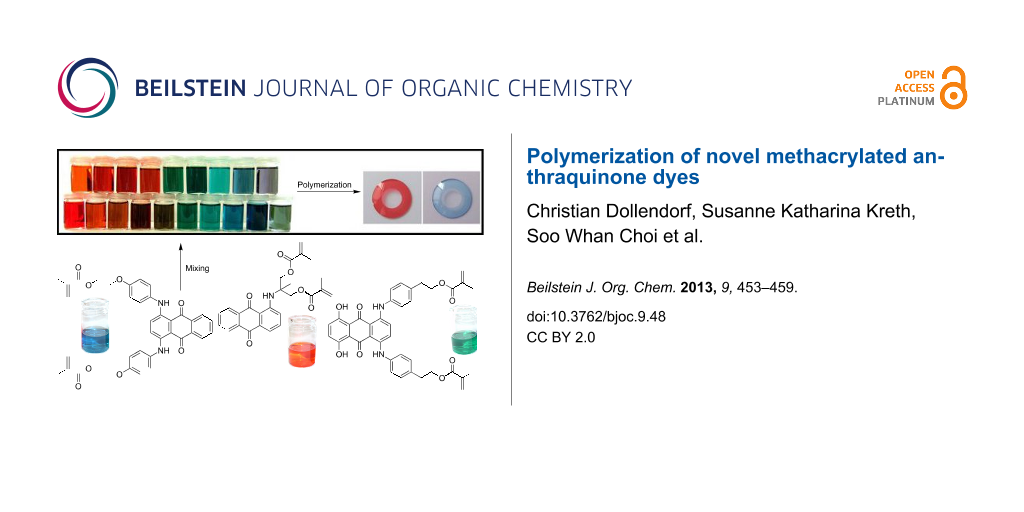
Abstract
A new series of polymerizable methacrylated anthraquinone dyes has been synthesized by nucleophilic aromatic substitution reactions and subsequent methacrylation. Thereby, green 5,8-bis(4-(2-methacryloxyethyl)phenylamino)-1,4-dihydroxyanthraquinone (2), blue 1,4-bis(4-((2-methacryloxyethyl)oxy)phenylamino)anthraquinone (6) and red 1-((2-methacryloxy-1,1-dimethylethyl)amino)anthraquinone (12), as well as 1-((1,3-dimethacryloxy-2-methylpropan-2-yl)amino)anthraquinone (15) were obtained. By mixing of these brilliant dyes in different ratios and concentrations, a broad color spectrum can be generated. After methacrylation, the monomeric dyes can be covalently emplaced into several copolymers. Due to two polymerizable functionalities, they can act as cross-linking agents. Thus, diffusion out of the polymer can be avoided, which increases the physiological compatibility and makes the dyes promising compounds for medical applications, such as iris implants.
Graphical Abstract
Introduction
Anthraquinone and its derivatives bearing hydroxy and amino moieties are of remarkable importance in pharmacological, biochemical and dye industries [1,2]. They have been described as important compounds for decades [3-6] and are used for the coloration of cotton and cellulose fibers as well as for synthetic materials such as polyamides [7]. Besides azo dyes, they represent the second largest class of textile pigments [8], and unlike these dyes, anthraquinone derivatives are resistant against degradation due to their aromatic structure [9]. As the syntheses of these dyes are more complicated, their production costs are higher in comparison to azo dyes. Therefore, anthraquinone dyes are only used if the required properties and colors are remarkable or when the desired colors cannot be obtained easily by the use of azo dyes, especially in the case of bright blue shades. Anthraquinone dyes can be obtained synthetically and by isolation from fungi such as Dermocybe sanguinea or other fungal species [5,10-13], to give them a higher compatibility with the environment and an improved availability [14-16].
Coloration of polymers is usually achieved by mixing dyes or pigments with (other) polymeric ingredients. In many cases, the colors rapidly fade or change as well as lose their mechanical properties when exposed to sunlight. More stable polymers can be prepared by the reaction of polymerizable dyes with appropriate comonomers. In the past, these materials have been widely used because of their photo- and thermostability, their strong fluorescence, or their resistance to water and other solvents [7,17,18].
