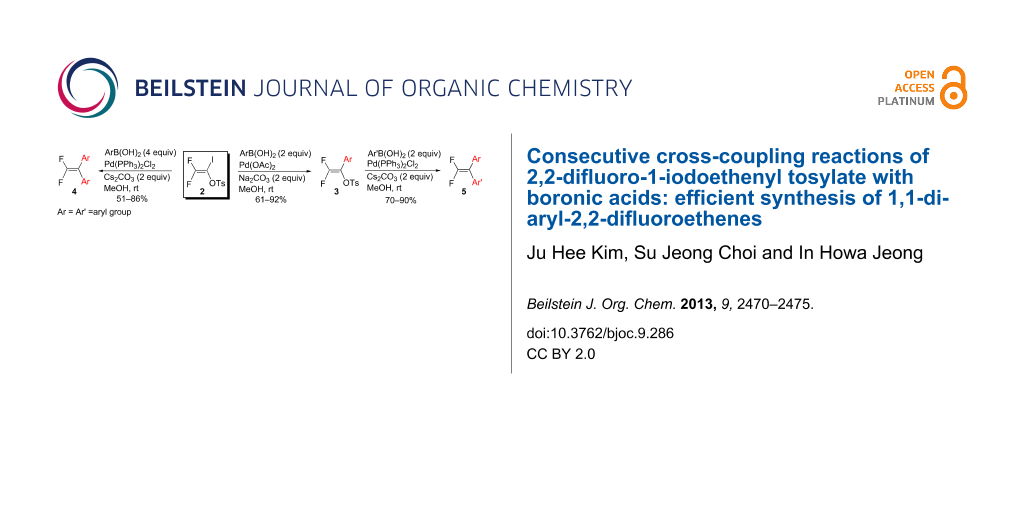
Abstract
The cross-coupling reactions of 2,2-difluoro-1-iodoethenyl tosylate (2) with 2 equiv of boronic acids in the presence of catalytic amounts of Pd(OAc)2 and Na2CO3 afforded the mono-coupled products 3 and 5 in high yields. The use of 4 equiv of boronic acids in the presence of catalytic amount of Pd(PPh3)2Cl2 and Na2CO3 in this reaction resulted in the formation of symmetrical di-coupled products 4 in high yields. Unsymmetrical di-coupled products 4 were obtained in high yields from the reactions of 3 with 2 equiv of boronic acids in the presence of catalytic amounts of Pd(OAc)2 and Na2CO3.
Graphical Abstract
Introduction
The synthesis of 2,2-disubstituted-1,1-difluoroethenes have received much attention to synthetic organofluorine chemists in recent years because of their unique chemical reactivities toward nucleophiles to produce monofluorinated organic compounds [1-4], and their biological activity, such as mechanism-based enzyme inhibitors, in the area of medicinal chemistry [5-8]. The 1,1-difluoroethenylidene functionality in these compounds is also known to act as a bioisostere for the carbonyl group of many biologically active compounds [9-12]. Although numerous methods for the preparation of 2,2-disubstituted-1,1-difluoroethenes have been reported in the previous literature [13-22], a consecutive cross-coupling reaction of a proper precursor such as a 1,1-difluoroethenylidene species bearing a metal functional group, a halogen substituent or a tosylate group at the vinyl carbon will provide a concise and efficient method for the synthesis of 2,2-disubstituted-1,1-difluoroethenes. Burton et al. reported a straightforward method for the preparation of 1,1-diaryl-2,2-difluoroethenes from the consecutive cross-coupling reaction of the 2,2-difluoro-1-bromoethenylzinc reagent with aryl iodides, followed by arylboronic acids [17]. Recently, we also prepared 2,2-difluoro-1-tributylstannylethenyl tosylate and (2,2-difluoroethenylidene)bis(tributylstannane) which were utilized in the palladium-catalyzed consecutive cross-coupling reactions with electrophilic aryl iodides or nucleophilic arylstannane reagents to afford the corresponding 1,1-diaryl-2,2-difluoroethenes [20,21]. However, these previous reagents still have some drawbacks such as the existence of the toxic tributylstannyl group, thermal instability of ethenylzinc reagents and the use of at least one nucleophilic reactive site for the coupling partner. In contrast to these reagents, the 1,1-difluoroethenylidene species bearing both an electrophilic halogen substituent and a tosylate group at the vinyl carbon have not been studied in the cross-coupling reaction with stable and less toxic nucleophilic metal reagents such as aryl- and alkenylboronic acids. Herein, we report a preparation of 2,2-difluoro-1-iodoethenyl tosylate and its cross-coupling reactions with aryl- and alkenylboronic acids to give the corresponding 1,1-difluoroalkenes.
