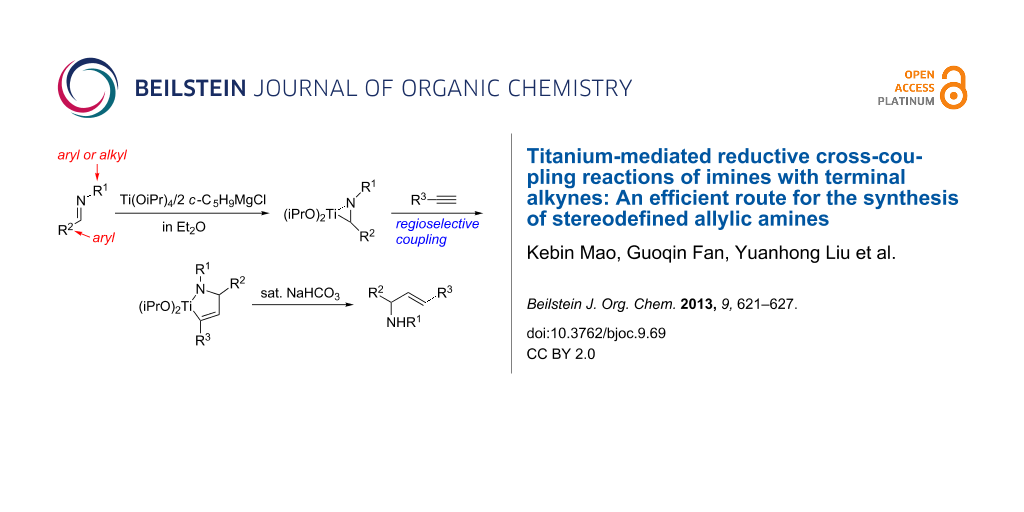
Abstract
Low-valency titanium species, generated in situ by using Ti(OiPr)4/2 c-C5H9MgCl reagent, react with imines to give a titanium-imine complex that can couple with terminal alkynes to provide azatitanacyclopentenes with excellent regioselectivity. Stereodefined allylic amines are obtained in good yields after hydrolysis or iodonolysis of the corresponding azatitanacyclopentenes. When ethynylcyclopropane is used as the coupling partner to react with imines in this reaction, the initially generated allylic amine undergoes an unexpected 1,3-amino migration on silica gel during the column chromatography.
Graphical Abstract
Introduction
Allylic amines are fundamental three-carbon building blocks in organic chemistry and their synthesis is an important industrial and synthetic goal [1-4]. The two functionalities in the allylic amine fragment, i.e., the nucleophilic amino group and the alkene, can ideally participate in cycloaddition reactions [5,6], condensation reactions [7], nucleophilic substitution reactions [8,9], radical reactions [10] and Pd-catalyzed reactions [11]. Thus, allylic amines have been used for the synthesis of numerous heterocycles and bioactive amines, such as α- and β-amino acids [12-15], different alkaloids [16], aminoallylsilanes [17], aminoepoxides [18], iodocyclocarbamates [19] and isoxazolines [20]. Although it has been reported that allylic amines can be synthesized by methods such as amination of allylic alcohols [21-24], direct allylic amination of simple alkenes [25-27], Morita–Baylis–Hillman reaction [28], alkenylation of imines [29-32], etc., it is still a great challenge to synthesize allylic amines with a stereodefined alkene moiety. The low-valency group 4 metal complexes (M = Ti or Zr) mediated reductive cross-coupling of imines with alkynes is one of the useful methods to construct stereodefined allylic amines. For example, Buchwald et al. reported that zirconocene-imine complexes, generated by treating Cp2ZrMeCl with lithium dialkylamide followed by elimination of methane from the resulting zirconocene(methyl) amide complex, coupled with alkynes to give geometrically pure allylic amines after hydrolysis [33]. They also developed an asymmetric variant of this reaction that proceeded to give allylic amine products with ee’s up to 99% by using chiral ansa-zirconocenes [34]. However, these reactions required a tedious multistep procedure for the preparation of zirconocene–imine complexes. In addition, the use of terminal alkynes produced an inseparable mixture of two regioisomers in some cases [33] or could not give the desired products [34]. Sato et al. reported that a divalent titanium reagent generated by the Ti(OiPr)4/2 iPrMgX system reacted with arylaldimines to provide the corresponding (η2-imine)Ti(OiPr)2 complex that, in turn, reacted with alkynes to give allylic amines after hydrolysis of the resulting azatitanacyclopentenes [35]. In this report, a terminal alkyne showed excellent regioselectivity and much better reactivity than internal alkynes. But only one successful example using a terminal alkyne appeared in this report (1-octyne). Sato’s group further applied this reaction for the synthesis of optically active allylic amines with chiral imines and terminal alkynes [36]. However, the imine substrates employed in their reactions were all N-alkyl substituted ones [35,36]. Until now the scope and limitations for titanium-mediated reductive cross-coupling reactions of imines with terminal alkynes have been far less studied.
