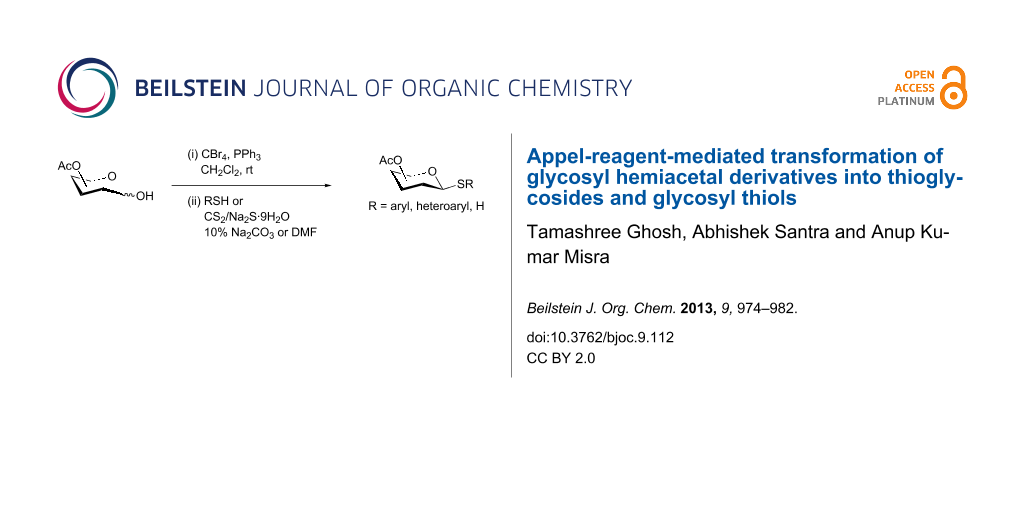
Abstract
A series of glycosyl hemiacetal derivatives have been transformed into thioglycosides and glycosyl thiols in a one-pot two-step reaction sequence mediated by Appel reagent (carbon tetrabromide and triphenylphosphine). 1,2-trans-Thioglycosides and β-glycosyl thiol derivatives were stereoselectively formed by the reaction of the in situ generated glycosyl bromides with thiols and sodium carbonotrithioate. The reaction conditions are reasonably simple and yields were very good.
Graphical Abstract
Introduction
Thioglycosides (1-thiosugar) are widely used glycosyl donors in glycosylation reactions [1-5]. Due to their thermal and chemical stability, they have been used as stable intermediates for functional-group transformations as well as stereoselective glycosylations. Thioglycosides can be transformed into various other glycosyl donors [6-10] (e.g., sulfoxide, sulfone, fluoride, bromide, hemiacetal, etc.) and hence the thio functionality is often used as a temporary anomeric protecting group. Thioglycosides can act as a glycosyl donor as well as glycosyl acceptor depending on the reaction conditions (orthogonal glycosylations) [11,12]. Due to their stability towards enzymatic hydrolysis, several thioglycosides have been evaluated as enzyme inhibitors [13,14]. As a consequence a large number of reports have appeared in the past for the preparation of thioglycosides. Conventionally, thioglycosides are prepared by the reaction of glycosyl acetates with thiols or trimethylsilylthiols in the presence of a Lewis acid (borontrifluoride diethyletherate, stannic chloride, trimethylsilyl trifluoromethanesulfonate, etc.) [15-20]. Other methods for the synthesis of thioglycosides include (a) reduction of disulfides using metallic salts [21,22] or nonmetallic reducing agents (triphenylphosphine or combination of triethylsilane and BF3·OEt2) followed by the reaction of the in situ generated thiolate ions with glycosyl bromides under phase-transfer conditions [23] or in ionic liquids [24]; (b) reaction of glycosyl bromides with thiols under phase-transfer conditions [25]; and (c) conversion of glycosyl acetates and bromides to isothiouronium salts followed by hydrolytic alkylation of isothiouronium salts with alkyl halide in the presence of a base [26,27]. Most of the reactions have several shortcomings, which include formation of an anomeric mixture of the products, instability of the starting materials (glycosyl bromides, etc.), multiple steps, unsatisfactory yields, use of metallic salts, use of expensive reagents, pregeneration of glycosyl isothiouronium salts, etc. Similar to thioglycosides, glycosyl thiol derivatives are useful intermediates for the synthesis of various thiooligosaccharides, glycoproteins and glycolipids [28-32]. The anomeric configurations of glycosyl thiols mostly remain unaffected in comparison to the glycosyl hemiacetal derivatives during their synthetic transformations [4]. Glycosyl thiol derivatives act as precursors for the preparation of several glycosyl donors such as thioglycosides [33,34], glycosyl sulfenamides [35] and sulfonamides [36], glycosyl disulfides [37], glycosyl thionolactones [38], etc. A number of reports are available for the preparation of glycosyl thiols, which include (a) a two-step reaction of glycosyl halide or acetate with thiourea or thioacetate and hydrolysis of the resulting intermediates [26,27]; (b) reaction of hydrogen sulfide gas with glycosyl halides in hydrogen fluoride [39]; (c) treatment of the glycosyl hemiacetal derivatives with Lawesson’s reagent [40] and (d) treatment of 1,6-anhydro sugar derivative [41] and glycosyl trichloroacetimidate derivatives [42] with bis(trimethylsilyl) sulfide. However, most of the reactions have several inherent shortcomings, such as the use of reactive starting materials, longer reaction time, multiple steps, unsatisfactory yield, formation an isomeric mixture, use of expensive reagents, use of hazardous gases, etc. Therefore, the development of convenient reaction conditions for the stereoselective preparation of thioglycosides and glycosyl thiols that are nonhazardous is pertinent. Recently, we reported the stereoselective preparation of β-glycosyl thiol derivatives by the treatment of glycosyl bromide derivatives with the in situ generated sodium carbonotrithioate (a combination of CS2 and Na2S·9H2O) [43]. Although, the reaction is highly stereoselective and high yielding it involves the handling of unstable glycosyl bromide. Therefore, as an extension of the earlier report [43], it was envisioned that the treatment of a stable glycosyl hemiacetal derivative with Appel reagent (carbon tetrabromide (CBr4) and triphenylphosphine (PPh3)) [44] could generate the glycosyl bromide in situ, [45,46] which, on reaction with thiol or sodium carbonotrithioate (generated in situ from CS2 and Na2S·9H2O) [43] in one-pot, could furnish thioglycosides and glycosyl thiol derivatives stereoselectively. We report herein, our findings on the Appel-reagent-mediated transformation of glycosyl hemiacetal derivatives to thioglycosides and glycosyl thiol derivatives (Scheme 1).