The purpose of this work was to create dyes of brilliant color modified with polymerizable groups, to enable the preparation of polymeric materials with covalently emplaced dyes. A broad range of the color spectrum can be produced by mixing three colors such as blue, green and red, which were synthesized in this work [19-24]. For example, colored polymers can be used for medical applications such as iris implants, where damaged irides are replenished with polymeric replications [25-27].
Results and Discussion
Starting from commercially available anthraquinone-derivatives, various polymerizable dyes were synthesized. Three colors (green, blue, red) were obtained (Scheme 1).
Scheme 1: Synthesis of red (9, 12, 15), blue (6, 10) and green (2) polymerizable dyes.
Scheme 1: Synthesis of red (9, 12, 15), blue (6, 10) and green (2) polymerizable dyes.
Polymerizable green dye 2 based on anthraquinone
The synthesis of the green anthraquinone based monomeric dye 2 is shown in Scheme 1, step a. 5,8-Dichloro-1,4-dihydroxyanthraquinone was first subjected to a nucleophilic substitution reaction with 2-(4-aminophenyl)ethanol to give a green-colored double-substituted intermediate 5,8-bis(4-(2-hydroxyethyl)phenylamino)-1,4-dihydroxyanthraquinone (1) in 50% overall yield. According to the literature, reactions can be accelerated with improved yields by microwave assistance [28,29]. After microwave-assisted methacrylation with methacrylic anhydride the polymerizable anthraquinone based monomeric dye 2 was obtained. Due to steric reasons, methacrylic anhydride is less reactive than the corresponding chloride and therefore selective for the methacrylation of the aliphatic hydroxy groups [25]. In this case, the use of methacryloyl chloride always leads to the formation of several side-products. As cross-linking agent, 2 can be copolymerized with other methacrylate- or acrylate-comonomers, to build up macromolecules bearing covalently emplaced dye derivatives.
The UV–vis spectra of 1 and 2 (Figure 1a) in each case show three absorption maxima (416, 636 and 684 nm), which can be found in the violet and red color ranges. A combination of these two colors appears purple, the complementary color to green. Methacrylation of 1 does not change the chromophore and therefore the absorption maxima. The change in the absorption behavior of 2 compared to 5,8-dichloro-1,4-dihydroxyanthraquinone (λmax = 500 nm) is a result of the enlargement of the π-electron system as aromatic moieties are introduced. This leads to absorption at longer wavelengths (bathochromic shift). Thus, the challenge of creating a green dye is to realize selective absorption in two different areas of the visible spectrum.
Figure 1: Visible spectra of the polymerizable dyes green 1/2 (a) and blue 6 (b).
Figure 1: Visible spectra of the polymerizable dyes green 1/2 (a) and blue 6 (b).
Polymerizable blue dye 6 based on anthraquinone
For the synthesis of the blue anthraquinone based monomeric dye 6, the precursor 1,4-dichloroanthraquinone (3) was prepared by the reaction of 1,4-dichlorobenzene with phthalic dichloride by Friedel–Crafts acylation followed by an acidic ring closure (Scheme 1, step b). In further reaction steps, 1,4-dichloroanthraquinone (3) and hydroxyaniline were reacted to obtain 1,4-bis(4-hydroxyphenylamino)anthraquinone (4). Subsequent reaction with 2-bromoethanol gave 1,4-bis(4-((2-hydroxyethyl)oxy)phenylamino)anthraquinone (5). In order to introduce polymerizable functionalities, 5 was treated with methacrylic chloride to give 1,4-bis(4-((2-methacryloxyethyl)oxy)phenylamino)anthraquinone (6) (Scheme 1, step c).
