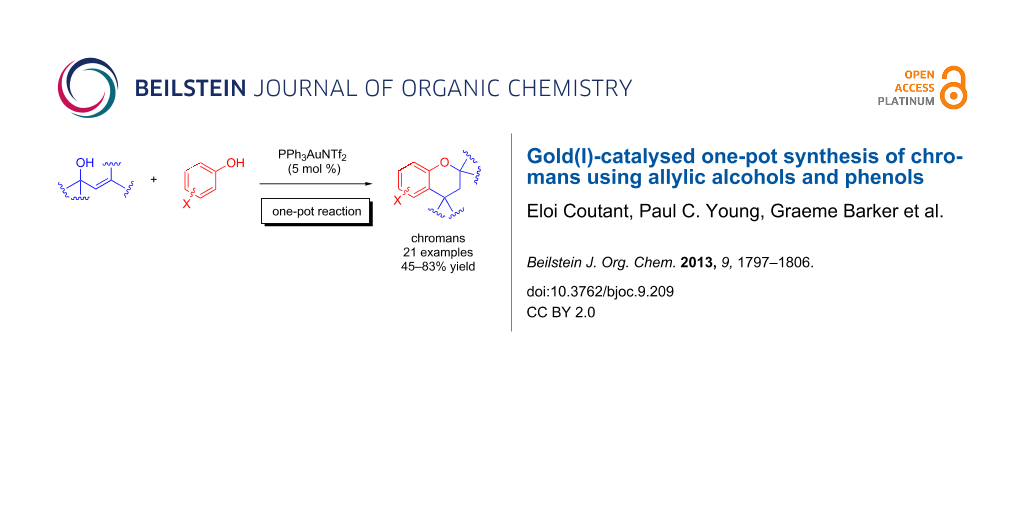
Abstract
A gold(I)-catalysed reaction of allylic alcohols and phenols produces chromans regioselectively via a one-pot Friedel–Crafts allylation/intramolecular hydroalkoxylation sequence. The reaction is mild, practical and tolerant of a wide variety of substituents on the phenol.
Graphical Abstract
Introduction
Chromans (dihydrobenzopyrans) are important and ubiquitous structural motifs found in a variety of important biologically active natural products such as vitamin E and flavanoids [1-5]. One approach towards chromans [6-12], which is biosynthetically inspired, is the Friedel–Crafts allylation [13] of phenols followed by cyclisation of the allylated phenol intermediate (via hydroalkoxylation). Initially, traditional allylating reagents such as allylic acetates were employed in Friedel–Crafts allylations [14], but more recently, there has been a distinct drive towards utilising more environmentally benign allylic alcohols (via a direct dehydrative coupling strategy) [15,16]. To this end, the use of molybdenum catalyst CpMoCl(CO)3 together with an oxidant, o-chloranil, has been documented to catalyse the reaction of allylic alcohols with phenols to form chromans [17,18]. Strong and superacids have also been utilised in the synthesis of chroman-containing targets [19-21]. Nevertheless, there are still a few drawbacks with these methods, for example, they usually require a large excess of substrate (e.g. ~30-fold excess), and in the case of acid catalysis, also poor yields when the phenol is not para-substituted. Therefore, it would be desirable to have a milder method which is compatible with a wide range of substituted phenols.
As part of our continued interest in developing new gold-catalysed [22-41] reactions [42-51], we have recently shown that gold(I) can catalyse a direct allylic etherification [52-59] of unactivated alcohols 2 with unactivated allylic alcohols 1 (Scheme 1, reaction 1) [60,61]. The reaction is mild, regioselective, and produces only water as a byproduct. During our studies, a wide range of primary, secondary and tertiary alcohols were successfully employed as nucleophiles [61-72], but our one attempt employing a phenol 5 as a nucleophile surprisingly produced chroman 6 instead (Scheme 1, reaction 2). Although gold(III)-catalysed Friedel–Crafts allylation of phenols has been reported by Chan and co-workers [73], there have been no reports on the direct synthesis of chromans [74] using gold catalysis [75] with phenols and allylic alcohols prior to our example shown in Scheme 1.
Scheme 1: Previous work on direct allylic etherification of allylic alcohols.
Scheme 1: Previous work on direct allylic etherification of allylic alcohols.
Since the reaction is very practical: distilled solvents and inert air atmosphere are not required, and the chroman is formed directly in a mild one-pot procedure with only water as the byproduct, we decided to investigate the chroman-forming reaction in more detail, beyond the sole example previously reported (Scheme 1, reaction 2). In this paper, we present our further studies on this one-pot chroman synthesis: improving the yield of the desired chroman and exploring the substrate scope.
Results and Discussion
To commence our studies, we first investigated the possibility of lowering the reaction temperature and used equivalents of phenol nucleophile. Suspecting that the moderate yield of 6 in Scheme 1 is due to the slight volatility of 6, an allylic alcohol 7 with a higher molecular weight was chosen as the model substrate in order to avoid any issues of volatility with the chroman products. As shown in Table 1, the standard conditions (50 °C, 5 equiv phenol 5) pleasingly provide a good 65% yield of the desired chroman 8 (Table 1, entry 1). Reducing the temperature is unfortunately detrimental to the formation of chroman: 40 °C gives a lower 59% yield of 8, as well as Friedel–Crafts allylation products 9 and 10, whereas 30 °C provides no chroman 8 at all, instead yielding only Friedel–Crafts products 9 and 10 (Table 1, entries 2 and 3). The ortho- and para-Friedel–Crafts products 9 and 10 are formed via formal SN2' regioselectivity and 9 is presumably the intermediate towards chroman 8 (vide infra). Thus, the higher temperature is clearly necessary to force the in situ cyclisation of 9 to 8.
Table 1: Initial temperature and equivalents screens, and control reactions.
|
|
||||||
| Entry | Equiv 5 | Temp. (°C) | Catalyst | 8 (%) | 9 (%) | 10 (%) |
|---|---|---|---|---|---|---|
| 1 | 5 | 50 | PPh3AuNTf2 | 65a | - | – |
| 2 | 5 | 40 | PPh3AuNTf2 | 59b | 10b | 20b |
| 3 | 5 | 30 | PPh3AuNTf2 | – | 66b | 27b |
| 4 | 2 | 50 | PPh3AuNTf2 | – | 63b | 22b |
| 5 | 1 | 50 | PPh3AuNTf2 | – | 50b | 19b |
| 6 | 3 | 60 | PPh3AuNTf2 | 63b | – | – |
| 7 | 4 | 60 | PPh3AuNTf2 | 60b | – | – |
| 8 | 5 | 50 | No catalyst | – | – | – |
| 9 | 5 | 50 | HNTf2 | 21a | – | – |
| 10 | 5 | 50 | AgNTf2 | – | 70b | 25b |
aIsolated yield. bYield obtained using 1H NMR analysis with 1,2,4,5-tetrachloro-3-nitrobenzene as internal standard.