Our group has developed a series of reactions using low-valency titanium reagents [37-39], including selective coupling of 1,3-butadiynes with aldehydes using Ti(OiPr)4/2 n-BuLi reagent [37] and titanium-mediated formation of cis-[3]cumulenes in the presence of a Lewis acid [38]. Very recently, we reported titanium mediated cross-coupling reactions of imines with ketones or aldehydes by the activation of imines with Ti(OiPr)4/2 c-C5H9MgCl reagent [40-42] leading to 1,2-amino alcohols [39]. These results prompted us to study the cross-coupling of imines with terminal alkynes by using the Ti(OiPr)4/2 c-C5H9MgCl reagent. In this paper, we describe the detailed results of these reactions (Scheme 1).
Scheme 1: Titanium-mediated cross-coupling of imines with terminal alkynes.
Scheme 1: Titanium-mediated cross-coupling of imines with terminal alkynes.
Results and Discussion
Synthesis of allylic amines by reductive cross-coupling using Ti(OiPr)4/2 c-C5H9MgCl. First, a typical example for the synthesis of allylic amines by reductive cross-coupling reactions using Ti(OiPr)4/2 c-C5H9MgCl reagent was studied by using imine 2a and 1-heptyne as model substrates (Scheme 2). Based on our previous report [39], Ti-imine complex 3a was generated in situ by the reaction of imine 2a with 1.3 equiv of Ti(OiPr)4/2 c-C5H9MgCl at −30 °C. It was found that Ti-imine complex 3a could smoothly couple with 1.5 equiv of 1-heptyne to give allylic amine 5a in 77% NMR yield after hydrolysis of the resulting azatitanacyclopentene complex 4a with saturated aqueous NaHCO3 solution. In this reaction, azatitanacyclopentene 4a, rather than its regioisomer 4a’, was formed preferentially, in which the pentyl group is situated adjacent to titanium (Scheme 3, reaction 1). Accordingly, the allylic amine 5a could be obtained after hydrolysis with excellent regioselectivity. There was no apparent formation of the regioisomer 4a’ and allylic amine 5a’ in this reaction, which may be due to the strong steric repulsion between the phenyl and pentyl groups during the coupling process (Scheme 3, reaction 2).
Scheme 2: Synthesis of allylic amine 5a by titanium-mediated coupling reaction of imine 2a with 1-heptyne.
Scheme 2: Synthesis of allylic amine 5a by titanium-mediated coupling reaction of imine 2a with 1-heptyne.
Scheme 3: The regiochemistry of titanium-mediated cross-coupling of imine with terminal alkyne.
Scheme 3: The regiochemistry of titanium-mediated cross-coupling of imine with terminal alkyne.