Scheme 1: Appel-reagent-mediated transformation of glycosyl hemiacetal derivatives into thioglycosides and β-glycosyl thiol derivatives in one pot.
Scheme 1: Appel-reagent-mediated transformation of glycosyl hemiacetal derivatives into thioglycosides and β-...
Results and Discussion
Initially, 2,3,4,6-tetra-O-acetyl-α,β-D-glucopyranose (1; 1.0 mmol) was treated with a mixture of CBr4 (1.5 mmol) and PPh3 (1.5 mmol) in CH2Cl2 (5 mL) at room temperature. The starting material was consumed in 6 h to give a faster-moving product (acetobromo-α-D-glucose). To the reaction mixture were added thiophenol (1.0 mmol), 10% aq Na2CO3 (5 mL) and tetrabutylammonium bromide (TBAB, catalytic) and the reaction mixture was stirred at room temperature for another 4 h. Aqueous work up of the biphasic reaction mixture furnished phenyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranoside (10) in 80% yield. After a series of experimentation it was observed that the use of 2.0 equiv each of CBr4 and PPh3 led to the full consumption of compound 1 in 4 h, and addition of 1.5 equiv thiophenol and 10% aq Na2CO3 under phase-transfer reaction conditions led to the formation of compound 10 in 88% yield in an additional 4 h (Scheme 1). The reaction was carried out in a set of commonly used organic solvents (e.g., toluene, EtOAc, CH3OH, CH3CN, THF, DMF, DMSO, etc.) and it was observed that the reaction proceeds smoothly in CH2Cl2 and DMF in a similar fashion to the first step. Since CH2Cl2 is a low-boiling-point solvent and can be used directly in the next step, this solvent was chosen in the first step of the reaction (Table 1). Increasing the quantity of the reagents (CBr4 and PPh3) did not change the reaction time and yield significantly. However, reducing the quantity of reagents led to a low yield of conversion in a longer reaction time (Table 2). Under the optimized reaction conditions a series of 1,2-trans-thioglycosides were prepared from various glycosyl hemiacetals in excellent yield (Table 3). A large-scale (5 g) preparation of compound 10 was also achieved under the reaction conditions in similar yield.
Table 1: Screening of solvents for the in situ conversion of glycosyl hemiacetal to glycosyl bromide.
|
|
|||
| Entry | Solvent | Time (h) | Conversion (%) |
|---|---|---|---|
| 1 | CH2Cl2 | 4 | 90 |
| 2 | CH3CN | 10 | 40a |
| 3 | THF | 10 | 40a |
| 4 | DMF | 4 | 85 |
| 5 | EtOAc | 12 | 20a |
| 6 | Toluene | 12 | 10a |
| 7 | DMSO | 5 | 80 |
| 8 | CH3OH | 4 | –b |
aThe rest of the starting material remained unreacted; bthe starting material was degraded.
Table 2: Optimization of the in situ conversion of glycosyl hemiacetal to glycosyl bromide.
|
|
||||
| Entry | CBr4 (equiv) | PPh3 (equiv) | Time (h) | Conversion (%) |
|---|---|---|---|---|
| 1 | 2.5 | 2.5 | 3.5 | 90 |
| 2 | 2.0 | 2.0 | 4 | 90 |
| 3 | 1.5 | 1.5 | 6 | 75a |
| 4 | 1.0 | 1.0 | 7 | 50a |
aThe rest of the starting material remained unreacted.
Table 3: Appel-reagent-mediated transformation of glycosyl hemiacetal derivatives to the thioglycosides at room temperature.
| Entry | Glycosyl hemiacetal | Thioglycoside | Time (h)a | Yield (%) | Ref |
|---|---|---|---|---|---|
| 1 |
1 |
10 |
4 | 88 | [49] |
| 2 |
1 |
11 |
4 | 90 | [50] |
| 3 |
1 |
12 |
4 | 86 | [51] |
| 4 |
1 |
13 |
8 | 82 | [52] |
| 5 |
1 |
14 |
6 | 80 | [23] |
| 6 |
2 |
15 |
4 | 90 | [53] |
| 7 |
2 |
16 |
4 | 88 | [51] |
| 8 |
2 |
17 |
8 | 84 | – |
| 9 |
2 |
18 |
8 | 77 | [54] |
| 10 |
2 |
19 |
6 | 80 | [23] |
| 11 |
3 |
20 |
8 | 76 | – |
| 12 |
3 |
21 |
4 | 88 | [53] |
| 13 |
4 |
22 |
4 | 90 | [55] |
| 14 |
4 |
23 |
8 | 88 | – |
| 15 |
5 |
24 |
4 | 92 | [56] |
| 16 |
6 |
25 |
6 | 85 | [57] |
| 17 |
7 |
26 |
4 | 88 | [25] |
| 18 |
7 |
27 |
4 | 85 | [50] |
| 19 |
8 |
28 |
4 | 88 | [25] |
| 20 |
9 |
29 |
4 | 85 | [25] |
aTime for the second steps after the formation of glycosyl bromides in situ.
