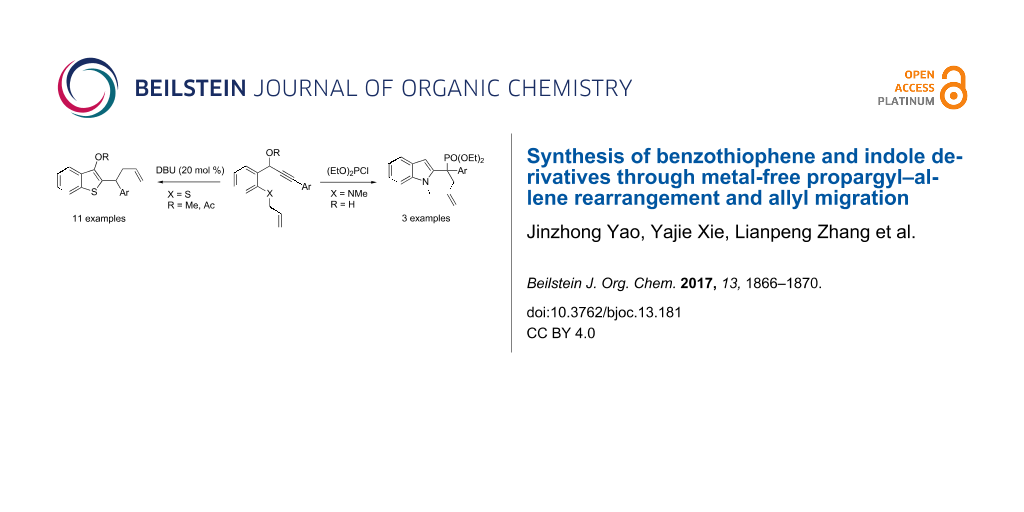
Abstract
An efficient base-catalyzed protocol for the synthesis of benzothiophene is described. The reaction proceeds via base-promoted propargyl–allenyl rearrangement followed by cyclization and allyl migration. Phosphine-substituted indoles can be synthesized by a similar strategy.
Graphical Abstract
Introduction
Heterocycles are frequently found in natural products and pharmacologically active compounds, thus economic and efficient methods to construct heterocycles are always highly desirable [1-6].
Benzothiophenes are important heterocycles that are one of the key motifs of anti-inflammatory, anti-estrogenic and anti-HIV drugs (Figure 1) [7-9]. Moreover, benzothiophenes have extensive applications in materials science. Besides the traditional methods of transition metal-catalyzed cyclization of alkyne substrates [10-12], the synthesis of benzothiophenes via metal-free conditions has recently aroused much attention [13-15]. For example, the preparation of C3-borylated benzothiophene by BCl3-induced borylative cyclization of arylalkynes was recently demonstrated by Ingleson [16].
Figure 1: Examples of biologically active benzothiophene derivatives.
Figure 1: Examples of biologically active benzothiophene derivatives.
Allene-mediated cyclization reactions are advantageous due to the convenient preparation of starting materials instead of the use of unstable or reactive polyfunctionalized allene substrates [17-27]. Although transition metal (e.g., Au, Pd)-catalysed propargyl–allenyl isomerization and cyclization reactions have been established [28,29], such transformations promoted by a base to construct heterocycles are not well-documented [30,31]. Recently, our group explored the utilization of β-sulfonium carbanions for the preparation of thiophene derivatives [19]. Alkynes were treated with acyl chloride under Sonogashira reaction conditions and the expected β-sulfonium carbanions were obtained in a one-pot process. Based on our understanding of organosulfur chemistry [20-22], we report herein a simple, metal-free method for the formation of benzothiophenes using an intramolecular addition of a sulfur atom (originated from a sulfide) to the electron-deficient allene moiety generated in situ by a propargyl–allenyl rearrangement [17-27] and an allyl migration [32-34] (Scheme 1). In addition, phosphine-substituted indole derivatives could also be conveniently constructed by a similar strategy. This method not only avoids the use of transition metal catalysts, but also provides the useful heterocycles which are not easily achieved through other protocols.
Scheme 1: Proposal of applicable β-sulfonium carbanion.