Results and Discussion
Although the chemistry of the 2,2-difluoroethenylidene species as a building block has been well established in recent years, 2,2-difluoro-1-iodoethenyl tosylate (2) was not previously prepared. However, we easily synthesized the starting material 2 from the reaction of 2,2,2-trifluoroethyl tosylate (1) with 2 equiv of LDA in THF at −78 °C, followed by treatment with 1 equiv of iodine (Scheme 1).
Scheme 1: Preparation of 2,2-difluoro-1-iodoethenyl tosylate (2).
Scheme 1: Preparation of 2,2-difluoro-1-iodoethenyl tosylate (2).
First, we attempted the consecutive palladium-catalyzed cross-coupling reaction of 2 with different arylboronic acids to afford unsymmetrical 1,1-diaryl-2,2-difluoroethenes. Since the use of a proper base in the Suzuki–Miyaura reaction is an important factor to increase the yield of coupled product, we screened bases to get the optimized reaction conditions. When 2 was reacted with 1 equiv of phenylboronic acid in the presence of 5 mol % of Pd(OAc)2 and Cs2CO3 (2 equiv) in methanol at room temperature for 15 h, mono- and di-coupled products 3a and 4a were obtained in 21% and 10% yields, respectively, along with a small amount of the self-coupled product (less than 5%) and reduced product. The use of 2 equiv of phenylboronic acid in the same reaction increased the yield of 3a (38%) and 4a (19%). However, the use of high molecular amounts of Pd catalyst did not improve the yield of 3. The same reaction was performed with K2CO3 instead of Cs2CO3 as a base to give 3a and 4a in 56% and 16% yields. The use of K3PO4 in this reaction provided similar results. Finally, the optimized reaction condition was achieved by using Na2CO3 as a base, in which only mono-coupled product 3a was obtained in 92% yield along with the self-coupled product derived from the excess boronic acid. When the reaction was performed in the presence of 5% Pd(PPh3)2Cl2 or Pd(CH3CN)2Cl2 instead of Pd(OAc)2, di-coupled product 4a was always formed in 6–13% yield. Optimization of the cross-coupling reaction of 2 with phenylboronic acid is summarized in Table 1.
Table 1: Optimization of the cross-coupling reaction of 2 with phenylboronic acid.
|
|
||||||
| Entry | Pd catalyst | X (equiv) | Y (mol %) | Base | Yield (%)a | |
|---|---|---|---|---|---|---|
| 3a | 4a | |||||
| 1 | Pd(OAc)2 | 1 | 5 | Cs2CO3 | 21 | 10 |
| 2 | Pd(OAc)2 | 2 | 5 | Cs2CO3 | 38 | 19 |
| 3 | Pd(OAc)2 | 3 | 5 | Cs2CO3 | 18 | 40 |
| 4 | Pd(OAc)2 | 2 | 10 | Cs2CO3 | 30 | 24 |
| 5 | Pd(OAc)2 | 2 | 5 | K2CO3 | 56 | 16 |
| 6 | Pd(OAc)2 | 2 | 5 | K3PO4 | 54 | 17 |
| 7 | Pd(OAc)2 | 2 | 5 | Na2CO3 | 92 | –b |
| 8 | Pd(PPh3)2Cl2 | 2 | 5 | Na2CO3 | 78 | 6 |
| 9 | Pd(CH3CN)2Cl2 | 2 | 5 | Na2CO3 | 55 | 13 |
aIsolated yield. bA trace amount of 4a was obtained.