In another experiment, compound 1 (1.0 mmol) was treated with a mixture of CBr4 (2.0 mmol) and PPh3 (2.0 mmol) in CH2Cl2 (5 mL) at room temperature for 4 h. Addition of a red-colored solution of CS2 (2.0 mmol) and Na2S·9H2O (2.0 mmol) (sodium carbonotrithioate) in DMF (2 mL, prepared separately) to the reaction mixture furnished 2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranose (30) in 90% yield instantly. After optimization of the reaction condition it was observed that the best yield of compound 30 can be achieved by using CS2 (1.5 mmol) and Na2S·9H2O (2.0 mmol) at room temperature. On reduction of the quantity of CS2 and Na2S·9H2O, the rate of the reaction became very slow and a considerable amount of undesired byproducts were formed. The optimized reaction conditions were applied for the preparation of a series of glycosyl thiols in excellent yield in a stereoselective manner (Table 4). The reaction conditions have several notable features, which include (a) excellent yield; (b) exceptionally high stereoselectivity; (c) one-pot two-step reaction conditions; (d) applicability for scaled-up synthesis. It is worth mentioning that 1,2-trans-thioglycosides and exclusively β-glycosyl thiols were formed under these conditions. The reaction conditions have been applied successfully for the preparation of thioglycosides and glycosyl thiols from D- and L-sugars as well as disaccharides. The stereochemistry of the anomeric centers of the thioglycosides and glycosyl thiols were confirmed from their NMR spectral analysis (coupling constant of the H-1 (J1,2)). Appearance of the coupling constant of the H-1 (J1,2) = 8–10 Hz and coupling constant of the SH group (JH-1,SH) = 9–10 Hz in the 1H NMR spectra of the glycosyl thiols confirmed the exclusive formation of β-glycosyl thiols. In the case of D-mannose and L-rhamnose, exclusive formation of β-products were unambiguously confirmed from the coupling constant at the anomeric center (JC-1,H-1) in the gated 1H coupled 13C NMR spectra. Coupling constant (JC-1,H-1) = 143 Hz and 142 Hz (less than 160 Hz) in the gated 1H coupled 13C NMR spectra of compounds 32 and 33 confirmed the exclusive formation of β-products [47,48]. It is presumed that the reaction of the glycosyl hemiacetal derivative with the combination of CBr4 and PPh3 furnished α-glycosyl bromide in the first step. Interconversion of the α-glycosyl bromide to the reactive β-glycosyl bromide in the presence of a catalytic bromide ion derived from TBAB, led to the formation of a 1,2-oxocarbonium ion by participation of the neighboring group, which finally furnished 1,2-trans-thioglycoside by the reaction of thiols under reasonably slow biphasic reaction conditions. The thioglycoside formation became very slow without the addition of TBAB and the same product was obtained in a poor yield over a much longer period of time. In contrast, rapid SN2-substitution of the bromide ion at the anomeric center in α-glycosyl bromide with a carbonotrithioate ion (derived from the reaction of CS2 and Na2S·9H2O) in homogeneous solution led to the exclusive formation of the β-glycosyl thiol derivative (Scheme 2).
Table 4: Appel-reagent-mediated transformation of glycosyl hemiacetal derivatives to glycosyl thiol derivatives at room temperature.
aTime for the second steps after formation of glycosyl bromides in situ.
Scheme 2: Plausible mechanistic pathways for the formation of 1,2-trans-thioglycoside and β-glycosyl thiol.
Scheme 2: Plausible mechanistic pathways for the formation of 1,2-trans-thioglycoside and β-glycosyl thiol.
Conclusion
In summary, treatment of glycosyl hemiacetal derivatives with Appel reagent followed by reaction with thiols and sodium carbonotrithioate (derived from the reaction of CS2 and Na2S·9H2O) furnished thioglycosides and glycosyl thiols in excellent yield with high stereoselectivity in a two-step, one-pot reaction condition. The reaction condition is operationally simple, mild, reproducible, high-yielding, highly stereoselective, and can be scaled up for large-scale preparation. These reaction conditions may be considered as a valuable addition to those existing in this area.
Experimental
General methods: All reactions were monitored by thin-layer chromatography over silica-gel-coated TLC plates. The spots on TLC were visualized by warming ceric sulfate (2% Ce(SO4)2 in 2 N H2SO4) sprayed plates on a hot plate. Silica gel 230–400 mesh was used for column chromatography. 1H and 13C NMR spectra were recorded on Bruker Avance 500 MHz by using CDCl3 as solvent and TMS as internal standard, unless stated otherwise. Chemical shift values are expressed as δ in parts per million. ESIMS were recorded on a Micromass mass spectrometer. Commercially available grades of organic solvents of adequate purity were used in all reactions.
General experimental conditions for the preparation of thioglycosides
To a solution of glycosyl hemiacetal (1.0 mmol) in dry CH2Cl2 (5 mL) were added CBr4 (2.0 mmol) and PPh3 (2.0 mmol) and the reaction mixture was stirred at room temperature for 4 h. After consumption of the starting material (TLC, hexane–EtOAc 2:1), thiophenol (1.5 mmol), 10% aq. Na2CO3 (5 mL) and TBAB (20 mg) were added to the reaction mixture, and it was stirred for the appropriate time mentioned in Table 3. The reaction mixture was diluted with water and extracted with CH2Cl2 (50mL). The organic layer was washed with water, dried (Na2SO4) and concentrated to give the crude product, which was purified over SiO2 by using hexane–EtOAc as eluant to give the pure product. Known compounds gave spectral data identical to the data reported in the cited references.
1-Phenyl-1H-tetrazol-5-yl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-galactopyranoside (17): Yellow oil; [α]D25 +15 (c 1.2, CHCl3); IR (neat): 3114, 2842, 1612, 1522, 1467, 912, 699 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.58–7.52 (m, 5H, Ar-H), 5.80 (d, J = 10.0 Hz, 1H, H-1), 5.46 (d, J = 3.0 Hz, 1H, H-4), 5.34 (t, J = 10.0 Hz each, 1H, H-2), 5.17 (dd, J = 10.0, 3.5 Hz, 1H, H-3), 4.12–4.10 (m, 2H, H-5, H-6a), 4.08–4.06 (m, 1H, H-6b), 2.15, 2.05, 2.01, 1.99 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3) δ 170.1, 169.9, 169.6, 169.5 (4 COCH3), 133.3–124.1 (Ar-C), 84.2 (C-1), 75.2 (C-3), 71.6 (C-4), 67.1 (C-5), 67.0 (C-2), 60.9 (C-6), 20.7, 20.6, 20.5, 20.4 (4 COCH3); ESIMS (m/z): 531.1 [M + Na]+; Anal. calcd for C21H24N4O9S (508.12): C, 49.60; H, 4.76; found: C, 49.45; H, 4.94.
