Search results
Search for "oxygen reduction" in Full Text gives 58 result(s) in Beilstein Journal of Nanotechnology.
Alloyed Pt3M (M = Co, Ni) nanoparticles supported on S- and N-doped carbon nanotubes for the oxygen reduction reaction
Beilstein J. Nanotechnol. 2019, 10, 1251–1269, doi:10.3762/bjnano.10.125

- ) alloyed nanoparticles that have a very homogeneous size distribution (in spite of the high metal loading of ≈40 wt % Pt), using an ionic liquid as a stabilizer. The electrochemical surface area, the activity for the oxygen reduction reaction and the amount of H2O2 generated during the oxygen reduction
- ; nickel; oxygen reduction reaction; platinum; proton exchange membrane fuel cell (PEMFC); Introduction Proton exchange membrane fuel cells (PEMFCs) convert chemical energy from the hydrogen oxidation reaction (HOR) and the oxygen reduction reaction (ORR) into electrical energy. PEMFCs are one of the most
- /RHE the fractions of H2O2 produced are below 1% for all catalysts. In this voltage region, the ORR occurs only through the four electron process. The H2O2 production is significantly higher at low potential, between 0.05 and 0.4 V/RHE, because of the oxygen reduction on carbon [80]. The amount of H2O2
Glucose-derived carbon materials with tailored properties as electrocatalysts for the oxygen reduction reaction
Beilstein J. Nanotechnol. 2019, 10, 1089–1102, doi:10.3762/bjnano.10.109

- -doped biomass-derived carbon materials were prepared by hydrothermal carbonization of glucose, and their textural and chemical properties were subsequently tailored to achieve materials with enhanced electrochemical performance towards the oxygen reduction reaction. Carbonization and physical activation
- of the nitrogen functionalities. Keywords: electrocatalysts; microporosity; nitrogen-doped carbon materials; oxygen reduction reaction; surface chemistry; Introduction Due to the recent increase in interest for more sustainable, renewable and cheaper energy, multiple conversion devices are being
- engines, as they are able to function as long as there is fuel, and for batteries, as they have similar characteristics under load conditions [1]. The performance of a fuel cell is mainly controlled by the oxygen reduction reaction (ORR) that takes place at the cathode [2], specifically by the
Hydrothermal-derived carbon as a stabilizing matrix for improved cycling performance of silicon-based anodes for lithium-ion full cells
Beilstein J. Nanotechnol. 2018, 9, 2381–2395, doi:10.3762/bjnano.9.223

- nanospheres was also reported by Xia et al. [62] during the synthesis of carbon spheres containing electrocatalysts for oxygen reduction reactions. Heckmann et al. [63] investigated the use of high-temperature-treated hydrothermal carbon spheres as cathode materials for dual-ion cells and found spherical
Metal-free catalysis based on nitrogen-doped carbon nanomaterials: a photoelectron spectroscopy point of view
Beilstein J. Nanotechnol. 2018, 9, 2015–2031, doi:10.3762/bjnano.9.191

- ; photoelectron spectroscopy; Introduction Catalytic processes are the basis of many important technologies in the chemical industry and for energy generation, especially in the context of renewable, clean and sustainable energy production [1]. The oxygen reduction reaction (ORR) and the electrocatalytic
- techniques, engineering and synthesis of the catalyst are to be optimized to yield an optimum concentration of active sites without inactive components. RRDE voltammograms for oxygen reduction in air-saturated 0.1 M KOH at the Pt–C/GC (curve 1), VA-CCNT/GC (curve 2), and VA-NCNT (curve 3) electrodes
Synthesis of rare-earth metal and rare-earth metal-fluoride nanoparticles in ionic liquids and propylene carbonate
Beilstein J. Nanotechnol. 2018, 9, 1881–1894, doi:10.3762/bjnano.9.180
- [23]. Rare-earth metal containing intermetallic nano-phases have been suggested as novel materials for various catalytic applications [24]. For example, Pt3Y and Pt5Gd were predicted to be more active as Pt in the oxygen reduction reaction (ORR) [25]. Nevertheless, the high reduction potentials of
Cyclodextrin inhibits zinc corrosion by destabilizing point defect formation in the oxide layer
Beilstein J. Nanotechnol. 2018, 9, 936–944, doi:10.3762/bjnano.9.86
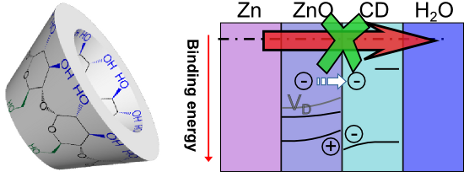
- tens of millivolts of the initial Ecorr in the presence of β-CD in the electrolyte. Lower values of Ecorr are an indication of a suppression of the cathodic process of oxygen reduction [16], Ecorr stablized quickly in the presence of the inhibitor, while reference measurements showed a slower decrease
- inhibition of the oxygen reduction and the concomitant shift in Ecorr leads to a significant decrease of the anodic dissolution, β-CD thus acts as a mixed corrosion inhibitor. It must be stressed that due to the difference in conditions –stagnant vs flowing electrolyte, chloride concentration– compared to
BN/Ag hybrid nanomaterials with petal-like surfaces as catalysts and antibacterial agents
Beilstein J. Nanotechnol. 2018, 9, 250–261, doi:10.3762/bjnano.9.27