After the successful coupling reaction of 2 with phenylboronic acid under the optimized reaction conditions, the same reaction was performed with other arylboronic acids bearing a proton, fluoro, chloro, methyl, methoxy and trifluoromethyl on the ortho-, meta- or para-position of the benzene ring. Reactions were successful with both electron-donating and electron-withdrawing arylboronic acids to produce the corresponding 2,2-difluoro-1-arylethenyl tosylates 3 in excellent isolated yields. Especially, the coupling reactions with arylboronic acids having an electron-donating group at the ortho-position of the benzene ring also provided the corresponding coupled products 3l–n in good yields. The cross-coupling reactions of 2 with arylboronic acids are summarized in Table 2.
Table 2: Preparation of 2,2-difluoro-1-arylethenyl tosylate 3.
|
|
|||
| Compound | R | t (h) | Yield (%)a |
|---|---|---|---|
| 3a | H | 15 | 92 |
| 3b | p-F | 15 | 81 |
| 3c | p-Cl | 14 | 84 |
| 3d | p-CH3 | 14 | 79 |
| 3e | p-OCH3 | 14 | 65 |
| 3f | p-CF3 | 15 | 85 |
| 3g | m-F | 16 | 72 |
| 3h | m-Cl | 16 | 76 |
| 3i | m-CH3 | 16 | 82 |
| 3j | m-OCH3 | 18 | 74 |
| 3k | m-CF3 | 18 | 80 |
| 3l | o-CH3 | 18 | 65 |
| 3m | o-OCH3 | 18 | 61 |
| 3n | o-Cl | 18 | 68 |
aIsolated yield.
Direct diarylation reactions of 2 with arylboronic acids were also performed because di-coupled product 4a was formed in a mono-arylation reaction (Table 1) and also a recent work showed a successful cross-coupling reaction of nonfluorinated enol tosylates with a variety of arylboronic acids [23]. We attempted a direct diarylation reaction of 2 with phenylboronic acid to establish the optimized reaction conditions. Initial reaction of 2 with 3 equiv of phenylboronic acid in the presence of 5 mol % of Pd(OAc)2 and 2 equiv of Na2CO3 in MeOH at room temperature for 22 h afforded the di-coupled product 4a in 30% yield. The yield of 4a was increased to 39% using 4 equiv of phenylboronic acid in this reaction. When the reaction was performed with K2CO3 or K3PO4 as a base under the same reaction conditions, the desired product 4a was obtained in up to 49–52% yield. The use of more soluble Cs2CO3 resulted in the formation of 4a in 61% yield. Increasing the reaction temperature did not improve the of yield of 4a. We also examined the effect of the palladium catalyst in this reaction. Therefore, the same reaction was performed in the presence of 5 mol % of Pd(PPh3)2Cl2 instead of Pd(OAc)2, in which 4a was obtained in 75% yield. Other palladium catalysts such as Pd(CH3CN)2Cl2 did not cause to improve the yield of 4a. Optimization of the di-coupling reaction of 2 with phenylboronic acid is summarized in Table 3.
Table 3: Optimization of the di-coupling reaction of 2 with phenylboronic acid.
|
|
|||||
| Entry | Pd catalyst | X (equiv) | Base | T (°C) | Yield (%)a |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | 3 | Na2CO3 | rt | 30 |
| 2 | Pd(OAc)2 | 4 | Na2CO3 | rt | 39 |
| 3 | Pd(OAc)2 | 4 | K2CO3 | rt | 52 |
| 4 | Pd(OAc)2 | 4 | K3PO4 | rt | 49 |
| 5 | Pd(OAc)2 | 4 | Cs2CO3 | rt | 61 |
| 6 | Pd(OAc)2 | 4 | Cs2CO3 | 50 | 58 |
| 7 | Pd(PPh3)2Cl2 | 4 | Cs2CO3 | rt | 75 |
| 8 | Pd(CH3CN)2Cl2 | 4 | Cs2CO3 | rt | 21 |
aIsolated yield of 4a.
