8 Hz, 2H, ArH), 7.76 (t, J = 7.2 Hz, 1H, ArH), 7.62 (d, J = 7.2 Hz, 1H, ArH), 7.55 (t, J = 7.8 Hz, 2H, ArH), 7.32 (d, J = 7.8 Hz, 1H, ArH), 7.14 (d, J = 7.8 Hz, 1H, ArH), 6.97 (d, J = 7.2 Hz, 1H, ArH), 5.28 (s, 1H, CH); 13C NMR (150 MHz, DMSO-d6) δ 191.0, 170.8, 144.0, 134.7, 134.4, 131.1, 129.1, 128.0, 123.2, 122.0, 119.3, 110.0, 63.6, 60.4; HRMS–ESI (m/z): [M + Na]+ calcd for C16H11NNaO3, 288.0631; found, 288.0628.
Typical procedure for the preparation of spiro[indoline-3,2'-oxiran]-2-ones 4a–e: A mixture of isatin (1.0 mmol), phenacyl bromide (2.2 mmol) and potassium carbonate (2.6 mmol) in 20.0 mL of chloroform was stirred at 50 °C for 10–24 h (TLC analysis). After cooling, the reaction was quenched with water. The solvent was evaporated under reduced pressure. The residue was recrystallized from ethanol to give the pure product for analysis.
3'-benzoyl-1-(2-oxo-2-phenylethyl)spiro[indoline-3,2'-oxiran]-2-one (4a): White solid, yield: 93%; mp 188–189 °C; IR (KBr) ν: 3449, 2929, 1733, 1696, 1613, 1597, 1467, 1351, 1229, 1186, 1101, 930, 786 cm−1; 1H NMR (600 MHz, DMSO-d6) δ 8.12 (d, J = 7.8 Hz, 2H, ArH), 7.91 (d, J = 7.2 Hz, 2H, ArH), 7.76 (t, J = 7.2 Hz, 1H, ArH), 7.69 (t, J = 7.2 Hz, 1H, ArH), 7.63 (t, J = 7.2 Hz, 2H, ArH), 7.55 (t, J = 7.8 Hz, 2H, ArH), 7.31 (t, J = 7.8 Hz, 1H, ArH), 7.14 (d, J = 7.8 Hz, 1H, ArH), 6.96 (t, J = 7.2 Hz, 1H, ArH), 6.90 (d, J = 7.2 Hz, 1H, ArH), 5.56–5.49 (m, 2H, CH2), 5.28 (s, 1H, CH); 13C NMR (150 MHz, DMSO-d6) δ 192.5, 190.9, 169.9, 144.9, 134.6, 134.5, 134.2, 134.1, 131.1, 129.1, 128.9, 128.3, 128.0, 122.9, 122.8, 118.7, 110.3, 64.1, 60.2, 47.3; HRMS–ESI (m/z): [M + K]+ calcd for C24H17KNO4, 422.0789; found, 422.0782.
Typical procedure for the preparation of spiro[indoline-3,2'-oxiran]-2-ones 5a–p: A mixture of isatin (1.0 mmol), phenacyl bromide (1.2 mmol) and potassium carbonate (1.5 mmol) in 20.0 mL of chloroform was stirred at 50 °C for 10–24 h (TLC analysis). After cooling, the reaction was quenched with water. The solvent was evaporated under reduced pressure. The residue was recrystallized with ethanol to give the pure product for analysis.
3'-benzoyl-1-benzylspiro[indoline-3,2'-oxiran]-2-one (5a) [35]: White solid, yield: 92%; mp 154–156 °C; IR (KBr) ν: 3034, 2923, 1730, 1691, 1610, 1463, 1360, 1232, 1185, 1104, 1007, 923, 870 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.94 (d, J = 7.8 Hz, 2H, ArH), 7.61 (t, J = 7.2 Hz, 1H, ArH), 7.47 (t, J = 7.8 Hz, 2H, ArH), 7.36–7.30 (m, 5H, ArH), 7.21 (t, J = 7.8 Hz, 1H, ArH), 7.12 (d, J = 7.2 Hz, 1H, ArH), 6.92 (t, J = 7.8 Hz, 1H, ArH), 6.78 (d, J = 7.8 Hz, 1H, ArH), 5.04 (s, 1H, CH), 5.01 (s, 2H, CH2); 13C NMR (150 MHz, CDCl3) δ 190.7, 170.4, 144.6, 135.2, 135.1, 134.5, 131.0, 129.0, 128.9, 128.3, 128.0, 127.3, 124.5, 123.3, 119.3, 110.0, 64.0, 61.0, 44.5; HRMS–ESI (m/z): [M + Na]+ calcd for C23H17NNaO3, 378.1101; found, 378.1103.