Scheme 1: Proposal of applicable β-sulfonium carbanion.
Results and Discussion
In the initial studies, we treated methyl 4-(3-(2-(allylthio)phenyl)-3-methoxyprop-1-yn-1-yl)benzoate (1a) with DBU (0.1 equiv) in THF at 50 °C under N2 for 12 h (Table 1, entry 1). Fortunately, the desired product 2a was obtained in 57% yield. No reaction was observed using TEA or DABCO, possibly because the allenic intermediate could not be formed by these comparatively weak bases (Table 1, entries 2 and 3), which was different from the previous work. Other bases, such as TBD, Cs2CO3, and t-BuOK were found to be less effective (Table 1, entries 4–6). To our delight, it was found that increasing the catalyst loading to 0.2 equiv resulted in an obviously higher yield of 83% (Table 1, entry 7). However, a higher catalyst loading had almost no influence on the reaction (Table 1, entry 8). It was found that THF was the best solvent after screening different solvents. Other solvents, such as DCE, toluene, and CH3CN were found to be less effective (Table 1, entries 9–11). The yield was reduced to 51% when the reaction time was decreased to 6 h (Table 1, entry 12). A lower temperature was found to be less effective for the reaction (Table 1, entry 13). Without the base, no reaction occurred, implying that the reaction proceeded exclusively through the allenic intermediate (Table 1, entry 14). Thus, the optimal reaction conditions were DBU (0.2 equiv) under nitrogen in THF at 50 °C for 12 h.
Table 1: Optimization of the reaction conditionsa.
|
|
||||
| Entry | Catalyst | x | Solvent | yield (%)b |
|---|---|---|---|---|
| 1 | DBU | 0.1 | THF | 57 |
| 2 | TEA | 0.1 | THF | N.D |
| 3 | DABCO | 0.1 | THF | N.D |
| 4 | TBD | 0.1 | THF | 22 |
| 5 | Cs2CO3 | 0.1 | THF | 23 |
| 6 | t-BuOK | 0.1 | THF | 27 |
| 7 | DBU | 0.2 | THF | 83 |
| 8 | DBU | 0.5 | THF | 82 |
| 9 | DBU | 0.2 | DCE | 62 |
| 10 | DBU | 0.2 | toluene | 68 |
| 11 | DBU | 0.2 | CH3CN | 58 |
| 12 | DBU | 0.2 | THF | 51c |
| 13 | DBU | 0.2 | THF | 32d |
| 14 | – | – | THF | N.D |
aReaction conditions: 1a (1.0 equiv), base (x equiv), 50 °C, 12 h, under N2. bIsolated yield. cThe reaction time was 6 h. dThe reaction was conducted at 25 °C. DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene. TBD: 1,5,7-triazabicyclo[4.4.0]dec-5-ene.
With the optimized reaction conditions in hand, we turned our attention to study the reaction scope and limitations of this reaction; the results are shown in Figure 2. A series of alkynes substituted with an electron-withdrawing group participated in this reaction smoothly to give the products in good yields (2a–k). A variety of substituents, such as p-COOEt, p-COCH3, dichloro, p-NO2, p-CF3 and p-CN were well-tolerated during the reaction, leading to 2a–f in 54–83% yield. The presence of methyl acrylate or pyridine was also well-tolerated, as exemplified in the formation of 2g,h in 48–57% yield. Besides methyl propargyl ethers, propargyl acetates were also tolerated under these conditions (2i–k). The presence of substituents on the aromatic ring, such as a methyl group or a chlorine atom, did not have much of an effect the reaction (2j,k).
Figure 2: Synthesis of benzothiophenes. Reaction conditions: 1 (0.5 mmol), DBU (0.1 mmol), THF (2.0 mL), 50 °C, 12 h, under N2. Yields are isolated yields.
Figure 2: Synthesis of benzothiophenes. Reaction conditions: 1 (0.5 mmol), DBU (0.1 mmol), THF (2.0 mL), 50 °...
