Abstract
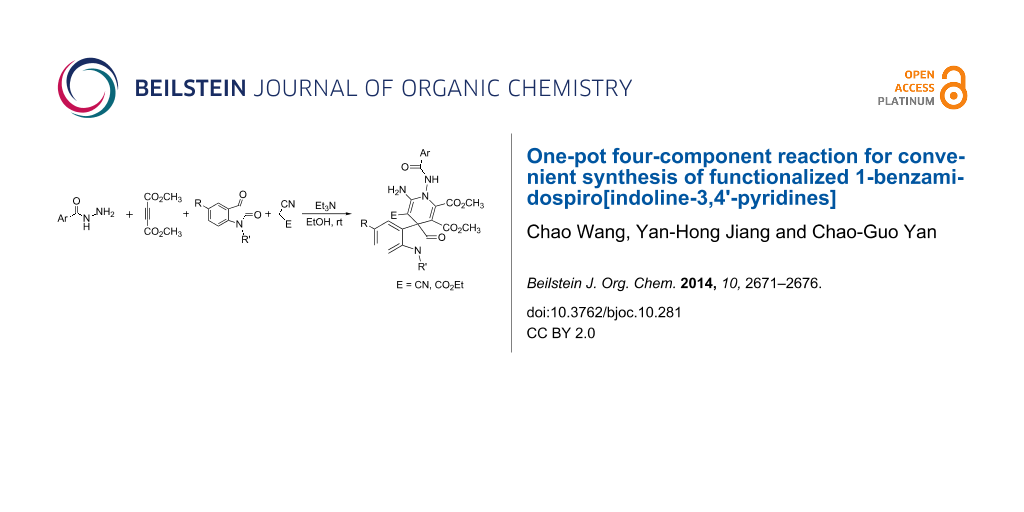
The one-pot four-component reaction of benzohydrazide (2-picolinohydrazide), acetylenedicarboxylate, isatins and malononitrile (ethyl cyanoacetate) with triethylamine as base catalyst afforded functionalized 1-benzamidospiro[indoline-3,4'-pyridines] in good yields. 1H NMR spectra indicated that an equilibrium of cis/trans-conformations exist in the obtained products.
Graphical Abstract
Introduction
The spirooxindole system is the core structure of many natural products and pharmaceutically important structures with notable structural complexity and biological activities of great interest [1-4]. Accordingly, many efficient and practical synthetic procedures have emerged for the synthesis of versatile spirooxindole-fused heterocycles [5-9]. In recent years, because of the emphasis on the development of green and sustainable chemistry, multicomponent reactions have been developed as efficient and potent tools for the preparation of structurally diverse molecules. Practically, multicomponent reactions based on the versatile reactivity of isatins and their 3-methylene derivatives have emerged in large numbers and become the new efficient protocols for the synthesis of various spirooxindoles [10-13].
On the other hand, the chemistry of Huisgen’s 1,4-dipoles, which are simply produced from the addition of nitrogen-containing nucleophiles to electron-deficient alkynes has aroused a lot of interest in organic synthesis due to its convenient formation and versatile reactivity [14,15]. The in situ formed Huisgen’s 1,4-dipoles were subsequently entrapped by various electrophiles and other reagents to finish a number of carbon–carbon bond formation reactions and heterocyclic construction processes [16-18]. A literature survey indicated that the common nitrogen-containing nucleophiles for generation of Huisgen’s 1,4-dipoles are aromatic heterocycles such as N-alkylimidazole, pyridine, quinoline, isoquinoline and primary aromatic amines. In recent years, other nitrogen-containing nucleophiles such as hydrazine and arylhydrazines are also used to generate Huisgen’s 1,4-dipoles in domino reactions [19-21]. Recently, we and Perumal have demonstrated that the four-component reaction of arylamine, acetylenedicarboxylate, isatin and malononitrile can afford the spiro[indoline-3,4’-pyridine] derivatives in satisfactory yields [22-24]. We envisioned that functionalized spiro[indoline-3,4’-pyridine] derivatives can be synthesized by employing other nitrogen-containing nucleophiles such as hydrazine and imines in the similar four-component reactions. In fact, the four-component reaction of hydrazine, acetylenedicarboxylate, isatin and malononitrile for the formation of spiro[indoline-3,4'-pyrano[2,3-c]pyrazoles] have been developed very recently by several groups [25-27]. Against this background and in continuation of our efforts toward the development of practical multicomponent reactions based on the reactivity of isatin and its derivatives [28-34], we herein wish to report the efficient synthesis of functionalized 1-benzamidospiro[indoline-3,4’-pyridines] via one-pot four-component reactions of benzohydrazide, acetylenedicarboxylate, isatin and malononitrile.