The obtained blue chromophore 6 shows absorption maxima at 408, 600 and 644 nm in the UV–vis spectrum (Figure 1b). These maxima are located in the purple, orange and red color ranges, which results in combination to give the color blue. Analogous to the green dye, methacrylation of 5 does not influence the absorption maxima.
Polymerizable red dyes 9, 12 and 15 based on anthraquinone
A red monomeric dye 9 with non-aromatic amines as functional moieties, which showed similar properties, was also synthesized starting from anthraquinone. 1,1-Dimethyl-2-hydroxyethylamine exhibits two methyl groups in the α-position to the nitrogen atom, which shields the chromophoric amine. The absence of hydrogen atoms in the α-position also protects the amino group from further reactions. In the cases of the green and blue monomeric dyes this effect was achieved by introducing a benzene ring in the α-position [25].
1,4-Dichloroanthraquinone was treated with 1,1-dimethyl-2-hydroxyethylamine to form two products, namely 1-chloro-4-((2-hydroxy-1,1-dimethylethyl)amino)anthraquinone (7) and 1,4-bis((2-hydroxy-1,1-dimethylethyl)amino)anthraquinone (8). Both products can be separated by column chromatography and esterified with methacryloyl chloride to obtain polymerizable monomeric dyes 9 and 10 (Scheme 1, step d). The monosubstituted product 9 shows a characteristic red color and an absorption maximum at 506 nm, whereas the disubstituted product 10 is colored blue with absorption maxima at 574 and 618 nm (Figure 2).
Figure 2: Visible spectra of red 9 and blue 10.
Figure 2: Visible spectra of red 9 and blue 10.
The synthesis of a red polymerizable dye 12 without any side-products can be accomplished by the use of 1-chloroanthraquinone and 1,1-dimethyl-2-hydroxyethylamine giving 1-((1-hydroxy-2-methylpropane-2-yl)amino)anthraquinone (11) in good yields (60%). Subsequent esterification with methacryloyl chloride gives 12 [26,27]. This dye shows a characteristic red color with an absorption maximum at 506 nm, which is located in the green color range, the complementary color to red.
By introduction of a second polymerizable functionality, diffusion of incompletely polymerized dyes out of the polymer network can be avoided, which increases the physiological compatibility. This monomer can also be used as a cross-linking agent. For this purpose, 1-chloroanthraquinone was coupled with 2-amino-2-methyl-1,3-propanediol under argon atmosphere and esterified with methacryloyl chloride to obtain 15 (Scheme 1, step g), which absorbs light at 495 nm.
In the absence of argon, the formation of a side-product can be observed. An oxidative ring closure takes place during the reaction of 1-chloroanthraquinone and 2-amino-2-methyl-1,3-propanediol, which gives 14 as a side-product in low yield (6%). The yield can be increased by microwave assistance and the use of higher temperatures [27]. It can be assumed that the driving force for the ring closure is the formation of a six-membered ring; however, the mechanism has not been investigated in detail so far. Since the ring closure does not affect the spectral properties, 14 can still be used as a polymerizable red dye.
In this work, side-products derived from ring-closure reactions were only found when aliphatic amines with two carbon units between the amine and hydroxy functionality were used. In the synthesis of green and blue dyes, aromatic amines that cannot build six-membered rings were utilized. However, the ring-closure can be suppressed by using inert gas or adequate compounds such as 5-aminopentane-1-ol, for example [27].
Polymerization and photosensitivity of the synthesized monomeric dyes 6, 9 and 10
By way of example for copolymerization of two monomeric dyes, 9 or 10 were copolymerized with 2-hydroxyethylmethacrylate (2-HEMA), tetrahydrofurfuryl methacrylate (THFMA) and ethylene glycol dimethacrylate (EGDMA) as cross-linking agents in order to obtain materials with duroplastic properties and high resistance against aggressive biological media [28,30-32]. When the polymers were placed in water, even after several weeks, no diffusion of monomeric dyes out of the network was observed, as examined by UV–vis spectroscopy. This proves the covalent emplacement of the monomeric dyes in the polymer network. Figure 3 shows polymers containing 9 and 10 after sharpening for further application as iris implants.