Indoles are also important heterocycles that are the key motif of many natural products and pharmaceuticals. Consequently, new and straightforward methods to access indoles are highly desirable [35,36]. We chose a propargyl phosphite rearrangement to achieve allenyl intermediates and aimed to synthetize indoles via allenyl phosphonates, which were versatile synthetic intermediates [37,38]. The N-methyl-N-allylpropargyl alcohol 3 was treated with (EtO)2PCl under alkaline conditions, then underwent a propargyl phosphite/allenyl phosphonate rearrangement and an intramolecular nucleophilic attack to form the indole moiety, followed by allyl migration (Scheme 2). Phosphine-substituted indole derivatives were obtained in moderate yield (Figure 3, 4a–c).
Scheme 2: Proposal of indole synthesis via allenylphosphonates.
Scheme 2: Proposal of indole synthesis via allenylphosphonates.
Figure 3: Synthesis of 1-methylindole phosphine oxides. Reaction conditions: 3 (0.5 mmol), (EtO)2PCl (0.6 mmol), Et3N (1.5 mmol), and THF (2.0 mL) at −78 °C. Yields are isolated yield.
Figure 3: Synthesis of 1-methylindole phosphine oxides. Reaction conditions: 3 (0.5 mmol), (EtO)2PCl (0.6 mmo...
Conclusion
In summary, we have developed an expedient route for the construction of benzothiophene and indole derivatives via propargyl–allene rearrangement and allyl migration. The reaction proceeded under mild conditions to produce useful benzothiophene and indole derivatives.
Supporting Information
| Supporting Information File 1: Experimental procedures and analytical data. | ||
| Format: PDF | Size: 1.1 MB | Download |
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (Project No. 21606080), Zhejiang Provincial Natural Science Foundation of China (No. LY14B020008), the Ph.D. Scientific Research Starting Foundation of Jiaxing University (No: 70515007) and Summit Program of Jiaxing University for Leading Talents.
References
-
Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872
Return to citation in text: [1] -
Zeni, G.; Larock, R. C. Chem. Rev. 2006, 106, 4644–4680. doi:10.1021/cr0683966
Return to citation in text: [1] -
Curran, D. P.; Solovyev, A.; Brahmi, M. M.; Fensterbank, L.; Malacria, M.; Lacôte, E. Angew. Chem., Int. Ed. 2011, 50, 10294–10317. doi:10.1002/anie.201102717
Return to citation in text: [1] -
Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666
Return to citation in text: [1] -
Wu, X.-F.; Neumann, H.; Beller, M. Chem. Rev. 2012, 113, 1–35. doi:10.1021/cr300100s
Return to citation in text: [1] -
Dubrovskiy, A. V.; Markina, N. A.; Larock, R. C. Org. Biomol. Chem. 2013, 11, 191–218. doi:10.1039/C2OB26673C
Return to citation in text: [1] -
Joshi, E. M.; Heasley, B. H.; Chordia, M. D.; Macdonald, T. L. Chem. Res. Toxicol. 2004, 17, 137–143. doi:10.1021/tx0341409