Next, lower equivalents of phenol 5 were investigated. Unfortunately, dropping the equivalents of phenol also appears to be detrimental to chroman formation: only Friedel–Crafts products 9 and 10 are observed with 2 or 1 equivalents of phenol (Table 1, entries 4 and 5). Pleasingly however, lower equivalents of phenol are tolerated if the temperature is increased to 60 °C (Table 1, entries 6 and 7).
In order to ascertain if the gold(I) catalyst is really necessary for the formation of chroman 8, several control reactions were carried out (Table 1, entries 8–10). Firstly, no reaction is observed in the absence of a catalyst (Table 1, entry 8). The Brønsted acid catalyst HNTf2 does form chroman 8, but in a poorer isolated yield (21%, Table 1, entry 9) and the silver salt [76] AgNTf2 as a catalyst does not provide any 8, yielding only 9 and 10 (Table 1, entry 10). The former is consistent with literature reports that Brønsted acid-catalysed reactions give poor yields when the phenol is not para-substituted [19]. Therefore, it seems that the gold(I) catalyst is most efficient in catalysing the one-pot formation of chroman 8.
With these results in hand, a phenol screen was carried out next (Table 2). Initially, the same conditions that were best for the formation of 8a were used (50 °C, 5 equiv 5a, Table 2, entry 1) with p-cresol (5b), but these conditions only produced the Friedel–Crafts intermediate 9b (Table 2, entry 2). Pleasingly, when the temperature was raised to 60 °C, the desired chroman 8b is successfully formed in 57% yield (Table 2, entry 3). Therefore, 60 °C was adopted as the new general conditions temperature. Additionally, it was found that the equivalents of phenol 5 can be lowered to 2 equivalents in some cases at this higher temperature (Table 2, entries 4, 7, 8 and 10). In contrast to the Brønsted acid procedure [19], the substitution position has limited effect on the efficiency of the gold-catalysed reaction, with p-cresol (5b), m-cresol (5c) and o-cresol (5d) all forming the desired chromans 8b–d in decent to good yields (Table 2, entries 3–5). The regioselectivity of the meta isomer is unsurprisingly poor (1:1 of 8c:8c'), and when both meta-positions are substituted (5e) the reaction proceeds to 8e well (Table 2, entry 6). A phenol with an electron-donating substituent 5f provides 8f in a good 71% yield (Table 2, entry 7) and one with an electron-withdrawing substituent 5g pleasingly also produces chroman 8g in a reasonable 54% yield (Table 2, entry 8). p-Bromo-substituted phenol 5h successfully yields chroman 8h which provides a handle for further functionalisation (Table 2, entry 9).
Table 2: Phenol nucleophile scope.
|
|
||||||
| Entry | Equiv 5 | Temp. (°C) | Time (h) | Phenol 5 | Product | Yield (%)a |
|---|---|---|---|---|---|---|
| 1 | 5 | 50 | 19 |
5a |
8a |
64 |
| 2 | 5 | 50 | 64 |
5b |
9b |
N/Db |
| 3 | 5 | 60 | 18 |
5b |
8b |
57 |
| 4 | 2 | 60 | 17 |
5c |
8c and 8c' |
71
(~1:1 8c:8c') |
| 5 | 5 | 60 | 18 |
5d |
8d |
69 |
| 6 | 5 | 60 | 17 |
5e |
8e |
63 |
| 7 | 2 | 60 | 17 |
5f |
8f |
71 |
| 8 | 2 | 60 | 18 |
5g |
8g |
54 |
| 9 | 5 | 60 | 17 |
5h |
8h |
58 |
| 10 | 2 | 60 | 17 |
5i |
8i |
57 |
| 11 | 5 | 70 | 43 |
5j |
8j |
83 |
| 12c | 5 |
70, 48 h;
80, 17 h |
65 |
5k |
8k |
69 |
| 13c,d | 5 | 90 | 65 |
5l |
8l |
83 |
aIsolated yield. bNot determined. cSolvent: dioxane. dReaction carried out in sealed tube.
Next, the effect of a larger substituent was probed (Table 2, entries 10 and 11). More hindered 2,4-di-tert-butylphenol (5j, Table 2, entry 11) requires a higher temperature (70 °C) and twice the reaction time (43 h) to go to completion compared to 4-tert-buylphenol (5i, Table 2, entry 10). Although phenol 5j exhibits the slowest reactivity of the phenols screened, it was chosen as the model phenol in the next allylic alcohol substrate screen (Table 3) since the extra molecular weight from the t-Bu substituents should reduce any volatility issues with the chroman products [77].
At this point, we observed that the general procedure does not work if the phenol reactant is insoluble in toluene, such as 5k. However, a simple change of solvent from toluene to dioxane provides the desired chroman 8k (Table 2, entry 12), although slightly higher temperatures (70–80 °C) and a longer reaction time (65 h) are required in this polar solvent to push the reaction to completion.
Finally, to show the synthetic utility of this procedure, a hydroquinone (trimethylhydroquinone TMHQ (5l)) was also evaluated, as TMHQ is commonly used towards the synthesis of vitamin E and its analogues [17,21]. For solubility issues, dioxane is used as the solvent. Initially, an oxidised side product 11 (Figure 1, formed by auto-oxidation of the Friedel–Crafts intermediate) is observed in 35% yield if the reaction is carried out in air (80 °C), resulting in a low 45% yield of 8l. When the reaction vessel is flushed with argon, the yield of 8l improves to 69%, but ultimately carrying out the reaction at 90 °C in a sealed tube provides a much better yield of 83% (Table 2, entry 13).
Figure 1: Initial side product with TMHQ.
Figure 1: Initial side product with TMHQ.