Reaction scope of various terminal alkynes and imines. With the optimized reaction conditions in hand, we next investigated the reaction scope by first performing the reaction of imine 2a with various terminal alkynes as shown in Table 1. When the terminal alkynes with n-hexyl or tert-butyl groups were used as coupling partners to react with imine 2a, the corresponding allylic amines 5b–c were obtained in 69–88% yields (Table 1, entries 2 and 3). The (E)-configuration of allylic amines 5 was confirmed by X-ray crystal analysis of 5c as shown in Figure 1 [43]. Terminal alkynes with chloro- or phenyl-functionalized alkyl chains were both compatible with this coupling reaction, furnishing the corresponding products 5d and 5e in 66% and 68% yields, respectively (Table 1, entries 4 and 5). Even terminal alkynes with trimethylsilyl or 2-pyridyl functionalities were tolerated well during the reaction to give allylic amines 5f and 5g in 80% and 81% yields, respectively (Table 1, entries 6 and 7).
Table 1: Synthesis of various allylic amines by titanium-mediated coupling reactions of imine 2a with different terminal alkynes.
|
|
|||
| entry | terminal alkyne | product | yield (%) of 2aa |
|---|---|---|---|
| 1 |
|
5a |
67 |
| 2 |
|
5b |
69 |
| 3 |
|
5c |
88 |
| 4 |
|
5d |
66 |
| 5 |
|
5e |
68 |
| 6 |
|
5f |
80 |
| 7 |
|
5g |
81 |
aIsolated yields.
![[1860-5397-9-69-1]](/bjoc/content/figures/1860-5397-9-69-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: X-ray crystal structure of compound 5c.
Figure 1: X-ray crystal structure of compound 5c.
A broad range of imine substrates were also examined for this reaction, as shown in Table 2. When the cross-coupling reactions of N-(p-bromophenyl)- or N-(p-methoxyphenyl)-substituted imines 2b and 2c were employed with 2-ethynylpyridine under the same conditions, the corresponding allylic amines 5h and 5i were obtained in 84% and 80% yields, respectively (Table 2, entries 1 and 2). The results indicated that electron-poor or -rich aryl substituents on the nitrogen atom of imines 2 had little influence on the yields of products 5. The reaction of imine 2d, with a bulky N-(1-naphthyl) group, with t-Bu-substituted alkyne also proceeded well to give allylic amine 5j in 67% yield (Table 2, entry 3). C-(p-bromophenyl)- or C-(p-methoxyphenyl)-substituted imines 2e and 2f reacted well with a series of terminal alkynes, furnishing 5k–5n in 75–84% yields (Table 2, entries 4–7). The results indicated that the electronic nature of the C-aryl ring also had little influence on the product yields. The reaction of N-propyl-substituted imine 2g with 2-ethynylpyridine produced the corresponding allylic amine 5o in 60% yield (Table 2, entry 8). In contrast to the results obtained by using Ti(OiPr)4/2 iPrMgX reagent [35], the coupling of imine 2g with 1-octyne could not afford the desired coupling product in our system (Table 2, entry 9). The structure of allylic amines was also determined by X-ray crystal analyses of compounds 5h and the acylated derivative (7) of 5l [43].
Table 2: Synthesis of various allylic amines by titanium-mediated coupling reactions of different imines with terminal alkynes.
|
|
|||||
| entry | imine | terminal alkyne | time (h)a | product | yield (%)b |
|---|---|---|---|---|---|
| 1 |
2b |
|
6 |
5h |
84 |
| 2 |
2c |
|
6 |
5i |
80 |
| 3c |
2d |
|
4 |
5j |
67 |
| 4 |
2e |
|
5 |
5k |
81 |
| 5 | 2e |
|
6 |
5l |
81 |
| 6 | 2e |
|
3 |
5m |
84 |
| 7 |
2f |
|
6 |
5n |
75 |
| 8 |
2g |
|
6 |
5o |
60 |
| 9 | 2g |
|
3 |
5p |
–d |
aReaction time for the second step. bIsolated yields. c1-Naph is 1-naphthyl. dThe desired product was not isolated.