Supporting Information
Single-crystal data for compounds 3c (CCDC 919779), 4a (CCDC 921900) and 5o (CCDC 919780) have been deposited in the Cambridge Crystallographic Data Centre. These data can be obtained free of charge via http://www.ccdc.ac.ck./data_request/cif.
| Supporting Information File 1: Experimental details and spectroscopic data. | ||
| Format: PDF | Size: 1.6 MB | Download |
References
-
Sundberg, R. J. The Chemistry of Indoles; Academic Press: New York, 1996.
Return to citation in text: [1] -
Abdel-Rahman, A. H.; Keshk, E. M.; Hanna, M. A.; El-Bady, S. M. Bioorg. Med. Chem. 2004, 12, 2483–2488. doi:10.1016/j.bmc.2003.10.063
Return to citation in text: [1] -
Koch, M. A.; Schuffenhauer, A.; Scheck, M.; Wetzel, S.; Casaulta, M.; Odermatt, A.; Ertl, P.; Waldmann, H. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 17272–17277. doi:10.1073/pnas.0503647102
Return to citation in text: [1] -
Ashimori, A.; Bachand, B.; Overman, L. E.; Poon, D. J. J. Am. Chem. Soc. 1998, 120, 6477–6487. doi:10.1021/ja980786p
Return to citation in text: [1] -
Sebahar, P. R.; Williams, R. M. J. Am. Chem. Soc. 2000, 122, 5666–5667. doi:10.1021/ja001133n
Return to citation in text: [1] -
Kotha, S.; Deb, A. C.; Lahiri, K.; Manivannan, E. Synthesis 2009, 165–193. doi:10.1055/s-0028-1083300
Return to citation in text: [1] -
Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003–3025. doi:10.1055/s-0029-1216975
Return to citation in text: [1] -
Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104–6155. doi:10.1021/cr300135y
Return to citation in text: [1] -
Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10, 5165–5181. doi:10.1039/c2ob25184a
Return to citation in text: [1] -
Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209–2219. doi:10.1002/ejoc.200300050
Return to citation in text: [1] -
Inoue, M.; Furuyama, H.; Sakazaki, H.; Hirama, M. Org. Lett. 2001, 3, 2863–2865. doi:10.1021/ol016303v
Return to citation in text: [1] -
Chouhan, M.; Senwar, K. R.; Sharma, R.; Grover, V.; Nair, V. A. Green Chem. 2011, 13, 2553–2560. doi:10.1039/c1gc15416h
Return to citation in text: [1] -
Dandia, A.; Singh, R.; Bhaskaran, S. Ultrason. Sonochem. 2010, 17, 399–402. doi:10.1016/j.ultsonch.2009.08.003
Return to citation in text: [1] [2] -
Schulz, V.; Davoust, M.; Lemarié, M.; Lohier, J.-F.; de Oliveira Santos, J. S.; Metzner, P.; Brière, J.-F. Org. Lett. 2007, 9, 1745–1748. doi:10.1021/ol070439x
Return to citation in text: [1] -
Muthusamy, S.; Karikalan, T.; Suresh, E. Tetrahedron Lett. 2011, 52, 1934–1937. doi:10.1016/j.tetlet.2011.02.052
Return to citation in text: [1] -
Dandia, A.; Singh, R.; Bhaskaran, S. Ultrason. Sonochem. 2011, 18, 1113–1117. doi:10.1016/j.ultsonch.2010.12.010
Return to citation in text: [1] -
Palumbo, C.; Mazzeo, G.; Mazziotta, A.; Gambacorta, A.; Loreto, M. A.; Migliorini, A.; Superchi, S.; Tofani, D.; Gasperi, T. Org. Lett. 2011, 13, 6248–6251. doi:10.1021/ol202646w
Return to citation in text: [1] -
Gasperi, T.; Loreto, M. A.; Migliorini, A.; Ventura, C. Eur. J. Org. Chem. 2011, 385–391. doi:10.1002/ejoc.201001021
Return to citation in text: [1] -
Shmidt, M. S.; Perillo, I. A.; González, M.; Blanco, M. M. Tetrahedron Lett. 2012, 53, 2514–2517. doi:10.1016/j.tetlet.2012.03.010
Return to citation in text: [1] [2] -