Results and Discussion
According to the reaction conditions of the previously reported four-component reaction for the efficient synthesis of the functionalized spiro[indoline-3,4’-pyridine] derivatives [23] a mixture of benzohydrazide and dimethyl acetylenedicarboxylate in ethanol was firstly stirred at room temperature for about fifteen minutes. Then isatin and malononitrile as well as triethylamine as the base catalyst were introduced into reaction system. The subsequent reaction proceeded very smoothly at room temperature to give the 1'-benzamidospiro[indoline-3,4’-pyridines] 1a–d in satisfactory yields (Table 1, entries 1–4). It is known that hydrazine reacts firstly with acetylenedicarboxylate to give the pyrazolone intermediate in the previously reported multicomponent reactions containing hydrazine and acetylenedicarboxylate [25-27]. Here, due to the protection of the benzoyl group, only the free amino group in benzohydrazide took part in the reaction to give the 1'-benzamido-substituted spiro[indoline-3,4’-pyridine]. It should be noted that pure products can be obtained by washing the formed precipitates from the reaction solution and no further purification process is needed. The similar reactions with ethyl cyanoacetate also produced the spiro compounds 1e–i in 68–74% (Table 1, entries 5–9). The substituents on the isatins showed little effect on the yields. Further, 2-picolinohydrazide can also be utilized in the four-component reactions to give the corresponding 1'-picolinamidospiro[indoline-3,4’-pyridines] 1j–m in satisfactory yields (Table 1, entries 10–13). These results indicate that this four-component reaction may have a widely variety of substrates.
Table 1: Synthesis of spiro[indoline-3,4'-pyridines] 1a–m via four-component reaction.a
|
|
||||||
| Entry | Compd | Ar | R | R’ | E | Yield (%, cis/trans)b |
|---|---|---|---|---|---|---|
| 1 | 1a | C6H5 | H | CH2C6H4 | CN | 72 (4:1) |
| 2 | 1b | C6H5 | F | CH2C6H4 | CN | 84 (6.5:1) |
| 3 | 1c | p-CH3C6H4 | H | H | CN | 78 |
| 4 | 1d | p-CH3C6H4 | F | CH2C6H4 | CN | 65 (6.5:1) |
| 5 | 1e | C6H5 | H | H | CO2Et | 74 (5:1) |
| 6 | 1f | C6H5 | Cl | H | CO2Et | 70 (5:1) |
| 7 | 1g | C6H5 | Cl | CH2C6H4 | CO2Et | 68 (6:1) |
| 8 | 1h | p-CH3C6H4 | Cl | H | CO2Et | 69 (5:1) |
| 9 | 1i | p-CH3C6H4 | Cl | CH2C6H4 | CO2Et | 70 (6.5:1) |
| 10 | 1j | 2-C5H4N | CH3 | H | CO2Et | 80 (5:1) |
| 11 | 1k | 2-C5H4N | Cl | H | CO2Et | 82 (4:1) |
| 12 | 1l | 2-C5H4N | Cl | CH2C6H4 | CO2Et | 68 (5:1) |
| 13 | 1m | 2-C5H4N | CH3 | CH2C6H4 | CO2Et | 81 (3:1) |
aReaction conditions: arylhydrazide (1.0 mmol), acetylenedicarboxylate (1.0 mmol) in EtOH (15.0 mL), rt, 15 min; isatin (1.0 mmol), malononitrile or ethyl cyanoacetate (1.0 mmol), Et3N (0.2 mmol), rt, 24 h; bIsolated yield.