Next, the allylic alcohol scope was investigated (Table 3). Firstly, going from more hindered Cy substituents (7) to less hindered n-hexyl substituents (12) allows the reaction to work smoothly at a lower temperature of 60 °C (Table 3. entries 1 and 2). Hindered 4 works equally well but requires extended reaction times (67 h) to achieve a good 83% yield (Table 3, entry 3). Less hindered 13 as well as 14 work smoothly to form 8o and spirocyclic chroman 8p (Table 3, entries 4 and 5). An aromatic substituent is also well tolerated (Table 3, entry 6). Next, the effect of substitution along the alkene was investigated (Table 3, entries 7–10). Substitution at the γ-position (16 and 17) seems to be tolerated, forming chromans 8r and 8s respectively albeit in moderate yields (Table 3, entries 7 and 8). Substitution at the β-position, however, is not tolerated: the reaction stops at the Friedel–Crafts stage (9t), and is reluctant to undergo further cyclisation to the desired chroman (Table 3, entry 9). Having investigated a series of tertiary allylic alcohols in entries 1–9, we next looked at selected primary and secondary allylic alcohols (Table 3, entries 10–12). The γ,γ-disubstituted primary allylic alcohol 19 forms the chroman 8o efficiently (Table 3, entry 10), which is the same product as from the tertiary allylic alcohol substrate 13 in entry 4. This implies that 19 undergoes the Friedel–Crafts allylation via opposite regioselectivity (a formal SN2 instead of SN2' observed in all other examples so far in Table 2 and Table 3) to form 9, followed by cyclisation to form the observed 8o. Using a γ,γ-disubstituted secondary allylic alcohol 20 also forms chroman 8u via an initial SN2 Friedel–Crafts regioselectivity, thus γ,γ-disubstitution on the alkene appears to be responsible for the switch in regioselectivity (Table 3, entry 11). This implies that the initial Friedel–Crafts allylation goes via Markovnikov selectivity. The unsubstituted secondary allylic alcohol 21, however, produces only the Friedel–Crafts allylation product 9v and is reluctant to undergo cyclisation to the chroman (Table 3, entry 12) even under more forcing conditions (80 °C, 65 h).
Table 3: Allylic alcohol scope.
|
|
|||||
| Entry | Time (h) | Temp. (°C) | Allylic alcohol | Product | Yield (%)a |
|---|---|---|---|---|---|
| 1 | 43 | 70 |
7 |
8j |
83 |
| 2 | 43 | 60 |
12 |
8m |
69 |
| 3 | 67 | 60 |
4 |
8n |
83 |
| 4 | 42 | 60 |
13 |
8o |
64 |
| 5 | 41 | 60 |
14 |
8p |
61 |
| 6 | 41 | 70 |
15 |
8q |
66 |
| 7 | 43 | 70 |
16 |
8r |
45 |
| 8 | 47 | 70 |
17 |
8s |
48b |
| 9 | 42 | 60 |
18 |
9t |
35 |
| 10 | 42 | 60 |
19 |
8o |
78 |
| 11 | 41 | 70 |
20 |
8u |
51 |
| 12c | 41 | 60 |
21 |
9v |
74 |
aIsolated yield. bApproximately 2:1 d.r. cHeating at 80 °C for 65 h still only gives 9v and no desired chroman.
Since 9 is always observed as a precursor towards 8 (i.e. at lower temperatures, shorter reactions times or when the reaction is analysed before completion), the most likely mechanism is the expected gold(I)-catalysed Friedel–Crafts allylation of the phenol (via Markovnikov regioselectivity), with allylic alcohol [15,73], followed by cyclisation of the intermediate 9 via hydroalkoxylation to form chroman 8 (Scheme 2) [13,15,16]. Chan and co-workers have previously proposed that the Friedel–Crafts mechanism could involve the activation of the allylic alcohol by the gold catalyst to turn the hydroxy group into a better leaving group [73]. The observed regioselectivities is then due to the subsequent attack at the less hindered position of this presumed activated intermediate [73,78].
Our subsequent investigations with the Friedel–Crafts intermediate 9a suggests that the second hydroalkoxylation step is not (or not solely) gold catalysed (Scheme 3). When isolated 9a is resubjected to the reaction conditions with or without additional phenol (5a), no cyclisation to the chroman 8a occurs (Scheme 3). Thus, the second cyclisation step is most likely Brønsted acid catalysed (or acid and gold(I) co-catalysed) [79], where the H+ required is being released in situ during the first Friedel–Crafts step to form 9. This would explain why 9 readily cyclises to chroman 8 in situ, but is reluctant to do so when it is isolated before being resubjected to more gold(I) catalyst, as in Scheme 3. Nevertheless, simply using the equivalent Brønsted acid HNTf2 is not as efficient as using gold(I), as shown in Table 1, entry 9.
If the hydroalkoxylation step is indeed H+ catalysed, addition of a Brønsted acid co-catalyst could force the chroman formation from substrates such as 21, which do not undergo the second hydroalkoxylation step under just gold(I)-catalysed conditions (Table 3, entry 12). Indeed, the reaction of 21 with 5j successfully produces the desired chroman 8v in 52% yield when HNTf2 is added as a co-catalyst (Scheme 4), further suggesting that the hydroalkoxylation step is most likely Brønsted acid catalysed or co-catalysed.
Scheme 4: Reaction of 21 with added Brønsted acid co-catalyst.
Scheme 4: Reaction of 21 with added Brønsted acid co-catalyst.
The suggested mechanism is presented in Scheme 5. Gold(I) catalysts are known to coordinate to alcohols [80], in this case turning the hydroxy group into a better leaving group (I), as previously suggested by Chan [73]. Attack at the less hindered position could occur either directly on I [SN2' shown, but in the case of γ,γ-disubstituted substrates (e.g. 19 and 20), this will occur via SN2] or via an allylic cation intermediate II. The intermediate 9 subsequently undergoes an acid-catalysed hydroalkoxylation to produce the desired chroman 8. Active catalyst LAu+ is presumably regenerated by protonolysis of LAuOH [81].
Conclusion
In conclusion, a simple one-pot procedure towards chromans is described via gold(I)-catalysed reaction of readily accessible phenols with allylic alcohols. This one-pot procedure involves a regioselective Friedel–Crafts allylation followed by cyclisation via hydroalkoxylation to form the chromans in good yields. At lower temperatures or shorter reaction times, the Friedel–Crafts allylation intermediates are usually observed. The reaction works with ortho-, meta- and para-substituents as well as electron donating and withdrawing substituents on the phenol, and hydroquinones (Table 2). A variety of allylic alcohol substrates work well, although substitution on the alkene is only tolerated at the γ-position, and not the β-position (Table 3). The procedure is mild, practically simple and regioselective. We therefore believe that it should find utility as a convenient method towards the synthesis of chroman targets.
Experimental
General procedure: The gold-catalysed reactions were all carried out in 1 dram screw-cap vials without the need for distilled solvents or inert atmosphere, unless otherwise stated. PPh3AuNTf2 (5 mol %) was added to a toluene solution (0.386 M) of allylic alcohol (1 equiv) and phenol (2 or 5 equiv). The reaction mixture was allowed to stir at 50–70 °C until the reaction is complete (16–67 h). The reaction was then filtered through a plug of silica (eluent: neat diethyl ether). The filtrate was concentrated under reduced pressure, and 1H NMR analysis of the crude mixture was used to determine the conversion to chroman 8. The crude material was then purified by flash column chromatography. [Note: If the starting materials are insoluble in toluene, dioxane is used as the solvent instead and the reaction temperature increased to 70–80 °C.]