Diarylation reactions of 2 with arylboronic acids substituted by fluoro, chloro, methyl, methoxy, nitro and trifluoromethyl substituent at the meta- or para-position of the benzene ring proceeded well under the optimized reaction conditions to give the corresponding symmetrical di-coupled products 4b–k in 51–86% yield. However, coupling reactions with arylboronic acids having a substituent such as chloro, methyl or a methoxy group at the ortho-position of the benzene ring to produce the di-coupled products 4l–n were unsuccessful. This result probably indicates that the coupling process could be affected by steric hindrance in the second coupling reaction step. The di-coupling reactions of 2 with arylboronic acids are summarized in Table 4.
Table 4: Preparation of symmetrical 1,1-diaryl-2,2-difluoroethenes 4.
|
|
||
| Compound No | R | Yield (%)a |
|---|---|---|
| 4a | H | 75 |
| 4b | p-F | 83 |
| 4c | p-Cl | 86 |
| 4d | p-CH3 | 74 |
| 4e | p-OCH3 | 60 |
| 4f | p-NO2 | 73 |
| 4g | m-F | 58 |
| 4h | m-Cl | 51 |
| 4i | m-CH3 | 56 |
| 4j | m-OCH3 | 53 |
| 4k | m-CF3 | 60 |
| 4l | o-CH3 | –b |
| 4m | o-OCH3 | –b |
| 4n | o-Cl | –b |
aIsolated yield. bA trace amount of product was obtained.
Unsymmetrical 1,1-diaryl-2,2-difluoroethenes can be prepared from the coupling reaction of mono-coupled tosylates 3 with arylboronic acids under similar reaction conditions. Therefore, the cross-coupling reaction of 3a with 2 equiv of p-chlorophenylboronic acid in the presence of 5 mol % Pd(PPh3)2Cl2 and 2 equiv of Cs2CO3 in MeOH at room temperature for 12 h afforded 1,1-diaryl-2,2-difluoroethene 4o in 89% yield. The similar reactions of 3a with arylboronic acids having a substituent such as fluoro, methyl, methoxy, and trifluoromethyl at the meta- or para-position of the benzene ring also provided the corresponding 1,1-diaryl-2,2-difluoroethenes 4p–v in 75–90% yields. The coupling reaction between 3c and m-methylphenylboronic acid also resulted in the formation of 4w in 79% yield. Similarly, 3k having an electron-withdrawing group on the benzene ring was also reacted with p-methylphenylboronic acid to yield 4x in 70% yield. Table 5 summarizes the results of the coupling reactions of 3 with several arylboronic acids to give unsymmetrical 1,1-diaryl-2,2-difluoroethenes.
Table 5: Preparation of unsymmetrical 1,1-diaryl-2,2-difluoroethenes 4.
|
|
||||
| Compound No | R | R’ | T (h) | Yield (%)a |
|---|---|---|---|---|
| 4o | H | p-Cl | 12 | 89 |
| 4p | H | p-F | 12 | 90 |
| 4q | H | p-CH3 | 12 | 81 |
| 4r | H | p-OCH3 | 14 | 79 |
| 4s | H | p-CF3 | 18 | 82 |
| 4t | H | m-Cl | 12 | 81 |
| 4u | H | m-CH3 | 14 | 77 |
| 4v | H | m-CF3 | 14 | 75 |
| 4w | p-Cl | m-CH3 | 12 | 79 |
| 4x | m-CF3 | p-CH3 | 12 | 70 |
aIsolated yield.