1-Phenyl-1H-tetrazol-5-yl 2,3,4,6-tetra-O-acetyl-1-thio-α-D-mannopyranoside (20): Yellow oil; [α]D25 −2 (c 1.2, CHCl3); IR (neat): 3104, 2838, 1610, 1520, 1472, 916, 697 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.61–7.50 (m, 5H, Ar-H), 6.12 (br s, 1H, H-1), 5.68 (d, J = 2.5 Hz, 1H, H-2), 5.27 (t, J = 10.0 Hz each, 1H, H-4), 5.19 (dd, J = 10.0, 3.0 Hz, 1H, H-3), 4.31 (dd, J = 12.5, 5.5 Hz, 1H, H-6a), 4.13 (d, J = 12.5 Hz, 1H, H-6b), 3.91–3.88 (m, 1H, H-5), 2.19, 2.07, 2.05, 1.99 (4 s, 12H, 4 COCH3); 13C NMR (125 MHz, CDCl3) δ 170.1, 170.0, 169.7, 169.6 (4 COCH3), 130.5–124.1 (Ar-C), 82.6 (C-1), 77.2 (C-3), 71.5 (C-4), 70.1 (C-5), 65.0 (C-2), 62.0 (C-6), 20.7, 20.6, 20.5, 20.4 (4 COCH3); ESIMS (m/z): 531.1 [M + Na]+; Anal. calcd for C21H24N4O9S (508.12): C, 49.60; H, 4.76; found: C, 49.42; H, 4.97.
1-Phenyl-1H-tetrazol-5-yl 2,3,4-tri-O-acetyl-1-thio-α-L-rhamnopyranoside (23): Yellow oil; [α]D25 +19 (c 1.2, CHCl3); IR (neat): 3106, 2836, 1600, 1502, 1457, 916, 699 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.59–7.50 (m, 5H, Ar-H), 6.10 (d, J = 1.0 Hz, 1H, H-1), 5.66 (d, J = 3.5, 1.0 Hz, 1H, H-2), 5.13 (dd, J = 10.0, 3.0 Hz, 1H, H-3), 5.06 (t, J = 9.5 Hz each, 1H, H-4), 3.80–3.75 (m, 1H, H-5), 2.18, 2.07, 1.97 (3 s, 9H, 3 COCH3), 1.28 (d, J = 6.0 Hz, 3H, CH3); 13C NMR (125 MHz, CDCl3) δ 170.1, 170.0, 169.9 (3 COCH3), 130.4–124.1 (Ar-C), 82.4 (C-1), 75.6 (C-5), 71.4 (C-3), 70.5 (C-4), 69.8 (C-2), 20.7, 20.6, 20.5 (3 COCH3), 17.6 (CH3); ESIMS (m/z): 473.1 [M + Na]+; Anal. calcd for C19H22N4O7S (450.12): C, 50.66; H, 4.92; found: C, 50.47; H, 5.15.
General experimental condition for the preparation of glycosyl thiol derivatives
To a solution of glycosyl hemiacetal (1.0 mmol) in dry CH2Cl2 (5 mL) were added CBr4 (2.0 mmol) and PPh3 (2.0 mmol), and the reaction mixture was stirred for 4 h at room temperature. After completion of the reaction (TLC; hexane–EtOAc 2:1), a red premixed solution of CS2 (1.5 mmol) and Na2S·9H2O (2.0 mmol) in DMF (2 mL) was added to the reaction mixture and it was stirred for the appropriate time mentioned in Table 4. The reaction mixture was diluted with water and extracted with CH2Cl2 (50 mL). The organic layer was washed with water, dried (Na2SO4) and concentrated to give the crude product, which was purified over SiO2 by using hexane–EtOAc as eluant to give the pure product. Known compounds gave spectral data identical to the data reported in the cited references.
Supporting Information
| Supporting Information File 1: Analytical data of compounds 10–16, 18, 19, 21, 22, 24-36 and 1H NMR, 13C NMR spectra of compounds 17, 20, and 23. | ||
| Format: PDF | Size: 1.0 MB | Download |
References
-
Kartha, K. P. R.; Field, R. A. In Best Synthetic Methods: Carbohydrates; Osborn, H. M. I., Ed.; Academic Press: Oxford, UK, 2003; pp 121–145.
Return to citation in text: [1] -
Nicolaou, K. C.; Ueno, H. In Preparative Carbohydrate Chemistry; Hanessian, S., Ed.; Marcel Dekker Inc.: New York, 1997; pp 313–338.
Return to citation in text: [1] -
Oscarson, S. In Carbohydrates in Chemistry and Biology; Ernst, B.; Hart, G. W.; Sinaÿ, P., Eds.; Wiley-VCH: Weinheim, Germany, 2000; Vol. 1, pp 93–116.
Return to citation in text: [1] -
Garegg, P. J. Adv. Carbohydr. Chem. Biochem. 1997, 52, 179–205. doi:10.1016/S0065-2318(08)60091-8
Return to citation in text: [1] [2] -
Toshima, K.; Tatsuta, K. Chem. Rev. 1993, 93, 1503–1531. doi:10.1021/cr00020a006
Return to citation in text: [1] -
Codée, J. D. C.; Litjens, R. E. J. N.; van den Bos, L. J.; Overkleeft, H. S.; van der Marel, G. A. Chem. Soc. Rev. 2005, 34, 769–782. doi:10.1039/b417138c
Return to citation in text: [1] -
Ferrier, R. J.; Furneaux, R. H. Methods of Carbohydrate Chemistry; Academic Press: New York, 1980; Vol. 8, pp 251–253.
Return to citation in text: [1] -
Fügedi, P.; Garegg, P. J.; Lönn, H.; Norberg, T. Glycoconjugate J. 1987, 4, 97–108. doi:10.1007/BF01049447
Return to citation in text: [1] -
Lear, M. J.; Yoshimura, F.; Hirama, M. Angew. Chem., Int. Ed. 2001, 40, 946–949. doi:10.1002/1521-3773(20010302)40:5<946::AID-ANIE946>3.0.CO;2-G
Return to citation in text: [1] -
Nicolaou, K. C.; Bockovich, N. J.; Carcanague, D. R.; Hummel, C. W.; Even, L. F. J. Am. Chem. Soc. 1992, 114, 8701–8702. doi:10.1021/ja00048a054
Return to citation in text: [1] -
Kanie, O.; Ito, Y.; Ogawa, T. J. Am. Chem. Soc. 1994, 116, 12073–12074. doi:10.1021/ja00105a066
Return to citation in text: [1] -
Kaeothip, S.; Demchenko, A. V. J. Org. Chem. 2011, 76, 7388–7398. doi:10.1021/jo201117s
Return to citation in text: [1] -
Khan, S. H.; Hindsgaul, O. In Molecular Glycobiology; Fukuda, M.; Hindsgaul, O., Eds.; IRL: Oxford, 1994; pp 206–229.