- ] that BN/Ag hybrid nanomaterials may possess high activity and stability as catalysts for oxidation of organic compounds. Moreover, N-impurities and other defects in thin layers of raw h-BN can act as active centers for oxygen reduction reactions [15]. Generally, the catalytic activity of BN/Ag hybrid
- treatment, which, as known, can withstand temperatures in excess of 900 °C without chemical degradation [17]. Since Ag2O decomposes at temperatures above 280 °C, it is reasonable to assume that Ag NPs (formed during decomposition) possess high activity in oxygen reduction reaction being catalysts at
Freestanding graphene/MnO2 cathodes for Li-ion batteries
Beilstein J. Nanotechnol. 2017, 8, 1932–1938, doi:10.3762/bjnano.8.193

- charge–discharge cycles [17]. MnO2 has many crystallographic polymorphs including α-, β-, δ-, γ-, ε-, and λ-MnO2. The electrochemical characteristics of MnO2, such as electrocatalytic activity, specific capacity and oxygen reduction reaction, vary according to its crystalline structure and morphology [18
Synthesis of graphene–transition metal oxide hybrid nanoparticles and their application in various fields
Beilstein J. Nanotechnol. 2017, 8, 688–714, doi:10.3762/bjnano.8.74

- and also to improve the volume change. In that context, graphene has been hybridised with Cr2O3 to improve the properties of the materials and the other strategy is to develop Cr2O3 materials on the nanoscale. Graphene–Cr2O3 hybrids have been explored with respect to the oxygen reduction reaction (ORR
- TMO–graphene materials, this hybrid is also used as an anode for LIBs [168][169] because it exhibits high oxygen reduction activity. Liang et al. showed that Co3O4–N-doped graphene exhibits similar catalytic activity like Co3O4–graphene hybrids but superior stability to Pt in alkaline solution [170
- micropipette tip which can detect nonenzymatic glucose [173]. N-doped graphene/Co3O4 has been used for selective oxidation of olefins and alcohols [174], LIBs [175], and oxygen reduction [176], and as a water electrolysis catalyst [177]. Co3O4 NPs were anchored on conducting graphene and used as an anode for
Orientation of FePt nanoparticles on top of a-SiO2/Si(001), MgO(001) and sapphire(0001): effect of thermal treatments and influence of substrate and particle size
Beilstein J. Nanotechnol. 2016, 7, 591–604, doi:10.3762/bjnano.7.52

- transmission electron microscopy (HRTEM); nanoparticles; reflection high-energy electron diffraction (RHEED); solid-phase epitaxy; texture; Introduction Due to their attractive catalytic properties for oxygen reduction reactions (ORR) [1][2] as well as their high magnetocrystalline anisotropy energy density
Nitrogen-doped graphene films from chemical vapor deposition of pyridine: influence of process parameters on the electrical and optical properties
Beilstein J. Nanotechnol. 2015, 6, 2028–2038, doi:10.3762/bjnano.6.206

- nitrogen might also confer useful chemical properties to graphene, e.g., rendering it catalytic to oxygen reduction reactions [24] or enhancing its lithium intercalation properties for battery applications [25]. Nitrogen doping was originally achieved ex situ by the post-growth treatment of pristine CVD
Comprehensive characterization and understanding of micro-fuel cells operating at high methanol concentrations
Beilstein J. Nanotechnol. 2015, 6, 2000–2006, doi:10.3762/bjnano.6.203

- cathode, at a given current density, decreases gradually, 0.88 to 0.6 V vs RHE, as the fuel concentration increases. The reason is that the fuel crossover effect (mixed-potential developed between the oxygen reduction reaction (ORR) and the methanol oxidation reaction (MOR)) is more important [31]. The
Scalable, high performance, enzymatic cathodes based on nanoimprint lithography
Beilstein J. Nanotechnol. 2015, 6, 1377–1384, doi:10.3762/bjnano.6.142