The structures of the prepared spiro[indoline-3,4’-pyridines] 1a–m were fully characterized with IR, 1H, 13C NMR, HRMS spectra and were further confirmed by single-crystal X-ray diffraction determination of the compound 1k (Figure 1). The 1H NMR spectra of compounds 1a–m usually showed that two diastereoisomers with a ratio in range of 3:1 to 6.5:1 exist in the obtained products. But there is only one diastereoisomer in the product 1c according to its 1H NMR spectrum. For an example, in the 1H NMR spectrum of compound 1a, the amido group displays two singlets with a ratio of 4:1 at 11.46 and 11.38 ppm. The two singlets at 3.65 and 3.25 ppm are the signals of the two methoxy groups in the major isomer and the two singlets at 3.60 and 3.17 ppm are characteristic for the two methoxy groups in the minor isomer. From the molecular structure of spiro compound 1k (Figure 1), it can be seen that the four carbon atoms and the nitrogen atom in the newly formed 1,4-dihydropyridyl ring exist nearly in one plane, while the C-4’ atom slightly deviates in this plane (0.318(4) Å). The phenyl group of the oxindole moiety and the 1'-picolinamido group exist in the same side of the 1,4-dihydropyridyl plane. By observing the crystal structure of spiro compound 1k, we could conclude that the 1'-picolinamido group might exist in cis- or trans-position of the phenyl group of the oxindole moiety. Thus, the cis/trans-conformations are in a dynamic equilibrium by inversion of the 1'-benzamido group (Scheme 1). The cis-conformation cannot be easily converted to the trans-conformation because the neighboring amino and methoxycarbonyl groups exhibit some steric hindrance for the inversion of the 1'-benzamido group. The 1H NMR spectra clearly indicated the existence of cis/trans-conformations.
![[1860-5397-10-281-1]](/bjoc/content/figures/1860-5397-10-281-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Molecular structure of spiro[indoline-3,4'-pyridine] 1k.
Figure 1: Molecular structure of spiro[indoline-3,4'-pyridine] 1k.
Scheme 1: The dynamic equilibrium of cis/trans-conformation of spiro[indoline-3,4'-pyridine].
Scheme 1: The dynamic equilibrium of cis/trans-conformation of spiro[indoline-3,4'-pyridine].
In order to explain the formation of the spiro[indoline-3,4'-pyridines], a rational reaction mechanism is briefly proposed on the basis of similar reactions of Huigen’s 1,4-dipoles [22-24] (Scheme 2). Firstly, the addition of benzohydrazide to acetylenedicarboxylate results in an active zwitterionic intermediate (A). In the meantime, the condensation of isatin with malononitrile or ethyl cyanoacetate in the presence of triethylamine affords isatylidenemalononitrile or its derivative (B). Then the nucleophilic addition of the zwitterionic intermediate (A) to isatylidenemalononitrile (B) produces the adduct (C), which in turn transferres to intermediate (D) by immigration of a proton from the nitrogen atom to the carbon atom. Thirdly, the intramolecular reaction of the amino group with the cyano group gives a cyclized intermediate (E). Finally, the imino–enamino tautomerization results in the final spiro compound 1. In this process, the initially formed zwitterrionic intermediate (A) does not cyclize to give pyrazolone intermediate as in the reaction of hydrazine with acetylenedicarboxylate. Thus, benzohydrazide shows very different reactivity to that of hydrazine in the four-component reaction.
Scheme 2: Proposed reaction mechanism for the four-component reaction.
Scheme 2: Proposed reaction mechanism for the four-component reaction.
Conclusion
In summary, we have investigated the four-component reactions of benzohydrazide, acetylenedicarboxylate, isatins and malononitrile or ethyl cyanoacetate and successfully developed an efficient synthetic procedure for the preparation of functionalized 1-benzamidospiro[indoline-3,4'-pyridines]. Furthermore, the reaction mechanism and the dynamic equilibrium of cis/trans-conformations were also briefly discussed. This reaction provided new examples for the development of potential applications of Huisgen’s 1,4-dipoles in synthetic chemistry.