Return to citation in text: [1] -
Witczak, Z. J.; Culhane, J. M. Appl. Microbiol. Biotechnol. 2005, 69, 237–244. doi:10.1007/s00253-005-0156-x
Return to citation in text: [1] -
Agnihotri, G.; Tiwari, P.; Misra, A. K. Carbohydr. Res. 2005, 340, 1393–1396. doi:10.1016/j.carres.2005.02.027
Return to citation in text: [1] -
Das, S. K.; Roy, N. Carbohydr. Res. 1996, 296, 275–277. doi:10.1016/S0008-6215(96)00235-2
Return to citation in text: [1] -
Pozsgay, V.; Jennings, H. J. Tetrahedron Lett. 1987, 28, 1375–1376. doi:10.1016/S0040-4039(00)95930-6
Return to citation in text: [1] -
Weng, S.-S.; Lin, Y.-D.; Chen, C.-T. Org. Lett. 2006, 8, 5633–5636. doi:10.1021/ol062375g
Return to citation in text: [1] -
Kihlberg, J. O.; Leigh, D. A.; Bundle, D. R. J. Org. Chem. 1990, 55, 2860–2863. doi:10.1021/jo00296a055
Return to citation in text: [1] -
Hanessian, S.; Guindon, Y. Carbohydr. Res. 1980, 86, C3–C6. doi:10.1016/S0008-6215(00)85913-3
Return to citation in text: [1] -
Mukherjee, C.; Tiwari, P.; Misra, A. K. Tetrahedron Lett. 2006, 47, 441–445. doi:10.1016/j.tetlet.2005.11.074
Return to citation in text: [1] -
Sridhar, P. R.; Prabhu, K. R.; Chandrasekaran, S. Eur. J. Org. Chem. 2004, 4809–4815. doi:10.1002/ejoc.200400360
Return to citation in text: [1] -
Mukherjee, C.; Misra, A. K. J. Carbohydr. Chem. 2007, 26, 213–221. doi:10.1080/07328300701410635
Return to citation in text: [1] [2] [3] -
Sau, A.; Misra, A. K. Synlett 2011, 1905–1911. doi:10.1055/s-0030-1260966
Return to citation in text: [1] -
Tropper, F. D.; Andersson, F. O.; Grand-Maître, C.; Roy, R. Synthesis 1991, 734–736. doi:10.1055/s-1991-26559
Return to citation in text: [1] [2] [3] [4] -
Ibatullin, F. M.; Shabalin, K. A.; Jänis, J. V.; Shavva, A. G. Tetrahedron Lett. 2003, 44, 7961–7964. doi:10.1016/j.tetlet.2003.08.120
Return to citation in text: [1] [2] -
Tiwari, P.; Agnihotri, G.; Misra, A. K. J. Carbohydr. Chem. 2005, 24, 723–732. doi:10.1080/07328300500256775
Return to citation in text: [1] [2] -
Rendle, P. M.; Seger, A.; Rodrigues, J.; Oldham, N. J.; Bott, R. R.; Jones, J. B.; Cowan, M. M.; Davis, B. G. J. Am. Chem. Soc. 2004, 126, 4750–4751. doi:10.1021/ja031698u
Return to citation in text: [1] -
Watt, G. M.; Lund, J.; Levens, M.; Kolli, V. S. K.; Jefferis, R.; Boons, G.-J. Chem. Biol. 2003, 10, 807–814. doi:10.1016/j.chembiol.2003.08.006
Return to citation in text: [1] -
Bernardes, G. J. L.; Chalker, J. M.; Errey, J. C.; Davis, B. G. J. Am. Chem. Soc. 2008, 130, 5052–5053. doi:10.1021/ja800800p
Return to citation in text: [1] -
Chalker, J. M.; Lercher, L.; Rose, N. R.; Schofield, C. J.; Davis, B. G. Angew. Chem., Int. Ed. 2012, 51, 1835–1839. doi:10.1002/anie.201106432
Return to citation in text: [1] -
Chalker, J. M.; Gunnoo, S. B.; Boutureira, O.; Gerstberger, S. C.; Fernández-González, M.; Bernardes, G. J. L.; Griffin, L.; Hailu, H.; Schofield, C. J.; Davis, B. G. Chem. Sci. 2011, 2, 1666–1676. doi:10.1039/c1sc00185j
Return to citation in text: [1] -
Ibatullin, F. M.; Selivanov, S. I.; Shavva, A. G. Synthesis 2001, 419–422. doi:10.1055/s-2001-11443
Return to citation in text: [1] -
Ibatullin, F. M.; Shabalin, K. A.; Jänis, J. V.; Selivanov, S. I. Tetrahedron Lett. 2001, 42, 4565–4567. doi:10.1016/S0040-4039(01)00775-4
Return to citation in text: [1] -
Lopez, M.; Drillaud, N.; Bornaghi, L. F.; Poulsen, S.-A. J. Org. Chem. 2009, 74, 2811–2816. doi:10.1021/jo9000367
Return to citation in text: [1] -
Colinas, P. A. Curr. Org. Chem. 2012, 16, 1670–1679. doi:10.2174/138527212800840892
Return to citation in text: [1] -
Bernardes, G. J. L.; Grayson, E. J.; Thompson, S.; Chalker, J. M.; Errey, J. C.; El Qualid, F.; Claridge, T. D. W.; Davis, B. G. Angew. Chem., Int. Ed. 2008, 47, 2244–2247. doi:10.1002/anie.200704381
Return to citation in text: [1] -
Wilkinson, B. L.; Fairbanks, A. J. Tetrahedron Lett. 2008, 49, 4941–4943. doi:10.1016/j.tetlet.2008.05.145
Return to citation in text: [1] -
Defaye, J.; Gadelle, A.; Pedersen, C. Carbohydr. Res. 1991, 217, 51–58. doi:10.1016/0008-6215(91)84116-V
Return to citation in text: [1] -
Bernardes, G. J. L.; Gamblin, D. P.; Davis, B. G. Angew. Chem., Int. Ed. 2006, 45, 4007–4011. doi:10.1002/anie.200600685