Sun, Y.; Sun, J.; Yan, C.-G. Tetrahedron Lett. 2012, 53, 3647–3649. doi:10.1016/j.tetlet.2012.05.023
Return to citation in text: [1] -
Han, Y.; Wu, Q.; Sun, J.; Yan, C.-G. Tetrahedron 2012, 68, 8539–8544. doi:10.1016/j.tet.2012.08.030
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2012, 1976–1983. doi:10.1002/ejoc.201101737
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Gong, H.; Xie, Y.-J.; Yan, C.-G. Org. Lett. 2012, 14, 5172–5175. doi:10.1021/ol302530m
Return to citation in text: [1] -
Wu, L.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2012, 10, 9452–9463. doi:10.1039/c2ob26849c
Return to citation in text: [1] -
Sun, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 8–14. doi:10.3762/bjoc.9.2
Return to citation in text: [1] -
Chuang, C.-P.; Chen, K.-P. Tetrahedron 2012, 68, 1401–1406. doi:10.1016/j.tet.2011.12.035
Return to citation in text: [1] -
Yan, C. G.; Song, X. K.; Wang, Q. F.; Sun, J.; Siemeling, U.; Bruhn, C. Chem. Commun. 2008, 1440–1442. doi:10.1039/b718171j
Return to citation in text: [1] -
Wang, Q.-F.; Song, X.-K.; Chen, J.; Yan, C.-G. J. Comb. Chem. 2009, 11, 1007–1010. doi:10.1021/cc900005v
Return to citation in text: [1] -
Wang, Q.-F.; Hou, H.; Hui, L.; Yan, C.-G. J. Org. Chem. 2009, 74, 7403–7406. doi:10.1021/jo901379h
Return to citation in text: [1] -
Wang, Q.-F.; Hui, L.; Hou, H.; Yan, C.-G. J. Comb. Chem. 2010, 12, 260–265. doi:10.1021/cc900161z
Return to citation in text: [1] -
Hou, H.; Zhang, Y.; Yan, C.-G. Chem. Commun. 2012, 48, 4492–4494. doi:10.1039/c2cc30708a
Return to citation in text: [1] -
Dandia, A.; Singh, R.; Saha, M.; Shivpuri, A. Pharmazie 2002, 57, 602–605.
Return to citation in text: [1] [2] [3] -
Kobayashi, G.; Furukawa, S.; Matsuda, Y. Yakugaku Zasshi 1966, 86, 1156–1159.
Return to citation in text: [1] -
Joshi, K. C.; Jain, R.; Garg, S. J. Heterocycl. Chem. 1984, 21, 977–979. doi:10.1002/jhet.5570210410
Return to citation in text: [1] [2] -
Chu, Y.; Liu, X.; Li, W.; Hu, X.; Lin, L.; Feng, X. Chem. Sci. 2012, 3, 1996–2000. doi:10.1039/c2sc20218b
Return to citation in text: [1] [2]
| 1. | Sundberg, R. J. The Chemistry of Indoles; Academic Press: New York, 1996. |
| 2. | Abdel-Rahman, A. H.; Keshk, E. M.; Hanna, M. A.; El-Bady, S. M. Bioorg. Med. Chem. 2004, 12, 2483–2488. doi:10.1016/j.bmc.2003.10.063 |
| 3. | Koch, M. A.; Schuffenhauer, A.; Scheck, M.; Wetzel, S.; Casaulta, M.; Odermatt, A.; Ertl, P.; Waldmann, H. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 17272–17277. doi:10.1073/pnas.0503647102 |
| 4. | Ashimori, A.; Bachand, B.; Overman, L. E.; Poon, D. J. J. Am. Chem. Soc. 1998, 120, 6477–6487. doi:10.1021/ja980786p |
| 5. | Sebahar, P. R.; Williams, R. M. J. Am. Chem. Soc. 2000, 122, 5666–5667. doi:10.1021/ja001133n |
| 20. | Sun, Y.; Sun, J.; Yan, C.-G. Tetrahedron Lett. 2012, 53, 3647–3649. doi:10.1016/j.tetlet.2012.05.023 |
| 21. | Han, Y.; Wu, Q.; Sun, J.; Yan, C.-G. Tetrahedron 2012, 68, 8539–8544. doi:10.1016/j.tet.2012.08.030 |
| 22. | Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2012, 1976–1983. doi:10.1002/ejoc.201101737 |
| 23. | Sun, J.; Sun, Y.; Gong, H.; Xie, Y.-J.; Yan, C.-G. Org. Lett. 2012, 14, 5172–5175. doi:10.1021/ol302530m |
| 24. | Wu, L.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2012, 10, 9452–9463. doi:10.1039/c2ob26849c |
| 25. | Sun, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 8–14. doi:10.3762/bjoc.9.2 |