Supporting Information
| Supporting Information File 1: Full experimental procedures, characterisation for all new compounds and copies of 1H and 13C NMR spectra. | ||
| Format: PDF | Size: 4.1 MB | Download |
References
-
Schneider, C. Mol. Nutr. Food Res. 2005, 49, 7–30. doi:10.1002/mnfr.200400049
Return to citation in text: [1] -
Brogden, P. J.; Gabbutt, C. D.; Hepworth, J. D. Pyrans and Fused Pyrans. In Comprehensive Heterocyclic Chemistry; Katrizky, A., Ed.; Pergamon Press: Oxford, 1984; Vol. 3, pp 573 ff. doi:10.1016/B978-008096519-2.00044-8
Return to citation in text: [1] -
Middleton, E., Jr.; Kandaswami, C.; Theoharides, T. C. Pharmacol. Rev. 2000, 52, 673–751.
Return to citation in text: [1] -
Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Med. Res. Rev. 2003, 23, 519–534. doi:10.1002/med.10033
Return to citation in text: [1] -
Shen, H. C. Tetrahedron 2009, 65, 3931–3952. doi:10.1016/j.tet.2009.02.002
Return to citation in text: [1] -
A related procedure towards chromans is the acid- or metal triflate-mediated cyclisation of phenols with 1,3-dienes. Using these methods, chromans and/or coumarans are obtained, depending on the structure of the 1,3-diene used. For selected recent examples, see references [7-10].
Return to citation in text: [1] -
Dang, T. T.; Boeck, F.; Hintermann, L. J. Org. Chem. 2011, 76, 9353–9361. doi:10.1021/jo201631x
Return to citation in text: [1] [2] -
Youn, S. W. Synlett 2007, 3050–3054. doi:10.1055/s-2007-990963
Return to citation in text: [1] [2] -
Youn, S. W.; Eom, J. I. J. Org. Chem. 2006, 71, 6705–6707. doi:10.1021/jo061221b
Return to citation in text: [1] [2] -
Adrio, L. A.; Hii, K. K. Chem. Commun. 2008, 2325–2327. doi:10.1039/b719465j
Return to citation in text: [1] [2] -
Bienaymé, H.; Ancel, J.-E.; Meilland, P.; Simonato, J.-P. Tetrahedron Lett. 2000, 41, 3339–3343. doi:10.1016/S0040-4039(00)00381-6
Return to citation in text: [1] -
Nguyen, R. V.; Yao, X.; Li, C. J. Org. Lett. 2006, 8, 2397–2399. doi:10.1021/ol0607692
Return to citation in text: [1] -
Rueping, M.; Nachtsheim, B. J. Beilstein J. Org. Chem. 2010, No. 6. doi:10.3762/bjoc.6.6
Return to citation in text: [1] [2] -
Malkov, A. V.; Davis, S. L.; Baxendale, I. R.; Mitchell, W. L.; Kočovský, P. J. Org. Chem. 1999, 64, 2751–2764. doi:10.1021/jo982178y
Return to citation in text: [1] -
Bandini, M.; Tragni, M. Org. Biomol. Chem. 2009, 7, 1501–1507. doi:10.1039/b823217b
Return to citation in text: [1] [2] [3] -
Kumar, R.; Van der Eycken, E. V. Chem. Soc. Rev. 2013, 42, 1121–1146. doi:10.1039/c2cs35397k
Return to citation in text: [1] [2] -
Yamamoto, Y.; Itonaga, K. Org. Lett. 2009, 11, 717–720. doi:10.1021/ol802800s
Return to citation in text: [1] [2] -
Malkov, A. V.; Spoor, P.; Vinader, V.; Kočovský, P. J. Org. Chem. 1999, 64, 5308–5311. doi:10.1021/jo990372u
Return to citation in text: [1] -
Ishino, Y.; Mihara, M.; Hayakawa, N.; Miyata, T.; Kaneko, Y.; Miyata, T. Synth. Commun. 2001, 31, 439–448. doi:10.1081/SCC-100000537
Return to citation in text: [1] [2] [3] -
Lee, J. H.; Bang, H. B.; Han, S. Y.; Jun, J.-G. Tetrahedron Lett. 2007, 48, 2889–2892. doi:10.1016/j.tetlet.2007.02.088
Return to citation in text: [1] -
Hasegawa, A.; Ishihara, K.; Yamamoto, H. Angew. Chem., Int. Ed. 2003, 42, 5731–5733. doi:10.1002/anie.200352382
Return to citation in text: [1] [2] -
Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395–403. doi:10.1038/nature05592
Return to citation in text: [1] -
Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335
Return to citation in text: [1] -
Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239–3265. doi:10.1021/cr068434l
Return to citation in text: [1] -
Shen, H. C. Tetrahedron 2008, 64, 3885–3903. doi:10.1016/j.tet.2008.01.081
Return to citation in text: [1] -
Shen, H. C. Tetrahedron 2008, 64, 7847–7870. doi:10.1016/j.tet.2008.05.082
Return to citation in text: [1] -
Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180–3211. doi:10.1021/cr000436x
Return to citation in text: [1] -
Marion, N.; Nolan, S. P. Chem. Soc. Rev. 2008, 37, 1776–1782. doi:10.1039/b711132k
Return to citation in text: [1] -
Jiménez-Núñez, E.; Echavarren, A. M. Chem. Commun. 2007, 333–346. doi:10.1039/b612008c
Return to citation in text: [1] -
Corma, A.; Leyva-Peréz, A.; Sabater, M. J. Chem. Rev. 2011, 111, 1657–1712. doi:10.1021/cr100414u
Return to citation in text: [1] -
Bandini, M. Chem. Soc. Rev. 2011, 40, 1358–1367. doi:10.1039/c0cs00041h
Return to citation in text: [1] -
Boorman, T. C.; Larrosa, I. Chem. Soc. Rev. 2011, 40, 1910–1925. doi:10.1039/c0cs00098a
Return to citation in text: [1] -
Hashmi, A. S. K.; Bührle, M. Aldrichimica Acta 2010, 43, 27–33.