Titanium-mediated reductive cross-coupling reaction of imines with ethynylcyclopropane. When ethynylcyclopropane was used as the coupling partner of imines 2 in the titanium-mediated reaction, 1,3-amino group migration occurred unexpectedly during the purification of the products by silica-gel chromatography (Scheme 4). For example, the reaction of azatitanacyclopropene 3e with 1.5 equiv of ethynylcyclopropane at −30 °C for 3 h afforded, after silica-gel chromatography, the amino-migration product of 1-cyclopropyl allylic amine 6q in 74% yield. The structure of 6q was confirmed unambiguously by X-ray crystal analysis of its amide derivative 8 ((E)-N-(3-(4-bromophenyl)-1-cyclopropylallyl)-3,5-dinitro-N-phenylbenzamide) as shown in Figure 2 [43]. Careful analysis of the crude reaction mixture before silica-gel purification revealed that the normal coupling product 5q was observed in 94% NMR yield. The result indicated that an isomerization of 5q to 6q occurred during the silica-gel isolation process. This isomerization may proceed via the formation of an allyl cationic intermediate promoted by silica gel due to its weak Lewis acidity [44,45].
Scheme 4: Synthesis of allylic amines 5q and 6q.
Scheme 4: Synthesis of allylic amines 5q and 6q.
Figure 2: X-ray crystal structure of compound 8.
Figure 2: X-ray crystal structure of compound 8.
Iodonolysis of azatitanacyclopentene. Furthermore, we found that iodinated allylic amine 9 could be obtained by iodonolysis of the azatitanacyclopentenes 4 (Scheme 5). For example, on treatment of azatitanacyclopentene 4g with two equiv of iodine at −30 °C followed by warming to −10 °C and stirring for 3 h, iodinated allylic amine 9 could be isolated in 81% yield. Compound 9 is highly valuable since further functionalization could be explored to synthesize a wide range of organic molecules.
Scheme 5: Synthesis of allylic amine 9 by iodonolysis of azatitanacyclopentene 4g.
Scheme 5: Synthesis of allylic amine 9 by iodonolysis of azatitanacyclopentene 4g.
Conclusion
In conclusion, we have developed efficient reductive cross-coupling reactions of imines with terminal alkynes by the activation of imines using Ti(OiPr)4/2 c-C5H9MgCl reagent. Various substituted allylic amine derivatives were obtained in good yields and with excellent regioselectivity after hydrolysis or iodonolysis of the resulting azatitanacyclopentenes. Further studies on the synthetic utility of the resulting titanacyclic intermediates and allylic amines are currently in progress.
Acknowledegments
We thank the National Natural Science Foundation of China (Grant No. 21072208, 21125210, 20821002), Chinese Academy of Science, and the Major State Basic Research Development Program (Grant No. 2011CB808700) for financial support.
Supporting Information
| Supporting Information File 1: Experimental section and NMR spectra. | ||
| Format: PDF | Size: 1.7 MB | Download |