Experimental
Reagents and apparatus: All reactions were monitored by TLC. Melting points were taken on a hot-plate microscope apparatus. IR spectra were obtained on a Bruker Tensor 27 spectrometer (KBr disc). 1H and 13C NMR spectra were recorded with a Bruker AV-600 spectrometer with DMSO-d6 as solvent and TMS as internal standard (600 and 150 MHz for 1H and 13C NMR spectra, respectively). HPLC/MS were measured at Bruker MicroTOF spectrometer. Single-crystal structure was determined on Bruker Smart-2 CCD diffractometer.
General procedure for the synthesis of 1,4-dihydropyridines 1a–m via four-component reactions: In a round bottom flask, a solution of benzohydrazide or 2-picolinohydrazide (1.0 mmol) and dimethyl acetylenedicarboxylate (1.0 mmol) in ethanol (15.0 mL) was stirred at room temperature for about fifteen minutes. Then, isatin (1.0 mmol), malononitrile or ethyl cyanoacetate (1.0 mmol) and triethylamine (0.2 mmol) was added. The mixture was stirred at room temperature for 24 hours. The resulting precipitates were collected by filtration and washed with cold alcohol to give the pure product for analysis.
Dimethyl 2'-amino-1'-benzamido-1-benzyl-3'-cyano-2-oxo-1'H-spiro[indoline-3,4'-pyridine]-5',6'-dicarboxylate (1a): white solid, 72%; mp 222–224 °C; 1H NMR (600 MHz, DMSO-d6) δ cis-isomer: 11.46 (s, 1H, NH), 7.90–7.89 (m, 2H, ArH), 7.65–7.62 (m, 2H, ArH), 7.55 (brs, 2H, ArH), 7.50 (brs, 2H, ArH), 7.34 (brs, 2H, ArH), 7.29–7.28 (m, 1H, ArH), 7.21 (brs, 1H, ArH), 7.10 (d, J = 7.2 Hz, 1H, ArH), 6.82 (d, J = 7.2 Hz, 1H, ArH), 6.72 (brs, 2H, NH2), 4.98 (d, J = 15.0 Hz, 1H, CH2), 4.82 (d, J = 15.0Hz, 1H, CH2), 3.65 (s, 3H, OCH3), 3.25 (s, 3H, OCH3); trans-isomer: 11.38 (s, 1H, NH), 7.85–7.84 (m, 2H, ArH), 6.79 (brs, 2H, NH2), 3.60 (s, 3H, OCH3), 3.17 (s, 3H, OCH3); cis/trans-isomers: 4:1; 13C NMR (150 MHz, DMSO-d6) δ 177.3, 166.8, 163.6, 161.9, 152.1, 145.2, 141.3, 136.2, 135.2, 132.6, 131.1, 129.5, 128.6, 128.5, 128.4, 127.9, 127.6, 127.3, 124.1, 123.5, 122.8, 118.5, 108.8, 58.4, 52.9, 51.9, 49.4, 43.5; IR (KBr) υ: 3456, 2952, 2186, 1708, 1654, 1613, 1575, 1482, 1432, 1302, 1224, 1183, 1133, 1091, 1029, 936, 754, 697 cm−1; HRMS (ESI) (m/z): [M + Na]+ calcd. for C31H25N5NaO6: 586.1697; found: 586.1703.
Supporting Information
Experimental details and detailed spectroscopic data of all new compounds are available as Supporting Information. Single crystal data for compounds 1k (CCDC 1000773) has been deposited in the Cambridge Crystallographic Data Center.
| Supporting Information File 1: Experimental details and spectroscopic data of all new compounds. | ||
| Format: PDF | Size: 752.3 KB | Download |
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21172189) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We thank Analysis and Test Center of Yangzhou University providing all analytical instruments.