Return to citation in text: [1] -
Shapiro, N. D.; Toste, F. D. Synlett 2010, 675–691. doi:10.1055/s-0029-1219369
Return to citation in text: [1] -
Sengupta, S.; Shi, X. ChemCatChem 2010, 2, 609–619. doi:10.1002/cctc.201000070
Return to citation in text: [1] -
Bongers, N.; Krause, N. Angew. Chem., Int. Ed. 2008, 47, 2178–2181. doi:10.1002/anie.200704729
Return to citation in text: [1] -
Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g
Return to citation in text: [1] -
Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326–3350. doi:10.1021/cr0684319
Return to citation in text: [1] -
Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454
Return to citation in text: [1] -
Rudolph, M.; Hashmi, A. S. K. Chem. Soc. Rev. 2012, 41, 2448–2462. doi:10.1039/c1cs15279c
Return to citation in text: [1] -
Muzart, J. Tetrahedron 2008, 64, 5815–5849. doi:10.1016/j.tet.2008.04.018
Return to citation in text: [1] -
Bauer, J. T.; Hadfield, M. S.; Lee, A.-L. Chem. Commun. 2008, 6405–6407. doi:10.1039/b815891f
Return to citation in text: [1] -
Hadfield, M. S.; Lee, A.-L. Org. Lett. 2010, 12, 484–487. doi:10.1021/ol902675k
Return to citation in text: [1] -
Hadfield, M. S.; Bauer, J. T.; Glen, P. E.; Lee, A.-L. Org. Biomol. Chem. 2010, 8, 4090–4095. doi:10.1039/c0ob00085j
Return to citation in text: [1] -
Heuer-Jungemann, A.; McLaren, R. G.; Hadfield, M. S.; Lee, A.-L. Tetrahedron 2011, 67, 1609–1616. doi:10.1016/j.tet.2011.01.021
Return to citation in text: [1] -
Hadfield, M. S.; Lee, A.-L. Chem. Commun. 2011, 47, 1333–1335. doi:10.1039/c0cc04217j
Return to citation in text: [1] -
Kilpin, K. J.; Paul, U. S. D.; Lee, A.-L.; Crowley, J. D. Chem. Commun. 2011, 47, 328–330. doi:10.1039/c0cc02185g
Return to citation in text: [1] -
Young, P. C.; Hadfield, M. S.; Arrowsmith, L.; Macleod, K. M.; Mudd, R. J.; Jordan-Hore, J. A.; Lee, A.-L. Org. Lett. 2012, 14, 898–901. doi:10.1021/ol203418u
Return to citation in text: [1] -
Hadfield, M. S.; Häller, L. J. L.; Lee, A.-L.; Macgregor, S. A.; O'Neill, J. A. T.; Watson, A. M. Org. Biomol. Chem. 2012, 10, 4433–4440. doi:10.1039/c2ob25183c
Return to citation in text: [1] -
Mudd, R. J.; Young, P. C.; Jordan-Hore, J. A.; Rosair, G. M.; Lee, A.-L. J. Org. Chem. 2012, 77, 7633–7639. doi:10.1021/jo300930c
Return to citation in text: [1] -
Young, P. C.; Green, S. L. J.; Rosair, G. M.; Lee, A.-L. Dalton Trans. 2013, 42, 9645–9653. doi:10.1039/c3dt50653c
Return to citation in text: [1] -
Aponick, A.; Biannic, B.; Jong, M. R. Chem. Commun. 2010, 46, 6849–6851. doi:10.1039/c0cc01961e
Return to citation in text: [1] -
Aponick, A.; Biannic, B. Org. Lett. 2011, 13, 1330–1333. doi:10.1021/ol200203k
Return to citation in text: [1] -
Aponick, A.; Li, C.-Y.; Biannic, B. Org. Lett. 2008, 10, 669–671. doi:10.1021/ol703002p
Return to citation in text: [1] -
Biannic, B.; Ghebreghiorgis, T.; Aponick, A. Beilstein J. Org. Chem. 2011, 7, 802–807. doi:10.3762/bjoc.7.91
Return to citation in text: [1] -
Bandini, M.; Monari, M.; Romaniello, A.; Tragni, M. Chem.–Eur. J. 2010, 16, 14272–14277. doi:10.1002/chem.201002606
Return to citation in text: [1] -
Unsworth, W. P.; Stevens, K.; Lamont, S. G.; Robertson, J. Chem. Commun. 2011, 47, 7659–7661. doi:10.1039/c1cc11805f
Return to citation in text: [1] -
Ghebreghiorgis, T.; Biannic, B.; Kirk, B. H.; Ess, D. H.; Aponick, A. J. Am. Chem. Soc. 2012, 134, 16307–16318. doi:10.1021/ja306333a
Return to citation in text: [1] -
Biannic, B.; Aponick, A. Eur. J. Org. Chem. 2011, 6605–6617. doi:10.1002/ejoc.201100858
Return to citation in text: [1] -
Young, P. C.; Schopf, N. A.; Lee, A.-L. Chem. Commun. 2013, 49, 4262–4264. doi:10.1039/c2cc36760b
Return to citation in text: [1] -
Mukherjee, P.; Widenhoefer, R. A. Chem.–Eur. J. 2013, 19, 3437–3444. doi:10.1002/chem.201203987
Return to citation in text: [1] [2] -
For an independent report using NHC gold(I) complexes, see reference [61]. For related reactions with N-nucleophiles instead, see references [63-67].
Return to citation in text: [1] -
Mukherjee, P.; Widenhoefer, R. A. Org. Lett. 2010, 12, 1184–1187. doi:10.1021/ol902923e
Return to citation in text: [1] [2] -
Ohshima, T.; Nakahara, Y.; Ipposhi, J.; Miyamoto, Y.; Mashima, K. Chem. Commun. 2011, 47, 8322–8324. doi:10.1039/c1cc12760h
Return to citation in text: [1] [2] -
Mukherjee, P.; Widenhoefer, R. A. Org. Lett. 2011, 13, 1334–1337. doi:10.1021/ol103175w
Return to citation in text: [1] [2] -
Kothandaraman, P.; Foo, S. J.; Chan, P. W. H. J. Org. Chem. 2009, 74, 5947–5952. doi:10.1021/jo900917q
Return to citation in text: [1] [2] -
Mukherjee, P.; Widenhoefer, R. A. Angew. Chem., Int. Ed. 2012, 51, 1405–1407. doi:10.1002/anie.201107877
Return to citation in text: [1] [2] -
Kothandaraman, P.; Mothe, S. R.; Toh, S. S. M.; Chan, P. W. H. J. Org. Chem. 2011, 76, 7633–7640. doi:10.1021/jo201208e
Return to citation in text: [1] -
Kothandaraman, P.; Huang, C.; Susanti, D.; Rao, W.; Chan, P. W. H. Chem.–Eur. J. 2011, 17, 10081–10088. doi:10.1002/chem.201101363
Return to citation in text: [1] -
Kothandaraman, P.; Rao, W.; Foo, S. J.; Chan, P. W. H. Angew. Chem., Int. Ed. 2010, 49, 4619–4623. doi:10.1002/anie.201000341
Return to citation in text: [1] -
Rao, W.; Chan, P. W. H. Chem.–Eur. J. 2008, 14, 10486–10495. doi:10.1002/chem.200801242
Return to citation in text: [1] -
Chen, Z.; Zhang, Y.-X.; Wang, Y.-H.; Zhu, L. L.; Liu, H.; Li, X. X.; Guo, L. Org. Lett. 2010, 12, 3468–3471. doi:10.1021/ol1012923
Return to citation in text: [1] -
Rao, W.; Chan, P. W. H. Org. Biomol. Chem. 2008, 6, 2426–2433. doi:10.1039/b805067h
Return to citation in text: [1] [2] [3] [4] [5] -
Jean, M.; van de Weghe, P. Tetrahedron Lett. 2011, 52, 3509–3513. doi:10.1016/j.tetlet.2011.04.122
Return to citation in text: [1] -
Rudolph, M.; Hashmi, A. S. K. Chem. Commun. 2011, 47, 6536–6544. doi:10.1039/C1CC10780A
Return to citation in text: [1] -
Wang, D.; Cai, S.; Sharma, S.; Jirak, J.; Thummanapelli, S. K.; Akhmedov, N. G.; Zhang, H.; Liu, X.; Peterson, J. L.; Shi, X. J. Am. Chem. Soc. 2012, 134, 9012–9019. doi:10.1021/ja303862z
Return to citation in text: [1] -
We have noted that the formation of chromans 8a–8j from 7 is also accompanied by the formation of a rearrangement side-product in approximately 15–25% yields (see Supporting Information File 1 for details). The formation of this rearrangement side product is only ever observed with cyclohexyl substituents (i.e. 7) and is not detected in any of the other allylic alcohols screened in Table 3. We have previously observed unusual behaviour in other substrates containing bis-cyclohexyl substituents in gold(I)-catalysed reactions, see reference [48].