References
-
Lawrence, S. A. Amines: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK, 2004.
Return to citation in text: [1] -
Cheikh, R. B.; Chaabouni, R.; Laurent, A.; Mison, P.; Nafti, A. Synthesis 1983, 685. doi:10.1055/s-1983-30473
Return to citation in text: [1] -
Johannsen, M.; Jørgensen, K. A. Chem. Rev. 1998, 98, 1689. doi:10.1021/cr970343o
Return to citation in text: [1] -
Nag, S.; Batra, S. Tetrahedron 2011, 67, 8959. doi:10.1016/j.tet.2011.07.087
Return to citation in text: [1] -
Sunderhaus, J. D.; Dockendorff, C.; Martin, S. F. Tetrahedron 2009, 65, 6454. doi:10.1016/j.tet.2009.05.009
Return to citation in text: [1] -
Pöverlein, C.; Breckle, G.; Lindel, T. Org. Lett. 2006, 8, 819. doi:10.1021/ol0526219
Return to citation in text: [1] -
Timoshchuk, V. A.; Hogrefe, R. I. Nucleosides, Nucleotides Nucleic Acids 2009, 28, 464. doi:10.1080/15257770903044598
Return to citation in text: [1] -
Warmus, J. S.; Dilley, G. J.; Meyers, A. I. J. Org. Chem. 1993, 58, 270. doi:10.1021/jo00053a053
Return to citation in text: [1] -
Monbaliu, J.-C.; Marchand-Brynaert, J. Tetrahedron Lett. 2008, 49, 1839. doi:10.1016/j.tetlet.2008.01.050
Return to citation in text: [1] -
Lee, H. S.; Kim, H. S.; Kim, J. M.; Kim, J. N. Tetrahedron 2008, 64, 2397. doi:10.1016/j.tet.2008.01.001
Return to citation in text: [1] -
Scarborough, C. C.; Stahl, S. S. Org. Lett. 2006, 8, 3251. doi:10.1021/ol061057e
Return to citation in text: [1] -
Hayashi, T.; Yamamoto, A.; Ito, Y.; Nishioka, E.; Miura, H.; Yanagi, K. J. Am. Chem. Soc. 1989, 111, 6301. doi:10.1021/ja00198a048
Return to citation in text: [1] -
Jumnah, R.; Williams, J. M. J.; Williams, A. C. Tetrahedron Lett. 1993, 34, 6619. doi:10.1016/0040-4039(93)88120-8
Return to citation in text: [1] -
Burgess, K.; Liu, L. T.; Pal, B. J. Org. Chem. 1993, 58, 4758. doi:10.1021/jo00069a052
Return to citation in text: [1] -
Bower, J. F.; Jumnah, R.; Williams, A. C.; Williams, J. M. J. J. Chem. Soc., Perkin Trans. 1 1997, 1411. doi:10.1039/A606586D
Return to citation in text: [1] -
Magnus, P.; Lacour, J.; Coldham, I.; Mugrage, B.; Bauta, W. B. Tetrahedron 1995, 51, 11087. doi:10.1016/0040-4020(95)00696-6
Return to citation in text: [1] -
Franciotti, M.; Mordini, A.; Taddei, M. Synlett 1992, 137. doi:10.1055/s-1992-21293
Return to citation in text: [1] -
Luly, J. R.; Dellaria, J. F.; Plattner, J. J.; Soderquist, J. L.; Yi, N. J. Org. Chem. 1987, 52, 1487. doi:10.1021/jo00384a020
Return to citation in text: [1] -
Kobayashi, S.; Isobe, T.; Ohno, M. Tetrahedron Lett. 1984, 25, 5079. doi:10.1016/S0040-4039(01)91124-4
Return to citation in text: [1] -
Nishi, T.; Morisawa, Y. Heterocycles 1989, 29, 1835. doi:10.3987/COM-89-5076
Return to citation in text: [1] -
Sen, S. E.; Roach, S. L. Synthesis 1995, 756. doi:10.1055/s-1995-4012
Return to citation in text: [1] -
Overman, L. E.; Zipp, G. G. J. Org. Chem. 1997, 62, 2288. doi:10.1021/jo962129q