Return to citation in text: [1] -
Li, H.; Xiao, H.; Lin, L.; Jou, D.; Kumari, V.; Lin, J.; Li, C. J. Med. Chem. 2014, 57, 632–641. doi:10.1021/jm401144z
Return to citation in text: [1] -
Xiong, R.; Patel, H. K.; Gutgesell, L. M.; Zhao, J.; Delgado-Rivera, L.; Pham, T. N. D.; Zhao, H.; Carlson, K.; Martin, T.; Katzenellenbogen, J. A.; Moore, T. W.; Tonetti, D. A.; Thatcher, G. R. J. J. Med. Chem. 2016, 59, 219–237. doi:10.1021/acs.jmedchem.5b01276
Return to citation in text: [1] -
Chen, C.-C.; Chen, C.-M.; Wu, M.-J. J. Org. Chem. 2014, 79, 4704–4711. doi:10.1021/jo500377v
Return to citation in text: [1] -
Dhage, Y. D.; Shirai, T.; Arima, M.; Nakazima, A.; Hikawa, H.; Taichi Kusakabe, I. A.; Takahashi, K.; Kato, K. RSC Adv. 2015, 5, 42623–42627. doi:10.1039/C5RA05263G
Return to citation in text: [1] -
Gabriele, B.; Mancuso, R.; Lupinacci, E.; Veltri, L.; Salerno, G.; Carfagna, C. J. Org. Chem. 2011, 76, 8277–8286. doi:10.1021/jo201471k
Return to citation in text: [1] -
Aurelio, L.; Volpe, R.; Halim, R.; Scammells, P. J.; Flynn, B. L. Adv. Synth. Catal. 2014, 356, 1974–1978. doi:10.1002/adsc.201400072
Return to citation in text: [1] -
Duan, Z.; Ranjit, S.; Liu, X. Org. Lett. 2010, 12, 2430–2433. doi:10.1021/ol100816g
Return to citation in text: [1] -
Matsumura, M.; Muranaka, A.; Kurihara, R.; Kanai, M.; Yoshida, K.; Kakusawa, N.; Hashizume, D.; Uchiyama, M.; Yasuike, S. Tetrahedron 2016, 72, 8085–8090. doi:10.1016/j.tet.2016.10.048
Return to citation in text: [1] -
Warner, A. J.; Churn, A.; McGough, J. S.; Ingleson, M. J. Angew. Chem., Int. Ed. 2017, 56, 354–358. doi:10.1002/anie.201610014
Return to citation in text: [1] -
Tejedor, D.; Méndez-Abt, G.; Cotos, L.; Garcia-Tellado, F. Chem. Soc. Rev. 2013, 42, 458–471. doi:10.1039/C2CS35311C
Return to citation in text: [1] [2] -
Braverman, S.; Cherkinsky, M. Top. Curr. Chem. 2007, 275, 67–101. doi:10.1007/128_047
Return to citation in text: [1] [2] -
Chen, D.; Xing, G.; Yao, J.; Zhou, H. Org. Chem. Front. 2017, 4, 1042–1045. doi:10.1039/C6QO00675B
Return to citation in text: [1] [2] [3] -
Chen, D.; Xing, G.; Yao, J.; Zhou, H. RSC Adv. 2016, 6, 103320–103323. doi:10.1039/C6RA21889J
Return to citation in text: [1] [2] [3] -
Chen, D.; Xing, G.; Zhou, H. Org. Chem. Front. 2015, 2, 947–950. doi:10.1039/C5QO00089K
Return to citation in text: [1] [2] [3] -
Chen, D.; Xing, G.; Chen, X.; Yao, J.; Zhou, H. Tetrahedron Lett. 2016, 57, 5124–5126. doi:10.1016/j.tetlet.2016.10.025
Return to citation in text: [1] [2] [3] -
Liu, L.; Wang, J.; Zhou, H. J. Org. Chem. 2015, 80, 4749–4753. doi:10.1021/acs.joc.5b00261
Return to citation in text: [1] [2] -
Liu, L.; Chen, D.; Zhou, H. Adv. Synth. Catal. 2015, 357, 389–394. doi:10.1002/adsc.201400713
Return to citation in text: [1] [2] -
Zhao, G.; Zhang, Q.; Zhou, H. J. Org. Chem. 2014, 79, 10867–10872. doi:10.1021/jo501867h