| 14. | Schulz, V.; Davoust, M.; Lemarié, M.; Lohier, J.-F.; de Oliveira Santos, J. S.; Metzner, P.; Brière, J.-F. Org. Lett. 2007, 9, 1745–1748. doi:10.1021/ol070439x |
| 15. | Muthusamy, S.; Karikalan, T.; Suresh, E. Tetrahedron Lett. 2011, 52, 1934–1937. doi:10.1016/j.tetlet.2011.02.052 |
| 16. | Dandia, A.; Singh, R.; Bhaskaran, S. Ultrason. Sonochem. 2011, 18, 1113–1117. doi:10.1016/j.ultsonch.2010.12.010 |
| 17. | Palumbo, C.; Mazzeo, G.; Mazziotta, A.; Gambacorta, A.; Loreto, M. A.; Migliorini, A.; Superchi, S.; Tofani, D.; Gasperi, T. Org. Lett. 2011, 13, 6248–6251. doi:10.1021/ol202646w |
| 18. | Gasperi, T.; Loreto, M. A.; Migliorini, A.; Ventura, C. Eur. J. Org. Chem. 2011, 385–391. doi:10.1002/ejoc.201001021 |
| 19. | Shmidt, M. S.; Perillo, I. A.; González, M.; Blanco, M. M. Tetrahedron Lett. 2012, 53, 2514–2517. doi:10.1016/j.tetlet.2012.03.010 |
| 35. | Chu, Y.; Liu, X.; Li, W.; Hu, X.; Lin, L.; Feng, X. Chem. Sci. 2012, 3, 1996–2000. doi:10.1039/c2sc20218b |
| 10. | Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209–2219. doi:10.1002/ejoc.200300050 |
| 11. | Inoue, M.; Furuyama, H.; Sakazaki, H.; Hirama, M. Org. Lett. 2001, 3, 2863–2865. doi:10.1021/ol016303v |
| 12. | Chouhan, M.; Senwar, K. R.; Sharma, R.; Grover, V.; Nair, V. A. Green Chem. 2011, 13, 2553–2560. doi:10.1039/c1gc15416h |
| 13. | Dandia, A.; Singh, R.; Bhaskaran, S. Ultrason. Sonochem. 2010, 17, 399–402. doi:10.1016/j.ultsonch.2009.08.003 |
| 34. | Joshi, K. C.; Jain, R.; Garg, S. J. Heterocycl. Chem. 1984, 21, 977–979. doi:10.1002/jhet.5570210410 |
| 6. | Kotha, S.; Deb, A. C.; Lahiri, K.; Manivannan, E. Synthesis 2009, 165–193. doi:10.1055/s-0028-1083300 |
| 7. | Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003–3025. doi:10.1055/s-0029-1216975 |
| 8. | Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104–6155. doi:10.1021/cr300135y |
| 9. | Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10, 5165–5181. doi:10.1039/c2ob25184a |
| 35. | Chu, Y.; Liu, X.; Li, W.; Hu, X.; Lin, L.; Feng, X. Chem. Sci. 2012, 3, 1996–2000. doi:10.1039/c2sc20218b |
| 33. | Kobayashi, G.; Furukawa, S.; Matsuda, Y. Yakugaku Zasshi 1966, 86, 1156–1159. |
| 34. | Joshi, K. C.; Jain, R.; Garg, S. J. Heterocycl. Chem. 1984, 21, 977–979. doi:10.1002/jhet.5570210410 |
| 13. | Dandia, A.; Singh, R.; Bhaskaran, S. Ultrason. Sonochem. 2010, 17, 399–402. doi:10.1016/j.ultsonch.2009.08.003 |
| 19. | Shmidt, M. S.; Perillo, I. A.; González, M.; Blanco, M. M. Tetrahedron Lett. 2012, 53, 2514–2517. doi:10.1016/j.tetlet.2012.03.010 |
| 26. | Chuang, C.-P.; Chen, K.-P. Tetrahedron 2012, 68, 1401–1406. doi:10.1016/j.tet.2011.12.035 |
| 27. | Yan, C. G.; Song, X. K.; Wang, Q. F.; Sun, J.; Siemeling, U.; Bruhn, C. Chem. Commun. 2008, 1440–1442. doi:10.1039/b718171j |
| 28. | Wang, Q.-F.; Song, X.-K.; Chen, J.; Yan, C.-G. J. Comb. Chem. 2009, 11, 1007–1010. doi:10.1021/cc900005v |
| 29. | Wang, Q.-F.; Hou, H.; Hui, L.; Yan, C.-G. J. Org. Chem. 2009, 74, 7403–7406. doi:10.1021/jo901379h |
| 30. | Wang, Q.-F.; Hui, L.; Hou, H.; Yan, C.-G. J. Comb. Chem. 2010, 12, 260–265. doi:10.1021/cc900161z |
| 31. | Hou, H.; Zhang, Y.; Yan, C.-G. Chem. Commun. 2012, 48, 4492–4494. doi:10.1039/c2cc30708a |
© 2013 Fu and Yan; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)