The successful cross-coupling reaction of 2 with arylboronic acids prompted us to examine similar coupling reactions with alkenylboronic acids. The same reaction conditions of the mono-coupling reaction of 2 with arylboronic acid was applied to the alkenylation of 2. Therefore, the cross-coupling reaction of 2 with trans-2-phenylethenylboronic acid in the presence of 5 mol % of Pd(OAc)2 and 2 equiv of Na2CO3 in MeOH at room temperature for 15 h provided the cross-coupled product 5a in 85% yield. The similar reaction of 2 with trans-oct-1-enylboronic acid afforded the cross-coupled product 5b in 81% yield. The cross-coupling reactions of 2 with alkenylboronic acids are summarized in Table 6.
Conclusion
In summary, we have successfully developed the consecutive cross-coupling reactions of 2,2-difluoro-1-iodoethenyl tosylate (2) with arylboronic or alkenylboronic acids in the presence of a suitable Pd catalyst and a base to afford 2,2-diaryl-1,1-difluoroethenes. The developed method provides synthetically useful advantages such as a straightforward procedure to give symmetrical and unsymmetrical 2,2-diaryl-1,1-difluoroethenes and the use of a less toxic reagent such as boronic acid.
Supporting Information
| Supporting Information File 1: Experimental details, full spectroscopic data and spectra. | ||
| Format: PDF | Size: 717.8 KB | Download |
References
-
Ichikawa, J.; Wada, Y.; Okauchi, T.; Minami, T. Chem. Commun. 1997, 1537–1538. doi:10.1039/a703110f
Return to citation in text: [1] -
Ichikawa, J.; Wada, Y.; Fujiwara, M.; Sakoda, K. Synthesis 2002, 1917–1936. doi:10.1055/s-2002-33912
Return to citation in text: [1] -
Ichikawa, J.; Miyazaki, H.; Sakoda, K.; Wada, Y. J. Fluorine Chem. 2004, 125, 585–593. doi:10.1016/j.jfluchem.2004.01.004
Return to citation in text: [1] -
Ichikawa, J.; Sakoda, K.; Moriyama, H.; Wada, Y. Synthesis 2006, 1590–1598. doi:10.1055/s-2006-926458
Return to citation in text: [1] -
McDonald, I. A.; Lacoste, J. M.; Bey, P.; Palfreyman, M. G.; Ziena, M. J. Med. Chem. 1985, 28, 186–193. doi:10.1021/jm00380a007
Return to citation in text: [1] -
Madden, B. A.; Prestwich, D. G. Bioorg. Med. Chem. Lett. 1997, 7, 309–314. doi:10.1016/S0960-894X(97)00008-5
Return to citation in text: [1] -
Weintraub, P. M.; Holland, A. K.; Gate, C. A.; Moore, W. R.; Resvick, R. J.; Bey, P.; Peet, N. P. Bioorg. Med. Chem. 2003, 11, 427–431. doi:10.1016/S0968-0896(02)00434-0
Return to citation in text: [1] -
Landelle, G.; Turcotte-Savard, M.-O.; Marterer, J.; Champagne, P. A.; Paquin, J.-F. Org. Lett. 2009, 11, 5406–5409. doi:10.1021/ol9022672
Return to citation in text: [1] -
Bobek, M.; Kavai, I.; De Clercq, E. J. Med. Chem. 1987, 30, 1494–1497. doi:10.1021/jm00391a036