Return to citation in text: [1] -
Georgy, M.; Boucard, V.; Campagne, J.-M. J. Am. Chem. Soc. 2005, 127, 14180–14181. doi:10.1021/ja0534147
Return to citation in text: [1] -
Hashmi, A. S. K. Catal. Today 2007, 122, 211–214. doi:10.1016/j.cattod.2006.10.006
Return to citation in text: [1] -
Zhdanko, A.; Ströbele, M.; Maier, M. E. Chem.–Eur. J. 2012, 18, 14732–14744. doi:10.1002/chem.201201215
Return to citation in text: [1] -
Gaillard, S.; Bosson, J.; Ramón, R. S.; Nun, P.; Slawin, A. M. Z.; Nolan, S. P. Chem.–Eur. J. 2010, 16, 13729–13740. doi:10.1002/chem.201001688
Return to citation in text: [1]
| 81. | Gaillard, S.; Bosson, J.; Ramón, R. S.; Nun, P.; Slawin, A. M. Z.; Nolan, S. P. Chem.–Eur. J. 2010, 16, 13729–13740. doi:10.1002/chem.201001688 |
| 7. | Dang, T. T.; Boeck, F.; Hintermann, L. J. Org. Chem. 2011, 76, 9353–9361. doi:10.1021/jo201631x |
| 8. | Youn, S. W. Synlett 2007, 3050–3054. doi:10.1055/s-2007-990963 |
| 9. | Youn, S. W.; Eom, J. I. J. Org. Chem. 2006, 71, 6705–6707. doi:10.1021/jo061221b |
| 10. | Adrio, L. A.; Hii, K. K. Chem. Commun. 2008, 2325–2327. doi:10.1039/b719465j |
| 61. | Mukherjee, P.; Widenhoefer, R. A. Chem.–Eur. J. 2013, 19, 3437–3444. doi:10.1002/chem.201203987 |
| 1. | Schneider, C. Mol. Nutr. Food Res. 2005, 49, 7–30. doi:10.1002/mnfr.200400049 |
| 2. | Brogden, P. J.; Gabbutt, C. D.; Hepworth, J. D. Pyrans and Fused Pyrans. In Comprehensive Heterocyclic Chemistry; Katrizky, A., Ed.; Pergamon Press: Oxford, 1984; Vol. 3, pp 573 ff. doi:10.1016/B978-008096519-2.00044-8 |
| 3. | Middleton, E., Jr.; Kandaswami, C.; Theoharides, T. C. Pharmacol. Rev. 2000, 52, 673–751. |
| 4. | Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Med. Res. Rev. 2003, 23, 519–534. doi:10.1002/med.10033 |
| 5. | Shen, H. C. Tetrahedron 2009, 65, 3931–3952. doi:10.1016/j.tet.2009.02.002 |
| 15. | Bandini, M.; Tragni, M. Org. Biomol. Chem. 2009, 7, 1501–1507. doi:10.1039/b823217b |
| 16. | Kumar, R.; Van der Eycken, E. V. Chem. Soc. Rev. 2013, 42, 1121–1146. doi:10.1039/c2cs35397k |
| 75. | Rudolph, M.; Hashmi, A. S. K. Chem. Commun. 2011, 47, 6536–6544. doi:10.1039/C1CC10780A |
| 14. | Malkov, A. V.; Davis, S. L.; Baxendale, I. R.; Mitchell, W. L.; Kočovský, P. J. Org. Chem. 1999, 64, 2751–2764. doi:10.1021/jo982178y |
| 76. | Wang, D.; Cai, S.; Sharma, S.; Jirak, J.; Thummanapelli, S. K.; Akhmedov, N. G.; Zhang, H.; Liu, X.; Peterson, J. L.; Shi, X. J. Am. Chem. Soc. 2012, 134, 9012–9019. doi:10.1021/ja303862z |
| 13. | Rueping, M.; Nachtsheim, B. J. Beilstein J. Org. Chem. 2010, No. 6. doi:10.3762/bjoc.6.6 |
| 73. | Rao, W.; Chan, P. W. H. Org. Biomol. Chem. 2008, 6, 2426–2433. doi:10.1039/b805067h |
| 6. | A related procedure towards chromans is the acid- or metal triflate-mediated cyclisation of phenols with 1,3-dienes. Using these methods, chromans and/or coumarans are obtained, depending on the structure of the 1,3-diene used. For selected recent examples, see references [7-10]. |
| 7. | Dang, T. T.; Boeck, F.; Hintermann, L. J. Org. Chem. 2011, 76, 9353–9361. doi:10.1021/jo201631x |
| 8. | Youn, S. W. Synlett 2007, 3050–3054. doi:10.1055/s-2007-990963 |
| 9. | Youn, S. W.; Eom, J. I. J. Org. Chem. 2006, 71, 6705–6707. doi:10.1021/jo061221b |
| 10. | Adrio, L. A.; Hii, K. K. Chem. Commun. 2008, 2325–2327. doi:10.1039/b719465j |
| 11. | Bienaymé, H.; Ancel, J.-E.; Meilland, P.; Simonato, J.-P. Tetrahedron Lett. 2000, 41, 3339–3343. doi:10.1016/S0040-4039(00)00381-6 |
| 12. | Nguyen, R. V.; Yao, X.; Li, C. J. Org. Lett. 2006, 8, 2397–2399. doi:10.1021/ol0607692 |
| 74. | Jean, M.; van de Weghe, P. Tetrahedron Lett. 2011, 52, 3509–3513. doi:10.1016/j.tetlet.2011.04.122 |
| 42. | Bauer, J. T.; Hadfield, M. S.; Lee, A.-L. Chem. Commun. 2008, 6405–6407. doi:10.1039/b815891f |
| 43. | Hadfield, M. S.; Lee, A.-L. Org. Lett. 2010, 12, 484–487. doi:10.1021/ol902675k |
| 44. | Hadfield, M. S.; Bauer, J. T.; Glen, P. E.; Lee, A.-L. Org. Biomol. Chem. 2010, 8, 4090–4095. doi:10.1039/c0ob00085j |
| 45. | Heuer-Jungemann, A.; McLaren, R. G.; Hadfield, M. S.; Lee, A.-L. Tetrahedron 2011, 67, 1609–1616. doi:10.1016/j.tet.2011.01.021 |
| 46. | Hadfield, M. S.; Lee, A.-L. Chem. Commun. 2011, 47, 1333–1335. doi:10.1039/c0cc04217j |
| 47. | Kilpin, K. J.; Paul, U. S. D.; Lee, A.-L.; Crowley, J. D. Chem. Commun. 2011, 47, 328–330. doi:10.1039/c0cc02185g |