- : bilirubin oxidase; bio-electrocatalysis; direct electron transfer; nanoimprint lithography; oxygen reduction reaction; Introduction Reduction of oxygen (O2) is the key reaction in many natural and artificial systems, and indeed, this reaction is one of the most interesting research issues in both academia
From lithium to sodium: cell chemistry of room temperature sodium–air and sodium–sulfur batteries
Beilstein J. Nanotechnol. 2015, 6, 1016–1055, doi:10.3762/bjnano.6.105

- , lithium is oxidized at the negative electrode and oxygen is reduced on the positive electrode. Similar to a fuel cell cathode, the positive electrode is a porous, electron-conducting support (gas diffusion layer, GDL) that enables oxygen transport, oxygen reduction (ORR) and oxygen evolution (OER) during
- made the same observation in 2002 and concluded that large particles could only grow if the oxide (Li2O2) is (a) soluble in the electrolyte (b) able to migrate on electrode surface or (c) capable of catalyzing the oxygen reduction [30]. Theoretical studies are particularly focused on possibility (c
Materials and characterization techniques for high-temperature polymer electrolyte membrane fuel cells
Beilstein J. Nanotechnol. 2015, 6, 68–83, doi:10.3762/bjnano.6.8

- exhibit high proton conductivity in low-hydration and even anhydrous states. Of special concern for phosphoric-acid-doped PBI-type membranes is the acid loss and management during operation. The slow oxygen reduction reaction in HT-PEMFCs remains a challenge. Phosphoric acid tends to adsorb onto the
- specific adsorption of the phosphoric acid electrolyte is known to hamper the oxygen reduction reaction activity on the cathode side. Moreover, the low solubility and diffusivity of oxygen in concentrated phosphoric acid has a negative effect on the ORR [14][15]. These problems are specific to phosphoric
- acid and not intrinsic to HT-PEMFCs. Alternative electrolytes such as ionic liquids or solid acids might solve the problem and accelerate the oxygen reduction reaction kinetics. The benefits of operating the fuel cell at elevated temperatures include improved catalyst activity, higher tolerance to
Manganese oxide phases and morphologies: A study on calcination temperature and atmospheric dependence
Beilstein J. Nanotechnol. 2015, 6, 47–59, doi:10.3762/bjnano.6.6

- the calcination temperature and the presence of an oxidizing or neutral atmosphere. Furthermore, to demonstrate the application potential of the synthesized MnOx species, we studied their catalytic activity for the oxygen reduction reaction in aprotic media. Linear sweep voltammetry revealed the best
- reaction intermediates, especially those involving oxygen. These properties are especially important for catalytic applications such as water oxidation [9][10][11] and the oxygen reduction and evolution reactions in metal/air battery systems [12][13][14][15][16]. Additionally, the advantages of manganese
- oxygen reduction reaction (ORR), linear sweep measurements were carried out. Figure 9 shows linear sweep measurements recorded at 50 mV/s comparing the activity of various 10% MnOx/carbon electrodes to a pure carbon electrode as a reference material for the ORR in aprotic electrolyte. The ORR peak
Nanocrystalline ceria coatings on solid oxide fuel cell anodes: the role of organic surfactant pretreatments on coating microstructures and sulfur tolerance
Beilstein J. Nanotechnol. 2014, 5, 1712–1724, doi:10.3762/bjnano.5.181

- , releasing electrons into an external circuit to do electrical work before they pass to the cathode for consumption in the oxygen reduction reaction. It is well known that the performance of SOFC anodes, typically composites of nickel metal with a zirconia or ceria ionic conductor, is degraded by sulfur
Donor–acceptor graphene-based hybrid materials facilitating photo-induced electron-transfer reactions
Beilstein J. Nanotechnol. 2014, 5, 1580–1589, doi:10.3762/bjnano.5.170

- EDTA and re-complexes after fresh addition of Fe(II) (Scheme 2). Furthermore, Fe–tpy–GO was tested as a catalyst for the oxygen reduction reaction (ORR) and found to be durable against carbon monoxide poisoning and to exhibit a higher fuel selectivity compared with commercially available Pt/C electro
Liquid fuel cells
Beilstein J. Nanotechnol. 2014, 5, 1399–1418, doi:10.3762/bjnano.5.153