Return to citation in text: [1] -
Zhu, X.; Dere, R. T.; Jiang, J.; Zhang, L.; Wang, X. J. Org. Chem. 2011, 76, 10187–10197. doi:10.1021/jo202069y
Return to citation in text: [1] [2] [3] -
Dere, R. T.; Kumar, A.; Kumar, V.; Zhu, X.; Schmidt, R. R. J. Org. Chem. 2011, 76, 7539–7545. doi:10.1021/jo200624e
Return to citation in text: [1] -
Jana, M.; Misra, A. K. J. Org. Chem. 2013, 78, 2680–2686. doi:10.1021/jo302115k
Return to citation in text: [1] [2] [3] -
Appel, R. Angew. Chem., Int. Ed. Engl. 1975, 14, 801–811. doi:10.1002/anie.197508011
Return to citation in text: [1] -
Nishida, Y.; Shingu, Y.; Dohi, H.; Kobayashi, K. Org. Lett. 2003, 5, 2377–2380. doi:10.1021/ol034269+
Return to citation in text: [1] -
Shingu, Y.; Nishida, Y.; Dohi, H.; Kobayashi, K. Org. Biomol. Chem. 2003, 1, 2518–2521. doi:10.1039/b303984f
Return to citation in text: [1] -
Bock, K.; Pederson, C. J. Chem. Soc., Perkin Trans. 2 1974, 293–297. doi:10.1039/p29740000293
Return to citation in text: [1] -
Crich, D.; Li, H. J. Org. Chem. 2002, 67, 4640–4646. doi:10.1021/jo0108818
Return to citation in text: [1] -
Dasgupta, F.; Garegg, P. J. Acta Chem. Scand. 1989, 43, 471–475. doi:10.3891/acta.chem.scand.43-0471
Return to citation in text: [1] -
Santra, A.; Sau, A.; Misra, A. K. J. Carbohydr. Chem. 2011, 30, 85–93. doi:10.1080/07328303.2011.605195
Return to citation in text: [1] [2] -
Tai, C.-A.; Kulkarni, S. S.; Hung, S.-C. J. Org. Chem. 2003, 68, 8719–8722. doi:10.1021/jo030073b
Return to citation in text: [1] [2] -
Couri, M. R.; Luduvico, I.; Santos, L.; Alves, R.; Prado, M. A.; Gil, R. F. Carbohydr. Res. 2007, 342, 1096–1100. doi:10.1016/j.carres.2007.02.007
Return to citation in text: [1] -
Khiar, N.; Martin-Lomas, M. J. Org. Chem. 1995, 60, 7017–7021. doi:10.1021/jo00126a065
Return to citation in text: [1] [2] -
Khodair, A. I.; Al-Masoudi, N. A.; Gesson, J.-P. Nucleosides, Nucleotides Nucleic Acids 2003, 22, 2061–2076. doi:10.1081/NCN-120026407
Return to citation in text: [1] -
Pozsgay, V.; Jennings, H. J. J. Org. Chem. 1988, 53, 4042–4052. doi:10.1021/jo00252a030
Return to citation in text: [1] -
Komba, S.; Ishida, H.; Kiso, M.; Hasegawa, A. Bioorg. Med. Chem. 1996, 4, 1833–1847. doi:10.1016/S0968-0896(96)00165-4
Return to citation in text: [1] -
Oturam, M. A.; Medebielle, M.; Patil, S. A.; Klein, R. S. Turk. J. Chem. 2002, 26, 317–322.
Return to citation in text: [1] -
Černy, M.; Staněk, J.; Pacák, J. Monatsh. Chem. 1963, 94, 290–294. doi:10.1007/BF00900251
Return to citation in text: [1] -
Fiore, M.; Marra, A.; Dondoni, A. J. Org. Chem. 2009, 74, 4422–4425. doi:10.1021/jo900514w
Return to citation in text: [1] -
Ponpipom, M. M.; Bugianesi, R. L.; Blake, T. J. J. Med. Chem. 1987, 30, 705–710. doi:10.1021/jm00387a021
Return to citation in text: [1] -
Meng, X.-B.; Yang, L.-D.; Li, H.; Li, Q.; Cheng, T.-M.; Cai, M.-S.; Li, Z.-J. Carbohydr. Res. 2002, 337, 977–981. doi:10.1016/S0008-6215(02)00094-0
Return to citation in text: [1] -
Fujihira, T.; Chida, M.; Kamijo, H.; Takido, T.; Seno, M. J. Carbohydr. Chem. 2002, 21, 287–292. doi:10.1081/CAR-120013495
Return to citation in text: [1]
| 49. | Dasgupta, F.; Garegg, P. J. Acta Chem. Scand. 1989, 43, 471–475. doi:10.3891/acta.chem.scand.43-0471 |
| 50. | Santra, A.; Sau, A.; Misra, A. K. J. Carbohydr. Chem. 2011, 30, 85–93. doi:10.1080/07328303.2011.605195 |
| 51. | Tai, C.-A.; Kulkarni, S. S.; Hung, S.-C. J. Org. Chem. 2003, 68, 8719–8722. doi:10.1021/jo030073b |
| 53. | Khiar, N.; Martin-Lomas, M. J. Org. Chem. 1995, 60, 7017–7021. doi:10.1021/jo00126a065 |
| 55. | Pozsgay, V.; Jennings, H. J. J. Org. Chem. 1988, 53, 4042–4052. doi:10.1021/jo00252a030 |
| 54. | Khodair, A. I.; Al-Masoudi, N. A.; Gesson, J.-P. Nucleosides, Nucleotides Nucleic Acids 2003, 22, 2061–2076. doi:10.1081/NCN-120026407 |
| 23. | Mukherjee, C.; Misra, A. K. J. Carbohydr. Chem. 2007, 26, 213–221. doi:10.1080/07328300701410635 |
| 53. | Khiar, N.; Martin-Lomas, M. J. Org. Chem. 1995, 60, 7017–7021. doi:10.1021/jo00126a065 |
| 51. | Tai, C.-A.; Kulkarni, S. S.; Hung, S.-C. J. Org. Chem. 2003, 68, 8719–8722. doi:10.1021/jo030073b |