Return to citation in text: [1] -
Guo, S.; Song, F.; Liu, Y. Synlett 2007, 964. doi:10.1055/s-2007-973865
Return to citation in text: [1] -
Defieber, C.; Ariger, M. A.; Moriel, P.; Carreira, E. M. Angew. Chem., Int. Ed. 2007, 46, 3139. doi:10.1002/anie.200700159
Return to citation in text: [1] -
Katz, T. J.; Shi, S. J. Org. Chem. 1994, 59, 8297. doi:10.1021/jo00105a063
Return to citation in text: [1] -
Brucko, M.; Khuong, T.-A. V.; Sharpless, K. B. Angew. Chem., Int. Ed. Engl. 1996, 35, 454. doi:10.1002/anie.199604541
Return to citation in text: [1] -
Shimizu, Y.; Obora, Y.; Ishii, Y. Org. Lett. 2010, 12, 1372. doi:10.1021/ol100292g
Return to citation in text: [1] -
Rastogi, N.; Mohan, R.; Panda, D.; Mobin, S. M.; Namboothiri, I. N. N. Org. Biomol. Chem. 2006, 4, 3211. doi:10.1039/b607537a
Return to citation in text: [1] -
Oi, S.; Moro, M.; Fukuhara, H.; Kawanishi, T.; Inoue, Y. Tetrahedron Lett. 1999, 40, 9259. doi:10.1016/S0040-4039(99)01857-2
Return to citation in text: [1] -
Wipf, P.; Kendall, C.; Stephenson, C. R. J. J. Am. Chem. Soc. 2003, 125, 761. doi:10.1021/ja028092a
Return to citation in text: [1] -
Brak, K.; Ellman, J. A. J. Am. Chem. Soc. 2009, 131, 3850. doi:10.1021/ja9002603
Return to citation in text: [1] -
Li, Y.; Xu, M.-H. Org. Lett. 2012, 14, 2062. doi:10.1021/ol300581n
Return to citation in text: [1] -
Buchwald, S. L.; Watson, B. T.; Wannamaker, M. W.; Dewan, J. C. J. Am. Chem. Soc. 1989, 111, 4486. doi:10.1021/ja00194a052
Return to citation in text: [1] [2] -
Grossman, R. B.; Davis, W. M.; Buchwald, S. L. J. Am. Chem. Soc. 1991, 113, 2321. doi:10.1021/ja00006a071
Return to citation in text: [1] [2] -
Gao, Y.; Yoshida, Y.; Sato, F. Synlett 1997, 1353. doi:10.1055/s-1997-1555
Return to citation in text: [1] [2] [3] -
Fukuhara, K.; Okamoto, S.; Sato, F. Org. Lett. 2003, 5, 2145. doi:10.1021/ol034599u
Return to citation in text: [1] [2] -
Chen, J.; Liu, Y. Tetrahedron Lett. 2008, 49, 6655. doi:10.1016/j.tetlet.2008.09.042
Return to citation in text: [1] [2] -
Chen, J.; Liu, Y. Organometallics 2010, 29, 505. doi:10.1021/om900941y
Return to citation in text: [1] [2] -
Fan, G.; Liu, Y. Tetrahedron Lett. 2012, 53, 5084. doi:10.1016/j.tetlet.2012.07.020
Return to citation in text: [1] [2] [3] -
Lecornué, F.; Ollivier, J. Chem. Commun. 2003, 584. doi:10.1039/b211642a
Return to citation in text: [1] -
McLaughlin, M.; Takahashi, M.; Micalizio, G. C. Angew. Chem., Int. Ed. 2007, 46, 3912. doi:10.1002/anie.200605060
Return to citation in text: [1] -
Chen, M. Z.; Micalizio, G. C. Org. Lett. 2009, 11, 4982. doi:10.1021/ol902169k
Return to citation in text: [1] -
CCDC-910581 (5c), 910580 (5h), 910575 (7), 910582 (8) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Return to citation in text: [1] [2] [3] -
Shao, L.-X.; Li, Y.-X.; Shi, M. Chem.–Eur. J. 2007, 13, 862. doi:10.1002/chem.200600722