Return to citation in text: [1] -
Moore, W. R.; Schatzman, G. L.; Jarvi, E. T.; Gross, R. S.; McCarthy, J. R. J. Am. Chem. Soc. 1992, 114, 360–361. doi:10.1021/ja00027a056
Return to citation in text: [1] -
Altenburger, J.-M.; Lassalle, G. Y.; Matrougui, M.; Galtier, D.; Jetha, J.-C.; Bocskei, Z.; Berry, C. N.; Lunven, C.; Lorrain, J.; Herault, J.-P.; Schaeffer, P.; O’Connor, S. E.; Herbert, J.-M. Bioorg. Med. Chem. 2004, 12, 1713–1730. doi:10.1016/j.bmc.2004.01.016
Return to citation in text: [1] -
Ichikawa, J. J. Synth. Org. Chem., Jpn. 2010, 68, 1175–1184. doi:10.5059/yukigoseikyokaishi.68.1175
Return to citation in text: [1] -
Lee, J.; Tsukazaki, M.; Snieckus, V. Tetrahedron Lett. 1993, 34, 415–418. doi:10.1016/0040-4039(93)85090-J
Return to citation in text: [1] -
Ichikawa, J. J. Fluorine Chem. 2000, 105, 257–263. doi:10.1016/S0022-1139(99)00268-7
Return to citation in text: [1] -
DeBoos, G. A.; Fullbrook, J. J.; Percy, J. M. Org. Lett. 2001, 3, 2859–2861. doi:10.1021/ol010135p
Return to citation in text: [1] -
Ichikawa, J.; Ishibashi, Y.; Fukui, H. Tetrahedron Lett. 2003, 44, 707–710. doi:10.1016/S0040-4039(02)02652-7
Return to citation in text: [1] -
Raghavanpillai, A.; Burton, D. J. J. Org. Chem. 2006, 71, 194–201. doi:10.1021/jo051842p
Return to citation in text: [1] [2] -
Choi, J. H.; Jeong, I. H. Tetrahedron Lett. 2008, 49, 952–955. doi:10.1016/j.tetlet.2007.12.028
Return to citation in text: [1] -
Zhao, Y.; Huang, W.; Zhu, L.; Hu, J. Org. Lett. 2010, 12, 1444–1447. doi:10.1021/ol100090r
Return to citation in text: [1] -
Han, S. Y.; Jeong, I. H. Org. Lett. 2010, 12, 5518–5521. doi:10.1021/ol1024037
Return to citation in text: [1] [2] -
Han, S. Y.; Lee, H. Y.; Jeon, J. H.; Jeong, I. H. Tetrahedron Lett. 2012, 53, 1833–1836. doi:10.1016/j.tetlet.2012.01.127
Return to citation in text: [1] [2] -
Turcotte-Savard, M.-O.; Paquin, J.-F. Org. Biomol. Chem. 2013, 11, 1367–1375. doi:10.1039/c2ob27221k
Return to citation in text: [1] -
Steinhuebel, D.; Baxter, J. M.; Palucki, M.; Davies, I. W. J. Org. Chem. 2005, 70, 10124–10127. doi:10.1021/jo051590s
Return to citation in text: [1]
| 1. | Ichikawa, J.; Wada, Y.; Okauchi, T.; Minami, T. Chem. Commun. 1997, 1537–1538. doi:10.1039/a703110f |
| 2. | Ichikawa, J.; Wada, Y.; Fujiwara, M.; Sakoda, K. Synthesis 2002, 1917–1936. doi:10.1055/s-2002-33912 |
| 3. | Ichikawa, J.; Miyazaki, H.; Sakoda, K.; Wada, Y. J. Fluorine Chem. 2004, 125, 585–593. doi:10.1016/j.jfluchem.2004.01.004 |
| 4. | Ichikawa, J.; Sakoda, K.; Moriyama, H.; Wada, Y. Synthesis 2006, 1590–1598. doi:10.1055/s-2006-926458 |
| 17. | Raghavanpillai, A.; Burton, D. J. J. Org. Chem. 2006, 71, 194–201. doi:10.1021/jo051842p |