![[1860-5397-9-48-3]](/bjoc/content/figures/1860-5397-9-48-3.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Sharpened blank of polymerized red 9 and blue 10 with HEMA, THFMA and EGDMA (left, right).
Figure 3: Sharpened blank of polymerized red 9 and blue 10 with HEMA, THFMA and EGDMA (left, right).
For use in medical applications like iris implants, the dyes and the corresponding polymers have to be biocompatible (ISO 11979-5) [33-35]. To analyze the photosensitivity of the materials, the dye-containing polymers were preserved in a sodium chloride solution at 35 °C and exposed to irradiation with visible light (3 mW/cm2 2500 h) for 100 days. As an example, Figure 4 shows the transmission spectrum for the polymer containing cross-linker 6. After 100 days, no changes in the transmission spectra could be observed, which proves the monomer dye stability and is also a hint toward its biocompatibility.
Mixture of polymerizable dyes 2, 6 and 15
By mixing the synthesized blue, red and green dyes (6, 15 and 2) in different ratios, individual color shades can be realized. This so-called RGB-color space can also be found in other color devices, such as monitors, scanners and color printers. A broad visible spectrum can be covered by mixing these three colors in different ratios [19-21].
The subjective color impression depends deeply on the used monomer concentration [36]. For example, the red dye 15 appears orange in high dilution, and the green dye 2 appears greenish-blue. At the same concentration, the blue dye 6 shows the lowest transparency. By mixing these colors, most of the blue-containing nuances only slightly differ. Due to the absorption of the broadest area in the visible spectrum and the lowest brilliance and transparency, the blue color masks the green and red one.
To avoid these effects, the concentrations of the monomer solutions had to be adapted. Each dye was diluted in acetone so as to show the same transparency and color brilliance. By mixing these solutions, a broad spectrum of colors can be created (Figure 5).
![[1860-5397-9-48-5]](/bjoc/content/figures/1860-5397-9-48-5.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Broad spectrum of colors, created by mixing of green 2, blue 6 and red 15.
Figure 5: Broad spectrum of colors, created by mixing of green 2, blue 6 and red 15.
In this way, it is possible to create all color shades and nuances that are needed to replicate human irides, such as blue, green, brown and even gray. It is possible to print different color shades by using the synthesized functional dyes, which can then be polymerized afterwards [37-40]. Thus, a color design can be permanently included into polymeric materials, such as materials used for iris implants or other medical applications.
Conclusion
In conclusion, a series of novel anthraquinone based polymerizable dyes has been synthesized by nucleophilic aromatic substitution reactions of anthraquinone derivatives with several amino alcohols. Depending on the moieties that were introduced, red-, blue- and green-colored dyes were prepared. After esterification with methacryloyl derivatives, polymerizable functionalities could be introduced. A broad color spectrum can be replicated by mixing these three dyes in different ratios and different concentrations. The dyes show no significant changes in their transmission spectra under sun-test conditions, and can be covalently emplaced into different copolymers. Thus, polymer materials that show no further diffusions of colored compounds out of the polymer networks were prepared. Containing two polymerizable functionalities, these monomeric dyes can also be used as cross-linking agents. Because of their stability, brilliance in color, and ability to cover a broad color spectrum, these monomers can be used for medical applications such as iris implants.
Supporting Information
Supporting Information features detailed data on syntheses.
| Supporting Information File 1: Experimental details. | ||
| Format: PDF | Size: 396.5 KB | Download |
References
-
Duan, Y.; Yu, J.; Liu, S.; Ji, M. Med. Chem. 2009, 5, 577–582. doi:10.2174/157340609790170498
Return to citation in text: [1] -
Abu-Darwish, S. M.; Ateyyat, A. M. World J. Agric. Sci. 2008, 4, 495–505.