Return to citation in text: [1] [2] -
Zhao, G.; Zhang, Q.; Zhou, H. Adv. Synth. Catal. 2013, 355, 3492–3496. doi:10.1002/adsc.201300573
Return to citation in text: [1] [2] -
Zhou, H.; Liu, L.; Xu, S. J. Org. Chem. 2012, 77, 9418–9421. doi:10.1021/jo3018186
Return to citation in text: [1] [2] -
Wang, C.; Kong, L.; Li, Y.; Li, Y. Eur. J. Org. Chem. 2014, 3556–3560. doi:10.1002/ejoc.201402059
Return to citation in text: [1] -
Thummanapelli, S. K.; Hosseyni, S.; Su, Y.; Akhmedov, N. G.; Shi, X. Chem. Commun. 2016, 52, 7687–7690. doi:10.1039/C6CC03032G
Return to citation in text: [1] -
You, X.; Xie, X.; Chen, H.; Li, Y.; Liu, Y. Chem. – Eur. J. 2015, 21, 18699–18705. doi:10.1002/chem.201503374
Return to citation in text: [1] -
Wang, T.; Naredla, R. R.; Thompson, S. K.; Hoye, T. R. Nature 2016, 532, 484–488. doi:10.1038/nature17429
Return to citation in text: [1] -
Gobé, V.; Dousset, M.; Retailleau, P.; Gandon, V.; Guinchard, X. Adv. Synth. Catal. 2016, 358, 3960–3965. doi:10.1002/adsc.201601075
Return to citation in text: [1] -
Li, D.-Y.; Wei, Y.; Marek, I.; Tang, X.-Y.; Shi, M. Chem. Sci. 2015, 6, 5519–5525. doi:10.1039/C5SC01806D
Return to citation in text: [1] -
Ji, K.-G.; Shu, X.-Z.; Chen, J.; Zhao, S.-C.; Zheng, Z.-J.; Lu, L.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2008, 10, 3919–3922. doi:10.1021/ol8015463
Return to citation in text: [1] -
Jin, H.; Huang, L.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2016, 55, 794–797. doi:10.1002/anie.201508309
Return to citation in text: [1] -
Yan, H.; Wang, H.; Li, X.; Xin, X.; Wang, C.; Wan, B. Angew. Chem., Int. Ed. 2015, 54, 10613–10617. doi:10.1002/anie.201503997
Return to citation in text: [1] -
Shen, R.; Luo, B.; Yang, J.; Zhang, L.; Han, L.-B. Chem. Commun. 2016, 52, 6451–6454. doi:10.1039/C6CC02563C
Return to citation in text: [1] -
Hu, G.; Shan, C.; Chen, W.; Xu, P.; Gao, Y.; Zhao, Y. Org. Lett. 2016, 18, 6066–6069. doi:10.1021/acs.orglett.6b03028
Return to citation in text: [1]
| 1. | Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872 |
| 2. | Zeni, G.; Larock, R. C. Chem. Rev. 2006, 106, 4644–4680. doi:10.1021/cr0683966 |
| 3. | Curran, D. P.; Solovyev, A.; Brahmi, M. M.; Fensterbank, L.; Malacria, M.; Lacôte, E. Angew. Chem., Int. Ed. 2011, 50, 10294–10317. doi:10.1002/anie.201102717 |
| 4. | Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666 |
| 5. | Wu, X.-F.; Neumann, H.; Beller, M. Chem. Rev. 2012, 113, 1–35. doi:10.1021/cr300100s |
| 6. | Dubrovskiy, A. V.; Markina, N. A.; Larock, R. C. Org. Biomol. Chem. 2013, 11, 191–218. doi:10.1039/C2OB26673C |
| 16. | Warner, A. J.; Churn, A.; McGough, J. S.; Ingleson, M. J. Angew. Chem., Int. Ed. 2017, 56, 354–358. doi:10.1002/anie.201610014 |