| 13. | Lee, J.; Tsukazaki, M.; Snieckus, V. Tetrahedron Lett. 1993, 34, 415–418. doi:10.1016/0040-4039(93)85090-J |
| 14. | Ichikawa, J. J. Fluorine Chem. 2000, 105, 257–263. doi:10.1016/S0022-1139(99)00268-7 |
| 15. | DeBoos, G. A.; Fullbrook, J. J.; Percy, J. M. Org. Lett. 2001, 3, 2859–2861. doi:10.1021/ol010135p |
| 16. | Ichikawa, J.; Ishibashi, Y.; Fukui, H. Tetrahedron Lett. 2003, 44, 707–710. doi:10.1016/S0040-4039(02)02652-7 |
| 17. | Raghavanpillai, A.; Burton, D. J. J. Org. Chem. 2006, 71, 194–201. doi:10.1021/jo051842p |
| 18. | Choi, J. H.; Jeong, I. H. Tetrahedron Lett. 2008, 49, 952–955. doi:10.1016/j.tetlet.2007.12.028 |
| 19. | Zhao, Y.; Huang, W.; Zhu, L.; Hu, J. Org. Lett. 2010, 12, 1444–1447. doi:10.1021/ol100090r |
| 20. | Han, S. Y.; Jeong, I. H. Org. Lett. 2010, 12, 5518–5521. doi:10.1021/ol1024037 |
| 21. | Han, S. Y.; Lee, H. Y.; Jeon, J. H.; Jeong, I. H. Tetrahedron Lett. 2012, 53, 1833–1836. doi:10.1016/j.tetlet.2012.01.127 |
| 22. | Turcotte-Savard, M.-O.; Paquin, J.-F. Org. Biomol. Chem. 2013, 11, 1367–1375. doi:10.1039/c2ob27221k |
| 9. | Bobek, M.; Kavai, I.; De Clercq, E. J. Med. Chem. 1987, 30, 1494–1497. doi:10.1021/jm00391a036 |
| 10. | Moore, W. R.; Schatzman, G. L.; Jarvi, E. T.; Gross, R. S.; McCarthy, J. R. J. Am. Chem. Soc. 1992, 114, 360–361. doi:10.1021/ja00027a056 |
| 11. | Altenburger, J.-M.; Lassalle, G. Y.; Matrougui, M.; Galtier, D.; Jetha, J.-C.; Bocskei, Z.; Berry, C. N.; Lunven, C.; Lorrain, J.; Herault, J.-P.; Schaeffer, P.; O’Connor, S. E.; Herbert, J.-M. Bioorg. Med. Chem. 2004, 12, 1713–1730. doi:10.1016/j.bmc.2004.01.016 |
| 12. | Ichikawa, J. J. Synth. Org. Chem., Jpn. 2010, 68, 1175–1184. doi:10.5059/yukigoseikyokaishi.68.1175 |
| 5. | McDonald, I. A.; Lacoste, J. M.; Bey, P.; Palfreyman, M. G.; Ziena, M. J. Med. Chem. 1985, 28, 186–193. doi:10.1021/jm00380a007 |
| 6. | Madden, B. A.; Prestwich, D. G. Bioorg. Med. Chem. Lett. 1997, 7, 309–314. doi:10.1016/S0960-894X(97)00008-5 |
| 7. | Weintraub, P. M.; Holland, A. K.; Gate, C. A.; Moore, W. R.; Resvick, R. J.; Bey, P.; Peet, N. P. Bioorg. Med. Chem. 2003, 11, 427–431. doi:10.1016/S0968-0896(02)00434-0 |
| 8. | Landelle, G.; Turcotte-Savard, M.-O.; Marterer, J.; Champagne, P. A.; Paquin, J.-F. Org. Lett. 2009, 11, 5406–5409. doi:10.1021/ol9022672 |
| 23. | Steinhuebel, D.; Baxter, J. M.; Palucki, M.; Davies, I. W. J. Org. Chem. 2005, 70, 10124–10127. doi:10.1021/jo051590s |
| 20. | Han, S. Y.; Jeong, I. H. Org. Lett. 2010, 12, 5518–5521. doi:10.1021/ol1024037 |
| 21. | Han, S. Y.; Lee, H. Y.; Jeon, J. H.; Jeong, I. H. Tetrahedron Lett. 2012, 53, 1833–1836. doi:10.1016/j.tetlet.2012.01.127 |
© 2013 Kim et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)