Return to citation in text: [1] -
Mishra, A. K.; Jacob, J.; Müllen, K. Dyes Pigm. 2007, 75, 1–10. doi:10.1016/j.dyepig.2006.05.025
Return to citation in text: [1] -
Agosti, G.; Birkinshaw, J. H.; Chaplen, P. Biochem. J. 1962, 85, 528–530.
Return to citation in text: [1] -
Bick, I. R. C.; Rhee, C. Biochem. J. 1966, 98, 112–116.
Return to citation in text: [1] [2] -
Dyes. In Encyclopedia of Chemical Technology; Kirk, R. E.; Othmer, D. F., Eds.; Wiley: Hoboken, NJ, 2005; Vol. 9, pp 300–347.
Return to citation in text: [1] -
Manson, J. S.; Ridyard, D. R. A. Anthraquinone Dyes. U.S. Patent 3935248, Jan 27, 1976.
Return to citation in text: [1] [2] -
Aspland, J. R. Textile Dyeing and Coloration; American Association of Chemists and Colorists: Research Triangle Park, NC, USA, 1997; pp 251 ff.
Return to citation in text: [1] -
Banat, I. M.; Nigam, P.; Singh, D.; Marchant, R. Bioresour. Technol. 1996, 58, 217–227. doi:10.1016/S0960-8524(96)00113-7
Return to citation in text: [1] -
Baker, R. A.; Tatum, J. H. J. Ferment. Bioeng. 1998, 85, 359–361. doi:10.1016/S0922-338X(98)80077-9
Return to citation in text: [1] -
Murakami, T.; Tanaka, N. Progress in the Chemistry of Organic Natural Products; Springer: Wien-New York, 1987; Vol. 54, pp 125–174.
Return to citation in text: [1] -
Hamlyn, P. F. British Mycological Society Newsletter 1998, May 17-18, http://fungus.org.uk/nwfg/fungbiot.htm.
Return to citation in text: [1] -
Hobson, D. K.; Wales, P. S. J. Soc. Dyers Colour. 1998, 114, 42–44. doi:10.1111/j.1478-4408.1998.tb01944.x
Return to citation in text: [1] -
Postsch, W. R. Melliand Textilber. 2002, 83, 35.
Return to citation in text: [1] -
Tiwari, V.; Vankar, P. S. Colourage 2001, 48, 25–28.
Return to citation in text: [1] -
Gupta, D.; Kumari, S.; Gulrajani, M. Color. Technol. 2001, 117, 333–336. doi:10.1111/j.1478-4408.2001.tb00085.x
Return to citation in text: [1] -
Marechal, E. Prog. Org. Coat. 1982, 10, 251–287. doi:10.1016/0300-9440(82)80022-2
Return to citation in text: [1] -
Miley, J. Pure Appl. Chem. 1996, 68, 1423–1428. doi:10.1351/pac199668071423
Return to citation in text: [1] -
Whelan, J. F.; Jackson, D. W. Arthroscop. Rel. Surg. 1992, 8, 311–319. doi:10.1016/0749-8063(92)90061-F
Return to citation in text: [1] [2] -
Arnold, M. S.; McGraw, G. J.; Forrest, S. R.; Lunt, R. R. Appl. Phys. Lett. 2008, 92, 053301–053304. doi:10.1063/1.2838457
Return to citation in text: [1] [2] -
Dong, G.; Xiao, X.; Zhang, L.; Ma, Z.; Bao, X.; Peng, M.; Zhanga, Q.; Qiu, J. J. Mater. Chem. 2011, 21, 2194–2203. doi:10.1039/c0jm02851g
Return to citation in text: [1] [2] -
Color Mixing. http://www.enchantedlearning.com/crafts/Colormixing.shtml (accessed Dec 20, 2012).
Return to citation in text: [1] -
Color Mixing. http://home.comcast.net/~RTRUSCIO/COLORSYS.htm (accessed Dec 20, 2012).
Return to citation in text: [1] -
Nassau, K., Ed. Fundamentals of color science; Azimuth; Elsevier, 1998.