References
-
Abdel-Rahman, A. H.; Keshk, E. M.; Hanna, M. A.; El-Bady, S. M. Bioorg. Med. Chem. 2004, 12, 2483–2488. doi:10.1016/j.bmc.2003.10.063
Return to citation in text: [1] -
Koch, M. A.; Schuffenhauer, A.; Scheck, M.; Wetzel, S.; Casaulta, M.; Odermatt, A.; Ertl, P.; Waldmann, H. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 17272–17277. doi:10.1073/pnas.0503647102
Return to citation in text: [1] -
Sebahar, P. R.; Williams, R. M. J. Am. Chem. Soc. 2000, 122, 5666–5667. doi:10.1021/ja001133n
Return to citation in text: [1] -
Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209–2219. doi:10.1002/ejoc.200300050
Return to citation in text: [1] -
Kotha, S.; Deb, A. C.; Lahiri, K.; Manivannan, E. Synthesis 2009, 165–193. doi:10.1055/s-0028-1083300
Return to citation in text: [1] -
Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003–3025. doi:10.1055/s-0029-1216975
Return to citation in text: [1] -
Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10, 5165–5181. doi:10.1039/c2ob25184a
Return to citation in text: [1] -
Hong, L.; Wang, R. Adv. Synth. Catal. 2013, 355, 1023–1052. doi:10.1002/adsc.201200808
Return to citation in text: [1] -
Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104–6155. doi:10.1021/cr300135y
Return to citation in text: [1] -
Mao, H.; Lin, A.; Tang, Y.; Shi, Y.; Hu, H.; Cheng, Y.; Zhu, C. Org. Lett. 2013, 15, 4062–4065. doi:10.1021/ol401595g
Return to citation in text: [1] -
Liu, Y.; Wang, H.; Wan, J. Asian J. Org. Chem. 2013, 2, 374–386. doi:10.1002/ajoc.201200180
Return to citation in text: [1] -
Cao, Z.-Y.; Wang, Y.-H.; Zeng, X.-P.; Zhou, J. Tetrahedron Lett. 2014, 55, 2571–2584. doi:10.1016/j.tetlet.2014.01.084
Return to citation in text: [1] -
Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, C. F., III. ACS Catal. 2014, 4, 743–762. doi:10.1021/cs401172r
Return to citation in text: [1] -
Huisgen, R.; Morikawa, M.; Herbig, K.; Brunn, E. Chem. Ber. 1967, 100, 1094–1106. doi:10.1002/cber.19671000406
Return to citation in text: [1] -
Huisgen, R. Z. Chem. 1968, 8, 290–298. doi:10.1002/zfch.19680080803
Return to citation in text: [1] -
Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p
Return to citation in text: [1] -
Nair, V.; Menon, R. S.; Sreekanth, A. R.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520–530. doi:10.1021/ar0502026
Return to citation in text: [1] -
Nair, V.; Deepthi, A.; Ashok, D.; Raveendran, A. E.; Paul, R. R. Tetrahedron 2014, 70, 3085–3105. doi:10.1016/j.tet.2014.03.014
Return to citation in text: [1] -
Kielland, N.; Lavilla, R. Top. Heterocycl. Chem. 2010, 25, 127–168. doi:10.1007/7081_2010_42
Return to citation in text: [1] -
Shaabani, A.; Maleki, A.; Rezayan, A. H.; Sarvary, A. Mol. Diversity 2011, 15, 41–68. doi:10.1007/s11030-010-9258-1
Return to citation in text: [1] -
Han, Y.; Sun, J.; Sun, Y.; Gao, H.; Yan, C. Chin. J. Org. Chem. 2012, 32, 1577–1586. doi:10.6023/cjoc201206008
Return to citation in text: [1] -
Kiruthika, S. E.; Lakshmi, N. V.; Banu, B. R.; Perumal, P. T. Tetrahedron Lett. 2011, 52, 6508–6511. doi:10.1016/j.tetlet.2011.09.119
Return to citation in text: [1] [2] -
Sun, J.; Wu, Q.; Zhang, L.; Yan, C. Chin. J. Chem. 2012, 30, 1548–1554. doi:10.1002/cjoc.201100657