| 48. | Young, P. C.; Hadfield, M. S.; Arrowsmith, L.; Macleod, K. M.; Mudd, R. J.; Jordan-Hore, J. A.; Lee, A.-L. Org. Lett. 2012, 14, 898–901. doi:10.1021/ol203418u |
| 49. | Hadfield, M. S.; Häller, L. J. L.; Lee, A.-L.; Macgregor, S. A.; O'Neill, J. A. T.; Watson, A. M. Org. Biomol. Chem. 2012, 10, 4433–4440. doi:10.1039/c2ob25183c |
| 50. | Mudd, R. J.; Young, P. C.; Jordan-Hore, J. A.; Rosair, G. M.; Lee, A.-L. J. Org. Chem. 2012, 77, 7633–7639. doi:10.1021/jo300930c |
| 51. | Young, P. C.; Green, S. L. J.; Rosair, G. M.; Lee, A.-L. Dalton Trans. 2013, 42, 9645–9653. doi:10.1039/c3dt50653c |
| 60. | Young, P. C.; Schopf, N. A.; Lee, A.-L. Chem. Commun. 2013, 49, 4262–4264. doi:10.1039/c2cc36760b |
| 61. | Mukherjee, P.; Widenhoefer, R. A. Chem.–Eur. J. 2013, 19, 3437–3444. doi:10.1002/chem.201203987 |
| 22. | Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395–403. doi:10.1038/nature05592 |
| 23. | Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335 |
| 24. | Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239–3265. doi:10.1021/cr068434l |
| 25. | Shen, H. C. Tetrahedron 2008, 64, 3885–3903. doi:10.1016/j.tet.2008.01.081 |
| 26. | Shen, H. C. Tetrahedron 2008, 64, 7847–7870. doi:10.1016/j.tet.2008.05.082 |
| 27. | Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180–3211. doi:10.1021/cr000436x |
| 28. | Marion, N.; Nolan, S. P. Chem. Soc. Rev. 2008, 37, 1776–1782. doi:10.1039/b711132k |
| 29. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Commun. 2007, 333–346. doi:10.1039/b612008c |
| 30. | Corma, A.; Leyva-Peréz, A.; Sabater, M. J. Chem. Rev. 2011, 111, 1657–1712. doi:10.1021/cr100414u |
| 31. | Bandini, M. Chem. Soc. Rev. 2011, 40, 1358–1367. doi:10.1039/c0cs00041h |
| 32. | Boorman, T. C.; Larrosa, I. Chem. Soc. Rev. 2011, 40, 1910–1925. doi:10.1039/c0cs00098a |
| 33. | Hashmi, A. S. K.; Bührle, M. Aldrichimica Acta 2010, 43, 27–33. |
| 34. | Shapiro, N. D.; Toste, F. D. Synlett 2010, 675–691. doi:10.1055/s-0029-1219369 |
| 35. | Sengupta, S.; Shi, X. ChemCatChem 2010, 2, 609–619. doi:10.1002/cctc.201000070 |
| 36. | Bongers, N.; Krause, N. Angew. Chem., Int. Ed. 2008, 47, 2178–2181. doi:10.1002/anie.200704729 |
| 37. | Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g |
| 38. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326–3350. doi:10.1021/cr0684319 |
| 39. | Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454 |
| 40. | Rudolph, M.; Hashmi, A. S. K. Chem. Soc. Rev. 2012, 41, 2448–2462. doi:10.1039/c1cs15279c |
| 41. | Muzart, J. Tetrahedron 2008, 64, 5815–5849. doi:10.1016/j.tet.2008.04.018 |
| 61. | Mukherjee, P.; Widenhoefer, R. A. Chem.–Eur. J. 2013, 19, 3437–3444. doi:10.1002/chem.201203987 |
| 62. | For an independent report using NHC gold(I) complexes, see reference [61]. For related reactions with N-nucleophiles instead, see references [63-67]. |
| 63. | Mukherjee, P.; Widenhoefer, R. A. Org. Lett. 2010, 12, 1184–1187. doi:10.1021/ol902923e |
| 64. | Ohshima, T.; Nakahara, Y.; Ipposhi, J.; Miyamoto, Y.; Mashima, K. Chem. Commun. 2011, 47, 8322–8324. doi:10.1039/c1cc12760h |
| 65. | Mukherjee, P.; Widenhoefer, R. A. Org. Lett. 2011, 13, 1334–1337. doi:10.1021/ol103175w |
| 66. | Kothandaraman, P.; Foo, S. J.; Chan, P. W. H. J. Org. Chem. 2009, 74, 5947–5952. doi:10.1021/jo900917q |
| 67. | Mukherjee, P.; Widenhoefer, R. A. Angew. Chem., Int. Ed. 2012, 51, 1405–1407. doi:10.1002/anie.201107877 |
| 68. | Kothandaraman, P.; Mothe, S. R.; Toh, S. S. M.; Chan, P. W. H. J. Org. Chem. 2011, 76, 7633–7640. doi:10.1021/jo201208e |
| 69. | Kothandaraman, P.; Huang, C.; Susanti, D.; Rao, W.; Chan, P. W. H. Chem.–Eur. J. 2011, 17, 10081–10088. doi:10.1002/chem.201101363 |
| 70. | Kothandaraman, P.; Rao, W.; Foo, S. J.; Chan, P. W. H. Angew. Chem., Int. Ed. 2010, 49, 4619–4623. doi:10.1002/anie.201000341 |
| 71. | Rao, W.; Chan, P. W. H. Chem.–Eur. J. 2008, 14, 10486–10495. doi:10.1002/chem.200801242 |
| 72. | Chen, Z.; Zhang, Y.-X.; Wang, Y.-H.; Zhu, L. L.; Liu, H.; Li, X. X.; Guo, L. Org. Lett. 2010, 12, 3468–3471. doi:10.1021/ol1012923 |
| 19. | Ishino, Y.; Mihara, M.; Hayakawa, N.; Miyata, T.; Kaneko, Y.; Miyata, T. Synth. Commun. 2001, 31, 439–448. doi:10.1081/SCC-100000537 |