Return to citation in text: [1] -
Kreth, K. Farbige Materialien für Irisimplantate. Ph.D. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2011.
Return to citation in text: [1] [2] [3] -
Dollendorf, C.; Kreth, K.; Choi, S. W.; Ritter, H. Patent 2012; not yet confirmed.
Return to citation in text: [1] [2] -
Dollendorf, C. Polymerisierbare Farbstoffe für Optische Anwendungen. Dipl. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2010.
Return to citation in text: [1] [2] [3] [4] -
Maatz, G.; Ritter, H. Macromol. Chem. Phys. 2012, 213, 1569–1574. doi:10.1002/macp.201200068
Return to citation in text: [1] [2] -
Theis, J.; Ritter, H.; Klee, J. E. Macromol. Rapid Commun. 2009, 30, 1424–1427. doi:10.1002/marc.200900157
Return to citation in text: [1] -
Odian, G. Principles of Polymerization; John Wiley & Sons: Hoboken, NJ, 2004. doi:10.1002/047147875X
Return to citation in text: [1] -
Kálal, J. Makromol. Chem. 1984, 7 (Suppl. 7), 31–39. doi:10.1002/macp.1984.020071984103
Return to citation in text: [1] -
Wichterle, O.; Lim, D. Nature 1960, 185, 117–118. doi:10.1038/185117a0
Return to citation in text: [1] -
Thiagalingam, S.; Tarongoy, P.; Hamrah, P.; Lobo, A.-M.; Nagao, K.; Barsam, C.; Bellows, R.; Pineda, R. J. Cataract Refractive Surg. 2008, 34, 1222–1224. doi:10.1016/j.jcrs.2008.02.035
Return to citation in text: [1] -
Langer, R.; Brem, H.; Tapper, D. J. Biomed. Mater. Res. 1981, 15, 267–277. doi:10.1002/jbm.820150212
Return to citation in text: [1] -
Smetana, K., Jr.; Šulc, J.; Krčová, Z.; Pitrová, Š. J. Biomed. Mater. Res. 1987, 21, 1247–1253. doi:10.1002/jbm.820211007
Return to citation in text: [1] -
Krazert, W.; Peichert, R., Eds. Farbstoffe; Quelle & Meyer: Heidelberg, Germany, 1981; pp 10–15.
Return to citation in text: [1] -
Bharathan, Y.; Yang, Y. Appl. Phys. Lett. 1998, 72, 2660–2662. doi:10.1063/1.121090
Return to citation in text: [1] -
Hebner, T. R.; Wu, C. C.; Marcy, D.; Lu, M. H.; Sturm, J. C. Appl. Phys. Lett. 1998, 72, 519–521. doi:10.1063/1.120807
Return to citation in text: [1] -
Sirringhaus, H.; Kawase, T.; Friend, R. H.; Shimoda, T.; Inbasekaran, M.; Wu, W.; Woo, E. P. Science 2000, 290, 2123–2126. doi:10.1126/science.290.5499.2123
Return to citation in text: [1] -
Shimoda, T.; Morii, K.; Seki, S.; Kiguchi, H. MRS Bull. 2003, 28, 821–827. doi:10.1557/mrs2003.231
Return to citation in text: [1]
| 19. | Whelan, J. F.; Jackson, D. W. Arthroscop. Rel. Surg. 1992, 8, 311–319. doi:10.1016/0749-8063(92)90061-F |
| 20. | Arnold, M. S.; McGraw, G. J.; Forrest, S. R.; Lunt, R. R. Appl. Phys. Lett. 2008, 92, 053301–053304. doi:10.1063/1.2838457 |
| 21. | Dong, G.; Xiao, X.; Zhang, L.; Ma, Z.; Bao, X.; Peng, M.; Zhanga, Q.; Qiu, J. J. Mater. Chem. 2011, 21, 2194–2203. doi:10.1039/c0jm02851g |