- reaction (HOR) and oxygen reduction reaction (ORR), but it is very expensive. To reduce the Pt loading and therefore the cost for the electrocatalyst, Pt-containing alloys and structured nanoparticles, e.g., “core–shell” materials with less expensive metals are being investigated. Alkaline fuel cells are
- promising fuels [71][73]. The operation of alkaline ethanol LFC has potential benefits compared with PEM LFC including faster kinetics of both ethanol oxidation and oxygen reduction in basic media and lower fuel crossover due to a reversed electro-osmotic effect of anion movement in the membrane. The major
Synthesis, characterization, and growth simulations of Cu–Pt bimetallic nanoclusters
Beilstein J. Nanotechnol. 2014, 5, 1371–1379, doi:10.3762/bjnano.5.150

- bimetallic (Pt–Co, Pt–Fe, Pt–Ni, Pt–Pd) nanocrystals with octahedral and cubic shape and examined their facet-dependent catalytic performance for the oxygen reduction reaction (ORR). Guo and co-workers [33] synthesized FePt and CoPt nanowires by organic-phase decomposition and demonstrated that these systems
Volcano plots in hydrogen electrocatalysis – uses and abuses
Beilstein J. Nanotechnol. 2014, 5, 846–854, doi:10.3762/bjnano.5.96

- of them has been successful [12]. For practical applications in fuel cells, the problem is not hydrogen oxidation but oxygen reduction, which is slow and inefficient. The full reduction involves four electron transfer steps, and possibly other chemical steps. The overall rate on a given substrate
- depends strongly on the pH value, and is also affected by anions. It is not surprising, that the details of the mechanism are still very much a subject of debate. Nevertheless, several attempts have been made to construct volcano plots for oxygen reduction as well. Really this topic is outside of the
- , which has to be compared with the standard potential for oxygen reduction at pH 0, 1.229 V SHE. Obviously, on a good catalyst for this reaction the adsorption energy must be of the order of 1 eV – which is exactly the energy of adsorption of OOH on Pt(111) [37]. We have replotted the theoretical
Carbon dioxide hydrogenation to aromatic hydrocarbons by using an iron/iron oxide nanocatalyst
Beilstein J. Nanotechnol. 2014, 5, 760–769, doi:10.3762/bjnano.5.88

- oxygen reduction reaction [46][47][48] have been reported. Synthesis of Fe/Fe3O4 nanoparticles Here we report the selective formation of aromatic hydrocarbons from CO2 hydrogenation reactions catalyzed by an Fe/Fe3O4 nanocatalyst. Recently, Sun’s group reported a facile method for synthesizing highly
Resonance of graphene nanoribbons doped with nitrogen and boron: a molecular dynamics study
Beilstein J. Nanotechnol. 2014, 5, 717–725, doi:10.3762/bjnano.5.84

- the electronic and quantum transport properties of graphene. Such doped graphene is envisioned with exciting applications as high-performance FET devices [8], and metal-free electrocatalyst for oxygen reduction fuel cells [9]. In addition to doping, various graphene derivatives have also been
Constant-distance mode SECM as a tool to visualize local electrocatalytic activity of oxygen reduction catalysts
Beilstein J. Nanotechnol. 2014, 5, 141–151, doi:10.3762/bjnano.5.14

- microscopy (4D SF/CD-SECM) was utilized for the investigation of the activity distribution of oxygen reduction catalysts. Carbon-supported Pt model catalyst powders have been immobilized in recessed microelectrodes and compared to a spot preparation technique. Microcavities serve as platform for the binder
- high-resolution SECM experiments to powder-based catalyst preparations. Keywords: electrocatalysis; oxygen reduction; recessed microelectrodes; redox-competition SECM; SECM; scanning electrochemical microscopy; shearforce-based constant-distance mode; Introduction In scanning electrochemical
- control mechanism. The concept of 4D CD-SECM was recently extended to intermittent contact-SECM [16]. Here, we describe the adaptation of the 4D SF/CD-SECM to the investigation of the activity of powdery oxygen reduction catalysts. In order to understand the properties of catalyst powders on a local scale
The role of oxygen and water on molybdenum nanoclusters for electro catalytic ammonia production
Beilstein J. Nanotechnol. 2014, 5, 111–120, doi:10.3762/bjnano.5.11

- therefore desorb thermally from the surface, except at very low oxygen coverages. The study of oxygen reduction produces adsorption energies for O, OH, H2O at different coverages. No apparent correlation is found between the adsorption energies of EO, EOH and EH2O on the cluster, see Figure S2 and Figure S3
- [2]. Instead of protonating the adsorbed nitrogen molecule, the potential could drive the reduction of oxygen. In Table 5, the potentials for driving the oxygen reduction processes are presented. Here, a potential of −0.77 V is required for the high oxygen coverage case, while the last reduction step


















































































































































































































