| 52. | Couri, M. R.; Luduvico, I.; Santos, L.; Alves, R.; Prado, M. A.; Gil, R. F. Carbohydr. Res. 2007, 342, 1096–1100. doi:10.1016/j.carres.2007.02.007 |
| 23. | Mukherjee, C.; Misra, A. K. J. Carbohydr. Chem. 2007, 26, 213–221. doi:10.1080/07328300701410635 |
| 56. | Komba, S.; Ishida, H.; Kiso, M.; Hasegawa, A. Bioorg. Med. Chem. 1996, 4, 1833–1847. doi:10.1016/S0968-0896(96)00165-4 |
| 57. | Oturam, M. A.; Medebielle, M.; Patil, S. A.; Klein, R. S. Turk. J. Chem. 2002, 26, 317–322. |
| 25. | Tropper, F. D.; Andersson, F. O.; Grand-Maître, C.; Roy, R. Synthesis 1991, 734–736. doi:10.1055/s-1991-26559 |
| 59. | Fiore, M.; Marra, A.; Dondoni, A. J. Org. Chem. 2009, 74, 4422–4425. doi:10.1021/jo900514w |
| 60. | Ponpipom, M. M.; Bugianesi, R. L.; Blake, T. J. J. Med. Chem. 1987, 30, 705–710. doi:10.1021/jm00387a021 |
| 41. | Zhu, X.; Dere, R. T.; Jiang, J.; Zhang, L.; Wang, X. J. Org. Chem. 2011, 76, 10187–10197. doi:10.1021/jo202069y |
| 58. | Černy, M.; Staněk, J.; Pacák, J. Monatsh. Chem. 1963, 94, 290–294. doi:10.1007/BF00900251 |
| 25. | Tropper, F. D.; Andersson, F. O.; Grand-Maître, C.; Roy, R. Synthesis 1991, 734–736. doi:10.1055/s-1991-26559 |
| 47. | Bock, K.; Pederson, C. J. Chem. Soc., Perkin Trans. 2 1974, 293–297. doi:10.1039/p29740000293 |
| 48. | Crich, D.; Li, H. J. Org. Chem. 2002, 67, 4640–4646. doi:10.1021/jo0108818 |
| 50. | Santra, A.; Sau, A.; Misra, A. K. J. Carbohydr. Chem. 2011, 30, 85–93. doi:10.1080/07328303.2011.605195 |
| 25. | Tropper, F. D.; Andersson, F. O.; Grand-Maître, C.; Roy, R. Synthesis 1991, 734–736. doi:10.1055/s-1991-26559 |
| 61. | Meng, X.-B.; Yang, L.-D.; Li, H.; Li, Q.; Cheng, T.-M.; Cai, M.-S.; Li, Z.-J. Carbohydr. Res. 2002, 337, 977–981. doi:10.1016/S0008-6215(02)00094-0 |
| 62. | Fujihira, T.; Chida, M.; Kamijo, H.; Takido, T.; Seno, M. J. Carbohydr. Chem. 2002, 21, 287–292. doi:10.1081/CAR-120013495 |
| 41. | Zhu, X.; Dere, R. T.; Jiang, J.; Zhang, L.; Wang, X. J. Org. Chem. 2011, 76, 10187–10197. doi:10.1021/jo202069y |
| 1. | Kartha, K. P. R.; Field, R. A. In Best Synthetic Methods: Carbohydrates; Osborn, H. M. I., Ed.; Academic Press: Oxford, UK, 2003; pp 121–145. |
| 2. | Nicolaou, K. C.; Ueno, H. In Preparative Carbohydrate Chemistry; Hanessian, S., Ed.; Marcel Dekker Inc.: New York, 1997; pp 313–338. |
| 3. | Oscarson, S. In Carbohydrates in Chemistry and Biology; Ernst, B.; Hart, G. W.; Sinaÿ, P., Eds.; Wiley-VCH: Weinheim, Germany, 2000; Vol. 1, pp 93–116. |
| 4. | Garegg, P. J. Adv. Carbohydr. Chem. Biochem. 1997, 52, 179–205. doi:10.1016/S0065-2318(08)60091-8 |
| 5. | Toshima, K.; Tatsuta, K. Chem. Rev. 1993, 93, 1503–1531. doi:10.1021/cr00020a006 |
| 15. | Agnihotri, G.; Tiwari, P.; Misra, A. K. Carbohydr. Res. 2005, 340, 1393–1396. doi:10.1016/j.carres.2005.02.027 |
| 16. | Das, S. K.; Roy, N. Carbohydr. Res. 1996, 296, 275–277. doi:10.1016/S0008-6215(96)00235-2 |
| 17. | Pozsgay, V.; Jennings, H. J. Tetrahedron Lett. 1987, 28, 1375–1376. doi:10.1016/S0040-4039(00)95930-6 |
| 18. | Weng, S.-S.; Lin, Y.-D.; Chen, C.-T. Org. Lett. 2006, 8, 5633–5636. doi:10.1021/ol062375g |
| 19. | Kihlberg, J. O.; Leigh, D. A.; Bundle, D. R. J. Org. Chem. 1990, 55, 2860–2863. doi:10.1021/jo00296a055 |
| 20. | Hanessian, S.; Guindon, Y. Carbohydr. Res. 1980, 86, C3–C6. doi:10.1016/S0008-6215(00)85913-3 |
| 36. | Colinas, P. A. Curr. Org. Chem. 2012, 16, 1670–1679. doi:10.2174/138527212800840892 |
| 13. | Khan, S. H.; Hindsgaul, O. In Molecular Glycobiology; Fukuda, M.; Hindsgaul, O., Eds.; IRL: Oxford, 1994; pp 206–229. |
| 14. | Witczak, Z. J.; Culhane, J. M. Appl. Microbiol. Biotechnol. 2005, 69, 237–244. doi:10.1007/s00253-005-0156-x |
| 37. | Bernardes, G. J. L.; Grayson, E. J.; Thompson, S.; Chalker, J. M.; Errey, J. C.; El Qualid, F.; Claridge, T. D. W.; Davis, B. G. Angew. Chem., Int. Ed. 2008, 47, 2244–2247. doi:10.1002/anie.200704381 |
| 11. | Kanie, O.; Ito, Y.; Ogawa, T. J. Am. Chem. Soc. 1994, 116, 12073–12074. doi:10.1021/ja00105a066 |
| 12. | Kaeothip, S.; Demchenko, A. V. J. Org. Chem. 2011, 76, 7388–7398. doi:10.1021/jo201117s |
| 33. | Ibatullin, F. M.; Selivanov, S. I.; Shavva, A. G. Synthesis 2001, 419–422. doi:10.1055/s-2001-11443 |