| 28. | Maatz, G.; Ritter, H. Macromol. Chem. Phys. 2012, 213, 1569–1574. doi:10.1002/macp.201200068 |
| 30. | Odian, G. Principles of Polymerization; John Wiley & Sons: Hoboken, NJ, 2004. doi:10.1002/047147875X |
| 31. | Kálal, J. Makromol. Chem. 1984, 7 (Suppl. 7), 31–39. doi:10.1002/macp.1984.020071984103 |
| 32. | Wichterle, O.; Lim, D. Nature 1960, 185, 117–118. doi:10.1038/185117a0 |
| 33. | Thiagalingam, S.; Tarongoy, P.; Hamrah, P.; Lobo, A.-M.; Nagao, K.; Barsam, C.; Bellows, R.; Pineda, R. J. Cataract Refractive Surg. 2008, 34, 1222–1224. doi:10.1016/j.jcrs.2008.02.035 |
| 34. | Langer, R.; Brem, H.; Tapper, D. J. Biomed. Mater. Res. 1981, 15, 267–277. doi:10.1002/jbm.820150212 |
| 35. | Smetana, K., Jr.; Šulc, J.; Krčová, Z.; Pitrová, Š. J. Biomed. Mater. Res. 1987, 21, 1247–1253. doi:10.1002/jbm.820211007 |
| 1. | Duan, Y.; Yu, J.; Liu, S.; Ji, M. Med. Chem. 2009, 5, 577–582. doi:10.2174/157340609790170498 |
| 2. | Abu-Darwish, S. M.; Ateyyat, A. M. World J. Agric. Sci. 2008, 4, 495–505. |
| 9. | Banat, I. M.; Nigam, P.; Singh, D.; Marchant, R. Bioresour. Technol. 1996, 58, 217–227. doi:10.1016/S0960-8524(96)00113-7 |
| 27. | Dollendorf, C. Polymerisierbare Farbstoffe für Optische Anwendungen. Dipl. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2010. |
| 8. | Aspland, J. R. Textile Dyeing and Coloration; American Association of Chemists and Colorists: Research Triangle Park, NC, USA, 1997; pp 251 ff. |
| 27. | Dollendorf, C. Polymerisierbare Farbstoffe für Optische Anwendungen. Dipl. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2010. |
| 7. | Manson, J. S.; Ridyard, D. R. A. Anthraquinone Dyes. U.S. Patent 3935248, Jan 27, 1976. |
| 25. | Kreth, K. Farbige Materialien für Irisimplantate. Ph.D. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2011. |
| 3. | Mishra, A. K.; Jacob, J.; Müllen, K. Dyes Pigm. 2007, 75, 1–10. doi:10.1016/j.dyepig.2006.05.025 |
| 4. | Agosti, G.; Birkinshaw, J. H.; Chaplen, P. Biochem. J. 1962, 85, 528–530. |
| 5. | Bick, I. R. C.; Rhee, C. Biochem. J. 1966, 98, 112–116. |
| 6. | Dyes. In Encyclopedia of Chemical Technology; Kirk, R. E.; Othmer, D. F., Eds.; Wiley: Hoboken, NJ, 2005; Vol. 9, pp 300–347. |
| 26. | Dollendorf, C.; Kreth, K.; Choi, S. W.; Ritter, H. Patent 2012; not yet confirmed. |
| 27. | Dollendorf, C. Polymerisierbare Farbstoffe für Optische Anwendungen. Dipl. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2010. |
| 19. | Whelan, J. F.; Jackson, D. W. Arthroscop. Rel. Surg. 1992, 8, 311–319. doi:10.1016/0749-8063(92)90061-F |
| 20. | Arnold, M. S.; McGraw, G. J.; Forrest, S. R.; Lunt, R. R. Appl. Phys. Lett. 2008, 92, 053301–053304. doi:10.1063/1.2838457 |
| 21. | Dong, G.; Xiao, X.; Zhang, L.; Ma, Z.; Bao, X.; Peng, M.; Zhanga, Q.; Qiu, J. J. Mater. Chem. 2011, 21, 2194–2203. doi:10.1039/c0jm02851g |