| 13. | Aurelio, L.; Volpe, R.; Halim, R.; Scammells, P. J.; Flynn, B. L. Adv. Synth. Catal. 2014, 356, 1974–1978. doi:10.1002/adsc.201400072 |
| 14. | Duan, Z.; Ranjit, S.; Liu, X. Org. Lett. 2010, 12, 2430–2433. doi:10.1021/ol100816g |
| 15. | Matsumura, M.; Muranaka, A.; Kurihara, R.; Kanai, M.; Yoshida, K.; Kakusawa, N.; Hashizume, D.; Uchiyama, M.; Yasuike, S. Tetrahedron 2016, 72, 8085–8090. doi:10.1016/j.tet.2016.10.048 |
| 10. | Chen, C.-C.; Chen, C.-M.; Wu, M.-J. J. Org. Chem. 2014, 79, 4704–4711. doi:10.1021/jo500377v |
| 11. | Dhage, Y. D.; Shirai, T.; Arima, M.; Nakazima, A.; Hikawa, H.; Taichi Kusakabe, I. A.; Takahashi, K.; Kato, K. RSC Adv. 2015, 5, 42623–42627. doi:10.1039/C5RA05263G |
| 12. | Gabriele, B.; Mancuso, R.; Lupinacci, E.; Veltri, L.; Salerno, G.; Carfagna, C. J. Org. Chem. 2011, 76, 8277–8286. doi:10.1021/jo201471k |
| 35. | Jin, H.; Huang, L.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2016, 55, 794–797. doi:10.1002/anie.201508309 |
| 36. | Yan, H.; Wang, H.; Li, X.; Xin, X.; Wang, C.; Wan, B. Angew. Chem., Int. Ed. 2015, 54, 10613–10617. doi:10.1002/anie.201503997 |
| 7. | Joshi, E. M.; Heasley, B. H.; Chordia, M. D.; Macdonald, T. L. Chem. Res. Toxicol. 2004, 17, 137–143. doi:10.1021/tx0341409 |
| 8. | Li, H.; Xiao, H.; Lin, L.; Jou, D.; Kumari, V.; Lin, J.; Li, C. J. Med. Chem. 2014, 57, 632–641. doi:10.1021/jm401144z |
| 9. | Xiong, R.; Patel, H. K.; Gutgesell, L. M.; Zhao, J.; Delgado-Rivera, L.; Pham, T. N. D.; Zhao, H.; Carlson, K.; Martin, T.; Katzenellenbogen, J. A.; Moore, T. W.; Tonetti, D. A.; Thatcher, G. R. J. J. Med. Chem. 2016, 59, 219–237. doi:10.1021/acs.jmedchem.5b01276 |
| 37. | Shen, R.; Luo, B.; Yang, J.; Zhang, L.; Han, L.-B. Chem. Commun. 2016, 52, 6451–6454. doi:10.1039/C6CC02563C |
| 38. | Hu, G.; Shan, C.; Chen, W.; Xu, P.; Gao, Y.; Zhao, Y. Org. Lett. 2016, 18, 6066–6069. doi:10.1021/acs.orglett.6b03028 |
| 19. | Chen, D.; Xing, G.; Yao, J.; Zhou, H. Org. Chem. Front. 2017, 4, 1042–1045. doi:10.1039/C6QO00675B |
| 17. | Tejedor, D.; Méndez-Abt, G.; Cotos, L.; Garcia-Tellado, F. Chem. Soc. Rev. 2013, 42, 458–471. doi:10.1039/C2CS35311C |
| 18. | Braverman, S.; Cherkinsky, M. Top. Curr. Chem. 2007, 275, 67–101. doi:10.1007/128_047 |
| 19. | Chen, D.; Xing, G.; Yao, J.; Zhou, H. Org. Chem. Front. 2017, 4, 1042–1045. doi:10.1039/C6QO00675B |
| 20. | Chen, D.; Xing, G.; Yao, J.; Zhou, H. RSC Adv. 2016, 6, 103320–103323. doi:10.1039/C6RA21889J |
| 21. | Chen, D.; Xing, G.; Zhou, H. Org. Chem. Front. 2015, 2, 947–950. doi:10.1039/C5QO00089K |
| 22. | Chen, D.; Xing, G.; Chen, X.; Yao, J.; Zhou, H. Tetrahedron Lett. 2016, 57, 5124–5126. doi:10.1016/j.tetlet.2016.10.025 |
| 23. | Liu, L.; Wang, J.; Zhou, H. J. Org. Chem. 2015, 80, 4749–4753. doi:10.1021/acs.joc.5b00261 |
| 24. | Liu, L.; Chen, D.; Zhou, H. Adv. Synth. Catal. 2015, 357, 389–394. doi:10.1002/adsc.201400713 |