Return to citation in text: [1] -
Eşsiz, S.; Şengül, M. E.; Şahin, E.; Daştan, A. Turk. J. Chem. 2011, 35, 587. doi:10.3906/kim-1101-991
Return to citation in text: [1]
| 39. | Fan, G.; Liu, Y. Tetrahedron Lett. 2012, 53, 5084. doi:10.1016/j.tetlet.2012.07.020 |
| 39. | Fan, G.; Liu, Y. Tetrahedron Lett. 2012, 53, 5084. doi:10.1016/j.tetlet.2012.07.020 |
| 43. | CCDC-910581 (5c), 910580 (5h), 910575 (7), 910582 (8) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 1. | Lawrence, S. A. Amines: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK, 2004. |
| 2. | Cheikh, R. B.; Chaabouni, R.; Laurent, A.; Mison, P.; Nafti, A. Synthesis 1983, 685. doi:10.1055/s-1983-30473 |
| 3. | Johannsen, M.; Jørgensen, K. A. Chem. Rev. 1998, 98, 1689. doi:10.1021/cr970343o |
| 4. | Nag, S.; Batra, S. Tetrahedron 2011, 67, 8959. doi:10.1016/j.tet.2011.07.087 |
| 10. | Lee, H. S.; Kim, H. S.; Kim, J. M.; Kim, J. N. Tetrahedron 2008, 64, 2397. doi:10.1016/j.tet.2008.01.001 |
| 28. | Rastogi, N.; Mohan, R.; Panda, D.; Mobin, S. M.; Namboothiri, I. N. N. Org. Biomol. Chem. 2006, 4, 3211. doi:10.1039/b607537a |
| 8. | Warmus, J. S.; Dilley, G. J.; Meyers, A. I. J. Org. Chem. 1993, 58, 270. doi:10.1021/jo00053a053 |
| 9. | Monbaliu, J.-C.; Marchand-Brynaert, J. Tetrahedron Lett. 2008, 49, 1839. doi:10.1016/j.tetlet.2008.01.050 |
| 29. | Oi, S.; Moro, M.; Fukuhara, H.; Kawanishi, T.; Inoue, Y. Tetrahedron Lett. 1999, 40, 9259. doi:10.1016/S0040-4039(99)01857-2 |
| 30. | Wipf, P.; Kendall, C.; Stephenson, C. R. J. J. Am. Chem. Soc. 2003, 125, 761. doi:10.1021/ja028092a |
| 31. | Brak, K.; Ellman, J. A. J. Am. Chem. Soc. 2009, 131, 3850. doi:10.1021/ja9002603 |
| 32. | Li, Y.; Xu, M.-H. Org. Lett. 2012, 14, 2062. doi:10.1021/ol300581n |
| 7. | Timoshchuk, V. A.; Hogrefe, R. I. Nucleosides, Nucleotides Nucleic Acids 2009, 28, 464. doi:10.1080/15257770903044598 |
| 21. | Sen, S. E.; Roach, S. L. Synthesis 1995, 756. doi:10.1055/s-1995-4012 |
| 22. | Overman, L. E.; Zipp, G. G. J. Org. Chem. 1997, 62, 2288. doi:10.1021/jo962129q |
| 23. | Guo, S.; Song, F.; Liu, Y. Synlett 2007, 964. doi:10.1055/s-2007-973865 |
| 24. | Defieber, C.; Ariger, M. A.; Moriel, P.; Carreira, E. M. Angew. Chem., Int. Ed. 2007, 46, 3139. doi:10.1002/anie.200700159 |
| 5. | Sunderhaus, J. D.; Dockendorff, C.; Martin, S. F. Tetrahedron 2009, 65, 6454. doi:10.1016/j.tet.2009.05.009 |
| 6. | Pöverlein, C.; Breckle, G.; Lindel, T. Org. Lett. 2006, 8, 819. doi:10.1021/ol0526219 |
| 25. | Katz, T. J.; Shi, S. J. Org. Chem. 1994, 59, 8297. doi:10.1021/jo00105a063 |
| 26. | Brucko, M.; Khuong, T.-A. V.; Sharpless, K. B. Angew. Chem., Int. Ed. Engl. 1996, 35, 454. doi:10.1002/anie.199604541 |
| 27. | Shimizu, Y.; Obora, Y.; Ishii, Y. Org. Lett. 2010, 12, 1372. doi:10.1021/ol100292g |
| 17. | Franciotti, M.; Mordini, A.; Taddei, M. Synlett 1992, 137. doi:10.1055/s-1992-21293 |