| 34. | Ibatullin, F. M.; Shabalin, K. A.; Jänis, J. V.; Selivanov, S. I. Tetrahedron Lett. 2001, 42, 4565–4567. doi:10.1016/S0040-4039(01)00775-4 |
| 6. | Codée, J. D. C.; Litjens, R. E. J. N.; van den Bos, L. J.; Overkleeft, H. S.; van der Marel, G. A. Chem. Soc. Rev. 2005, 34, 769–782. doi:10.1039/b417138c |
| 7. | Ferrier, R. J.; Furneaux, R. H. Methods of Carbohydrate Chemistry; Academic Press: New York, 1980; Vol. 8, pp 251–253. |
| 8. | Fügedi, P.; Garegg, P. J.; Lönn, H.; Norberg, T. Glycoconjugate J. 1987, 4, 97–108. doi:10.1007/BF01049447 |
| 9. | Lear, M. J.; Yoshimura, F.; Hirama, M. Angew. Chem., Int. Ed. 2001, 40, 946–949. doi:10.1002/1521-3773(20010302)40:5<946::AID-ANIE946>3.0.CO;2-G |
| 10. | Nicolaou, K. C.; Bockovich, N. J.; Carcanague, D. R.; Hummel, C. W.; Even, L. F. J. Am. Chem. Soc. 1992, 114, 8701–8702. doi:10.1021/ja00048a054 |
| 35. | Lopez, M.; Drillaud, N.; Bornaghi, L. F.; Poulsen, S.-A. J. Org. Chem. 2009, 74, 2811–2816. doi:10.1021/jo9000367 |
| 25. | Tropper, F. D.; Andersson, F. O.; Grand-Maître, C.; Roy, R. Synthesis 1991, 734–736. doi:10.1055/s-1991-26559 |
| 28. | Rendle, P. M.; Seger, A.; Rodrigues, J.; Oldham, N. J.; Bott, R. R.; Jones, J. B.; Cowan, M. M.; Davis, B. G. J. Am. Chem. Soc. 2004, 126, 4750–4751. doi:10.1021/ja031698u |
| 29. | Watt, G. M.; Lund, J.; Levens, M.; Kolli, V. S. K.; Jefferis, R.; Boons, G.-J. Chem. Biol. 2003, 10, 807–814. doi:10.1016/j.chembiol.2003.08.006 |
| 30. | Bernardes, G. J. L.; Chalker, J. M.; Errey, J. C.; Davis, B. G. J. Am. Chem. Soc. 2008, 130, 5052–5053. doi:10.1021/ja800800p |
| 31. | Chalker, J. M.; Lercher, L.; Rose, N. R.; Schofield, C. J.; Davis, B. G. Angew. Chem., Int. Ed. 2012, 51, 1835–1839. doi:10.1002/anie.201106432 |
| 32. | Chalker, J. M.; Gunnoo, S. B.; Boutureira, O.; Gerstberger, S. C.; Fernández-González, M.; Bernardes, G. J. L.; Griffin, L.; Hailu, H.; Schofield, C. J.; Davis, B. G. Chem. Sci. 2011, 2, 1666–1676. doi:10.1039/c1sc00185j |
| 4. | Garegg, P. J. Adv. Carbohydr. Chem. Biochem. 1997, 52, 179–205. doi:10.1016/S0065-2318(08)60091-8 |
| 23. | Mukherjee, C.; Misra, A. K. J. Carbohydr. Chem. 2007, 26, 213–221. doi:10.1080/07328300701410635 |
| 21. | Mukherjee, C.; Tiwari, P.; Misra, A. K. Tetrahedron Lett. 2006, 47, 441–445. doi:10.1016/j.tetlet.2005.11.074 |
| 22. | Sridhar, P. R.; Prabhu, K. R.; Chandrasekaran, S. Eur. J. Org. Chem. 2004, 4809–4815. doi:10.1002/ejoc.200400360 |
| 26. | Ibatullin, F. M.; Shabalin, K. A.; Jänis, J. V.; Shavva, A. G. Tetrahedron Lett. 2003, 44, 7961–7964. doi:10.1016/j.tetlet.2003.08.120 |
| 27. | Tiwari, P.; Agnihotri, G.; Misra, A. K. J. Carbohydr. Chem. 2005, 24, 723–732. doi:10.1080/07328300500256775 |
| 39. | Defaye, J.; Gadelle, A.; Pedersen, C. Carbohydr. Res. 1991, 217, 51–58. doi:10.1016/0008-6215(91)84116-V |
| 38. | Wilkinson, B. L.; Fairbanks, A. J. Tetrahedron Lett. 2008, 49, 4941–4943. doi:10.1016/j.tetlet.2008.05.145 |
| 26. | Ibatullin, F. M.; Shabalin, K. A.; Jänis, J. V.; Shavva, A. G. Tetrahedron Lett. 2003, 44, 7961–7964. doi:10.1016/j.tetlet.2003.08.120 |
| 27. | Tiwari, P.; Agnihotri, G.; Misra, A. K. J. Carbohydr. Chem. 2005, 24, 723–732. doi:10.1080/07328300500256775 |
| 45. | Nishida, Y.; Shingu, Y.; Dohi, H.; Kobayashi, K. Org. Lett. 2003, 5, 2377–2380. doi:10.1021/ol034269+ |
| 46. | Shingu, Y.; Nishida, Y.; Dohi, H.; Kobayashi, K. Org. Biomol. Chem. 2003, 1, 2518–2521. doi:10.1039/b303984f |
| 43. | Jana, M.; Misra, A. K. J. Org. Chem. 2013, 78, 2680–2686. doi:10.1021/jo302115k |
| 43. | Jana, M.; Misra, A. K. J. Org. Chem. 2013, 78, 2680–2686. doi:10.1021/jo302115k |
| 44. | Appel, R. Angew. Chem., Int. Ed. Engl. 1975, 14, 801–811. doi:10.1002/anie.197508011 |
| 42. | Dere, R. T.; Kumar, A.; Kumar, V.; Zhu, X.; Schmidt, R. R. J. Org. Chem. 2011, 76, 7539–7545. doi:10.1021/jo200624e |
| 43. | Jana, M.; Misra, A. K. J. Org. Chem. 2013, 78, 2680–2686. doi:10.1021/jo302115k |
| 40. | Bernardes, G. J. L.; Gamblin, D. P.; Davis, B. G. Angew. Chem., Int. Ed. 2006, 45, 4007–4011. doi:10.1002/anie.200600685 |
| 41. | Zhu, X.; Dere, R. T.; Jiang, J.; Zhang, L.; Wang, X. J. Org. Chem. 2011, 76, 10187–10197. doi:10.1021/jo202069y |
© 2013 Ghosh et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)