| 25. | Zhao, G.; Zhang, Q.; Zhou, H. J. Org. Chem. 2014, 79, 10867–10872. doi:10.1021/jo501867h |
| 26. | Zhao, G.; Zhang, Q.; Zhou, H. Adv. Synth. Catal. 2013, 355, 3492–3496. doi:10.1002/adsc.201300573 |
| 27. | Zhou, H.; Liu, L.; Xu, S. J. Org. Chem. 2012, 77, 9418–9421. doi:10.1021/jo3018186 |
| 30. | You, X.; Xie, X.; Chen, H.; Li, Y.; Liu, Y. Chem. – Eur. J. 2015, 21, 18699–18705. doi:10.1002/chem.201503374 |
| 31. | Wang, T.; Naredla, R. R.; Thompson, S. K.; Hoye, T. R. Nature 2016, 532, 484–488. doi:10.1038/nature17429 |
| 32. | Gobé, V.; Dousset, M.; Retailleau, P.; Gandon, V.; Guinchard, X. Adv. Synth. Catal. 2016, 358, 3960–3965. doi:10.1002/adsc.201601075 |
| 33. | Li, D.-Y.; Wei, Y.; Marek, I.; Tang, X.-Y.; Shi, M. Chem. Sci. 2015, 6, 5519–5525. doi:10.1039/C5SC01806D |
| 34. | Ji, K.-G.; Shu, X.-Z.; Chen, J.; Zhao, S.-C.; Zheng, Z.-J.; Lu, L.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2008, 10, 3919–3922. doi:10.1021/ol8015463 |
| 28. | Wang, C.; Kong, L.; Li, Y.; Li, Y. Eur. J. Org. Chem. 2014, 3556–3560. doi:10.1002/ejoc.201402059 |
| 29. | Thummanapelli, S. K.; Hosseyni, S.; Su, Y.; Akhmedov, N. G.; Shi, X. Chem. Commun. 2016, 52, 7687–7690. doi:10.1039/C6CC03032G |
| 17. | Tejedor, D.; Méndez-Abt, G.; Cotos, L.; Garcia-Tellado, F. Chem. Soc. Rev. 2013, 42, 458–471. doi:10.1039/C2CS35311C |
| 18. | Braverman, S.; Cherkinsky, M. Top. Curr. Chem. 2007, 275, 67–101. doi:10.1007/128_047 |
| 19. | Chen, D.; Xing, G.; Yao, J.; Zhou, H. Org. Chem. Front. 2017, 4, 1042–1045. doi:10.1039/C6QO00675B |
| 20. | Chen, D.; Xing, G.; Yao, J.; Zhou, H. RSC Adv. 2016, 6, 103320–103323. doi:10.1039/C6RA21889J |
| 21. | Chen, D.; Xing, G.; Zhou, H. Org. Chem. Front. 2015, 2, 947–950. doi:10.1039/C5QO00089K |
| 22. | Chen, D.; Xing, G.; Chen, X.; Yao, J.; Zhou, H. Tetrahedron Lett. 2016, 57, 5124–5126. doi:10.1016/j.tetlet.2016.10.025 |
| 23. | Liu, L.; Wang, J.; Zhou, H. J. Org. Chem. 2015, 80, 4749–4753. doi:10.1021/acs.joc.5b00261 |
| 24. | Liu, L.; Chen, D.; Zhou, H. Adv. Synth. Catal. 2015, 357, 389–394. doi:10.1002/adsc.201400713 |
| 25. | Zhao, G.; Zhang, Q.; Zhou, H. J. Org. Chem. 2014, 79, 10867–10872. doi:10.1021/jo501867h |
| 26. | Zhao, G.; Zhang, Q.; Zhou, H. Adv. Synth. Catal. 2013, 355, 3492–3496. doi:10.1002/adsc.201300573 |
| 27. | Zhou, H.; Liu, L.; Xu, S. J. Org. Chem. 2012, 77, 9418–9421. doi:10.1021/jo3018186 |
| 20. | Chen, D.; Xing, G.; Yao, J.; Zhou, H. RSC Adv. 2016, 6, 103320–103323. doi:10.1039/C6RA21889J |
| 21. | Chen, D.; Xing, G.; Zhou, H. Org. Chem. Front. 2015, 2, 947–950. doi:10.1039/C5QO00089K |
| 22. | Chen, D.; Xing, G.; Chen, X.; Yao, J.; Zhou, H. Tetrahedron Lett. 2016, 57, 5124–5126. doi:10.1016/j.tetlet.2016.10.025 |
© 2017 Yao et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)