Return to citation in text: [1] [2] [3] -
Zhang, L.-J.; Wu, Q.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 846–851. doi:10.3762/bjoc.9.97
Return to citation in text: [1] [2] -
Pal, S.; Khan, M. N.; Karamthulla, S.; Abbas, S. J.; Choudhury, L. H. Tetrahedron Lett. 2013, 54, 5434–5440. doi:10.1016/j.tetlet.2013.07.117
Return to citation in text: [1] [2] -
Pore, D. M.; Patil, P. B.; Gaikwad, D. S.; Hegade, P. G.; Patil, J. D.; Undale, K. A. Tetrahedron Lett. 2013, 54, 5876–5878. doi:10.1016/j.tetlet.2013.08.084
Return to citation in text: [1] [2] -
Feng, J.; Ablajan, K.; Sali, A. Tetrahedron 2014, 70, 484–489. doi:10.1016/j.tet.2013.11.019
Return to citation in text: [1] [2] -
Sun, Y.; Sun, J.; Yan, C.-G. Tetrahedron Lett. 2012, 53, 3647–3649. doi:10.1016/j.tetlet.2012.05.023
Return to citation in text: [1] -
Han, Y.; Wu, Q.; Sun, J.; Yan, C.-G. Tetrahedron 2012, 68, 8539–8544. doi:10.1016/j.tet.2012.08.030
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2012, 1976–1983. doi:10.1002/ejoc.201101737
Return to citation in text: [1] -
Wu, L.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2012, 10, 9452–9463. doi:10.1039/c2ob26849c
Return to citation in text: [1] -
Sun, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 8–14. doi:10.3762/bjoc.9.2
Return to citation in text: [1] -
Fu, Q.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 918–924. doi:10.3762/bjoc.9.105
Return to citation in text: [1] -
Gao, H.; Sun, J.; Yan, C.-G. J. Org. Chem. 2014, 79, 4131–4136. doi:10.1021/jo500144z
Return to citation in text: [1]
| 1. | Abdel-Rahman, A. H.; Keshk, E. M.; Hanna, M. A.; El-Bady, S. M. Bioorg. Med. Chem. 2004, 12, 2483–2488. doi:10.1016/j.bmc.2003.10.063 |
| 2. | Koch, M. A.; Schuffenhauer, A.; Scheck, M.; Wetzel, S.; Casaulta, M.; Odermatt, A.; Ertl, P.; Waldmann, H. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 17272–17277. doi:10.1073/pnas.0503647102 |
| 3. | Sebahar, P. R.; Williams, R. M. J. Am. Chem. Soc. 2000, 122, 5666–5667. doi:10.1021/ja001133n |
| 4. | Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209–2219. doi:10.1002/ejoc.200300050 |
| 16. | Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p |
| 17. | Nair, V.; Menon, R. S.; Sreekanth, A. R.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520–530. doi:10.1021/ar0502026 |
| 18. | Nair, V.; Deepthi, A.; Ashok, D.; Raveendran, A. E.; Paul, R. R. Tetrahedron 2014, 70, 3085–3105. doi:10.1016/j.tet.2014.03.014 |
| 14. | Huisgen, R.; Morikawa, M.; Herbig, K.; Brunn, E. Chem. Ber. 1967, 100, 1094–1106. doi:10.1002/cber.19671000406 |
| 15. | Huisgen, R. Z. Chem. 1968, 8, 290–298. doi:10.1002/zfch.19680080803 |
| 10. | Mao, H.; Lin, A.; Tang, Y.; Shi, Y.; Hu, H.; Cheng, Y.; Zhu, C. Org. Lett. 2013, 15, 4062–4065. doi:10.1021/ol401595g |
| 11. | Liu, Y.; Wang, H.; Wan, J. Asian J. Org. Chem. 2013, 2, 374–386. doi:10.1002/ajoc.201200180 |
| 12. | Cao, Z.-Y.; Wang, Y.-H.; Zeng, X.-P.; Zhou, J. Tetrahedron Lett. 2014, 55, 2571–2584. doi:10.1016/j.tetlet.2014.01.084 |
| 13. | Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, C. F., III. ACS Catal. 2014, 4, 743–762. doi:10.1021/cs401172r |
| 5. | Kotha, S.; Deb, A. C.; Lahiri, K.; Manivannan, E. Synthesis 2009, 165–193. doi:10.1055/s-0028-1083300 |