| 19. | Kobayashi, S.; Isobe, T.; Ohno, M. Tetrahedron Lett. 1984, 25, 5079. doi:10.1016/S0040-4039(01)91124-4 |
| 43. | CCDC-910581 (5c), 910580 (5h), 910575 (7), 910582 (8) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 16. | Magnus, P.; Lacour, J.; Coldham, I.; Mugrage, B.; Bauta, W. B. Tetrahedron 1995, 51, 11087. doi:10.1016/0040-4020(95)00696-6 |
| 44. | Shao, L.-X.; Li, Y.-X.; Shi, M. Chem.–Eur. J. 2007, 13, 862. doi:10.1002/chem.200600722 |
| 45. | Eşsiz, S.; Şengül, M. E.; Şahin, E.; Daştan, A. Turk. J. Chem. 2011, 35, 587. doi:10.3906/kim-1101-991 |
| 12. | Hayashi, T.; Yamamoto, A.; Ito, Y.; Nishioka, E.; Miura, H.; Yanagi, K. J. Am. Chem. Soc. 1989, 111, 6301. doi:10.1021/ja00198a048 |
| 13. | Jumnah, R.; Williams, J. M. J.; Williams, A. C. Tetrahedron Lett. 1993, 34, 6619. doi:10.1016/0040-4039(93)88120-8 |
| 14. | Burgess, K.; Liu, L. T.; Pal, B. J. Org. Chem. 1993, 58, 4758. doi:10.1021/jo00069a052 |
| 15. | Bower, J. F.; Jumnah, R.; Williams, A. C.; Williams, J. M. J. J. Chem. Soc., Perkin Trans. 1 1997, 1411. doi:10.1039/A606586D |
| 11. | Scarborough, C. C.; Stahl, S. S. Org. Lett. 2006, 8, 3251. doi:10.1021/ol061057e |
| 18. | Luly, J. R.; Dellaria, J. F.; Plattner, J. J.; Soderquist, J. L.; Yi, N. J. Org. Chem. 1987, 52, 1487. doi:10.1021/jo00384a020 |
| 43. | CCDC-910581 (5c), 910580 (5h), 910575 (7), 910582 (8) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 33. | Buchwald, S. L.; Watson, B. T.; Wannamaker, M. W.; Dewan, J. C. J. Am. Chem. Soc. 1989, 111, 4486. doi:10.1021/ja00194a052 |
| 33. | Buchwald, S. L.; Watson, B. T.; Wannamaker, M. W.; Dewan, J. C. J. Am. Chem. Soc. 1989, 111, 4486. doi:10.1021/ja00194a052 |
| 34. | Grossman, R. B.; Davis, W. M.; Buchwald, S. L. J. Am. Chem. Soc. 1991, 113, 2321. doi:10.1021/ja00006a071 |
| 40. | Lecornué, F.; Ollivier, J. Chem. Commun. 2003, 584. doi:10.1039/b211642a |
| 41. | McLaughlin, M.; Takahashi, M.; Micalizio, G. C. Angew. Chem., Int. Ed. 2007, 46, 3912. doi:10.1002/anie.200605060 |
| 42. | Chen, M. Z.; Micalizio, G. C. Org. Lett. 2009, 11, 4982. doi:10.1021/ol902169k |
| 37. | Chen, J.; Liu, Y. Tetrahedron Lett. 2008, 49, 6655. doi:10.1016/j.tetlet.2008.09.042 |
| 38. | Chen, J.; Liu, Y. Organometallics 2010, 29, 505. doi:10.1021/om900941y |
| 39. | Fan, G.; Liu, Y. Tetrahedron Lett. 2012, 53, 5084. doi:10.1016/j.tetlet.2012.07.020 |
| 37. | Chen, J.; Liu, Y. Tetrahedron Lett. 2008, 49, 6655. doi:10.1016/j.tetlet.2008.09.042 |
| 36. | Fukuhara, K.; Okamoto, S.; Sato, F. Org. Lett. 2003, 5, 2145. doi:10.1021/ol034599u |
| 35. | Gao, Y.; Yoshida, Y.; Sato, F. Synlett 1997, 1353. doi:10.1055/s-1997-1555 |
| 36. | Fukuhara, K.; Okamoto, S.; Sato, F. Org. Lett. 2003, 5, 2145. doi:10.1021/ol034599u |
| 34. | Grossman, R. B.; Davis, W. M.; Buchwald, S. L. J. Am. Chem. Soc. 1991, 113, 2321. doi:10.1021/ja00006a071 |
© 2013 Mao et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)