| 20. | Lee, J. H.; Bang, H. B.; Han, S. Y.; Jun, J.-G. Tetrahedron Lett. 2007, 48, 2889–2892. doi:10.1016/j.tetlet.2007.02.088 |
| 21. | Hasegawa, A.; Ishihara, K.; Yamamoto, H. Angew. Chem., Int. Ed. 2003, 42, 5731–5733. doi:10.1002/anie.200352382 |
| 63. | Mukherjee, P.; Widenhoefer, R. A. Org. Lett. 2010, 12, 1184–1187. doi:10.1021/ol902923e |
| 64. | Ohshima, T.; Nakahara, Y.; Ipposhi, J.; Miyamoto, Y.; Mashima, K. Chem. Commun. 2011, 47, 8322–8324. doi:10.1039/c1cc12760h |
| 65. | Mukherjee, P.; Widenhoefer, R. A. Org. Lett. 2011, 13, 1334–1337. doi:10.1021/ol103175w |
| 66. | Kothandaraman, P.; Foo, S. J.; Chan, P. W. H. J. Org. Chem. 2009, 74, 5947–5952. doi:10.1021/jo900917q |
| 67. | Mukherjee, P.; Widenhoefer, R. A. Angew. Chem., Int. Ed. 2012, 51, 1405–1407. doi:10.1002/anie.201107877 |
| 17. | Yamamoto, Y.; Itonaga, K. Org. Lett. 2009, 11, 717–720. doi:10.1021/ol802800s |
| 18. | Malkov, A. V.; Spoor, P.; Vinader, V.; Kočovský, P. J. Org. Chem. 1999, 64, 5308–5311. doi:10.1021/jo990372u |
| 52. | Aponick, A.; Biannic, B.; Jong, M. R. Chem. Commun. 2010, 46, 6849–6851. doi:10.1039/c0cc01961e |
| 53. | Aponick, A.; Biannic, B. Org. Lett. 2011, 13, 1330–1333. doi:10.1021/ol200203k |
| 54. | Aponick, A.; Li, C.-Y.; Biannic, B. Org. Lett. 2008, 10, 669–671. doi:10.1021/ol703002p |
| 55. | Biannic, B.; Ghebreghiorgis, T.; Aponick, A. Beilstein J. Org. Chem. 2011, 7, 802–807. doi:10.3762/bjoc.7.91 |
| 56. | Bandini, M.; Monari, M.; Romaniello, A.; Tragni, M. Chem.–Eur. J. 2010, 16, 14272–14277. doi:10.1002/chem.201002606 |
| 57. | Unsworth, W. P.; Stevens, K.; Lamont, S. G.; Robertson, J. Chem. Commun. 2011, 47, 7659–7661. doi:10.1039/c1cc11805f |
| 58. | Ghebreghiorgis, T.; Biannic, B.; Kirk, B. H.; Ess, D. H.; Aponick, A. J. Am. Chem. Soc. 2012, 134, 16307–16318. doi:10.1021/ja306333a |
| 59. | Biannic, B.; Aponick, A. Eur. J. Org. Chem. 2011, 6605–6617. doi:10.1002/ejoc.201100858 |
| 48. | Young, P. C.; Hadfield, M. S.; Arrowsmith, L.; Macleod, K. M.; Mudd, R. J.; Jordan-Hore, J. A.; Lee, A.-L. Org. Lett. 2012, 14, 898–901. doi:10.1021/ol203418u |
| 77. | We have noted that the formation of chromans 8a–8j from 7 is also accompanied by the formation of a rearrangement side-product in approximately 15–25% yields (see Supporting Information File 1 for details). The formation of this rearrangement side product is only ever observed with cyclohexyl substituents (i.e. 7) and is not detected in any of the other allylic alcohols screened in Table 3. We have previously observed unusual behaviour in other substrates containing bis-cyclohexyl substituents in gold(I)-catalysed reactions, see reference [48]. |
| 19. | Ishino, Y.; Mihara, M.; Hayakawa, N.; Miyata, T.; Kaneko, Y.; Miyata, T. Synth. Commun. 2001, 31, 439–448. doi:10.1081/SCC-100000537 |
| 19. | Ishino, Y.; Mihara, M.; Hayakawa, N.; Miyata, T.; Kaneko, Y.; Miyata, T. Synth. Commun. 2001, 31, 439–448. doi:10.1081/SCC-100000537 |
| 80. | Zhdanko, A.; Ströbele, M.; Maier, M. E. Chem.–Eur. J. 2012, 18, 14732–14744. doi:10.1002/chem.201201215 |
| 73. | Rao, W.; Chan, P. W. H. Org. Biomol. Chem. 2008, 6, 2426–2433. doi:10.1039/b805067h |
| 73. | Rao, W.; Chan, P. W. H. Org. Biomol. Chem. 2008, 6, 2426–2433. doi:10.1039/b805067h |
| 78. | Georgy, M.; Boucard, V.; Campagne, J.-M. J. Am. Chem. Soc. 2005, 127, 14180–14181. doi:10.1021/ja0534147 |
| 79. | Hashmi, A. S. K. Catal. Today 2007, 122, 211–214. doi:10.1016/j.cattod.2006.10.006 |
| 13. | Rueping, M.; Nachtsheim, B. J. Beilstein J. Org. Chem. 2010, No. 6. doi:10.3762/bjoc.6.6 |
| 15. | Bandini, M.; Tragni, M. Org. Biomol. Chem. 2009, 7, 1501–1507. doi:10.1039/b823217b |
| 16. | Kumar, R.; Van der Eycken, E. V. Chem. Soc. Rev. 2013, 42, 1121–1146. doi:10.1039/c2cs35397k |
| 73. | Rao, W.; Chan, P. W. H. Org. Biomol. Chem. 2008, 6, 2426–2433. doi:10.1039/b805067h |
| 17. | Yamamoto, Y.; Itonaga, K. Org. Lett. 2009, 11, 717–720. doi:10.1021/ol802800s |
| 21. | Hasegawa, A.; Ishihara, K.; Yamamoto, H. Angew. Chem., Int. Ed. 2003, 42, 5731–5733. doi:10.1002/anie.200352382 |
| 15. | Bandini, M.; Tragni, M. Org. Biomol. Chem. 2009, 7, 1501–1507. doi:10.1039/b823217b |
| 73. | Rao, W.; Chan, P. W. H. Org. Biomol. Chem. 2008, 6, 2426–2433. doi:10.1039/b805067h |
© 2013 Coutant et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)