| 22. | Color Mixing. http://www.enchantedlearning.com/crafts/Colormixing.shtml (accessed Dec 20, 2012). |
| 23. | Color Mixing. http://home.comcast.net/~RTRUSCIO/COLORSYS.htm (accessed Dec 20, 2012). |
| 24. | Nassau, K., Ed. Fundamentals of color science; Azimuth; Elsevier, 1998. |
| 28. | Maatz, G.; Ritter, H. Macromol. Chem. Phys. 2012, 213, 1569–1574. doi:10.1002/macp.201200068 |
| 29. | Theis, J.; Ritter, H.; Klee, J. E. Macromol. Rapid Commun. 2009, 30, 1424–1427. doi:10.1002/marc.200900157 |
| 7. | Manson, J. S.; Ridyard, D. R. A. Anthraquinone Dyes. U.S. Patent 3935248, Jan 27, 1976. |
| 17. | Marechal, E. Prog. Org. Coat. 1982, 10, 251–287. doi:10.1016/0300-9440(82)80022-2 |
| 18. | Miley, J. Pure Appl. Chem. 1996, 68, 1423–1428. doi:10.1351/pac199668071423 |
| 25. | Kreth, K. Farbige Materialien für Irisimplantate. Ph.D. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2011. |
| 14. | Postsch, W. R. Melliand Textilber. 2002, 83, 35. |
| 15. | Tiwari, V.; Vankar, P. S. Colourage 2001, 48, 25–28. |
| 16. | Gupta, D.; Kumari, S.; Gulrajani, M. Color. Technol. 2001, 117, 333–336. doi:10.1111/j.1478-4408.2001.tb00085.x |
| 36. | Krazert, W.; Peichert, R., Eds. Farbstoffe; Quelle & Meyer: Heidelberg, Germany, 1981; pp 10–15. |
| 5. | Bick, I. R. C.; Rhee, C. Biochem. J. 1966, 98, 112–116. |
| 10. | Baker, R. A.; Tatum, J. H. J. Ferment. Bioeng. 1998, 85, 359–361. doi:10.1016/S0922-338X(98)80077-9 |
| 11. | Murakami, T.; Tanaka, N. Progress in the Chemistry of Organic Natural Products; Springer: Wien-New York, 1987; Vol. 54, pp 125–174. |
| 12. | Hamlyn, P. F. British Mycological Society Newsletter 1998, May 17-18, http://fungus.org.uk/nwfg/fungbiot.htm. |
| 13. | Hobson, D. K.; Wales, P. S. J. Soc. Dyers Colour. 1998, 114, 42–44. doi:10.1111/j.1478-4408.1998.tb01944.x |
| 25. | Kreth, K. Farbige Materialien für Irisimplantate. Ph.D. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2011. |
| 26. | Dollendorf, C.; Kreth, K.; Choi, S. W.; Ritter, H. Patent 2012; not yet confirmed. |
| 27. | Dollendorf, C. Polymerisierbare Farbstoffe für Optische Anwendungen. Dipl. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2010. |
| 37. | Bharathan, Y.; Yang, Y. Appl. Phys. Lett. 1998, 72, 2660–2662. doi:10.1063/1.121090 |
| 38. | Hebner, T. R.; Wu, C. C.; Marcy, D.; Lu, M. H.; Sturm, J. C. Appl. Phys. Lett. 1998, 72, 519–521. doi:10.1063/1.120807 |
| 39. | Sirringhaus, H.; Kawase, T.; Friend, R. H.; Shimoda, T.; Inbasekaran, M.; Wu, W.; Woo, E. P. Science 2000, 290, 2123–2126. doi:10.1126/science.290.5499.2123 |
| 40. | Shimoda, T.; Morii, K.; Seki, S.; Kiguchi, H. MRS Bull. 2003, 28, 821–827. doi:10.1557/mrs2003.231 |
© 2013 Dollendorf et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)