| 6. | Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003–3025. doi:10.1055/s-0029-1216975 |
| 7. | Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10, 5165–5181. doi:10.1039/c2ob25184a |
| 8. | Hong, L.; Wang, R. Adv. Synth. Catal. 2013, 355, 1023–1052. doi:10.1002/adsc.201200808 |
| 9. | Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104–6155. doi:10.1021/cr300135y |
| 28. | Sun, Y.; Sun, J.; Yan, C.-G. Tetrahedron Lett. 2012, 53, 3647–3649. doi:10.1016/j.tetlet.2012.05.023 |
| 29. | Han, Y.; Wu, Q.; Sun, J.; Yan, C.-G. Tetrahedron 2012, 68, 8539–8544. doi:10.1016/j.tet.2012.08.030 |
| 30. | Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2012, 1976–1983. doi:10.1002/ejoc.201101737 |
| 31. | Wu, L.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2012, 10, 9452–9463. doi:10.1039/c2ob26849c |
| 32. | Sun, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 8–14. doi:10.3762/bjoc.9.2 |
| 33. | Fu, Q.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 918–924. doi:10.3762/bjoc.9.105 |
| 34. | Gao, H.; Sun, J.; Yan, C.-G. J. Org. Chem. 2014, 79, 4131–4136. doi:10.1021/jo500144z |
| 25. | Pal, S.; Khan, M. N.; Karamthulla, S.; Abbas, S. J.; Choudhury, L. H. Tetrahedron Lett. 2013, 54, 5434–5440. doi:10.1016/j.tetlet.2013.07.117 |
| 26. | Pore, D. M.; Patil, P. B.; Gaikwad, D. S.; Hegade, P. G.; Patil, J. D.; Undale, K. A. Tetrahedron Lett. 2013, 54, 5876–5878. doi:10.1016/j.tetlet.2013.08.084 |
| 27. | Feng, J.; Ablajan, K.; Sali, A. Tetrahedron 2014, 70, 484–489. doi:10.1016/j.tet.2013.11.019 |
| 25. | Pal, S.; Khan, M. N.; Karamthulla, S.; Abbas, S. J.; Choudhury, L. H. Tetrahedron Lett. 2013, 54, 5434–5440. doi:10.1016/j.tetlet.2013.07.117 |
| 26. | Pore, D. M.; Patil, P. B.; Gaikwad, D. S.; Hegade, P. G.; Patil, J. D.; Undale, K. A. Tetrahedron Lett. 2013, 54, 5876–5878. doi:10.1016/j.tetlet.2013.08.084 |
| 27. | Feng, J.; Ablajan, K.; Sali, A. Tetrahedron 2014, 70, 484–489. doi:10.1016/j.tet.2013.11.019 |
| 22. | Kiruthika, S. E.; Lakshmi, N. V.; Banu, B. R.; Perumal, P. T. Tetrahedron Lett. 2011, 52, 6508–6511. doi:10.1016/j.tetlet.2011.09.119 |
| 23. | Sun, J.; Wu, Q.; Zhang, L.; Yan, C. Chin. J. Chem. 2012, 30, 1548–1554. doi:10.1002/cjoc.201100657 |
| 24. | Zhang, L.-J.; Wu, Q.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 846–851. doi:10.3762/bjoc.9.97 |
| 22. | Kiruthika, S. E.; Lakshmi, N. V.; Banu, B. R.; Perumal, P. T. Tetrahedron Lett. 2011, 52, 6508–6511. doi:10.1016/j.tetlet.2011.09.119 |
| 23. | Sun, J.; Wu, Q.; Zhang, L.; Yan, C. Chin. J. Chem. 2012, 30, 1548–1554. doi:10.1002/cjoc.201100657 |
| 24. | Zhang, L.-J.; Wu, Q.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2013, 9, 846–851. doi:10.3762/bjoc.9.97 |
| 19. | Kielland, N.; Lavilla, R. Top. Heterocycl. Chem. 2010, 25, 127–168. doi:10.1007/7081_2010_42 |
| 20. | Shaabani, A.; Maleki, A.; Rezayan, A. H.; Sarvary, A. Mol. Diversity 2011, 15, 41–68. doi:10.1007/s11030-010-9258-1 |
| 21. | Han, Y.; Sun, J.; Sun, Y.; Gao, H.; Yan, C. Chin. J. Org. Chem. 2012, 32, 1577–1586. doi:10.6023/cjoc201206008 |
| 23. | Sun, J.; Wu, Q.; Zhang, L.; Yan, C. Chin. J. Chem. 2012, 30, 1548–1554. doi:10.1002/cjoc.201100657 |
© 2014 Wang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)